User login
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Head and Neck Cancer: VA Dietitian Advocates Whole Foods Over Supplements
PHOENIX — Patients with head and neck cancer face high rates of malnutrition during treatment, and oral supplements are often recommended. But they are not the entire answer, a dietician told colleagues at the Association of Veterans Affairs (VA) Hematology/Oncology annual meeting.
“Patients should consume the most liberal diet possible throughout treatment,” said advanced practice oncology dietician Brittany Leneweaver, RD, CSO, CES, at the VA Washington DC Healthcare System. “This means not solely relying on oral nutrition supplements like Ensure if possible.”
While Leneweaver said many patients will need supplements, she stressed these products “are meant to supplement the diet and not be the sole source of nutrition, ideally.” Encouraging the intake of whole foods “is really key to make the transition back to solid foods after they’re done with treatment. This makes it so much easier when they’re already swallowing those thicker textures, rather than just liquid the entire time.”
Malnutrition: Common and Damaging
As Leneweaver noted, malnutrition is common in patients with head and neck cancer, and can lead to “increased treatment toxicity, increased risk of infection, decreased survival, increased surgical complication, delayed healing, decreased physical function, and decreased quality of life.”
Malnutrition data in patients with head and neck cancer in the US is sparse. However, a 2024 study found malnutrition in 20% of patients undergoing head and neck cancer surgery and linked the condition to increased length of stay (β, 5.20 additional days), higher costs (β, $15,722) higher odds of potentially preventable complications (adjusted odds ratio [aOR], 2.04), and lower odds of discharge to home (aOR, 0.34).
Leneweaver said her role involves addressing “nutrition impact symptoms” that reduce veteran food intake such as difficulty swallowing, taste disorders, dry mouth, and inflammation of the mucus membranes.
“I can’t tell you how much time I spend just talking to the patient about their medication regimens, making sure they have antiemetics on board, letting the radiation oncologist know, ‘Hey, it’s probably time for medicine,’” she said. “We’re constantly looking at side effects and addressing to alert the team as quickly as possible so that we can prevent further weight loss.”
Better Diets Lead to Better Outcomes
Leneweaver noted that “many times, patients will continue to rely on oral supplements as their primary source of nutrition over the long term. They may be missing out on several health benefits as a result.”
Research shows that high-quality diets matter in this patient group, she said. They’re associated with “decreased symptoms during treatment, reduced head and neck cancer risk, and reduced risk of those chronic nutrition impact symptoms,” Leneweaver said.
Diets before and after cancer diagnosis can make a difference. A 2019 study examined patient diets prior to diagnosis of head and neck cancer. It found that patients with better diet quality were less likely to experience overall nutrition impact symptoms (OR 0.45). However, “studies have found that the majority of our patients with head and neck cancer have an inadequate diet prior to diagnosis,” Leneweaver said.
As for postdiagnosis nutrition, a 2022 study linked healthier diets in patients with head and neck cancer to 93% lower 3-year risk of all-cause mortality and 85% lower risk of cancer-specific mortality.
What’s in a High-Quality Diet?
Regarding specific food recommendations, Leneweaver prefers the American Institute for Cancer Research (AICR) nutrition guidelines over the US Department of Agriculture’s Dietary Guidelines for Americans. The AICR “more clearly recommends plant-based diet with at least two-thirds of each meal coming from a variety of plant sources” and recommends avoiding alcohol entirely and limiting red meat, she said.
Leneweaver said she recognizes that dietary change can be gradual.
“It’s not going to happen overnight,” she said. “We know that lifestyle change takes a lot of work.”
Basic interventions can be effective, she said: “This can be just as simple as recommending a plant-based diet to your patient or recommending they eat the rainbow. And I don’t mean Skittles, I mean actual plants. If you just mention these couple of things to the patients, this can really go a long way, especially if they’re hearing that consistent messaging.”
Team-Based Follow-Up Is Key
Leneweaver emphasized the importance of following up over time even if patients do not initially accept referrals to nutritional services. Dieticians ideally see patients before or during initial treatment and then weekly during radiation therapy. Posttreatment follow-up continues “until they’re nutritionally stable. This can be anywhere from weekly to monthly.”
Leneweaver emphasized collaborating with other team members. For example, she works with a speech pathologist at joint visits, either weekly or monthly, “so that they can get off of that feeding tube or get back to a solid consistency diet, typically before that 3-month PET scan.”
It is also important to understand barriers to healthy eating in the veteran population, including transportation challenges and poor access to healthy food, Leneweaver said.
“Make sure you’re utilizing your social worker, your psychologist, other resources, and food pantries, if you have them.”
Even when the most ideal choices are not available, she said, “if they only have access to canned vegetables, I’d much rather them eat that than have nothing.”
No disclosures for Leneweaver were provided.
PHOENIX — Patients with head and neck cancer face high rates of malnutrition during treatment, and oral supplements are often recommended. But they are not the entire answer, a dietician told colleagues at the Association of Veterans Affairs (VA) Hematology/Oncology annual meeting.
“Patients should consume the most liberal diet possible throughout treatment,” said advanced practice oncology dietician Brittany Leneweaver, RD, CSO, CES, at the VA Washington DC Healthcare System. “This means not solely relying on oral nutrition supplements like Ensure if possible.”
While Leneweaver said many patients will need supplements, she stressed these products “are meant to supplement the diet and not be the sole source of nutrition, ideally.” Encouraging the intake of whole foods “is really key to make the transition back to solid foods after they’re done with treatment. This makes it so much easier when they’re already swallowing those thicker textures, rather than just liquid the entire time.”
Malnutrition: Common and Damaging
As Leneweaver noted, malnutrition is common in patients with head and neck cancer, and can lead to “increased treatment toxicity, increased risk of infection, decreased survival, increased surgical complication, delayed healing, decreased physical function, and decreased quality of life.”
Malnutrition data in patients with head and neck cancer in the US is sparse. However, a 2024 study found malnutrition in 20% of patients undergoing head and neck cancer surgery and linked the condition to increased length of stay (β, 5.20 additional days), higher costs (β, $15,722) higher odds of potentially preventable complications (adjusted odds ratio [aOR], 2.04), and lower odds of discharge to home (aOR, 0.34).
Leneweaver said her role involves addressing “nutrition impact symptoms” that reduce veteran food intake such as difficulty swallowing, taste disorders, dry mouth, and inflammation of the mucus membranes.
“I can’t tell you how much time I spend just talking to the patient about their medication regimens, making sure they have antiemetics on board, letting the radiation oncologist know, ‘Hey, it’s probably time for medicine,’” she said. “We’re constantly looking at side effects and addressing to alert the team as quickly as possible so that we can prevent further weight loss.”
Better Diets Lead to Better Outcomes
Leneweaver noted that “many times, patients will continue to rely on oral supplements as their primary source of nutrition over the long term. They may be missing out on several health benefits as a result.”
Research shows that high-quality diets matter in this patient group, she said. They’re associated with “decreased symptoms during treatment, reduced head and neck cancer risk, and reduced risk of those chronic nutrition impact symptoms,” Leneweaver said.
Diets before and after cancer diagnosis can make a difference. A 2019 study examined patient diets prior to diagnosis of head and neck cancer. It found that patients with better diet quality were less likely to experience overall nutrition impact symptoms (OR 0.45). However, “studies have found that the majority of our patients with head and neck cancer have an inadequate diet prior to diagnosis,” Leneweaver said.
As for postdiagnosis nutrition, a 2022 study linked healthier diets in patients with head and neck cancer to 93% lower 3-year risk of all-cause mortality and 85% lower risk of cancer-specific mortality.
What’s in a High-Quality Diet?
Regarding specific food recommendations, Leneweaver prefers the American Institute for Cancer Research (AICR) nutrition guidelines over the US Department of Agriculture’s Dietary Guidelines for Americans. The AICR “more clearly recommends plant-based diet with at least two-thirds of each meal coming from a variety of plant sources” and recommends avoiding alcohol entirely and limiting red meat, she said.
Leneweaver said she recognizes that dietary change can be gradual.
“It’s not going to happen overnight,” she said. “We know that lifestyle change takes a lot of work.”
Basic interventions can be effective, she said: “This can be just as simple as recommending a plant-based diet to your patient or recommending they eat the rainbow. And I don’t mean Skittles, I mean actual plants. If you just mention these couple of things to the patients, this can really go a long way, especially if they’re hearing that consistent messaging.”
Team-Based Follow-Up Is Key
Leneweaver emphasized the importance of following up over time even if patients do not initially accept referrals to nutritional services. Dieticians ideally see patients before or during initial treatment and then weekly during radiation therapy. Posttreatment follow-up continues “until they’re nutritionally stable. This can be anywhere from weekly to monthly.”
Leneweaver emphasized collaborating with other team members. For example, she works with a speech pathologist at joint visits, either weekly or monthly, “so that they can get off of that feeding tube or get back to a solid consistency diet, typically before that 3-month PET scan.”
It is also important to understand barriers to healthy eating in the veteran population, including transportation challenges and poor access to healthy food, Leneweaver said.
“Make sure you’re utilizing your social worker, your psychologist, other resources, and food pantries, if you have them.”
Even when the most ideal choices are not available, she said, “if they only have access to canned vegetables, I’d much rather them eat that than have nothing.”
No disclosures for Leneweaver were provided.
PHOENIX — Patients with head and neck cancer face high rates of malnutrition during treatment, and oral supplements are often recommended. But they are not the entire answer, a dietician told colleagues at the Association of Veterans Affairs (VA) Hematology/Oncology annual meeting.
“Patients should consume the most liberal diet possible throughout treatment,” said advanced practice oncology dietician Brittany Leneweaver, RD, CSO, CES, at the VA Washington DC Healthcare System. “This means not solely relying on oral nutrition supplements like Ensure if possible.”
While Leneweaver said many patients will need supplements, she stressed these products “are meant to supplement the diet and not be the sole source of nutrition, ideally.” Encouraging the intake of whole foods “is really key to make the transition back to solid foods after they’re done with treatment. This makes it so much easier when they’re already swallowing those thicker textures, rather than just liquid the entire time.”
Malnutrition: Common and Damaging
As Leneweaver noted, malnutrition is common in patients with head and neck cancer, and can lead to “increased treatment toxicity, increased risk of infection, decreased survival, increased surgical complication, delayed healing, decreased physical function, and decreased quality of life.”
Malnutrition data in patients with head and neck cancer in the US is sparse. However, a 2024 study found malnutrition in 20% of patients undergoing head and neck cancer surgery and linked the condition to increased length of stay (β, 5.20 additional days), higher costs (β, $15,722) higher odds of potentially preventable complications (adjusted odds ratio [aOR], 2.04), and lower odds of discharge to home (aOR, 0.34).
Leneweaver said her role involves addressing “nutrition impact symptoms” that reduce veteran food intake such as difficulty swallowing, taste disorders, dry mouth, and inflammation of the mucus membranes.
“I can’t tell you how much time I spend just talking to the patient about their medication regimens, making sure they have antiemetics on board, letting the radiation oncologist know, ‘Hey, it’s probably time for medicine,’” she said. “We’re constantly looking at side effects and addressing to alert the team as quickly as possible so that we can prevent further weight loss.”
Better Diets Lead to Better Outcomes
Leneweaver noted that “many times, patients will continue to rely on oral supplements as their primary source of nutrition over the long term. They may be missing out on several health benefits as a result.”
Research shows that high-quality diets matter in this patient group, she said. They’re associated with “decreased symptoms during treatment, reduced head and neck cancer risk, and reduced risk of those chronic nutrition impact symptoms,” Leneweaver said.
Diets before and after cancer diagnosis can make a difference. A 2019 study examined patient diets prior to diagnosis of head and neck cancer. It found that patients with better diet quality were less likely to experience overall nutrition impact symptoms (OR 0.45). However, “studies have found that the majority of our patients with head and neck cancer have an inadequate diet prior to diagnosis,” Leneweaver said.
As for postdiagnosis nutrition, a 2022 study linked healthier diets in patients with head and neck cancer to 93% lower 3-year risk of all-cause mortality and 85% lower risk of cancer-specific mortality.
What’s in a High-Quality Diet?
Regarding specific food recommendations, Leneweaver prefers the American Institute for Cancer Research (AICR) nutrition guidelines over the US Department of Agriculture’s Dietary Guidelines for Americans. The AICR “more clearly recommends plant-based diet with at least two-thirds of each meal coming from a variety of plant sources” and recommends avoiding alcohol entirely and limiting red meat, she said.
Leneweaver said she recognizes that dietary change can be gradual.
“It’s not going to happen overnight,” she said. “We know that lifestyle change takes a lot of work.”
Basic interventions can be effective, she said: “This can be just as simple as recommending a plant-based diet to your patient or recommending they eat the rainbow. And I don’t mean Skittles, I mean actual plants. If you just mention these couple of things to the patients, this can really go a long way, especially if they’re hearing that consistent messaging.”
Team-Based Follow-Up Is Key
Leneweaver emphasized the importance of following up over time even if patients do not initially accept referrals to nutritional services. Dieticians ideally see patients before or during initial treatment and then weekly during radiation therapy. Posttreatment follow-up continues “until they’re nutritionally stable. This can be anywhere from weekly to monthly.”
Leneweaver emphasized collaborating with other team members. For example, she works with a speech pathologist at joint visits, either weekly or monthly, “so that they can get off of that feeding tube or get back to a solid consistency diet, typically before that 3-month PET scan.”
It is also important to understand barriers to healthy eating in the veteran population, including transportation challenges and poor access to healthy food, Leneweaver said.
“Make sure you’re utilizing your social worker, your psychologist, other resources, and food pantries, if you have them.”
Even when the most ideal choices are not available, she said, “if they only have access to canned vegetables, I’d much rather them eat that than have nothing.”
No disclosures for Leneweaver were provided.
Epidemiology and Survival of Parotid Gland Malignancies With Brain Metastases: A Population- Based Study
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Analysis of the Frequency of level 1 OncoKB Genomic Alterations in Veterans With Various Solid Organ Malignancies
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
GATA3-Positive Metastatic Esophageal Adenocarcinoma: A Rare Diagnostic Challenge
Background
GATA3 is a zinc finger transcription factor most commonly used as an immunohistochemical marker in breast and urothelial carcinomas, though it has also been detected—albeit infrequently—in other malignancies. While GATA3 expression is well established in esophageal squamous cell carcinoma, it is rarely documented in esophageal adenocarcinoma. We present a unique case of widely metastatic, strongly GATA3-positive esophageal adenocarcinoma.
Case Presentation
A 59-year-old male smoker with gastroesophageal reflux disease and a history of alcohol use disorder presented with progressive right hip pain following a fall. Imaging revealed multiple lytic bone lesions and a soft tissue mass near the right acetabulum. PET-CT demonstrated widespread osseous metastases and FDG-avid uptake in the distal esophagus and perigastric lymph nodes. Laboratory findings included a PSA of 14. Biopsy of the right pubic ramus revealed adenocarcinoma positive for GATA3, CK7, CK20 (patchy), CK40, and P63, and negative for PSA. Given the unexpected GATA3 positivity, the differential diagnosis included primary urothelial cancer. However, cystoscopy and urine cytology were unremarkable. EGD revealed Barrett’s esophagus without a discrete mass. Biopsy of the distal esophagus confirmed poorly differentiated adenocarcinoma with underlying dysplasia. Molecular profiling showed HER2-negative, microsatellite stable (MSS) disease with PD-L1 expression of 10%. Due to an ECOG performance status ≥3 and extensive metastatic burden, the patient was not a candidate for systemic therapy and was transitioned to hospice care.
Discussion
A large tissue microarray study of over 16,000 tumors found weak GATA3 expression in only 2.4% of esophageal adenocarcinomas, with strong or diffuse positivity virtually undocumented. In this case, the unusual immunoprofile initially raised concern for a primary urothelial tumor, but endoscopic biopsy confirmed an esophageal origin. Aberrant GATA3 expression in poorly differentiated gastrointestinal tumors may complicate diagnosis and has been associated with worse prognosis. Awareness of such atypical patterns is critical for accurate tumor classification and appropriate management in metastatic disease.
Conclusions
This case highlights a rare presentation of GATA3-positive esophageal adenocarcinoma. Further reporting may improve diagnostic accuracy and inform future therapeutic strategies for this unique subset of tumors.
Background
GATA3 is a zinc finger transcription factor most commonly used as an immunohistochemical marker in breast and urothelial carcinomas, though it has also been detected—albeit infrequently—in other malignancies. While GATA3 expression is well established in esophageal squamous cell carcinoma, it is rarely documented in esophageal adenocarcinoma. We present a unique case of widely metastatic, strongly GATA3-positive esophageal adenocarcinoma.
Case Presentation
A 59-year-old male smoker with gastroesophageal reflux disease and a history of alcohol use disorder presented with progressive right hip pain following a fall. Imaging revealed multiple lytic bone lesions and a soft tissue mass near the right acetabulum. PET-CT demonstrated widespread osseous metastases and FDG-avid uptake in the distal esophagus and perigastric lymph nodes. Laboratory findings included a PSA of 14. Biopsy of the right pubic ramus revealed adenocarcinoma positive for GATA3, CK7, CK20 (patchy), CK40, and P63, and negative for PSA. Given the unexpected GATA3 positivity, the differential diagnosis included primary urothelial cancer. However, cystoscopy and urine cytology were unremarkable. EGD revealed Barrett’s esophagus without a discrete mass. Biopsy of the distal esophagus confirmed poorly differentiated adenocarcinoma with underlying dysplasia. Molecular profiling showed HER2-negative, microsatellite stable (MSS) disease with PD-L1 expression of 10%. Due to an ECOG performance status ≥3 and extensive metastatic burden, the patient was not a candidate for systemic therapy and was transitioned to hospice care.
Discussion
A large tissue microarray study of over 16,000 tumors found weak GATA3 expression in only 2.4% of esophageal adenocarcinomas, with strong or diffuse positivity virtually undocumented. In this case, the unusual immunoprofile initially raised concern for a primary urothelial tumor, but endoscopic biopsy confirmed an esophageal origin. Aberrant GATA3 expression in poorly differentiated gastrointestinal tumors may complicate diagnosis and has been associated with worse prognosis. Awareness of such atypical patterns is critical for accurate tumor classification and appropriate management in metastatic disease.
Conclusions
This case highlights a rare presentation of GATA3-positive esophageal adenocarcinoma. Further reporting may improve diagnostic accuracy and inform future therapeutic strategies for this unique subset of tumors.
Background
GATA3 is a zinc finger transcription factor most commonly used as an immunohistochemical marker in breast and urothelial carcinomas, though it has also been detected—albeit infrequently—in other malignancies. While GATA3 expression is well established in esophageal squamous cell carcinoma, it is rarely documented in esophageal adenocarcinoma. We present a unique case of widely metastatic, strongly GATA3-positive esophageal adenocarcinoma.
Case Presentation
A 59-year-old male smoker with gastroesophageal reflux disease and a history of alcohol use disorder presented with progressive right hip pain following a fall. Imaging revealed multiple lytic bone lesions and a soft tissue mass near the right acetabulum. PET-CT demonstrated widespread osseous metastases and FDG-avid uptake in the distal esophagus and perigastric lymph nodes. Laboratory findings included a PSA of 14. Biopsy of the right pubic ramus revealed adenocarcinoma positive for GATA3, CK7, CK20 (patchy), CK40, and P63, and negative for PSA. Given the unexpected GATA3 positivity, the differential diagnosis included primary urothelial cancer. However, cystoscopy and urine cytology were unremarkable. EGD revealed Barrett’s esophagus without a discrete mass. Biopsy of the distal esophagus confirmed poorly differentiated adenocarcinoma with underlying dysplasia. Molecular profiling showed HER2-negative, microsatellite stable (MSS) disease with PD-L1 expression of 10%. Due to an ECOG performance status ≥3 and extensive metastatic burden, the patient was not a candidate for systemic therapy and was transitioned to hospice care.
Discussion
A large tissue microarray study of over 16,000 tumors found weak GATA3 expression in only 2.4% of esophageal adenocarcinomas, with strong or diffuse positivity virtually undocumented. In this case, the unusual immunoprofile initially raised concern for a primary urothelial tumor, but endoscopic biopsy confirmed an esophageal origin. Aberrant GATA3 expression in poorly differentiated gastrointestinal tumors may complicate diagnosis and has been associated with worse prognosis. Awareness of such atypical patterns is critical for accurate tumor classification and appropriate management in metastatic disease.
Conclusions
This case highlights a rare presentation of GATA3-positive esophageal adenocarcinoma. Further reporting may improve diagnostic accuracy and inform future therapeutic strategies for this unique subset of tumors.
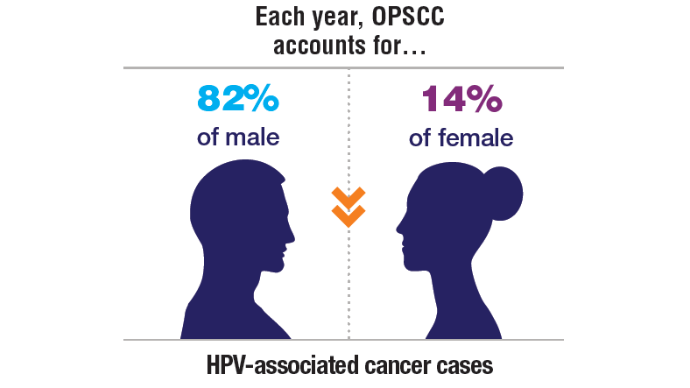
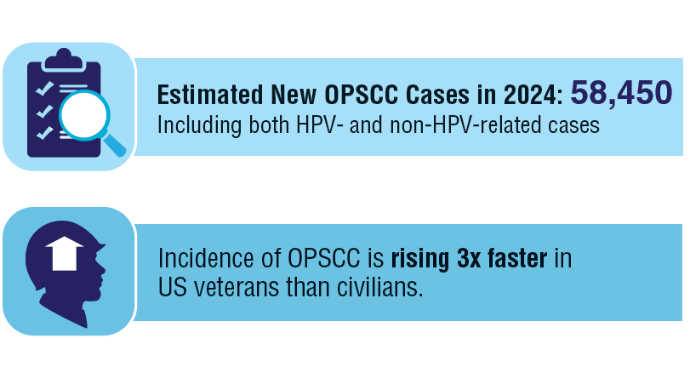
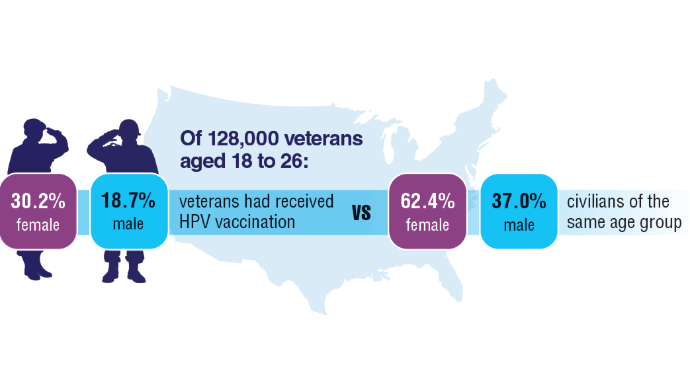
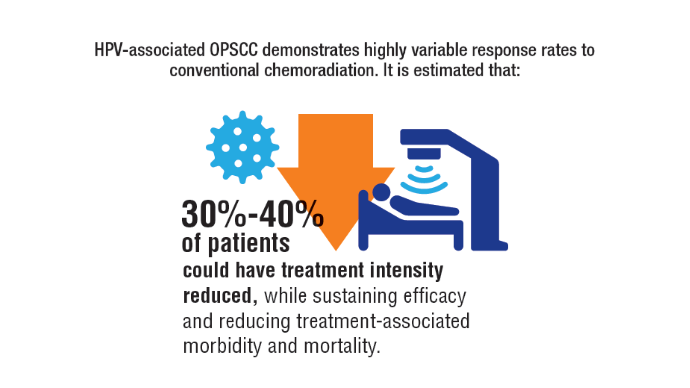
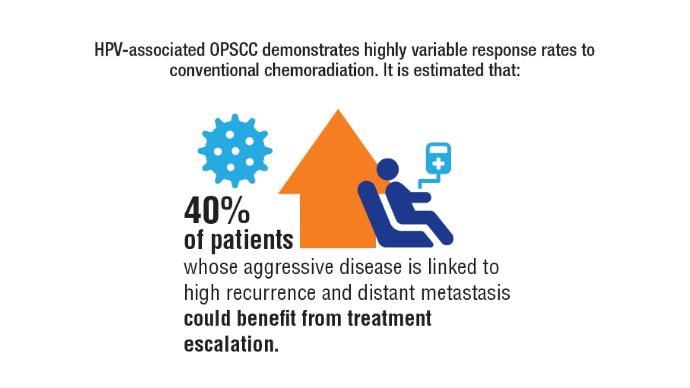
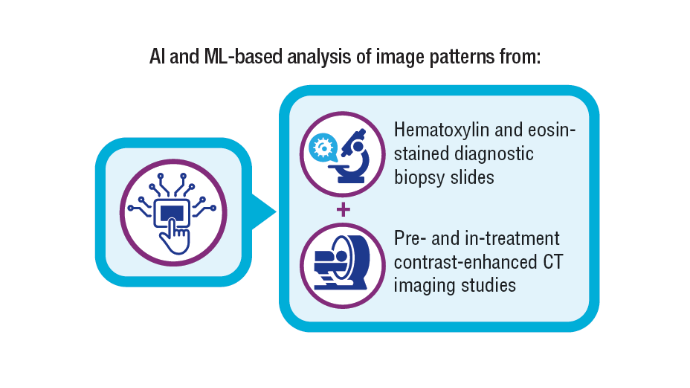
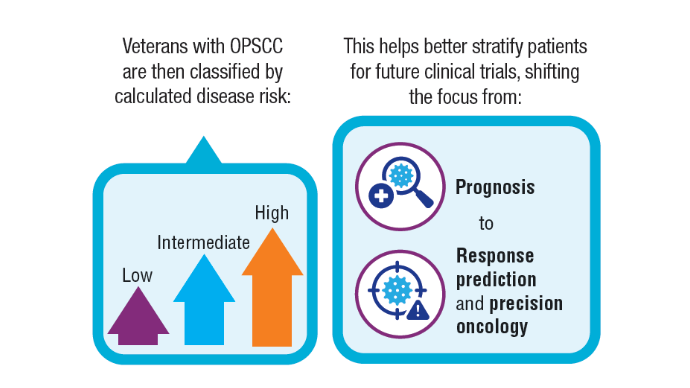
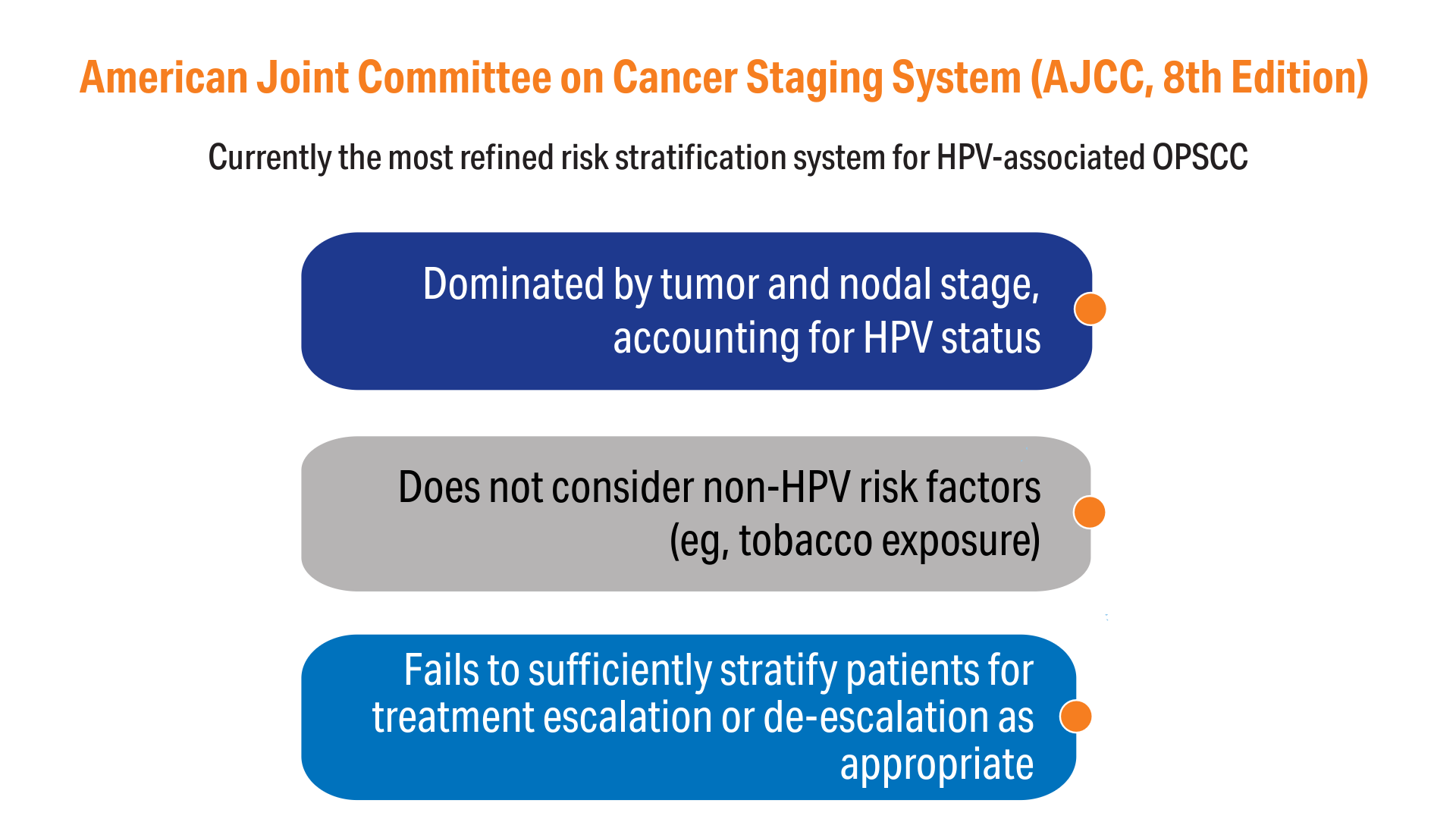
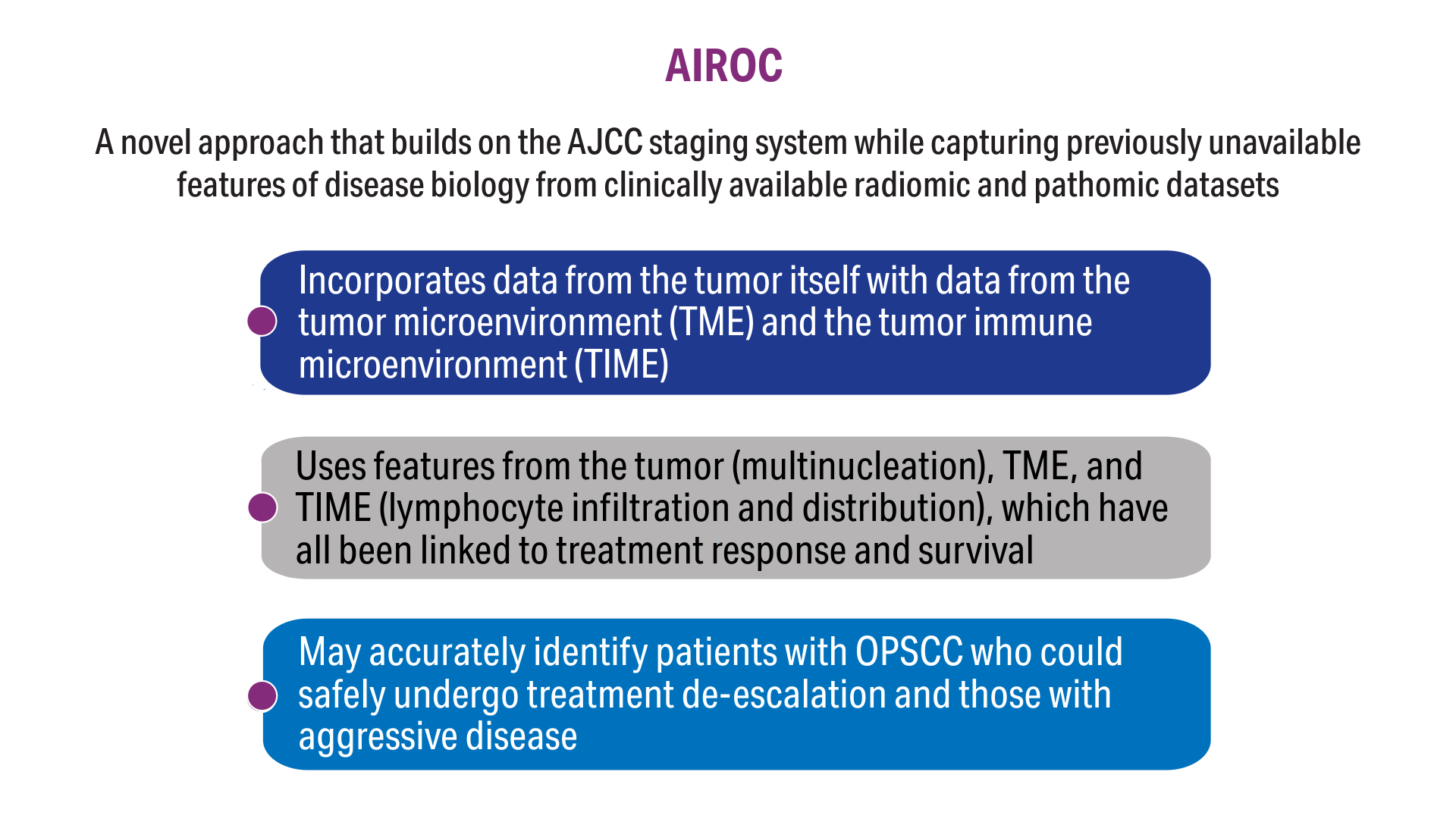
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
Click here to view more from Cancer Data Trends 2025.
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
Can Adjuvant Immunotherapy Boost Survival Outcomes in Advanced Nasopharyngeal Cancer?
TOPLINE:
Adjuvant therapy with camrelizumab significantly improved 3-year event-free survival in patients with locoregionally advanced nasopharyngeal carcinoma compared with observation, according to findings from the phase 3 DIPPER trial.
METHODOLOGY:
- About 20%-30% of patients with locoregionally advanced nasopharyngeal carcinoma experience disease relapse after definitive chemoradiotherapy. Camrelizumab plus chemotherapy can improve progression-free survival in patients with recurrent or metastatic nasopharyngeal carcinoma, but its effectiveness as adjuvant therapy in locoregionally advanced disease remains unclear.
- Researchers conducted the randomized phase 3 DIPPER trial at 11 centers in China, enrolling 450 patients with T4N1M0 or T1-4N2-3M0 nasopharyngeal carcinoma who had completed induction-concurrent chemoradiotherapy.
- Participants were randomly assigned to receive either adjuvant camrelizumab (200 mg intravenously every 3 weeks for 12 cycles; n = 226) or observation (n = 224). The median follow-up duration was 39 months.
- The primary endpoint was event-free survival, defined as freedom from distant metastasis, locoregional relapse, or death due to any cause; secondary endpoints included distant metastasis–free survival, locoregional relapse–free survival, overall survival, and safety.
TAKEAWAY:
- Patients who received camrelizumab had a higher 3-year event-free survival rate than those who underwent observation (86.9% vs 77.3%; stratified hazard ratio [HR], 0.56; P = .01).
- The 3-year distant metastasis–free survival was also higher in the camrelizumab group (92.4% vs 84.5%; stratified HR, 0.54; P = .04).
- Patients in the camrelizumab group had higher locoregional relapse–free survival at 3 years than those in the observation group (92.8% vs 87.0%; stratified HR, 0.53; P = .046). However, the difference in overall survival between the groups was not significant.
- The safety analysis included 426 patients; 97.1% of those who received camrelizumab experienced at least one adverse event of any grade, the most common being reactive capillary endothelial proliferation compared with 85.5% of those in the observation group. Further, 11.2% of patients taking camrelizumab reported grade 3 or 4 events, including leukopenia and neutropenia compared with 3% in the observation group.
IN PRACTICE:
“The DIPPER trial demonstrated that adjuvant camrelizumab following induction-concurrent chemoradiotherapy significantly improved event-free survival by 9.6% with a favorable safety profile in patients with locoregionally advanced [nasopharyngeal carcinoma],” the authors wrote.
“If survival is eventually proven to be improved with induction chemoimmunotherapy, can we begin asking about de-escalation of chemoradiotherapy” for patients with nasopharyngeal carcinoma? “This question is exceptionally important, given the significant long-term consequences of radiotherapy on survivors,” the author of an accompanying editorial wrote.
SOURCE:
The study was led by Ye-Lin Liang, MD, Sun Yat-sen University Cancer Center in Guangzhou, China, and was published online in JAMA.
LIMITATIONS:
The study included patients from an endemic region where nasopharyngeal carcinoma is predominantly linked to Epstein-Barr virus infection, potentially affecting the generalizability of the findings to nonendemic populations. The open-label design may have introduced bias. Additionally, combined positive scores for programmed cell death ligand 1 (PD-L1) were unavailable for some patients, potentially affecting the analysis of the correlation between PD-L1 expression and clinical outcomes.
DISCLOSURES:
The study was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project, National Natural Science Foundation of China, Guangzhou Municipal Health Commission, Key Area Research and Development Program of Guangdong Province, Overseas Expertise Introduction Project for Discipline Innovation, and Cancer Innovative Research Program of Sun Yat-sen University Cancer Center. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adjuvant therapy with camrelizumab significantly improved 3-year event-free survival in patients with locoregionally advanced nasopharyngeal carcinoma compared with observation, according to findings from the phase 3 DIPPER trial.
METHODOLOGY:
- About 20%-30% of patients with locoregionally advanced nasopharyngeal carcinoma experience disease relapse after definitive chemoradiotherapy. Camrelizumab plus chemotherapy can improve progression-free survival in patients with recurrent or metastatic nasopharyngeal carcinoma, but its effectiveness as adjuvant therapy in locoregionally advanced disease remains unclear.
- Researchers conducted the randomized phase 3 DIPPER trial at 11 centers in China, enrolling 450 patients with T4N1M0 or T1-4N2-3M0 nasopharyngeal carcinoma who had completed induction-concurrent chemoradiotherapy.
- Participants were randomly assigned to receive either adjuvant camrelizumab (200 mg intravenously every 3 weeks for 12 cycles; n = 226) or observation (n = 224). The median follow-up duration was 39 months.
- The primary endpoint was event-free survival, defined as freedom from distant metastasis, locoregional relapse, or death due to any cause; secondary endpoints included distant metastasis–free survival, locoregional relapse–free survival, overall survival, and safety.
TAKEAWAY:
- Patients who received camrelizumab had a higher 3-year event-free survival rate than those who underwent observation (86.9% vs 77.3%; stratified hazard ratio [HR], 0.56; P = .01).
- The 3-year distant metastasis–free survival was also higher in the camrelizumab group (92.4% vs 84.5%; stratified HR, 0.54; P = .04).
- Patients in the camrelizumab group had higher locoregional relapse–free survival at 3 years than those in the observation group (92.8% vs 87.0%; stratified HR, 0.53; P = .046). However, the difference in overall survival between the groups was not significant.
- The safety analysis included 426 patients; 97.1% of those who received camrelizumab experienced at least one adverse event of any grade, the most common being reactive capillary endothelial proliferation compared with 85.5% of those in the observation group. Further, 11.2% of patients taking camrelizumab reported grade 3 or 4 events, including leukopenia and neutropenia compared with 3% in the observation group.
IN PRACTICE:
“The DIPPER trial demonstrated that adjuvant camrelizumab following induction-concurrent chemoradiotherapy significantly improved event-free survival by 9.6% with a favorable safety profile in patients with locoregionally advanced [nasopharyngeal carcinoma],” the authors wrote.
“If survival is eventually proven to be improved with induction chemoimmunotherapy, can we begin asking about de-escalation of chemoradiotherapy” for patients with nasopharyngeal carcinoma? “This question is exceptionally important, given the significant long-term consequences of radiotherapy on survivors,” the author of an accompanying editorial wrote.
SOURCE:
The study was led by Ye-Lin Liang, MD, Sun Yat-sen University Cancer Center in Guangzhou, China, and was published online in JAMA.
LIMITATIONS:
The study included patients from an endemic region where nasopharyngeal carcinoma is predominantly linked to Epstein-Barr virus infection, potentially affecting the generalizability of the findings to nonendemic populations. The open-label design may have introduced bias. Additionally, combined positive scores for programmed cell death ligand 1 (PD-L1) were unavailable for some patients, potentially affecting the analysis of the correlation between PD-L1 expression and clinical outcomes.
DISCLOSURES:
The study was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project, National Natural Science Foundation of China, Guangzhou Municipal Health Commission, Key Area Research and Development Program of Guangdong Province, Overseas Expertise Introduction Project for Discipline Innovation, and Cancer Innovative Research Program of Sun Yat-sen University Cancer Center. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adjuvant therapy with camrelizumab significantly improved 3-year event-free survival in patients with locoregionally advanced nasopharyngeal carcinoma compared with observation, according to findings from the phase 3 DIPPER trial.
METHODOLOGY:
- About 20%-30% of patients with locoregionally advanced nasopharyngeal carcinoma experience disease relapse after definitive chemoradiotherapy. Camrelizumab plus chemotherapy can improve progression-free survival in patients with recurrent or metastatic nasopharyngeal carcinoma, but its effectiveness as adjuvant therapy in locoregionally advanced disease remains unclear.
- Researchers conducted the randomized phase 3 DIPPER trial at 11 centers in China, enrolling 450 patients with T4N1M0 or T1-4N2-3M0 nasopharyngeal carcinoma who had completed induction-concurrent chemoradiotherapy.
- Participants were randomly assigned to receive either adjuvant camrelizumab (200 mg intravenously every 3 weeks for 12 cycles; n = 226) or observation (n = 224). The median follow-up duration was 39 months.
- The primary endpoint was event-free survival, defined as freedom from distant metastasis, locoregional relapse, or death due to any cause; secondary endpoints included distant metastasis–free survival, locoregional relapse–free survival, overall survival, and safety.
TAKEAWAY:
- Patients who received camrelizumab had a higher 3-year event-free survival rate than those who underwent observation (86.9% vs 77.3%; stratified hazard ratio [HR], 0.56; P = .01).
- The 3-year distant metastasis–free survival was also higher in the camrelizumab group (92.4% vs 84.5%; stratified HR, 0.54; P = .04).
- Patients in the camrelizumab group had higher locoregional relapse–free survival at 3 years than those in the observation group (92.8% vs 87.0%; stratified HR, 0.53; P = .046). However, the difference in overall survival between the groups was not significant.
- The safety analysis included 426 patients; 97.1% of those who received camrelizumab experienced at least one adverse event of any grade, the most common being reactive capillary endothelial proliferation compared with 85.5% of those in the observation group. Further, 11.2% of patients taking camrelizumab reported grade 3 or 4 events, including leukopenia and neutropenia compared with 3% in the observation group.
IN PRACTICE:
“The DIPPER trial demonstrated that adjuvant camrelizumab following induction-concurrent chemoradiotherapy significantly improved event-free survival by 9.6% with a favorable safety profile in patients with locoregionally advanced [nasopharyngeal carcinoma],” the authors wrote.
“If survival is eventually proven to be improved with induction chemoimmunotherapy, can we begin asking about de-escalation of chemoradiotherapy” for patients with nasopharyngeal carcinoma? “This question is exceptionally important, given the significant long-term consequences of radiotherapy on survivors,” the author of an accompanying editorial wrote.
SOURCE:
The study was led by Ye-Lin Liang, MD, Sun Yat-sen University Cancer Center in Guangzhou, China, and was published online in JAMA.
LIMITATIONS:
The study included patients from an endemic region where nasopharyngeal carcinoma is predominantly linked to Epstein-Barr virus infection, potentially affecting the generalizability of the findings to nonendemic populations. The open-label design may have introduced bias. Additionally, combined positive scores for programmed cell death ligand 1 (PD-L1) were unavailable for some patients, potentially affecting the analysis of the correlation between PD-L1 expression and clinical outcomes.
DISCLOSURES:
The study was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project, National Natural Science Foundation of China, Guangzhou Municipal Health Commission, Key Area Research and Development Program of Guangdong Province, Overseas Expertise Introduction Project for Discipline Innovation, and Cancer Innovative Research Program of Sun Yat-sen University Cancer Center. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Postoperative RT Plus Cetuximab Showed Mixed Results in Head and Neck Cancer Trial
TOPLINE:
Adding cetuximab (C) to postoperative radiotherapy (RT) improves disease-free survival (DFS) but not overall survival (OS) in intermediate-risk squamous cell carcinoma of the head and neck. Benefits were specifically observed in human papillomavirus–negative patients, who represented 80.2% of the study participants.
METHODOLOGY:
- Previous research showed that adding high-dose cisplatin to postoperative RT in patients with squamous cell carcinoma of the head and neck (SCCHN) improved outcomes in those with positive margins and nodal extracapsular extension of the tumor.
- The new phase 3 randomized trial, which enrolled patients from November 2009 to March 2018 included 577 individuals with resected SCCHN of the oral cavity, oropharynx, or larynx, specifically.
- Participants were randomly assigned 1:1 to receive either intensity-modulated RT (60-66 Gy) with weekly C (400 mg/m2 loading dose, followed by 250 mg/m2 weekly) or RT alone.
- The primary endpoint was OS in randomly assigned eligible patients, with DFS and toxicity as secondary endpoints.
- Analysis included stratified log-rank test for OS and DFS, while toxicity was compared using Fisher’s exact test.
TAKEAWAY:
- OS was not significantly improved with RT plus C vs RT alone (hazard ratio [HR], 0.81; 95% CI, 0.60-1.08; P = .0747), though 5-year OS rates were 76.5% vs 68.7%.
- DFS showed significant improvement with combined therapy (HR, 0.75; 95% CI, 0.57-0.98; P = .0168), with 5-year rates of 71.7% vs 63.6%.
- Grade 3-4 acute toxicity rates were significantly higher with combination therapy (70.3% vs 39.7%; P < .0001), primarily affecting skin and mucosa, though late toxicity rates were similar (33.2% vs 29.0%; P = .3101).
- The benefit of RT plus C was only observed in those patients who were human papillomavirus–negative, which comprised 80.2% of trial participants.
IN PRACTICE:
RT plus C “significantly improved DFS, but not OS, with no increase in long-term toxicity, compared with RT alone for resected, intermediate-risk SCCHN. RT plus C is an appropriate option for carefully selected patients with HPV-negative disease,” the authors of the study wrote.
SOURCE:
This study was led by Mitchell Machtay, MD, Penn State Health Milton S. Hershey Medical Center in Hershey, Pennsylvania. It was published online in Journal of Clinical Oncology.
LIMITATIONS:
According to the researchers, the OS in the control group was higher than predicted, resulting in the trial being underpowered to detect the initial targeted HR. The authors noted that the better-than-expected survival rates could be attributed to improvements in surgical and radiotherapeutic techniques, including mandatory intensity-modulated RT for all patients. Additionally, the researchers pointed out that patient selection may have influenced outcomes, as only 30% of study participants had more than two risk factors.
DISCLOSURES:
This study was supported by grants from the US National Cancer Institute and Eli Lilly Inc. Machtay received grants from Lilly/ImClone, AstraZeneca, and Merck Sharp & Dohme. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Adding cetuximab (C) to postoperative radiotherapy (RT) improves disease-free survival (DFS) but not overall survival (OS) in intermediate-risk squamous cell carcinoma of the head and neck. Benefits were specifically observed in human papillomavirus–negative patients, who represented 80.2% of the study participants.
METHODOLOGY:
- Previous research showed that adding high-dose cisplatin to postoperative RT in patients with squamous cell carcinoma of the head and neck (SCCHN) improved outcomes in those with positive margins and nodal extracapsular extension of the tumor.
- The new phase 3 randomized trial, which enrolled patients from November 2009 to March 2018 included 577 individuals with resected SCCHN of the oral cavity, oropharynx, or larynx, specifically.
- Participants were randomly assigned 1:1 to receive either intensity-modulated RT (60-66 Gy) with weekly C (400 mg/m2 loading dose, followed by 250 mg/m2 weekly) or RT alone.
- The primary endpoint was OS in randomly assigned eligible patients, with DFS and toxicity as secondary endpoints.
- Analysis included stratified log-rank test for OS and DFS, while toxicity was compared using Fisher’s exact test.
TAKEAWAY:
- OS was not significantly improved with RT plus C vs RT alone (hazard ratio [HR], 0.81; 95% CI, 0.60-1.08; P = .0747), though 5-year OS rates were 76.5% vs 68.7%.
- DFS showed significant improvement with combined therapy (HR, 0.75; 95% CI, 0.57-0.98; P = .0168), with 5-year rates of 71.7% vs 63.6%.
- Grade 3-4 acute toxicity rates were significantly higher with combination therapy (70.3% vs 39.7%; P < .0001), primarily affecting skin and mucosa, though late toxicity rates were similar (33.2% vs 29.0%; P = .3101).
- The benefit of RT plus C was only observed in those patients who were human papillomavirus–negative, which comprised 80.2% of trial participants.
IN PRACTICE:
RT plus C “significantly improved DFS, but not OS, with no increase in long-term toxicity, compared with RT alone for resected, intermediate-risk SCCHN. RT plus C is an appropriate option for carefully selected patients with HPV-negative disease,” the authors of the study wrote.
SOURCE:
This study was led by Mitchell Machtay, MD, Penn State Health Milton S. Hershey Medical Center in Hershey, Pennsylvania. It was published online in Journal of Clinical Oncology.
LIMITATIONS:
According to the researchers, the OS in the control group was higher than predicted, resulting in the trial being underpowered to detect the initial targeted HR. The authors noted that the better-than-expected survival rates could be attributed to improvements in surgical and radiotherapeutic techniques, including mandatory intensity-modulated RT for all patients. Additionally, the researchers pointed out that patient selection may have influenced outcomes, as only 30% of study participants had more than two risk factors.
DISCLOSURES:
This study was supported by grants from the US National Cancer Institute and Eli Lilly Inc. Machtay received grants from Lilly/ImClone, AstraZeneca, and Merck Sharp & Dohme. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Adding cetuximab (C) to postoperative radiotherapy (RT) improves disease-free survival (DFS) but not overall survival (OS) in intermediate-risk squamous cell carcinoma of the head and neck. Benefits were specifically observed in human papillomavirus–negative patients, who represented 80.2% of the study participants.
METHODOLOGY:
- Previous research showed that adding high-dose cisplatin to postoperative RT in patients with squamous cell carcinoma of the head and neck (SCCHN) improved outcomes in those with positive margins and nodal extracapsular extension of the tumor.
- The new phase 3 randomized trial, which enrolled patients from November 2009 to March 2018 included 577 individuals with resected SCCHN of the oral cavity, oropharynx, or larynx, specifically.
- Participants were randomly assigned 1:1 to receive either intensity-modulated RT (60-66 Gy) with weekly C (400 mg/m2 loading dose, followed by 250 mg/m2 weekly) or RT alone.
- The primary endpoint was OS in randomly assigned eligible patients, with DFS and toxicity as secondary endpoints.
- Analysis included stratified log-rank test for OS and DFS, while toxicity was compared using Fisher’s exact test.
TAKEAWAY:
- OS was not significantly improved with RT plus C vs RT alone (hazard ratio [HR], 0.81; 95% CI, 0.60-1.08; P = .0747), though 5-year OS rates were 76.5% vs 68.7%.
- DFS showed significant improvement with combined therapy (HR, 0.75; 95% CI, 0.57-0.98; P = .0168), with 5-year rates of 71.7% vs 63.6%.
- Grade 3-4 acute toxicity rates were significantly higher with combination therapy (70.3% vs 39.7%; P < .0001), primarily affecting skin and mucosa, though late toxicity rates were similar (33.2% vs 29.0%; P = .3101).
- The benefit of RT plus C was only observed in those patients who were human papillomavirus–negative, which comprised 80.2% of trial participants.
IN PRACTICE:
RT plus C “significantly improved DFS, but not OS, with no increase in long-term toxicity, compared with RT alone for resected, intermediate-risk SCCHN. RT plus C is an appropriate option for carefully selected patients with HPV-negative disease,” the authors of the study wrote.
SOURCE:
This study was led by Mitchell Machtay, MD, Penn State Health Milton S. Hershey Medical Center in Hershey, Pennsylvania. It was published online in Journal of Clinical Oncology.
LIMITATIONS:
According to the researchers, the OS in the control group was higher than predicted, resulting in the trial being underpowered to detect the initial targeted HR. The authors noted that the better-than-expected survival rates could be attributed to improvements in surgical and radiotherapeutic techniques, including mandatory intensity-modulated RT for all patients. Additionally, the researchers pointed out that patient selection may have influenced outcomes, as only 30% of study participants had more than two risk factors.
DISCLOSURES:
This study was supported by grants from the US National Cancer Institute and Eli Lilly Inc. Machtay received grants from Lilly/ImClone, AstraZeneca, and Merck Sharp & Dohme. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Thyroid Cancer Detections Due to More Screenings With GLP-1s
New research, with the caveat of a relatively short follow-up, adds to encouraging data showing an overall low risk of the development of thyroid cancer associated with glucagon-like peptide 1 receptor agonist (GLP-1 RA) treatment.
However, a notably increased rate of thyroid cancer detections limited only to the first year after drug initiation — but not later, coupled with an increased rate of thyroid cancer screening during that period, raises the suggestion that a thyroid cancer concern among the millions of patients taking the immensely popular drugs could exacerbate the already problematic over-detection of incidental thyroid cancers.
“Our study confirms the increased likelihood of being diagnosed with what appears to be low-risk thyroid cancer after treatment with GLP-1 RAs — but not because patients treated with GLP-1 RAs are more likely to develop thyroid cancer but rather because they are more likely to be diagnosed with it because we, as clinicians, are more likely to look for it,” senior author Rozalina G. McCoy, MD, associate professor and associate division chief for clinical research in the Division of Endocrinology, Diabetes, and Nutrition, Department of Medicine at the University of Maryland School of Medicine, in Baltimore, told this news organization.
The findings were published in JAMA Otolaryngology-Head & Neck Surgery.
Thyroid cancer concerns surrounding GLP-1 RAs stem from preclinical studies showing a risk for medullary thyroid cancer in rodents, resulting in a boxed warning from the US Food and Drug Administration recommending that those with a family history of the condition avoid the drugs.
More recent studies showing GLP-1 receptors in human papillary thyroid cancer cells has further raised concerns of a risk beyond medullary thyroid cancer, however, recent studies evaluating the risk have been inconclusive.
To further investigate, McCoy and colleagues evaluated claims data on 351,913 adult enrollees in Medicare Advantage and fee-for-service plans in the United States who had type 2 diabetes, a moderate risk for cardiovascular disease, and no history of thyroid cancer.
Of the patients, who were about half women, 41,112 started treatments with GLP-1 RAs; 76,093, started with dipeptidyl peptidase-4 (DPP-4) inhibitors; 43,499, with sodium-glucose cotransporter 2 (SGLT2i) inhibitors; and 191,209 with sulfonylurea therapy between January 2014 and December 2021.
After inverse probability weighting, baseline characteristics in the drug groups were well-balanced.
Overall, with median follow-up times of 660 days (range, 254-1157 days) in the GLP-1 RA group; approximately 4 years in the sulfonylurea and DPP-4 inhibitor groups; and about 2.5 years in the SGLT2 inhibitor group, the absolute risk for thyroid cancer diagnoses in the treatment groups were low, with a rate of 0.17% in the GLP-1 RA group, 0.23% in the DPP-4 inhibitor group, 0.17% in the SGLT2 inhibitor group, and 0.20% in the sulfonylurea group.
The modified intention to treat analysis concluded no increase with GLP-1 RA use in thyroid cancer risk compared with the other three diabetes drugs (hazard ratio [HR], 1.24).
Higher Cancers Detected in First Year Likely From Higher Screening
Importantly, the risk for thyroid cancer was significantly higher within the first year after GLP-1 RA initiation (HR, 1.85) and was also higher among patients who stayed on the medication and didn’t add other medication (HR, 2.07).
With the latency period for the development of thyroid cancer from external exposures taking several years (such as radiation, taking approximately 2.5 years), some studies in fact exclude patients diagnosed with cancers within a year of starting GLP-1s, as it is implausible for a cancer to develop so soon.
Cancers detected that early therefore are considered to likely have been found due to stepped up screening resulting from an increased concern of thyroid cancer with GLP-1 use.
In support of that theory, the study showed that those in the GLP-1 group had a significantly higher rate of receiving thyroid ultrasounds than those in the non−GLP-1 group, at 1.2% at 6 months for GLP-1RA initiators vs 0.8% for non−GLP-1 RA initiators, and an estimated 2.1% at 12 months for GLP-1RA initiators vs 1.5% for non−GLP-1RA initiators (P < .01).
Though the percentages were small, in relative terms, the 6-month rate of screening in the GLP-1 RA group is a 50% increase, and the 12-month rate represents a 40% increase, McCoy pointed out.
“If we think about 1.2% of people getting an ultrasound in the context of over 41,000 patients, that is a lot of ultrasounds,” she said.
“To my knowledge, this is the first study to actually look at rates of thyroid ultrasounds with GLP-1 RA initiation, so it will definitely be important to look at the trends more broadly,” McCoy added.
McCoy noted that a key caveat of the study is the relatively short follow-up of only about 2 years.
“We have not been able to follow patients for decades to know if there is risk of new thyroid cancers developing after prolonged exposure,” she said. “But we generally don’t know this for any medication until it is used by many millions of people for many decades.”
Consistent Findings in Recent Research
In another recent study evaluating the risk for thyroid cancer among patients receiving GLP-1s, with a longer follow-up of 3.9 years, Bjorn Pasternak, MD, PhD, of the Division of Clinical Epidemiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, and colleagues reported similar findings — the overall risk for thyroid cancer with GLP-1s was not increased (HR, 0.93), and the risk beginning 1 year after treatment initiation was lower, at 0.83.
However, the risk in that study only looking at the first year alone was again higher, at HR 1.47.
“Our analyses indicating a nominally increased risk restricted to the first year after starting treatment might be consistent with an increased detection of thyroid cancer among patients using GLP-1 RAs,” he told this news organization.
In addition to the short follow-up of the current study, Pasternak noted the caveat of the study’s inclusion mainly of patients who initiated the four diabetes drug groups, which is a limitation “because a high proportion of the patients with type 2 diabetes in routine care who start GLP-1 RAs have started it as their third, fourth, or even fifth diabetes drug, especially if they have had diabetes for some time.”
“To capture a larger proportion of the full population of GLP-1 RA users, it would have been preferable to create three different cohorts for the purpose of this study and investigate them separately, including GLP-1 vs DPP4i; GLP-1 vs SGLT2i; GLP-1 vs sulfonylurea,” he said.
Rise in Incidental Thyroid Cancer Detection, An Ongoing Concern
The advent of more advanced imaging and thyroid cancer testing in recent years has already led to a significant problem of over-detection and sometimes over-treatment of incidental, small thyroid cancers that will likely remain harmless in the majority of patients.
With approximately 64 million prescriptions of GLP-1 RAs dispensed between 2000 and 2015 and the percentage increasing at a rate of about 10%-30% per year, increased thyroid testing among those patients could indeed add to the problem.
Meanwhile, thyroid testing is currently not recommended for patients on GLP-1s who do not have a thyroid cancer risk, and the results from the current and Pasternak’s studies, as well as several other recent studies, appear to support those recommendations.
“Collectively, these reassuring data could potentially help reduce [thyroid cancer] over-detection and screening,” Pasternak said.
McCoy echoed that the evidence indicates that “for the vast majority of people there is no indication that GLP-1 RA causes thyroid cancer.”
She underscored that overdiagnosis can have important implications, sharing her own personal experience on the receiving end of a cancer diagnosis.
“Overdiagnosis of these low-risk thyroid cancers may ultimately lead to more harm for the patient than not making the diagnosis, since surgery for thyroid cancer carries real risks (including of death) and there are downstream consequences to having your thyroid removed and relying on lifelong hormone replacement,” McCoy said.
“As a cancer survivor myself, though of lymphoma, not thyroid cancer, I also know the profound impact that a cancer diagnosis has on the individual and their loved ones,” McCoy said.
“It is not something I would wish for anyone, which is why I always speak to my patients about searching for something we may not want to — or need to — find.”
The authors and Pasternak had no disclosures to report.
A version of this article first appeared on Medscape.com.
New research, with the caveat of a relatively short follow-up, adds to encouraging data showing an overall low risk of the development of thyroid cancer associated with glucagon-like peptide 1 receptor agonist (GLP-1 RA) treatment.
However, a notably increased rate of thyroid cancer detections limited only to the first year after drug initiation — but not later, coupled with an increased rate of thyroid cancer screening during that period, raises the suggestion that a thyroid cancer concern among the millions of patients taking the immensely popular drugs could exacerbate the already problematic over-detection of incidental thyroid cancers.
“Our study confirms the increased likelihood of being diagnosed with what appears to be low-risk thyroid cancer after treatment with GLP-1 RAs — but not because patients treated with GLP-1 RAs are more likely to develop thyroid cancer but rather because they are more likely to be diagnosed with it because we, as clinicians, are more likely to look for it,” senior author Rozalina G. McCoy, MD, associate professor and associate division chief for clinical research in the Division of Endocrinology, Diabetes, and Nutrition, Department of Medicine at the University of Maryland School of Medicine, in Baltimore, told this news organization.
The findings were published in JAMA Otolaryngology-Head & Neck Surgery.
Thyroid cancer concerns surrounding GLP-1 RAs stem from preclinical studies showing a risk for medullary thyroid cancer in rodents, resulting in a boxed warning from the US Food and Drug Administration recommending that those with a family history of the condition avoid the drugs.
More recent studies showing GLP-1 receptors in human papillary thyroid cancer cells has further raised concerns of a risk beyond medullary thyroid cancer, however, recent studies evaluating the risk have been inconclusive.
To further investigate, McCoy and colleagues evaluated claims data on 351,913 adult enrollees in Medicare Advantage and fee-for-service plans in the United States who had type 2 diabetes, a moderate risk for cardiovascular disease, and no history of thyroid cancer.
Of the patients, who were about half women, 41,112 started treatments with GLP-1 RAs; 76,093, started with dipeptidyl peptidase-4 (DPP-4) inhibitors; 43,499, with sodium-glucose cotransporter 2 (SGLT2i) inhibitors; and 191,209 with sulfonylurea therapy between January 2014 and December 2021.
After inverse probability weighting, baseline characteristics in the drug groups were well-balanced.
Overall, with median follow-up times of 660 days (range, 254-1157 days) in the GLP-1 RA group; approximately 4 years in the sulfonylurea and DPP-4 inhibitor groups; and about 2.5 years in the SGLT2 inhibitor group, the absolute risk for thyroid cancer diagnoses in the treatment groups were low, with a rate of 0.17% in the GLP-1 RA group, 0.23% in the DPP-4 inhibitor group, 0.17% in the SGLT2 inhibitor group, and 0.20% in the sulfonylurea group.
The modified intention to treat analysis concluded no increase with GLP-1 RA use in thyroid cancer risk compared with the other three diabetes drugs (hazard ratio [HR], 1.24).
Higher Cancers Detected in First Year Likely From Higher Screening
Importantly, the risk for thyroid cancer was significantly higher within the first year after GLP-1 RA initiation (HR, 1.85) and was also higher among patients who stayed on the medication and didn’t add other medication (HR, 2.07).
With the latency period for the development of thyroid cancer from external exposures taking several years (such as radiation, taking approximately 2.5 years), some studies in fact exclude patients diagnosed with cancers within a year of starting GLP-1s, as it is implausible for a cancer to develop so soon.
Cancers detected that early therefore are considered to likely have been found due to stepped up screening resulting from an increased concern of thyroid cancer with GLP-1 use.
In support of that theory, the study showed that those in the GLP-1 group had a significantly higher rate of receiving thyroid ultrasounds than those in the non−GLP-1 group, at 1.2% at 6 months for GLP-1RA initiators vs 0.8% for non−GLP-1 RA initiators, and an estimated 2.1% at 12 months for GLP-1RA initiators vs 1.5% for non−GLP-1RA initiators (P < .01).
Though the percentages were small, in relative terms, the 6-month rate of screening in the GLP-1 RA group is a 50% increase, and the 12-month rate represents a 40% increase, McCoy pointed out.
“If we think about 1.2% of people getting an ultrasound in the context of over 41,000 patients, that is a lot of ultrasounds,” she said.
“To my knowledge, this is the first study to actually look at rates of thyroid ultrasounds with GLP-1 RA initiation, so it will definitely be important to look at the trends more broadly,” McCoy added.
McCoy noted that a key caveat of the study is the relatively short follow-up of only about 2 years.
“We have not been able to follow patients for decades to know if there is risk of new thyroid cancers developing after prolonged exposure,” she said. “But we generally don’t know this for any medication until it is used by many millions of people for many decades.”
Consistent Findings in Recent Research
In another recent study evaluating the risk for thyroid cancer among patients receiving GLP-1s, with a longer follow-up of 3.9 years, Bjorn Pasternak, MD, PhD, of the Division of Clinical Epidemiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, and colleagues reported similar findings — the overall risk for thyroid cancer with GLP-1s was not increased (HR, 0.93), and the risk beginning 1 year after treatment initiation was lower, at 0.83.
However, the risk in that study only looking at the first year alone was again higher, at HR 1.47.
“Our analyses indicating a nominally increased risk restricted to the first year after starting treatment might be consistent with an increased detection of thyroid cancer among patients using GLP-1 RAs,” he told this news organization.
In addition to the short follow-up of the current study, Pasternak noted the caveat of the study’s inclusion mainly of patients who initiated the four diabetes drug groups, which is a limitation “because a high proportion of the patients with type 2 diabetes in routine care who start GLP-1 RAs have started it as their third, fourth, or even fifth diabetes drug, especially if they have had diabetes for some time.”
“To capture a larger proportion of the full population of GLP-1 RA users, it would have been preferable to create three different cohorts for the purpose of this study and investigate them separately, including GLP-1 vs DPP4i; GLP-1 vs SGLT2i; GLP-1 vs sulfonylurea,” he said.
Rise in Incidental Thyroid Cancer Detection, An Ongoing Concern
The advent of more advanced imaging and thyroid cancer testing in recent years has already led to a significant problem of over-detection and sometimes over-treatment of incidental, small thyroid cancers that will likely remain harmless in the majority of patients.
With approximately 64 million prescriptions of GLP-1 RAs dispensed between 2000 and 2015 and the percentage increasing at a rate of about 10%-30% per year, increased thyroid testing among those patients could indeed add to the problem.
Meanwhile, thyroid testing is currently not recommended for patients on GLP-1s who do not have a thyroid cancer risk, and the results from the current and Pasternak’s studies, as well as several other recent studies, appear to support those recommendations.
“Collectively, these reassuring data could potentially help reduce [thyroid cancer] over-detection and screening,” Pasternak said.
McCoy echoed that the evidence indicates that “for the vast majority of people there is no indication that GLP-1 RA causes thyroid cancer.”
She underscored that overdiagnosis can have important implications, sharing her own personal experience on the receiving end of a cancer diagnosis.
“Overdiagnosis of these low-risk thyroid cancers may ultimately lead to more harm for the patient than not making the diagnosis, since surgery for thyroid cancer carries real risks (including of death) and there are downstream consequences to having your thyroid removed and relying on lifelong hormone replacement,” McCoy said.
“As a cancer survivor myself, though of lymphoma, not thyroid cancer, I also know the profound impact that a cancer diagnosis has on the individual and their loved ones,” McCoy said.
“It is not something I would wish for anyone, which is why I always speak to my patients about searching for something we may not want to — or need to — find.”
The authors and Pasternak had no disclosures to report.
A version of this article first appeared on Medscape.com.
New research, with the caveat of a relatively short follow-up, adds to encouraging data showing an overall low risk of the development of thyroid cancer associated with glucagon-like peptide 1 receptor agonist (GLP-1 RA) treatment.
However, a notably increased rate of thyroid cancer detections limited only to the first year after drug initiation — but not later, coupled with an increased rate of thyroid cancer screening during that period, raises the suggestion that a thyroid cancer concern among the millions of patients taking the immensely popular drugs could exacerbate the already problematic over-detection of incidental thyroid cancers.
“Our study confirms the increased likelihood of being diagnosed with what appears to be low-risk thyroid cancer after treatment with GLP-1 RAs — but not because patients treated with GLP-1 RAs are more likely to develop thyroid cancer but rather because they are more likely to be diagnosed with it because we, as clinicians, are more likely to look for it,” senior author Rozalina G. McCoy, MD, associate professor and associate division chief for clinical research in the Division of Endocrinology, Diabetes, and Nutrition, Department of Medicine at the University of Maryland School of Medicine, in Baltimore, told this news organization.
The findings were published in JAMA Otolaryngology-Head & Neck Surgery.
Thyroid cancer concerns surrounding GLP-1 RAs stem from preclinical studies showing a risk for medullary thyroid cancer in rodents, resulting in a boxed warning from the US Food and Drug Administration recommending that those with a family history of the condition avoid the drugs.
More recent studies showing GLP-1 receptors in human papillary thyroid cancer cells has further raised concerns of a risk beyond medullary thyroid cancer, however, recent studies evaluating the risk have been inconclusive.
To further investigate, McCoy and colleagues evaluated claims data on 351,913 adult enrollees in Medicare Advantage and fee-for-service plans in the United States who had type 2 diabetes, a moderate risk for cardiovascular disease, and no history of thyroid cancer.
Of the patients, who were about half women, 41,112 started treatments with GLP-1 RAs; 76,093, started with dipeptidyl peptidase-4 (DPP-4) inhibitors; 43,499, with sodium-glucose cotransporter 2 (SGLT2i) inhibitors; and 191,209 with sulfonylurea therapy between January 2014 and December 2021.
After inverse probability weighting, baseline characteristics in the drug groups were well-balanced.
Overall, with median follow-up times of 660 days (range, 254-1157 days) in the GLP-1 RA group; approximately 4 years in the sulfonylurea and DPP-4 inhibitor groups; and about 2.5 years in the SGLT2 inhibitor group, the absolute risk for thyroid cancer diagnoses in the treatment groups were low, with a rate of 0.17% in the GLP-1 RA group, 0.23% in the DPP-4 inhibitor group, 0.17% in the SGLT2 inhibitor group, and 0.20% in the sulfonylurea group.
The modified intention to treat analysis concluded no increase with GLP-1 RA use in thyroid cancer risk compared with the other three diabetes drugs (hazard ratio [HR], 1.24).
Higher Cancers Detected in First Year Likely From Higher Screening
Importantly, the risk for thyroid cancer was significantly higher within the first year after GLP-1 RA initiation (HR, 1.85) and was also higher among patients who stayed on the medication and didn’t add other medication (HR, 2.07).
With the latency period for the development of thyroid cancer from external exposures taking several years (such as radiation, taking approximately 2.5 years), some studies in fact exclude patients diagnosed with cancers within a year of starting GLP-1s, as it is implausible for a cancer to develop so soon.
Cancers detected that early therefore are considered to likely have been found due to stepped up screening resulting from an increased concern of thyroid cancer with GLP-1 use.
In support of that theory, the study showed that those in the GLP-1 group had a significantly higher rate of receiving thyroid ultrasounds than those in the non−GLP-1 group, at 1.2% at 6 months for GLP-1RA initiators vs 0.8% for non−GLP-1 RA initiators, and an estimated 2.1% at 12 months for GLP-1RA initiators vs 1.5% for non−GLP-1RA initiators (P < .01).
Though the percentages were small, in relative terms, the 6-month rate of screening in the GLP-1 RA group is a 50% increase, and the 12-month rate represents a 40% increase, McCoy pointed out.
“If we think about 1.2% of people getting an ultrasound in the context of over 41,000 patients, that is a lot of ultrasounds,” she said.
“To my knowledge, this is the first study to actually look at rates of thyroid ultrasounds with GLP-1 RA initiation, so it will definitely be important to look at the trends more broadly,” McCoy added.
McCoy noted that a key caveat of the study is the relatively short follow-up of only about 2 years.
“We have not been able to follow patients for decades to know if there is risk of new thyroid cancers developing after prolonged exposure,” she said. “But we generally don’t know this for any medication until it is used by many millions of people for many decades.”
Consistent Findings in Recent Research
In another recent study evaluating the risk for thyroid cancer among patients receiving GLP-1s, with a longer follow-up of 3.9 years, Bjorn Pasternak, MD, PhD, of the Division of Clinical Epidemiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, and colleagues reported similar findings — the overall risk for thyroid cancer with GLP-1s was not increased (HR, 0.93), and the risk beginning 1 year after treatment initiation was lower, at 0.83.
However, the risk in that study only looking at the first year alone was again higher, at HR 1.47.
“Our analyses indicating a nominally increased risk restricted to the first year after starting treatment might be consistent with an increased detection of thyroid cancer among patients using GLP-1 RAs,” he told this news organization.
In addition to the short follow-up of the current study, Pasternak noted the caveat of the study’s inclusion mainly of patients who initiated the four diabetes drug groups, which is a limitation “because a high proportion of the patients with type 2 diabetes in routine care who start GLP-1 RAs have started it as their third, fourth, or even fifth diabetes drug, especially if they have had diabetes for some time.”
“To capture a larger proportion of the full population of GLP-1 RA users, it would have been preferable to create three different cohorts for the purpose of this study and investigate them separately, including GLP-1 vs DPP4i; GLP-1 vs SGLT2i; GLP-1 vs sulfonylurea,” he said.
Rise in Incidental Thyroid Cancer Detection, An Ongoing Concern
The advent of more advanced imaging and thyroid cancer testing in recent years has already led to a significant problem of over-detection and sometimes over-treatment of incidental, small thyroid cancers that will likely remain harmless in the majority of patients.
With approximately 64 million prescriptions of GLP-1 RAs dispensed between 2000 and 2015 and the percentage increasing at a rate of about 10%-30% per year, increased thyroid testing among those patients could indeed add to the problem.
Meanwhile, thyroid testing is currently not recommended for patients on GLP-1s who do not have a thyroid cancer risk, and the results from the current and Pasternak’s studies, as well as several other recent studies, appear to support those recommendations.
“Collectively, these reassuring data could potentially help reduce [thyroid cancer] over-detection and screening,” Pasternak said.
McCoy echoed that the evidence indicates that “for the vast majority of people there is no indication that GLP-1 RA causes thyroid cancer.”
She underscored that overdiagnosis can have important implications, sharing her own personal experience on the receiving end of a cancer diagnosis.
“Overdiagnosis of these low-risk thyroid cancers may ultimately lead to more harm for the patient than not making the diagnosis, since surgery for thyroid cancer carries real risks (including of death) and there are downstream consequences to having your thyroid removed and relying on lifelong hormone replacement,” McCoy said.
“As a cancer survivor myself, though of lymphoma, not thyroid cancer, I also know the profound impact that a cancer diagnosis has on the individual and their loved ones,” McCoy said.
“It is not something I would wish for anyone, which is why I always speak to my patients about searching for something we may not want to — or need to — find.”
The authors and Pasternak had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY-HEAD & NECK SURGERY
Red Wine May Not Be a Health Tonic, But Is It a Cancer Risk?
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.