User login
Antibody shows early promise in AML/MDS trial
MADRID—Interim results of a phase 1 study suggest flotetuzumab, a CD123 and CD3 bispecific antibody, may be a feasible treatment option for relapsed or refractory acute myeloid leukemia (AML) or intermediate/high-risk myelodysplastic syndromes (MDS).
Researchers said flotetuzumab demonstrated acceptable tolerability in the dose-escalation portion of the study, with infusion-related reactions (IRRs) and cytokine release syndrome (CRS) being the most common adverse events (AEs).
In addition, flotetuzumab exhibited anti-leukemic activity in 8 of 14 response-evaluable patients, with 6 patients achieving a response.
Norbert Vey, MD, of Institut Paoli-Calmettes in Marseille, France, presented these results at the ESMO 2017 Congress (abstract 995O*). The study is sponsored by MacroGenics, Inc., the company developing flotetuzumab.
Flotetuzumab (MGD006) recognizes CD123 and CD3. The primary mechanism of flotetuzumab is thought to be its ability to redirect T cells to kill CD123-expressing cells. To achieve this, the molecule combines a portion of an antibody recognizing CD3 (an activating molecule expressed by T cells) with an arm that recognizes CD123 on the target cancer cells.
In this ongoing phase 1 study of flotetuzumab, researchers have enrolled 47 patients with a median age of 64 (range, 29-84). About 89% of these patients had AML (n=42), and the rest (n=5) had MDS.
Twenty-four percent had relapsed AML (n=10), 55% had refractory AML (n=23), and 21% had failed treatment with hypomethylating agents (n=9). One patient had intermediate-1-risk MDS, 2 had intermediate-2-risk, and 2 had high-risk MDS.
Treatment
The study began with single patients receiving flotetuzumab at escalating doses—3 ng/kg/day, 10 ng/kg/day, 30 ng/kg/day, and 100 ng/kg/day.
Then, patients received a range of doses on 2 different schedules for cycle 1. One group received treatment 7 days a week. The other had a 4-days-on/3-days-off schedule.
All patients received a lead-in dose during the first week of cycle 1. They received 30 ng/kg/day for 3 days, then 100 ng/kg/day for 4 days.
For the rest of cycle 1, patients in the 4 days/3 days group received doses of 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg. Patients in the daily dosing group received doses of 300 ng/kg, 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg.
For cycle 2 and beyond, all patients were on the 4-days-on/3-days-off schedule.
Safety
The maximum tolerated dose and schedule was 500 ng/kg/day for 7 days.
Dose-limiting toxicities occurring at the 700 ng/kg/day dose included grade 2 IRRs/CRS in 2 patients and grade 3 myalgia in 1 patient. There was 1 drug-related central nervous system AE that led to treatment discontinuation.
IRRs/CRS occurred in 77% of patients, with 13% of patients having grade 3 events and 8.5% of patients discontinuing treatment due to IRRs/CRS.
The researchers said they found ways to decrease the incidence and severity of CRS. One is early intervention with tocilizumab. The other is a 2-step lead-in dose during week 1. So patients first receive 30 ng/kg, then 100 ng/kg, and then their target dose.
Other grade 3 AEs occurring in this trial include febrile neutropenia (11%), anemia (11%), and decreases in platelets (13%), white blood cells (11%), and lymphocytes (13%).
Efficacy
The researchers said they observed encouraging anti-leukemic activity in patients treated at 500 ng/kg/day or greater.
As of the data cut-off, 14 patients treated at this dose were evaluable for response. Eight (57%) patients had anti-leukemic activity, with 6 (43%) of these patients experiencing an objective response.
One patient achieved a complete response (CR), 2 had a CR with incomplete count recovery, and 1 had a molecular CR.
In most responders, anti-leukemic activity was observed after a single cycle of therapy.
MacroGenics is currently enrolling patients in dose-expansion cohorts. The company plans to present updated results from this trial at another scientific conference later this year. ![]()
*Slides from this presentation are available on the MacroGenics website at http://ir.macrogenics.com/events.cfm.
MADRID—Interim results of a phase 1 study suggest flotetuzumab, a CD123 and CD3 bispecific antibody, may be a feasible treatment option for relapsed or refractory acute myeloid leukemia (AML) or intermediate/high-risk myelodysplastic syndromes (MDS).
Researchers said flotetuzumab demonstrated acceptable tolerability in the dose-escalation portion of the study, with infusion-related reactions (IRRs) and cytokine release syndrome (CRS) being the most common adverse events (AEs).
In addition, flotetuzumab exhibited anti-leukemic activity in 8 of 14 response-evaluable patients, with 6 patients achieving a response.
Norbert Vey, MD, of Institut Paoli-Calmettes in Marseille, France, presented these results at the ESMO 2017 Congress (abstract 995O*). The study is sponsored by MacroGenics, Inc., the company developing flotetuzumab.
Flotetuzumab (MGD006) recognizes CD123 and CD3. The primary mechanism of flotetuzumab is thought to be its ability to redirect T cells to kill CD123-expressing cells. To achieve this, the molecule combines a portion of an antibody recognizing CD3 (an activating molecule expressed by T cells) with an arm that recognizes CD123 on the target cancer cells.
In this ongoing phase 1 study of flotetuzumab, researchers have enrolled 47 patients with a median age of 64 (range, 29-84). About 89% of these patients had AML (n=42), and the rest (n=5) had MDS.
Twenty-four percent had relapsed AML (n=10), 55% had refractory AML (n=23), and 21% had failed treatment with hypomethylating agents (n=9). One patient had intermediate-1-risk MDS, 2 had intermediate-2-risk, and 2 had high-risk MDS.
Treatment
The study began with single patients receiving flotetuzumab at escalating doses—3 ng/kg/day, 10 ng/kg/day, 30 ng/kg/day, and 100 ng/kg/day.
Then, patients received a range of doses on 2 different schedules for cycle 1. One group received treatment 7 days a week. The other had a 4-days-on/3-days-off schedule.
All patients received a lead-in dose during the first week of cycle 1. They received 30 ng/kg/day for 3 days, then 100 ng/kg/day for 4 days.
For the rest of cycle 1, patients in the 4 days/3 days group received doses of 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg. Patients in the daily dosing group received doses of 300 ng/kg, 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg.
For cycle 2 and beyond, all patients were on the 4-days-on/3-days-off schedule.
Safety
The maximum tolerated dose and schedule was 500 ng/kg/day for 7 days.
Dose-limiting toxicities occurring at the 700 ng/kg/day dose included grade 2 IRRs/CRS in 2 patients and grade 3 myalgia in 1 patient. There was 1 drug-related central nervous system AE that led to treatment discontinuation.
IRRs/CRS occurred in 77% of patients, with 13% of patients having grade 3 events and 8.5% of patients discontinuing treatment due to IRRs/CRS.
The researchers said they found ways to decrease the incidence and severity of CRS. One is early intervention with tocilizumab. The other is a 2-step lead-in dose during week 1. So patients first receive 30 ng/kg, then 100 ng/kg, and then their target dose.
Other grade 3 AEs occurring in this trial include febrile neutropenia (11%), anemia (11%), and decreases in platelets (13%), white blood cells (11%), and lymphocytes (13%).
Efficacy
The researchers said they observed encouraging anti-leukemic activity in patients treated at 500 ng/kg/day or greater.
As of the data cut-off, 14 patients treated at this dose were evaluable for response. Eight (57%) patients had anti-leukemic activity, with 6 (43%) of these patients experiencing an objective response.
One patient achieved a complete response (CR), 2 had a CR with incomplete count recovery, and 1 had a molecular CR.
In most responders, anti-leukemic activity was observed after a single cycle of therapy.
MacroGenics is currently enrolling patients in dose-expansion cohorts. The company plans to present updated results from this trial at another scientific conference later this year. ![]()
*Slides from this presentation are available on the MacroGenics website at http://ir.macrogenics.com/events.cfm.
MADRID—Interim results of a phase 1 study suggest flotetuzumab, a CD123 and CD3 bispecific antibody, may be a feasible treatment option for relapsed or refractory acute myeloid leukemia (AML) or intermediate/high-risk myelodysplastic syndromes (MDS).
Researchers said flotetuzumab demonstrated acceptable tolerability in the dose-escalation portion of the study, with infusion-related reactions (IRRs) and cytokine release syndrome (CRS) being the most common adverse events (AEs).
In addition, flotetuzumab exhibited anti-leukemic activity in 8 of 14 response-evaluable patients, with 6 patients achieving a response.
Norbert Vey, MD, of Institut Paoli-Calmettes in Marseille, France, presented these results at the ESMO 2017 Congress (abstract 995O*). The study is sponsored by MacroGenics, Inc., the company developing flotetuzumab.
Flotetuzumab (MGD006) recognizes CD123 and CD3. The primary mechanism of flotetuzumab is thought to be its ability to redirect T cells to kill CD123-expressing cells. To achieve this, the molecule combines a portion of an antibody recognizing CD3 (an activating molecule expressed by T cells) with an arm that recognizes CD123 on the target cancer cells.
In this ongoing phase 1 study of flotetuzumab, researchers have enrolled 47 patients with a median age of 64 (range, 29-84). About 89% of these patients had AML (n=42), and the rest (n=5) had MDS.
Twenty-four percent had relapsed AML (n=10), 55% had refractory AML (n=23), and 21% had failed treatment with hypomethylating agents (n=9). One patient had intermediate-1-risk MDS, 2 had intermediate-2-risk, and 2 had high-risk MDS.
Treatment
The study began with single patients receiving flotetuzumab at escalating doses—3 ng/kg/day, 10 ng/kg/day, 30 ng/kg/day, and 100 ng/kg/day.
Then, patients received a range of doses on 2 different schedules for cycle 1. One group received treatment 7 days a week. The other had a 4-days-on/3-days-off schedule.
All patients received a lead-in dose during the first week of cycle 1. They received 30 ng/kg/day for 3 days, then 100 ng/kg/day for 4 days.
For the rest of cycle 1, patients in the 4 days/3 days group received doses of 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg. Patients in the daily dosing group received doses of 300 ng/kg, 500 ng/kg, 700 ng/kg, 900 ng/kg, or 1000 ng/kg.
For cycle 2 and beyond, all patients were on the 4-days-on/3-days-off schedule.
Safety
The maximum tolerated dose and schedule was 500 ng/kg/day for 7 days.
Dose-limiting toxicities occurring at the 700 ng/kg/day dose included grade 2 IRRs/CRS in 2 patients and grade 3 myalgia in 1 patient. There was 1 drug-related central nervous system AE that led to treatment discontinuation.
IRRs/CRS occurred in 77% of patients, with 13% of patients having grade 3 events and 8.5% of patients discontinuing treatment due to IRRs/CRS.
The researchers said they found ways to decrease the incidence and severity of CRS. One is early intervention with tocilizumab. The other is a 2-step lead-in dose during week 1. So patients first receive 30 ng/kg, then 100 ng/kg, and then their target dose.
Other grade 3 AEs occurring in this trial include febrile neutropenia (11%), anemia (11%), and decreases in platelets (13%), white blood cells (11%), and lymphocytes (13%).
Efficacy
The researchers said they observed encouraging anti-leukemic activity in patients treated at 500 ng/kg/day or greater.
As of the data cut-off, 14 patients treated at this dose were evaluable for response. Eight (57%) patients had anti-leukemic activity, with 6 (43%) of these patients experiencing an objective response.
One patient achieved a complete response (CR), 2 had a CR with incomplete count recovery, and 1 had a molecular CR.
In most responders, anti-leukemic activity was observed after a single cycle of therapy.
MacroGenics is currently enrolling patients in dose-expansion cohorts. The company plans to present updated results from this trial at another scientific conference later this year. ![]()
*Slides from this presentation are available on the MacroGenics website at http://ir.macrogenics.com/events.cfm.
New toolkit can help nurses use genomics in patient care
The National Human Genome Research Institute (NHGRI) has created an online toolkit to help nurses integrate genomics into patient care.
The Method for Introducing a New Competency Genomics (MINC) website provides resources for nursing leaders at all levels of genomics competency, ranging from basic knowledge about genomics to its practical impact on healthcare systems and policies.
The resources are intended to help practicing nurses care for patients undergoing genomic testing and treatments, build awareness in their communities, and understand how to prepare their workforce for emerging clinical applications.
“The MINC toolkit is a starting point for healthcare providers who want to promote genomic integration into practice to benefit their patients,” said Laura Lyman Rodriguez, PhD, director of the Division of Policy, Communication and Education at NHGRI.
“It was designed based on the efforts of magnet hospital nurses whose experiences were used in the design and foundation for the toolkit.”
The toolkit is structured in a question and answer format, allowing users to tailor their interventions based on the resources that will work best for them in their clinical setting.
A key feature of the toolkit is “Champion Stories.” These video testimonials from health administrators and educators describe how they overcame barriers as they developed the necessary genomics knowledge to offer personalized care to their patients. ![]()
The National Human Genome Research Institute (NHGRI) has created an online toolkit to help nurses integrate genomics into patient care.
The Method for Introducing a New Competency Genomics (MINC) website provides resources for nursing leaders at all levels of genomics competency, ranging from basic knowledge about genomics to its practical impact on healthcare systems and policies.
The resources are intended to help practicing nurses care for patients undergoing genomic testing and treatments, build awareness in their communities, and understand how to prepare their workforce for emerging clinical applications.
“The MINC toolkit is a starting point for healthcare providers who want to promote genomic integration into practice to benefit their patients,” said Laura Lyman Rodriguez, PhD, director of the Division of Policy, Communication and Education at NHGRI.
“It was designed based on the efforts of magnet hospital nurses whose experiences were used in the design and foundation for the toolkit.”
The toolkit is structured in a question and answer format, allowing users to tailor their interventions based on the resources that will work best for them in their clinical setting.
A key feature of the toolkit is “Champion Stories.” These video testimonials from health administrators and educators describe how they overcame barriers as they developed the necessary genomics knowledge to offer personalized care to their patients. ![]()
The National Human Genome Research Institute (NHGRI) has created an online toolkit to help nurses integrate genomics into patient care.
The Method for Introducing a New Competency Genomics (MINC) website provides resources for nursing leaders at all levels of genomics competency, ranging from basic knowledge about genomics to its practical impact on healthcare systems and policies.
The resources are intended to help practicing nurses care for patients undergoing genomic testing and treatments, build awareness in their communities, and understand how to prepare their workforce for emerging clinical applications.
“The MINC toolkit is a starting point for healthcare providers who want to promote genomic integration into practice to benefit their patients,” said Laura Lyman Rodriguez, PhD, director of the Division of Policy, Communication and Education at NHGRI.
“It was designed based on the efforts of magnet hospital nurses whose experiences were used in the design and foundation for the toolkit.”
The toolkit is structured in a question and answer format, allowing users to tailor their interventions based on the resources that will work best for them in their clinical setting.
A key feature of the toolkit is “Champion Stories.” These video testimonials from health administrators and educators describe how they overcame barriers as they developed the necessary genomics knowledge to offer personalized care to their patients. ![]()
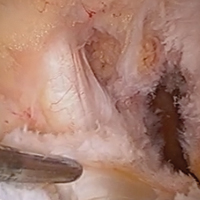
Medial Oblique Meniscomeniscal Ligament
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
FDA approves triple-therapy inhaler for COPD
The Food and Drug Administration has approved Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol), a triple-therapy inhaler for the treatment of chronic obstructive pulmonary disease (COPD) in adult patients, according to a press release from GlaxoSmithKline and Innoviva.
Trelegy Ellipta combines an inhaled corticosteroid, a long-acting muscarinic antagonist, and a long-acting beta2-adrenergic agonist into an inhaler meant for once-daily use in people with COPD. Chronic bronchitis and/or emphysema patients are also indicated for treatment. The FDA-approved dosage is 100 mcg of fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol.
“This approval represents a significant therapeutic convenience for those appropriate patients already on Breo Ellipta, that require additional bronchodilation or for those patients already on a combination of Breo Ellipta and Incruse Ellipta,” Mike Aguiar, CEO of Innoviva said in the press release.
In results supporting the FDA approval, the IMPACT study, a 52-week phase 3 clinical trial including 10,355 COPD patients sponsored by GSK, found that patients receiving Trelegy Ellipta experienced a 25% reduction in moderate to severe exacerbations compared to patients receiving Anoro Ellipta, and a 15% reduction in moderate to severe exacerbations, compared with patients receiving Relvar/Breo Ellipta. Change from baseline FEV1, change from baseline scores on the St George’s Respiratory Questionnaire, and time to first moderate/severe COPD exacerbation also were improved in the Trelegy Ellipta study group compared to the others.
“This is the first study to report a comparison of a single inhaler triple therapy with two dual therapies, providing much needed clinical evidence about the ability of a single inhaler triple therapy to reduce exacerbations,” Patrick Vallance, President of R&D at GSK, noted in a press release announcing the results of the IMPACT study.
The Food and Drug Administration has approved Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol), a triple-therapy inhaler for the treatment of chronic obstructive pulmonary disease (COPD) in adult patients, according to a press release from GlaxoSmithKline and Innoviva.
Trelegy Ellipta combines an inhaled corticosteroid, a long-acting muscarinic antagonist, and a long-acting beta2-adrenergic agonist into an inhaler meant for once-daily use in people with COPD. Chronic bronchitis and/or emphysema patients are also indicated for treatment. The FDA-approved dosage is 100 mcg of fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol.
“This approval represents a significant therapeutic convenience for those appropriate patients already on Breo Ellipta, that require additional bronchodilation or for those patients already on a combination of Breo Ellipta and Incruse Ellipta,” Mike Aguiar, CEO of Innoviva said in the press release.
In results supporting the FDA approval, the IMPACT study, a 52-week phase 3 clinical trial including 10,355 COPD patients sponsored by GSK, found that patients receiving Trelegy Ellipta experienced a 25% reduction in moderate to severe exacerbations compared to patients receiving Anoro Ellipta, and a 15% reduction in moderate to severe exacerbations, compared with patients receiving Relvar/Breo Ellipta. Change from baseline FEV1, change from baseline scores on the St George’s Respiratory Questionnaire, and time to first moderate/severe COPD exacerbation also were improved in the Trelegy Ellipta study group compared to the others.
“This is the first study to report a comparison of a single inhaler triple therapy with two dual therapies, providing much needed clinical evidence about the ability of a single inhaler triple therapy to reduce exacerbations,” Patrick Vallance, President of R&D at GSK, noted in a press release announcing the results of the IMPACT study.
The Food and Drug Administration has approved Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol), a triple-therapy inhaler for the treatment of chronic obstructive pulmonary disease (COPD) in adult patients, according to a press release from GlaxoSmithKline and Innoviva.
Trelegy Ellipta combines an inhaled corticosteroid, a long-acting muscarinic antagonist, and a long-acting beta2-adrenergic agonist into an inhaler meant for once-daily use in people with COPD. Chronic bronchitis and/or emphysema patients are also indicated for treatment. The FDA-approved dosage is 100 mcg of fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol.
“This approval represents a significant therapeutic convenience for those appropriate patients already on Breo Ellipta, that require additional bronchodilation or for those patients already on a combination of Breo Ellipta and Incruse Ellipta,” Mike Aguiar, CEO of Innoviva said in the press release.
In results supporting the FDA approval, the IMPACT study, a 52-week phase 3 clinical trial including 10,355 COPD patients sponsored by GSK, found that patients receiving Trelegy Ellipta experienced a 25% reduction in moderate to severe exacerbations compared to patients receiving Anoro Ellipta, and a 15% reduction in moderate to severe exacerbations, compared with patients receiving Relvar/Breo Ellipta. Change from baseline FEV1, change from baseline scores on the St George’s Respiratory Questionnaire, and time to first moderate/severe COPD exacerbation also were improved in the Trelegy Ellipta study group compared to the others.
“This is the first study to report a comparison of a single inhaler triple therapy with two dual therapies, providing much needed clinical evidence about the ability of a single inhaler triple therapy to reduce exacerbations,” Patrick Vallance, President of R&D at GSK, noted in a press release announcing the results of the IMPACT study.
VIDEO: Educational intervention boosts A fib anticoagulation
BARCELONA – A program promoting broader anticoagulation of patients with atrial fibrillation that used education and feedback from practice audits produced a substantial increase in sustained anticoagulant use and cut strokes in a multinational study with almost 2,300 patients in 48 practices.
Among atrial fibrillation (AF) patients who were not on an anticoagulant at baseline (34% of the enrolled group) 48% of patients in the intervention group began anticoagulant treatment and remained on it for a year with intervention compared with 18% of patients in the control arm without the intervention, Christopher B. Granger, MD, said at the annual congress of the European Society of Cardiology.
The intervention, which highlighted to health care providers the opportunity to start their AF patients on anticoagulant treatment, “transforms how care is provided to this population” of AF patients, Dr. Granger said in a video interview. “Doing something like this can have enormous public health implications.”
IMPACT AF (The Clinical Trial to Improve Treatment With Blood Thinners in Patients With Atrial Fibrillation) randomized 2,281 AF patients in 48 practices in five middle-income countries: Argentina, Brazil, China, India, and Romania. Randomization was by practice, and patients were assigned to either usual care or to an intervention that ran educational sessions for patients and providers on the benefits of and best practices for using anticoagulants. The intervention also monitored anticoagulant use by the patients in each practice and gave providers case-by-case feedback on the care patients received. The educational component customized the feedback to focus on overcoming treatment barriers specific for each patient. This audit and feedback process was a key part of the intervention, Dr. Granger said.
In an adjusted analysis, among patients not on an anticoagulant at baseline, the ones managed in practices that received the intervention had a greater than fourfold likelihood of receiving anticoagulant treatment, compared with patients in practices with no intervention. The intervention was especially successful in transitioning patients off of aspirin treatment, considered ineffective for AF stroke prevention, and onto an anticoagulant, most commonly warfarin.
Overall, anticoagulant use rose by 12 percentage points from baseline among patients in the intervention practices and by 3 percentage points over baseline among the control patients, a statistically significant difference for the study’s primary endpoint.
During 1-year follow-up, 11 strokes occurred among patients managed in practices that received the intervention and 21 in those in control practices, a 52% relative hazard reduction linked with the intervention that was statistically significant, Dr. Granger reported. Concurrently with his talk, the results also appeared online (Lancet. 2017. doi: 10.1016/S0140-6736[17]32165-7).
“How will we take what we have learned [in IMPACT AF] and have it available to people who want to replicate this?” asked Dr. Granger. “We have partnered with several national cardiology societies, and we are working with them to optimize the tools and provide the tools we’ve used,” he said. “We will develop a website for people who want to take this information and use it in their practices.” Dr. Granger and his associates also are working with the Food and Drug Administration and other groups to come up with interventions specially designed for U.S. practice.
IMPACT AF received partial funding from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. Dr. Granger has received honoraria and research funding from all of these companies, and also from Janssen and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
IMPACT AF is an important study, with impressive results that confirm the value of integrated atrial fibrillation care. In the study, a comprehensive and continuous educational intervention with 11 distinct components aimed at health care professionals and patients increased the initiation of and adherence to oral anticoagulation in patients with AF. This effect linked with a significantly reduced incidence of strokes.
Digital tools are an important part of the intervention. They provide both information and feedback, and they create a platform that can involve all stakeholders in management of atrial fibrillation. Informing AF patients about their treatment can result in patients who take responsibility for their management. Integrated AF management models can improve continued delivery of chronic care.
Paulus Kirchhof, MD , is a professor and deputy director of the Institute of Cardiovascular Sciences at the University of Birmingham, England. He has received honoraria and research funding from several drug companies. He made these comments as designated discussant for the report.
IMPACT AF is an important study, with impressive results that confirm the value of integrated atrial fibrillation care. In the study, a comprehensive and continuous educational intervention with 11 distinct components aimed at health care professionals and patients increased the initiation of and adherence to oral anticoagulation in patients with AF. This effect linked with a significantly reduced incidence of strokes.
Digital tools are an important part of the intervention. They provide both information and feedback, and they create a platform that can involve all stakeholders in management of atrial fibrillation. Informing AF patients about their treatment can result in patients who take responsibility for their management. Integrated AF management models can improve continued delivery of chronic care.
Paulus Kirchhof, MD , is a professor and deputy director of the Institute of Cardiovascular Sciences at the University of Birmingham, England. He has received honoraria and research funding from several drug companies. He made these comments as designated discussant for the report.
IMPACT AF is an important study, with impressive results that confirm the value of integrated atrial fibrillation care. In the study, a comprehensive and continuous educational intervention with 11 distinct components aimed at health care professionals and patients increased the initiation of and adherence to oral anticoagulation in patients with AF. This effect linked with a significantly reduced incidence of strokes.
Digital tools are an important part of the intervention. They provide both information and feedback, and they create a platform that can involve all stakeholders in management of atrial fibrillation. Informing AF patients about their treatment can result in patients who take responsibility for their management. Integrated AF management models can improve continued delivery of chronic care.
Paulus Kirchhof, MD , is a professor and deputy director of the Institute of Cardiovascular Sciences at the University of Birmingham, England. He has received honoraria and research funding from several drug companies. He made these comments as designated discussant for the report.
BARCELONA – A program promoting broader anticoagulation of patients with atrial fibrillation that used education and feedback from practice audits produced a substantial increase in sustained anticoagulant use and cut strokes in a multinational study with almost 2,300 patients in 48 practices.
Among atrial fibrillation (AF) patients who were not on an anticoagulant at baseline (34% of the enrolled group) 48% of patients in the intervention group began anticoagulant treatment and remained on it for a year with intervention compared with 18% of patients in the control arm without the intervention, Christopher B. Granger, MD, said at the annual congress of the European Society of Cardiology.
The intervention, which highlighted to health care providers the opportunity to start their AF patients on anticoagulant treatment, “transforms how care is provided to this population” of AF patients, Dr. Granger said in a video interview. “Doing something like this can have enormous public health implications.”
IMPACT AF (The Clinical Trial to Improve Treatment With Blood Thinners in Patients With Atrial Fibrillation) randomized 2,281 AF patients in 48 practices in five middle-income countries: Argentina, Brazil, China, India, and Romania. Randomization was by practice, and patients were assigned to either usual care or to an intervention that ran educational sessions for patients and providers on the benefits of and best practices for using anticoagulants. The intervention also monitored anticoagulant use by the patients in each practice and gave providers case-by-case feedback on the care patients received. The educational component customized the feedback to focus on overcoming treatment barriers specific for each patient. This audit and feedback process was a key part of the intervention, Dr. Granger said.
In an adjusted analysis, among patients not on an anticoagulant at baseline, the ones managed in practices that received the intervention had a greater than fourfold likelihood of receiving anticoagulant treatment, compared with patients in practices with no intervention. The intervention was especially successful in transitioning patients off of aspirin treatment, considered ineffective for AF stroke prevention, and onto an anticoagulant, most commonly warfarin.
Overall, anticoagulant use rose by 12 percentage points from baseline among patients in the intervention practices and by 3 percentage points over baseline among the control patients, a statistically significant difference for the study’s primary endpoint.
During 1-year follow-up, 11 strokes occurred among patients managed in practices that received the intervention and 21 in those in control practices, a 52% relative hazard reduction linked with the intervention that was statistically significant, Dr. Granger reported. Concurrently with his talk, the results also appeared online (Lancet. 2017. doi: 10.1016/S0140-6736[17]32165-7).
“How will we take what we have learned [in IMPACT AF] and have it available to people who want to replicate this?” asked Dr. Granger. “We have partnered with several national cardiology societies, and we are working with them to optimize the tools and provide the tools we’ve used,” he said. “We will develop a website for people who want to take this information and use it in their practices.” Dr. Granger and his associates also are working with the Food and Drug Administration and other groups to come up with interventions specially designed for U.S. practice.
IMPACT AF received partial funding from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. Dr. Granger has received honoraria and research funding from all of these companies, and also from Janssen and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BARCELONA – A program promoting broader anticoagulation of patients with atrial fibrillation that used education and feedback from practice audits produced a substantial increase in sustained anticoagulant use and cut strokes in a multinational study with almost 2,300 patients in 48 practices.
Among atrial fibrillation (AF) patients who were not on an anticoagulant at baseline (34% of the enrolled group) 48% of patients in the intervention group began anticoagulant treatment and remained on it for a year with intervention compared with 18% of patients in the control arm without the intervention, Christopher B. Granger, MD, said at the annual congress of the European Society of Cardiology.
The intervention, which highlighted to health care providers the opportunity to start their AF patients on anticoagulant treatment, “transforms how care is provided to this population” of AF patients, Dr. Granger said in a video interview. “Doing something like this can have enormous public health implications.”
IMPACT AF (The Clinical Trial to Improve Treatment With Blood Thinners in Patients With Atrial Fibrillation) randomized 2,281 AF patients in 48 practices in five middle-income countries: Argentina, Brazil, China, India, and Romania. Randomization was by practice, and patients were assigned to either usual care or to an intervention that ran educational sessions for patients and providers on the benefits of and best practices for using anticoagulants. The intervention also monitored anticoagulant use by the patients in each practice and gave providers case-by-case feedback on the care patients received. The educational component customized the feedback to focus on overcoming treatment barriers specific for each patient. This audit and feedback process was a key part of the intervention, Dr. Granger said.
In an adjusted analysis, among patients not on an anticoagulant at baseline, the ones managed in practices that received the intervention had a greater than fourfold likelihood of receiving anticoagulant treatment, compared with patients in practices with no intervention. The intervention was especially successful in transitioning patients off of aspirin treatment, considered ineffective for AF stroke prevention, and onto an anticoagulant, most commonly warfarin.
Overall, anticoagulant use rose by 12 percentage points from baseline among patients in the intervention practices and by 3 percentage points over baseline among the control patients, a statistically significant difference for the study’s primary endpoint.
During 1-year follow-up, 11 strokes occurred among patients managed in practices that received the intervention and 21 in those in control practices, a 52% relative hazard reduction linked with the intervention that was statistically significant, Dr. Granger reported. Concurrently with his talk, the results also appeared online (Lancet. 2017. doi: 10.1016/S0140-6736[17]32165-7).
“How will we take what we have learned [in IMPACT AF] and have it available to people who want to replicate this?” asked Dr. Granger. “We have partnered with several national cardiology societies, and we are working with them to optimize the tools and provide the tools we’ve used,” he said. “We will develop a website for people who want to take this information and use it in their practices.” Dr. Granger and his associates also are working with the Food and Drug Administration and other groups to come up with interventions specially designed for U.S. practice.
IMPACT AF received partial funding from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. Dr. Granger has received honoraria and research funding from all of these companies, and also from Janssen and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Anticoagulation rose by 12 percentage points from baseline with intervention and by 3 percentage points among controls.
Data source: IMPACT AF, which randomized 2,281 AF patients for 1 year at 48 centers in five middle-income countries.
Disclosures: IMPACT AF received partial funding from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. Dr. Granger has received honoraria and research funding from all of these companies, and also from Janssen and Medtronic.
E-cigarettes most popular among youngest adults
Over 15% of adults have used electronic cigarettes at some time, and about 3% reported current use when they were surveyed in 2016, according to the Centers for Disease Control and Prevention.
When those numbers are broken down by age group, the youngest adults are the most likely e-cigarette users: 23.5% of those aged 18-24 years had ever vaped and 4.5% were currently vaping either every day or on some days, the CDC reported (MMWR. 2017;66[33]:892).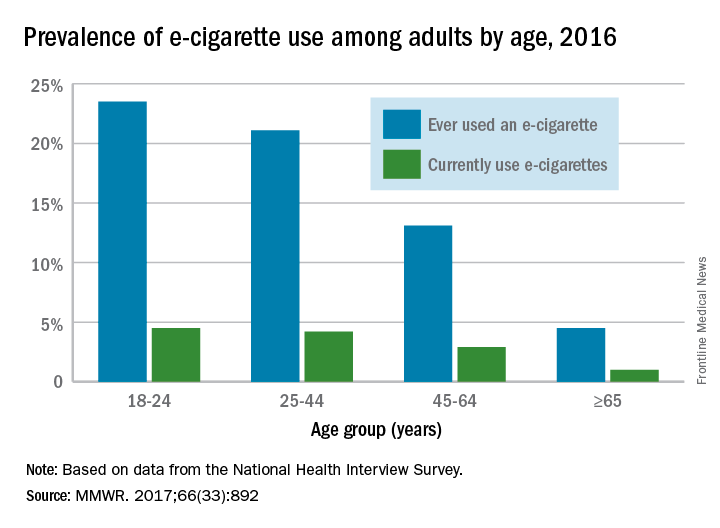
Over 15% of adults have used electronic cigarettes at some time, and about 3% reported current use when they were surveyed in 2016, according to the Centers for Disease Control and Prevention.
When those numbers are broken down by age group, the youngest adults are the most likely e-cigarette users: 23.5% of those aged 18-24 years had ever vaped and 4.5% were currently vaping either every day or on some days, the CDC reported (MMWR. 2017;66[33]:892).
Over 15% of adults have used electronic cigarettes at some time, and about 3% reported current use when they were surveyed in 2016, according to the Centers for Disease Control and Prevention.
When those numbers are broken down by age group, the youngest adults are the most likely e-cigarette users: 23.5% of those aged 18-24 years had ever vaped and 4.5% were currently vaping either every day or on some days, the CDC reported (MMWR. 2017;66[33]:892).
FROM MMWR
Wound expert: Consider hyperbaric oxygen therapy for diabetic foot ulcers
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
EXPERT ANALYSIS AT THE ADA ANNUAL SCIENTIFIC SESSIONS
MONARCH 3: Abemaciclib plus AI boosts PFS in HR+/HER2- breast cancer
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
Madrid – A combination of the investigational cyclin-dependent kinase 4/6 (CDK4/6) agent abemaciclib and a nonsteroidal aromatase inhibitor (AI) was associated with a near doubling of progression-free survival in postmenopausal women with previously untreated hormone-receptor positive, human epidermal growth factor receptor 2–negative (HR+/HER2-) advanced breast cancer.
At a planned 18-month interim analysis of the MONARCH 3 trial, the median investigator-assessed progression free survival (PFS), the primary endpoint, had not been reached for 328 patients assigned to receive abemaciclib with either anastrozole (Arimidex) or letrozole (Femara). In contrast, the median PFS for 165 patients assigned to an AI and a placebo was 14.7 months, translating into a hazard ratio (HR) of 0.543 (P = .000021), reported Angelo Di Leo, MD, of Hospital of Prato, Istituto Toscano Tumori, Prato, Italy.
“Abemaciclib in combination with a nonsteroidal aromatase inhibitor is superior to a nonsteroidal aromatase inhibitor alone in terms of progression-free survival, but also in terms of the objective response rate as the initial treatment of HER2-negative, endocrine sensitive advanced breast cancer,” he said at a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
The efficacy of abemaciclib was consistently seen across all subgroups.
“However, we have observed that the patients deriving the largest benefit from abemaciclib are those who have adverse prognostic factors such as, for instance, the presence of liver metastases, or the fact the disease has relapsed only after a few years from the end of adjuvant endocrine therapy,” he added.
The study was stopped for efficacy at the interim analysis.
Abemaciclib has previously been shown to be active as a monotherapy in treatment-refractory HR+/HER2- breast cancer, and in combination with fulvestrant (Faslodex) in patients who had disease progression on endocrine therapy.
Dr. Di Leo and his colleagues enrolled 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer who had not received systemic therapy in this setting. Patients who had prior neoadjuvant or adjuvant endocrine therapy were allowed if they had a disease-free interval of more than 1 year since completing endocrine therapy, The patients also had to have good performance status (Eastern Cooperative Oncology Group PS score 1 or less).
They were randomly assigned on a 2:1 basis to receive abemaciclib 150 mg b.i.d. on a continuous schedule plus either anastrozole 1 mg or letrozole 2.5 mg daily until disease progression, or to placebo plus either of the two AIs.
In addition to the superior PFS with abemaciclib added to an AI, as noted before, the CDK4/6 inhibitor was associated with a significantly better objective response rate (ORR), at 48.2% compared with 34.5% for placebo (P = .002). Among patients with measurable disease at baseline, the respective ORRs were 59.2% and 43.8% (P = .004). The clinical benefit rate in this subgroup was also better with abemaciclib, at 79.3% vs. 69.2% (P = .024).
In exploratory subgroup analyses, the investigators found that patients who had indicators of poor prognosis seemed to derive “substantial” benefit from the addition of abemaciclib. However, in an exploratory analysis in patients with disease only in bone, the investigators found that adding abemaciclib did not appear to improve PFS, suggesting that this subgroup could be treated effectively with endocrine therapy alone. Dr. Di Leo cautioned against overinterpreting this finding however, as only 109 patients had bone-only disease.
The safety analysis showed that patients were able to tolerate the combination fairly well. The incidence of grade 3 or 4 neutropenia was 21.1% with the combination compared with 1.2% with placebo, and grade 3 diarrhea occurred in 9.5% vs. 1.2% (no grade 4 diarrhea in either arm). The diarrhea tended to occur early in therapy and could be managed with dose adjustments and antidiarrheal medications, Dr. Di Leo said.
“What we would like to ask is, is this a practice-changing study? Do the results change standard first-line endocrine-based therapy, and then do these results change who we give endocrine therapy to?,” said invited discussant Nicholas Turner, PhD, of The Royal Marsden Hospital in London.
“The study stopped at the reported interim analysis, so at the moment the abemaciclib arm hasn’t reached the median PFS, but we can anticipate that with further follow-up we will see approximately a year improvement in median PFS by the addition of abemaciclib, which is really a substantial improvement in PFS for these patients. And importantly, this benefit was confirmed by a blinded independent central review of the investigator PFS,” he said.
Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
AT ESMO 2017
Key clinical point: Adding the CDK4/6 inhibitor abemaciclib to an aromatase inhibitor significantly improved progression-free survival in the frontline for postmenopausal women with HR+/HER2- breast cancer.
Major finding: Median PFS was not reached with abemaciclib and letrozole or anastrozole, vs. 14.7 months for a placebo plus aromatase inhibitor.
Data source: Randomized phase 3 trial of 493 postmenopausal women with metastatic or locally recurrent HR+/HER2- breast cancer.
Disclosures: Eli Lilly funded MONARCH 3. Dr. Di Leo and Dr. Turner reported receiving honoraria from the company.
Alopecia patients share their struggles
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
AT AN FDA PUBLIC MEETING
Calcitonin-to-CEA ratio predicts medullary thyroid cancer survival
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT WCTC 2017
Key clinical point:
Major finding: Presurgery, a calcitonin-to-CEA ratio below 18 was linked with superior survival in patients whose CEA was at least 25 ng/Ml.
Data source: A single-center, retrospective study with 164 patients assessed before thyroidectomy and 187 assessed after surgery.
Disclosures: Dr. Jaber had no disclosures.