User login
Brain Cancer: Epidemiology, TBI, and New Treatments
Brain Cancer: Epidemiology, TBI, and New Treatments
Click to view more from Cancer Data Trends 2025.
- Bihn JR, Cioffi G, Waite KA, et al. Brain tumors in United States military veterans.
Neuro Oncol. 2024;26(2):387-396. doi:10.1093/neuonc/noad182 - Stewart IJ, Howard JT, Poltavsky E, et al. Traumatic Brain Injury and Subsequent
Risk of Brain Cancer in US Veterans of the Iraq and Afghanistan Wars. JAMA Netw
Open. 2024;7(2):e2354588. doi:10.1001/jamanetworkopen.2023.54588 - DoD/USU Brain Tissue Repository. December 15, 2023. Accessed December 11,
2024. https://researchbraininjury.org/ - Munch TN, Gørtz S, Wohlfahrt J, Melbye M. The long-term risk of malignant
astrocytic tumors after structural brain injury--a nationwide cohort study. Neuro
Oncol. 2015;17(5):718-724. doi:10.1093/neuonc/nou312 - Strowd RE, Dunbar EM, Gan HK, et al. Practical guidance for telemedicine use in
neuro-oncology. Neurooncol Pract. 2022;9(2):91-104. doi:10.1093/nop/npac002 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
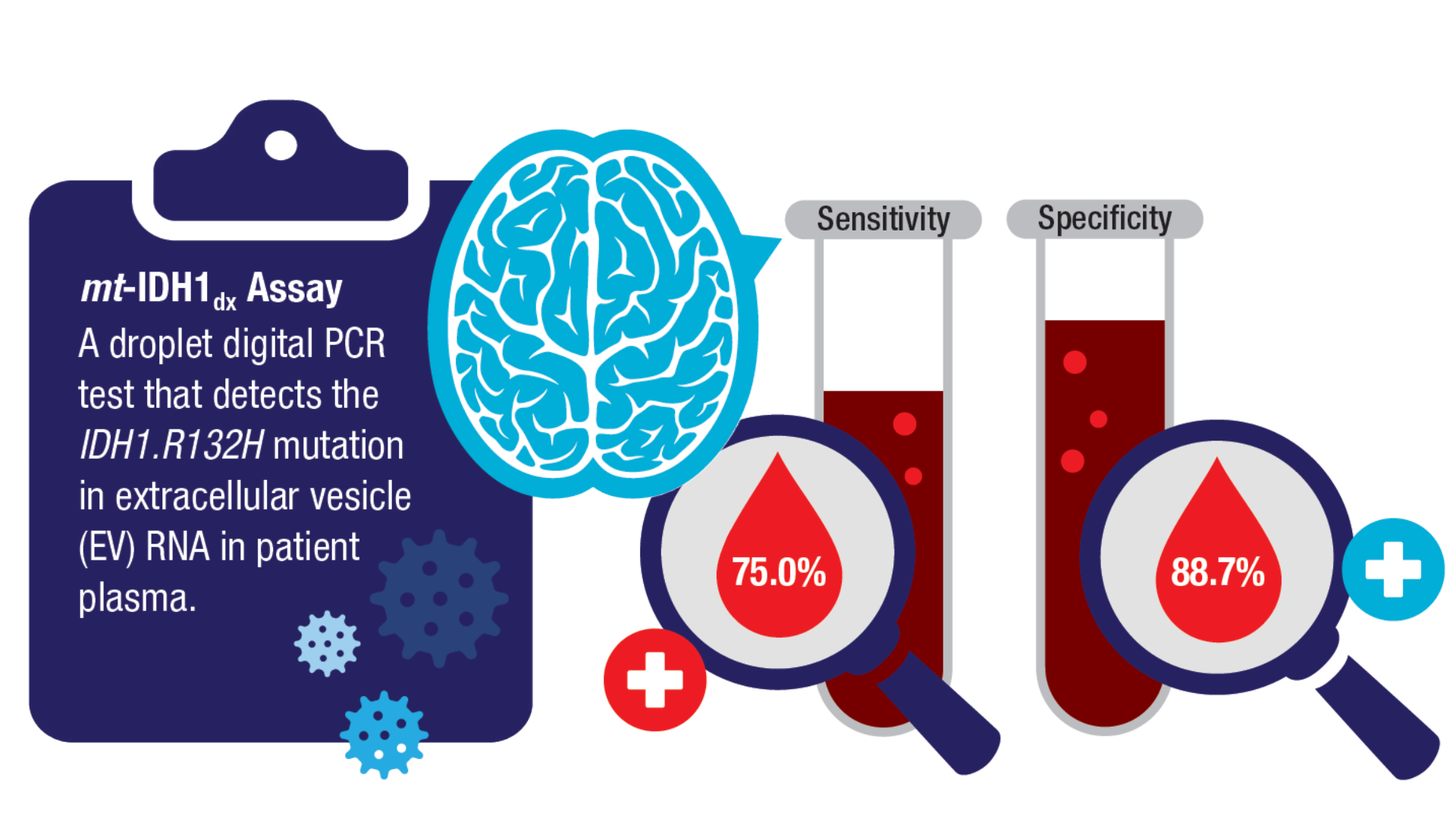
Book. 2024;44(e100042. doi:10.1200/EDBK_100042 - Batool SM, Escobedo AK, Hsia T, et al. Clinical utility of a blood based assay for
the detection of IDH1.R132H-mutant gliomas. Nat Commun. 2024;15(1):7074.
doi:10.1038/s41467-024-51332-7 - Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al; INDIGO Trial Investigators.
Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med.
2023;389(7):589-601. doi:10.1056/NEJMoa2304194 - FDA. US Food and Drug Administration. FDA approves vorasidenib for Grade 2
astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation.
Accessed December 11, 2024. https://www.fda.gov/drugs/resourcesinformation-
approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-oroligodendroglioma-
susceptible-idh1-or-idh2-mutation - NIH. National Cancer Institute. Tovorafenib Approved for Some Children with Low-
Grade Glioma. Accessed December 11, 2024. https://www.cancer.gov/news-events/
cancer-currents-blog/2024/pediatric-low-grade-glioma-tovorafenib-braf - The Veteran Population. Accessed December 11, 2024. https://www.va.gov/vetdata/
docs/surveysandstudies/vetpop.pdf - Miller AM, Szalontay L, Bouvier N, et al. Next-generation sequencing of
cerebrospinal fluid for clinical molecular diagnostics in pediatric, adolescent
and young adult brain tumor patients. Neuro Oncol. 2022;24(10):1763-1772.
doi:10.1093/neuonc/noac035
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Bihn JR, Cioffi G, Waite KA, et al. Brain tumors in United States military veterans.
Neuro Oncol. 2024;26(2):387-396. doi:10.1093/neuonc/noad182 - Stewart IJ, Howard JT, Poltavsky E, et al. Traumatic Brain Injury and Subsequent
Risk of Brain Cancer in US Veterans of the Iraq and Afghanistan Wars. JAMA Netw
Open. 2024;7(2):e2354588. doi:10.1001/jamanetworkopen.2023.54588 - DoD/USU Brain Tissue Repository. December 15, 2023. Accessed December 11,
2024. https://researchbraininjury.org/ - Munch TN, Gørtz S, Wohlfahrt J, Melbye M. The long-term risk of malignant
astrocytic tumors after structural brain injury--a nationwide cohort study. Neuro
Oncol. 2015;17(5):718-724. doi:10.1093/neuonc/nou312 - Strowd RE, Dunbar EM, Gan HK, et al. Practical guidance for telemedicine use in
neuro-oncology. Neurooncol Pract. 2022;9(2):91-104. doi:10.1093/nop/npac002 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(e100042. doi:10.1200/EDBK_100042 - Batool SM, Escobedo AK, Hsia T, et al. Clinical utility of a blood based assay for
the detection of IDH1.R132H-mutant gliomas. Nat Commun. 2024;15(1):7074.
doi:10.1038/s41467-024-51332-7 - Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al; INDIGO Trial Investigators.
Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med.
2023;389(7):589-601. doi:10.1056/NEJMoa2304194 - FDA. US Food and Drug Administration. FDA approves vorasidenib for Grade 2
astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation.
Accessed December 11, 2024. https://www.fda.gov/drugs/resourcesinformation-
approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-oroligodendroglioma-
susceptible-idh1-or-idh2-mutation - NIH. National Cancer Institute. Tovorafenib Approved for Some Children with Low-
Grade Glioma. Accessed December 11, 2024. https://www.cancer.gov/news-events/
cancer-currents-blog/2024/pediatric-low-grade-glioma-tovorafenib-braf - The Veteran Population. Accessed December 11, 2024. https://www.va.gov/vetdata/
docs/surveysandstudies/vetpop.pdf - Miller AM, Szalontay L, Bouvier N, et al. Next-generation sequencing of
cerebrospinal fluid for clinical molecular diagnostics in pediatric, adolescent
and young adult brain tumor patients. Neuro Oncol. 2022;24(10):1763-1772.
doi:10.1093/neuonc/noac035
- Bihn JR, Cioffi G, Waite KA, et al. Brain tumors in United States military veterans.
Neuro Oncol. 2024;26(2):387-396. doi:10.1093/neuonc/noad182 - Stewart IJ, Howard JT, Poltavsky E, et al. Traumatic Brain Injury and Subsequent
Risk of Brain Cancer in US Veterans of the Iraq and Afghanistan Wars. JAMA Netw
Open. 2024;7(2):e2354588. doi:10.1001/jamanetworkopen.2023.54588 - DoD/USU Brain Tissue Repository. December 15, 2023. Accessed December 11,
2024. https://researchbraininjury.org/ - Munch TN, Gørtz S, Wohlfahrt J, Melbye M. The long-term risk of malignant
astrocytic tumors after structural brain injury--a nationwide cohort study. Neuro
Oncol. 2015;17(5):718-724. doi:10.1093/neuonc/nou312 - Strowd RE, Dunbar EM, Gan HK, et al. Practical guidance for telemedicine use in
neuro-oncology. Neurooncol Pract. 2022;9(2):91-104. doi:10.1093/nop/npac002 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(e100042. doi:10.1200/EDBK_100042 - Batool SM, Escobedo AK, Hsia T, et al. Clinical utility of a blood based assay for
the detection of IDH1.R132H-mutant gliomas. Nat Commun. 2024;15(1):7074.
doi:10.1038/s41467-024-51332-7 - Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al; INDIGO Trial Investigators.
Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med.
2023;389(7):589-601. doi:10.1056/NEJMoa2304194 - FDA. US Food and Drug Administration. FDA approves vorasidenib for Grade 2
astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation.
Accessed December 11, 2024. https://www.fda.gov/drugs/resourcesinformation-
approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-oroligodendroglioma-
susceptible-idh1-or-idh2-mutation - NIH. National Cancer Institute. Tovorafenib Approved for Some Children with Low-
Grade Glioma. Accessed December 11, 2024. https://www.cancer.gov/news-events/
cancer-currents-blog/2024/pediatric-low-grade-glioma-tovorafenib-braf - The Veteran Population. Accessed December 11, 2024. https://www.va.gov/vetdata/
docs/surveysandstudies/vetpop.pdf - Miller AM, Szalontay L, Bouvier N, et al. Next-generation sequencing of
cerebrospinal fluid for clinical molecular diagnostics in pediatric, adolescent
and young adult brain tumor patients. Neuro Oncol. 2022;24(10):1763-1772.
doi:10.1093/neuonc/noac035
Brain Cancer: Epidemiology, TBI, and New Treatments
Brain Cancer: Epidemiology, TBI, and New Treatments
MRI-Invisible Prostate Lesions: Are They Dangerous?
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
Cellular Therapies for Solid Tumors: The Next Big Thing?
The cutting edge of treating solid tumors with cell therapies got notably sharper in 2024.
First came the US Food and Drug Administration (FDA) approval in February 2024 of the tumor-infiltrating lymphocyte (TIL) therapy lifileucel in unresectable or metastatic melanoma that had progressed on prior immunotherapy, the first cellular therapy for any solid tumor. Then came the August FDA approval of afamitresgene autoleucel in unresectable or metastatic synovial sarcoma with failed chemotherapy, the first engineered T-cell therapy for cancers in soft tissue.
“This was a pipe dream just a decade ago,” Alison Betof Warner, MD, PhD, lead author of a lifileucel study (NCT05640193), said in an interview with Medscape Medical News. “At the start of 2024, we had no approvals of these kinds of products in solid cancers. Now we have two.”
As the director of Solid Tumor Cell Therapy and leader of Stanford Medicine’s Melanoma and Cutaneous Oncology Clinical Research Group, Betof Warner has been at the forefront of developing commercial cell therapy using tumor-infiltrating lymphocytes (TILs).
“The approval of lifileucel increases confidence that we can get these therapies across the regulatory finish line and to patients,” Betof Warner said during the interview. She was not involved in the development of afamitresgene autoleucel.
‘Reverse Engineering’
In addition to her contributions to the work that led to lifileucel’s approval, Betof Warner was the lead author on the first consensus guidelines on management and best practices for tumor-infiltrating lymphocyte cell therapy.
Betof Warner began studying TILs after doing research with her mentors in immuno-oncology, Jedd D. Wolchok and Michael A. Postow. Their investigations — including one that Betof Warner coauthored — into how monoclonal antibodies and checkpoint inhibitors, such as ipilimumab or nivolumab, might extend the lives of people with advanced unresectable or metastatic melanoma inspired her to push further to find ways to minimize treatment while maximizing outcomes for patients. Betof Warner’s interest overall, she said in the interview, is in capitalizing on what can be learned about how the immune system controls cancer.
“What we know is that the immune system has the ability to kill cancer,” Betof Warner said. “Therefore we need to be thinking about how we can increase immune surveillance. How can we enhance that before a patient develops advanced cancer?
Betof Warner said that although TILs are now standard treatment in melanoma, there is about a 30% response rate compared with about a 50% response rate in immunotherapy, and the latter is easier for the patient to withstand.
“Antibodies on the frontline are better than going through a surgery and then waiting weeks to get your therapy,” Betof Warner said in the interview. “You can come into my clinic and get an antibody therapy in 30 minutes and go straight to work. TILs require patients to be in the hospital for weeks at a time and out of work for months at a time.”
In an effort to combine therapies to maximize best outcomes, a phase 3 trial (NCT05727904) is currently recruiting. The TILVANCE-301 trial will compare immunotherapy plus adoptive cell therapy vs immunotherapy alone in untreated unresectable or metastatic melanoma. Betof Warner is not a part of this study.
Cell Therapies Include CAR T Cells and TCRT
In general, adoptive T-cell therapies such as TILs involve the isolation of autologous immune cells that are removed from the body and either expanded or modified to optimize their efficacy in fighting antigens, before their transfer to the patient as a living drug by infusion.
In addition to TILs, adoptive cell therapies for antitumor therapeutics include chimeric antigen receptor (CAR) T cells and engineered T-cell receptor therapy (TCRT).
In CAR T-cell therapy and TCRT, naive T cells are harvested from the patient’s blood then engineered to target a tumor. In TIL therapy, tumor-specific T cells are taken from the patient’s tumor. Once extracted, the respective cells are expanded billions of times and then delivered back to the patient’s body, said Betof Warner.
“The main promise of this approach is to generate responses in what we know as ‘cold’ tumors, or tumors that do not have a lot of endogenous T-cell infiltration or where the T cells are not working well, to bring in tumor targeting T cells and then trigger an immune response,” Betof Warner told an audience at the American Society of Clinical Oncology (ASCO) 2024 annual meeting.
TIL patients also receive interleukin (IL)-2 infusions to further stimulate the cells. In patients being treated with TCRT, they either receive low or no IL-2, Betof Warner said in her ASCO presentation, “Adopting Cutting-Edge Cell Therapies in Melanoma,” part of the session Beyond the Tip of the Iceberg: Next-Generation Cell-Based Therapies.
Decades in the Making
The National Cancer Institute began investigating TILs in the late 1980s, with the current National Cancer Institute (NCI) surgery chief, Steven Rosenberg, MD, PhD, leading the first-ever trials that showed TILs could shrink tumors in people with advanced melanoma.
Since then, NCI staff and others have also investigated TILs beyond melanoma and additional cell therapies based on CAR T cells and TCRT for antitumor therapeutics.
“TCRs are different from CAR Ts because they go after intracellular antigens instead of extracellular antigens,” said Betof Warner. “That has appeal because many of the tumor antigens we’re looking for will be intracellular.”
Because CAR T cells only target extracellular antigens, their utility is somewhat limited. Although several CAR T-cell therapies exist for blood cancers, there currently are no approved CAR T-cell therapies for solid tumors. However, several trials of CAR T cells in gastrointestinal cancers and melanoma are ongoing, said Betof Warner, who is not a part of these studies.
“We are starting to see early-phase efficacy in pediatric gliomas,” Betof Warner said, mentioning a study conducted by colleagues at Stanford who demonstrated potential for anti-GD2 CAR T-cell therapy in deadly pediatric diffuse midline gliomas, tumors on the spine and brain.
In their study, nine out of 11 participants (median age, 15 years) showed benefit from the cell therapy, with one participant’s tumors resolving completely. The results paved the way for the FDA to grant a Regenerative Medicine Advanced Therapy designation for use of anti-GD2 CAR T cells in H3K27M-positive diffuse midline gliomas.
The investigators are now recruiting for a phase 1 trial (NCT04196413). Results of the initial study were published in Nature last month.
Another lesser-known cell therapy expected to advance at some point in the future for solid tumors is use of the body’s natural killer (NK) cells. “They’ve been known about for a long time, but they are more difficult to regulate, which is one reason why it has taken longer to make NK cell therapies,” said Betof Warner, who is not involved in the study of NK cells. “One of their advantages is that, potentially, there could be an ‘off the shelf’ NK product. They don’t necessarily have to be made with autologous cells.”
Risk-Benefit Profiles Depend on Mechanism of Action
If the corresponding TCR sequence of a tumor antigen is known, said Betof Warner, it is possible to use leukapheresis to generate naive circulating lymphocytes. Once infused, the manufactured TCRTs will activate in the body the same as native cells because the signaling is the same.
An advantage to TCRT compared with CAR T-cell therapy is that it targets intracellular proteins, which are significantly present in the tumor, Betof Warner said in her presentation at ASCO 2024. She clarified that tumors will usually be screened for the presence of this antigen before a patient is selected for treatment with that particular therapy, because not all antigens are highly expressed in every tumor.
“Furthermore, the tumor antigen has to be presented by a major histocompatibility complex, meaning there are human leukocyte antigen restrictions, which impacts patient selection,” she said.
A risk with both TCRT and CAR T-cell therapy, according to Betof Warner, is that because there are often shared antigens between tumor and normal tissues, on-target/off-tumor toxicity is a risk.
“TILs are different because they are nonengineered, at least not for antigen recognition. They are polyclonal and go after multiple targets,” Betof Warner said. “TCRs and CARs are engineered to go after one target. So, TILs have much lower rates of on-tumor/off-target effects, vs when you engineer a very high affinity receptor like a TCR or CAR.”
A good example of how this amplification of TCR affinity can lead to poor outcomes is in metastatic melanoma, said Betof Warner.
In investigations (NCI-07-C-0174 and NCI-07-C-0175) of TCRT in metastatic melanoma, for example, the researchers were targeting MART-1 or gp100, which are expressed in melanocytes.
“The problem was that these antigens are also expressed in the eyes and ears, so it caused eye inflammation and hearing loss in a number of patients because it wasn’t specific enough for the tumor,” said Betof Warner. “So, if that target is highly expressed on normal tissue, then you have a high risk.”
Promise of PRAME
Betof Warner said the most promising TCRT at present is the investigational autologous cell therapy IMA203 (NCT03688124), which targets the preferentially expressed antigen (PRAME). Although PRAME is found in many tumors, this testis antigen does not tend to express in normal, healthy adult tissues. Betof Warner is not affiliated with this study.
“It’s maybe the most exciting TCRT cell in melanoma,” Betof Warner told her audience at the ASCO 2024 meeting. Because the expression rate of PRAME in cutaneous and uveal melanoma is at or above 95% and 90%, respectively, she said “it is a really good target in melanoma.”
Phase 1a results reported in late 2023 from a first-in-human trial of IMA203 involving 13 persons with highly advanced melanoma and a median of 5.5 previous treatments showed a 50% objective response rate in the 12 evaluable results. The duration of response ranged between 2.2 and 14.7 months (median follow-up, 14 months).
The safety profile of the treatment was favorable, with no grade 3 adverse events occurring in more than 10% of the cohort, and no grade 5 adverse events at all.
Phase 1b results published in October by maker Immatics showed that in 28 heavily pretreated metastatic melanoma patients, IMA203 had a confirmed objective response rate of 54% with a median duration of response of 12.1 months, while maintaining a favorable tolerability profile.
Accelerated Approvals, Boxed Warnings
The FDA granted accelerated approvals for both lifileucel, the TIL therapy, and afamitresgene autoleucel, the TCRT.
Both were approved with boxed warnings. Lifileucel’s warning is for treatment-related mortality, prolonged severe cytopenia, severe infection, and cardiopulmonary and renal impairment. Afamitresgene autoleucel’s boxed warning is for serious or fatal cytokine release syndrome, which may be severe or life-threatening.
With these approvals, the bar is now raised on TILs and TCRTs, said Betof Warner.
The lifileucel trial studied 73 patients whose melanoma had continued to metastasize despite treatment with a programmed cell death protein (PD-1)/ programmed death-ligand (PD-L1)–targeted immune checkpoint inhibitor and a BRAF inhibitor (if appropriate based on tumor mutation status), and whose lifileucel dose was at least 7.5 billion cells (the approved dose). The cohort also received a median of six IL-2 (aldesleukin) doses.
The objective response rate was 31.5% (95% CI, 21.1-43.4), and median duration of response was not reached (lower bound of 95% CI, 4.1).
In the afamitresgene autoleucel study, 44 of 52 patients with synovial sarcoma received leukapheresis and a single infusion of afamitresgene autoleucel.
The overall response rate was 43.2% (95% CI, 28.4-59.0). The median time to response was 4.9 weeks (95% CI, 4.4-8), and the median duration of response was 6 months (lower bound of 95% CI, 4.6). Among patients who were responsive to the treatment, 45.6% and 39.0% had a duration of response of 6 months or longer and 12 months or longer, respectively.
New Hope for Patients
Betof Warner and her colleagues are now recruiting for an open-label, phase 1/2 investigation of the safety and efficacy of the TIL therapy OBX-115 in adult advanced solid tumors in melanoma or non–small cell lung cancer. The first-in-human results of a previous trial were presented at the ASCO 2024 meeting, and OBX-115 received FDA fast track designation in July.
“I think the results are really promising,” said Betof Warner. “This is an engineered TIL that does not require administering IL-2 to the patient. There were four out of the nine patients who responded to the treatment and there were no dose-limiting toxicities, no cytokine and no intracranial — all of which is excellent.”
For Betof Warner, the possibility that by using their own immune system, patients with advanced and refractory cancers could soon have a one-time treatment with a cell therapy rather than innumerable bouts of chemotherapy pushes her onward.
“The idea that we can treat cancer one time and have it not recur for years — that’s pushing the start of saying there’s a cure of cancer. That a person could move on from cancer like they move on from an infection. That is the potential of this work. We’re not there yet, but that’s where we need to think and dream big,” she said.
Betof Warner disclosed consulting/advisory roles with BluePath Solutions, Bristol-Myers Squibb/Medarex, Immatics, Instil Bio, Iovance Biotherapeutics, Lyell Immunopharma, Merck, Novartis, and Pfizer and research funding and travel expenses from Iovance Biotherapeutics.
A version of this article appeared on Medscape.com.
The cutting edge of treating solid tumors with cell therapies got notably sharper in 2024.
First came the US Food and Drug Administration (FDA) approval in February 2024 of the tumor-infiltrating lymphocyte (TIL) therapy lifileucel in unresectable or metastatic melanoma that had progressed on prior immunotherapy, the first cellular therapy for any solid tumor. Then came the August FDA approval of afamitresgene autoleucel in unresectable or metastatic synovial sarcoma with failed chemotherapy, the first engineered T-cell therapy for cancers in soft tissue.
“This was a pipe dream just a decade ago,” Alison Betof Warner, MD, PhD, lead author of a lifileucel study (NCT05640193), said in an interview with Medscape Medical News. “At the start of 2024, we had no approvals of these kinds of products in solid cancers. Now we have two.”
As the director of Solid Tumor Cell Therapy and leader of Stanford Medicine’s Melanoma and Cutaneous Oncology Clinical Research Group, Betof Warner has been at the forefront of developing commercial cell therapy using tumor-infiltrating lymphocytes (TILs).
“The approval of lifileucel increases confidence that we can get these therapies across the regulatory finish line and to patients,” Betof Warner said during the interview. She was not involved in the development of afamitresgene autoleucel.
‘Reverse Engineering’
In addition to her contributions to the work that led to lifileucel’s approval, Betof Warner was the lead author on the first consensus guidelines on management and best practices for tumor-infiltrating lymphocyte cell therapy.
Betof Warner began studying TILs after doing research with her mentors in immuno-oncology, Jedd D. Wolchok and Michael A. Postow. Their investigations — including one that Betof Warner coauthored — into how monoclonal antibodies and checkpoint inhibitors, such as ipilimumab or nivolumab, might extend the lives of people with advanced unresectable or metastatic melanoma inspired her to push further to find ways to minimize treatment while maximizing outcomes for patients. Betof Warner’s interest overall, she said in the interview, is in capitalizing on what can be learned about how the immune system controls cancer.
“What we know is that the immune system has the ability to kill cancer,” Betof Warner said. “Therefore we need to be thinking about how we can increase immune surveillance. How can we enhance that before a patient develops advanced cancer?
Betof Warner said that although TILs are now standard treatment in melanoma, there is about a 30% response rate compared with about a 50% response rate in immunotherapy, and the latter is easier for the patient to withstand.
“Antibodies on the frontline are better than going through a surgery and then waiting weeks to get your therapy,” Betof Warner said in the interview. “You can come into my clinic and get an antibody therapy in 30 minutes and go straight to work. TILs require patients to be in the hospital for weeks at a time and out of work for months at a time.”
In an effort to combine therapies to maximize best outcomes, a phase 3 trial (NCT05727904) is currently recruiting. The TILVANCE-301 trial will compare immunotherapy plus adoptive cell therapy vs immunotherapy alone in untreated unresectable or metastatic melanoma. Betof Warner is not a part of this study.
Cell Therapies Include CAR T Cells and TCRT
In general, adoptive T-cell therapies such as TILs involve the isolation of autologous immune cells that are removed from the body and either expanded or modified to optimize their efficacy in fighting antigens, before their transfer to the patient as a living drug by infusion.
In addition to TILs, adoptive cell therapies for antitumor therapeutics include chimeric antigen receptor (CAR) T cells and engineered T-cell receptor therapy (TCRT).
In CAR T-cell therapy and TCRT, naive T cells are harvested from the patient’s blood then engineered to target a tumor. In TIL therapy, tumor-specific T cells are taken from the patient’s tumor. Once extracted, the respective cells are expanded billions of times and then delivered back to the patient’s body, said Betof Warner.
“The main promise of this approach is to generate responses in what we know as ‘cold’ tumors, or tumors that do not have a lot of endogenous T-cell infiltration or where the T cells are not working well, to bring in tumor targeting T cells and then trigger an immune response,” Betof Warner told an audience at the American Society of Clinical Oncology (ASCO) 2024 annual meeting.
TIL patients also receive interleukin (IL)-2 infusions to further stimulate the cells. In patients being treated with TCRT, they either receive low or no IL-2, Betof Warner said in her ASCO presentation, “Adopting Cutting-Edge Cell Therapies in Melanoma,” part of the session Beyond the Tip of the Iceberg: Next-Generation Cell-Based Therapies.
Decades in the Making
The National Cancer Institute began investigating TILs in the late 1980s, with the current National Cancer Institute (NCI) surgery chief, Steven Rosenberg, MD, PhD, leading the first-ever trials that showed TILs could shrink tumors in people with advanced melanoma.
Since then, NCI staff and others have also investigated TILs beyond melanoma and additional cell therapies based on CAR T cells and TCRT for antitumor therapeutics.
“TCRs are different from CAR Ts because they go after intracellular antigens instead of extracellular antigens,” said Betof Warner. “That has appeal because many of the tumor antigens we’re looking for will be intracellular.”
Because CAR T cells only target extracellular antigens, their utility is somewhat limited. Although several CAR T-cell therapies exist for blood cancers, there currently are no approved CAR T-cell therapies for solid tumors. However, several trials of CAR T cells in gastrointestinal cancers and melanoma are ongoing, said Betof Warner, who is not a part of these studies.
“We are starting to see early-phase efficacy in pediatric gliomas,” Betof Warner said, mentioning a study conducted by colleagues at Stanford who demonstrated potential for anti-GD2 CAR T-cell therapy in deadly pediatric diffuse midline gliomas, tumors on the spine and brain.
In their study, nine out of 11 participants (median age, 15 years) showed benefit from the cell therapy, with one participant’s tumors resolving completely. The results paved the way for the FDA to grant a Regenerative Medicine Advanced Therapy designation for use of anti-GD2 CAR T cells in H3K27M-positive diffuse midline gliomas.
The investigators are now recruiting for a phase 1 trial (NCT04196413). Results of the initial study were published in Nature last month.
Another lesser-known cell therapy expected to advance at some point in the future for solid tumors is use of the body’s natural killer (NK) cells. “They’ve been known about for a long time, but they are more difficult to regulate, which is one reason why it has taken longer to make NK cell therapies,” said Betof Warner, who is not involved in the study of NK cells. “One of their advantages is that, potentially, there could be an ‘off the shelf’ NK product. They don’t necessarily have to be made with autologous cells.”
Risk-Benefit Profiles Depend on Mechanism of Action
If the corresponding TCR sequence of a tumor antigen is known, said Betof Warner, it is possible to use leukapheresis to generate naive circulating lymphocytes. Once infused, the manufactured TCRTs will activate in the body the same as native cells because the signaling is the same.
An advantage to TCRT compared with CAR T-cell therapy is that it targets intracellular proteins, which are significantly present in the tumor, Betof Warner said in her presentation at ASCO 2024. She clarified that tumors will usually be screened for the presence of this antigen before a patient is selected for treatment with that particular therapy, because not all antigens are highly expressed in every tumor.
“Furthermore, the tumor antigen has to be presented by a major histocompatibility complex, meaning there are human leukocyte antigen restrictions, which impacts patient selection,” she said.
A risk with both TCRT and CAR T-cell therapy, according to Betof Warner, is that because there are often shared antigens between tumor and normal tissues, on-target/off-tumor toxicity is a risk.
“TILs are different because they are nonengineered, at least not for antigen recognition. They are polyclonal and go after multiple targets,” Betof Warner said. “TCRs and CARs are engineered to go after one target. So, TILs have much lower rates of on-tumor/off-target effects, vs when you engineer a very high affinity receptor like a TCR or CAR.”
A good example of how this amplification of TCR affinity can lead to poor outcomes is in metastatic melanoma, said Betof Warner.
In investigations (NCI-07-C-0174 and NCI-07-C-0175) of TCRT in metastatic melanoma, for example, the researchers were targeting MART-1 or gp100, which are expressed in melanocytes.
“The problem was that these antigens are also expressed in the eyes and ears, so it caused eye inflammation and hearing loss in a number of patients because it wasn’t specific enough for the tumor,” said Betof Warner. “So, if that target is highly expressed on normal tissue, then you have a high risk.”
Promise of PRAME
Betof Warner said the most promising TCRT at present is the investigational autologous cell therapy IMA203 (NCT03688124), which targets the preferentially expressed antigen (PRAME). Although PRAME is found in many tumors, this testis antigen does not tend to express in normal, healthy adult tissues. Betof Warner is not affiliated with this study.
“It’s maybe the most exciting TCRT cell in melanoma,” Betof Warner told her audience at the ASCO 2024 meeting. Because the expression rate of PRAME in cutaneous and uveal melanoma is at or above 95% and 90%, respectively, she said “it is a really good target in melanoma.”
Phase 1a results reported in late 2023 from a first-in-human trial of IMA203 involving 13 persons with highly advanced melanoma and a median of 5.5 previous treatments showed a 50% objective response rate in the 12 evaluable results. The duration of response ranged between 2.2 and 14.7 months (median follow-up, 14 months).
The safety profile of the treatment was favorable, with no grade 3 adverse events occurring in more than 10% of the cohort, and no grade 5 adverse events at all.
Phase 1b results published in October by maker Immatics showed that in 28 heavily pretreated metastatic melanoma patients, IMA203 had a confirmed objective response rate of 54% with a median duration of response of 12.1 months, while maintaining a favorable tolerability profile.
Accelerated Approvals, Boxed Warnings
The FDA granted accelerated approvals for both lifileucel, the TIL therapy, and afamitresgene autoleucel, the TCRT.
Both were approved with boxed warnings. Lifileucel’s warning is for treatment-related mortality, prolonged severe cytopenia, severe infection, and cardiopulmonary and renal impairment. Afamitresgene autoleucel’s boxed warning is for serious or fatal cytokine release syndrome, which may be severe or life-threatening.
With these approvals, the bar is now raised on TILs and TCRTs, said Betof Warner.
The lifileucel trial studied 73 patients whose melanoma had continued to metastasize despite treatment with a programmed cell death protein (PD-1)/ programmed death-ligand (PD-L1)–targeted immune checkpoint inhibitor and a BRAF inhibitor (if appropriate based on tumor mutation status), and whose lifileucel dose was at least 7.5 billion cells (the approved dose). The cohort also received a median of six IL-2 (aldesleukin) doses.
The objective response rate was 31.5% (95% CI, 21.1-43.4), and median duration of response was not reached (lower bound of 95% CI, 4.1).
In the afamitresgene autoleucel study, 44 of 52 patients with synovial sarcoma received leukapheresis and a single infusion of afamitresgene autoleucel.
The overall response rate was 43.2% (95% CI, 28.4-59.0). The median time to response was 4.9 weeks (95% CI, 4.4-8), and the median duration of response was 6 months (lower bound of 95% CI, 4.6). Among patients who were responsive to the treatment, 45.6% and 39.0% had a duration of response of 6 months or longer and 12 months or longer, respectively.
New Hope for Patients
Betof Warner and her colleagues are now recruiting for an open-label, phase 1/2 investigation of the safety and efficacy of the TIL therapy OBX-115 in adult advanced solid tumors in melanoma or non–small cell lung cancer. The first-in-human results of a previous trial were presented at the ASCO 2024 meeting, and OBX-115 received FDA fast track designation in July.
“I think the results are really promising,” said Betof Warner. “This is an engineered TIL that does not require administering IL-2 to the patient. There were four out of the nine patients who responded to the treatment and there were no dose-limiting toxicities, no cytokine and no intracranial — all of which is excellent.”
For Betof Warner, the possibility that by using their own immune system, patients with advanced and refractory cancers could soon have a one-time treatment with a cell therapy rather than innumerable bouts of chemotherapy pushes her onward.
“The idea that we can treat cancer one time and have it not recur for years — that’s pushing the start of saying there’s a cure of cancer. That a person could move on from cancer like they move on from an infection. That is the potential of this work. We’re not there yet, but that’s where we need to think and dream big,” she said.
Betof Warner disclosed consulting/advisory roles with BluePath Solutions, Bristol-Myers Squibb/Medarex, Immatics, Instil Bio, Iovance Biotherapeutics, Lyell Immunopharma, Merck, Novartis, and Pfizer and research funding and travel expenses from Iovance Biotherapeutics.
A version of this article appeared on Medscape.com.
The cutting edge of treating solid tumors with cell therapies got notably sharper in 2024.
First came the US Food and Drug Administration (FDA) approval in February 2024 of the tumor-infiltrating lymphocyte (TIL) therapy lifileucel in unresectable or metastatic melanoma that had progressed on prior immunotherapy, the first cellular therapy for any solid tumor. Then came the August FDA approval of afamitresgene autoleucel in unresectable or metastatic synovial sarcoma with failed chemotherapy, the first engineered T-cell therapy for cancers in soft tissue.
“This was a pipe dream just a decade ago,” Alison Betof Warner, MD, PhD, lead author of a lifileucel study (NCT05640193), said in an interview with Medscape Medical News. “At the start of 2024, we had no approvals of these kinds of products in solid cancers. Now we have two.”
As the director of Solid Tumor Cell Therapy and leader of Stanford Medicine’s Melanoma and Cutaneous Oncology Clinical Research Group, Betof Warner has been at the forefront of developing commercial cell therapy using tumor-infiltrating lymphocytes (TILs).
“The approval of lifileucel increases confidence that we can get these therapies across the regulatory finish line and to patients,” Betof Warner said during the interview. She was not involved in the development of afamitresgene autoleucel.
‘Reverse Engineering’
In addition to her contributions to the work that led to lifileucel’s approval, Betof Warner was the lead author on the first consensus guidelines on management and best practices for tumor-infiltrating lymphocyte cell therapy.
Betof Warner began studying TILs after doing research with her mentors in immuno-oncology, Jedd D. Wolchok and Michael A. Postow. Their investigations — including one that Betof Warner coauthored — into how monoclonal antibodies and checkpoint inhibitors, such as ipilimumab or nivolumab, might extend the lives of people with advanced unresectable or metastatic melanoma inspired her to push further to find ways to minimize treatment while maximizing outcomes for patients. Betof Warner’s interest overall, she said in the interview, is in capitalizing on what can be learned about how the immune system controls cancer.
“What we know is that the immune system has the ability to kill cancer,” Betof Warner said. “Therefore we need to be thinking about how we can increase immune surveillance. How can we enhance that before a patient develops advanced cancer?
Betof Warner said that although TILs are now standard treatment in melanoma, there is about a 30% response rate compared with about a 50% response rate in immunotherapy, and the latter is easier for the patient to withstand.
“Antibodies on the frontline are better than going through a surgery and then waiting weeks to get your therapy,” Betof Warner said in the interview. “You can come into my clinic and get an antibody therapy in 30 minutes and go straight to work. TILs require patients to be in the hospital for weeks at a time and out of work for months at a time.”
In an effort to combine therapies to maximize best outcomes, a phase 3 trial (NCT05727904) is currently recruiting. The TILVANCE-301 trial will compare immunotherapy plus adoptive cell therapy vs immunotherapy alone in untreated unresectable or metastatic melanoma. Betof Warner is not a part of this study.
Cell Therapies Include CAR T Cells and TCRT
In general, adoptive T-cell therapies such as TILs involve the isolation of autologous immune cells that are removed from the body and either expanded or modified to optimize their efficacy in fighting antigens, before their transfer to the patient as a living drug by infusion.
In addition to TILs, adoptive cell therapies for antitumor therapeutics include chimeric antigen receptor (CAR) T cells and engineered T-cell receptor therapy (TCRT).
In CAR T-cell therapy and TCRT, naive T cells are harvested from the patient’s blood then engineered to target a tumor. In TIL therapy, tumor-specific T cells are taken from the patient’s tumor. Once extracted, the respective cells are expanded billions of times and then delivered back to the patient’s body, said Betof Warner.
“The main promise of this approach is to generate responses in what we know as ‘cold’ tumors, or tumors that do not have a lot of endogenous T-cell infiltration or where the T cells are not working well, to bring in tumor targeting T cells and then trigger an immune response,” Betof Warner told an audience at the American Society of Clinical Oncology (ASCO) 2024 annual meeting.
TIL patients also receive interleukin (IL)-2 infusions to further stimulate the cells. In patients being treated with TCRT, they either receive low or no IL-2, Betof Warner said in her ASCO presentation, “Adopting Cutting-Edge Cell Therapies in Melanoma,” part of the session Beyond the Tip of the Iceberg: Next-Generation Cell-Based Therapies.
Decades in the Making
The National Cancer Institute began investigating TILs in the late 1980s, with the current National Cancer Institute (NCI) surgery chief, Steven Rosenberg, MD, PhD, leading the first-ever trials that showed TILs could shrink tumors in people with advanced melanoma.
Since then, NCI staff and others have also investigated TILs beyond melanoma and additional cell therapies based on CAR T cells and TCRT for antitumor therapeutics.
“TCRs are different from CAR Ts because they go after intracellular antigens instead of extracellular antigens,” said Betof Warner. “That has appeal because many of the tumor antigens we’re looking for will be intracellular.”
Because CAR T cells only target extracellular antigens, their utility is somewhat limited. Although several CAR T-cell therapies exist for blood cancers, there currently are no approved CAR T-cell therapies for solid tumors. However, several trials of CAR T cells in gastrointestinal cancers and melanoma are ongoing, said Betof Warner, who is not a part of these studies.
“We are starting to see early-phase efficacy in pediatric gliomas,” Betof Warner said, mentioning a study conducted by colleagues at Stanford who demonstrated potential for anti-GD2 CAR T-cell therapy in deadly pediatric diffuse midline gliomas, tumors on the spine and brain.
In their study, nine out of 11 participants (median age, 15 years) showed benefit from the cell therapy, with one participant’s tumors resolving completely. The results paved the way for the FDA to grant a Regenerative Medicine Advanced Therapy designation for use of anti-GD2 CAR T cells in H3K27M-positive diffuse midline gliomas.
The investigators are now recruiting for a phase 1 trial (NCT04196413). Results of the initial study were published in Nature last month.
Another lesser-known cell therapy expected to advance at some point in the future for solid tumors is use of the body’s natural killer (NK) cells. “They’ve been known about for a long time, but they are more difficult to regulate, which is one reason why it has taken longer to make NK cell therapies,” said Betof Warner, who is not involved in the study of NK cells. “One of their advantages is that, potentially, there could be an ‘off the shelf’ NK product. They don’t necessarily have to be made with autologous cells.”
Risk-Benefit Profiles Depend on Mechanism of Action
If the corresponding TCR sequence of a tumor antigen is known, said Betof Warner, it is possible to use leukapheresis to generate naive circulating lymphocytes. Once infused, the manufactured TCRTs will activate in the body the same as native cells because the signaling is the same.
An advantage to TCRT compared with CAR T-cell therapy is that it targets intracellular proteins, which are significantly present in the tumor, Betof Warner said in her presentation at ASCO 2024. She clarified that tumors will usually be screened for the presence of this antigen before a patient is selected for treatment with that particular therapy, because not all antigens are highly expressed in every tumor.
“Furthermore, the tumor antigen has to be presented by a major histocompatibility complex, meaning there are human leukocyte antigen restrictions, which impacts patient selection,” she said.
A risk with both TCRT and CAR T-cell therapy, according to Betof Warner, is that because there are often shared antigens between tumor and normal tissues, on-target/off-tumor toxicity is a risk.
“TILs are different because they are nonengineered, at least not for antigen recognition. They are polyclonal and go after multiple targets,” Betof Warner said. “TCRs and CARs are engineered to go after one target. So, TILs have much lower rates of on-tumor/off-target effects, vs when you engineer a very high affinity receptor like a TCR or CAR.”
A good example of how this amplification of TCR affinity can lead to poor outcomes is in metastatic melanoma, said Betof Warner.
In investigations (NCI-07-C-0174 and NCI-07-C-0175) of TCRT in metastatic melanoma, for example, the researchers were targeting MART-1 or gp100, which are expressed in melanocytes.
“The problem was that these antigens are also expressed in the eyes and ears, so it caused eye inflammation and hearing loss in a number of patients because it wasn’t specific enough for the tumor,” said Betof Warner. “So, if that target is highly expressed on normal tissue, then you have a high risk.”
Promise of PRAME
Betof Warner said the most promising TCRT at present is the investigational autologous cell therapy IMA203 (NCT03688124), which targets the preferentially expressed antigen (PRAME). Although PRAME is found in many tumors, this testis antigen does not tend to express in normal, healthy adult tissues. Betof Warner is not affiliated with this study.
“It’s maybe the most exciting TCRT cell in melanoma,” Betof Warner told her audience at the ASCO 2024 meeting. Because the expression rate of PRAME in cutaneous and uveal melanoma is at or above 95% and 90%, respectively, she said “it is a really good target in melanoma.”
Phase 1a results reported in late 2023 from a first-in-human trial of IMA203 involving 13 persons with highly advanced melanoma and a median of 5.5 previous treatments showed a 50% objective response rate in the 12 evaluable results. The duration of response ranged between 2.2 and 14.7 months (median follow-up, 14 months).
The safety profile of the treatment was favorable, with no grade 3 adverse events occurring in more than 10% of the cohort, and no grade 5 adverse events at all.
Phase 1b results published in October by maker Immatics showed that in 28 heavily pretreated metastatic melanoma patients, IMA203 had a confirmed objective response rate of 54% with a median duration of response of 12.1 months, while maintaining a favorable tolerability profile.
Accelerated Approvals, Boxed Warnings
The FDA granted accelerated approvals for both lifileucel, the TIL therapy, and afamitresgene autoleucel, the TCRT.
Both were approved with boxed warnings. Lifileucel’s warning is for treatment-related mortality, prolonged severe cytopenia, severe infection, and cardiopulmonary and renal impairment. Afamitresgene autoleucel’s boxed warning is for serious or fatal cytokine release syndrome, which may be severe or life-threatening.
With these approvals, the bar is now raised on TILs and TCRTs, said Betof Warner.
The lifileucel trial studied 73 patients whose melanoma had continued to metastasize despite treatment with a programmed cell death protein (PD-1)/ programmed death-ligand (PD-L1)–targeted immune checkpoint inhibitor and a BRAF inhibitor (if appropriate based on tumor mutation status), and whose lifileucel dose was at least 7.5 billion cells (the approved dose). The cohort also received a median of six IL-2 (aldesleukin) doses.
The objective response rate was 31.5% (95% CI, 21.1-43.4), and median duration of response was not reached (lower bound of 95% CI, 4.1).
In the afamitresgene autoleucel study, 44 of 52 patients with synovial sarcoma received leukapheresis and a single infusion of afamitresgene autoleucel.
The overall response rate was 43.2% (95% CI, 28.4-59.0). The median time to response was 4.9 weeks (95% CI, 4.4-8), and the median duration of response was 6 months (lower bound of 95% CI, 4.6). Among patients who were responsive to the treatment, 45.6% and 39.0% had a duration of response of 6 months or longer and 12 months or longer, respectively.
New Hope for Patients
Betof Warner and her colleagues are now recruiting for an open-label, phase 1/2 investigation of the safety and efficacy of the TIL therapy OBX-115 in adult advanced solid tumors in melanoma or non–small cell lung cancer. The first-in-human results of a previous trial were presented at the ASCO 2024 meeting, and OBX-115 received FDA fast track designation in July.
“I think the results are really promising,” said Betof Warner. “This is an engineered TIL that does not require administering IL-2 to the patient. There were four out of the nine patients who responded to the treatment and there were no dose-limiting toxicities, no cytokine and no intracranial — all of which is excellent.”
For Betof Warner, the possibility that by using their own immune system, patients with advanced and refractory cancers could soon have a one-time treatment with a cell therapy rather than innumerable bouts of chemotherapy pushes her onward.
“The idea that we can treat cancer one time and have it not recur for years — that’s pushing the start of saying there’s a cure of cancer. That a person could move on from cancer like they move on from an infection. That is the potential of this work. We’re not there yet, but that’s where we need to think and dream big,” she said.
Betof Warner disclosed consulting/advisory roles with BluePath Solutions, Bristol-Myers Squibb/Medarex, Immatics, Instil Bio, Iovance Biotherapeutics, Lyell Immunopharma, Merck, Novartis, and Pfizer and research funding and travel expenses from Iovance Biotherapeutics.
A version of this article appeared on Medscape.com.
Treatment of Glioblastoma: A Potential Shift in Paradigm
Treatment of Glioblastoma: A Potential Shift in Paradigm
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
Treatment of Glioblastoma: A Potential Shift in Paradigm
Treatment of Glioblastoma: A Potential Shift in Paradigm
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
New Cancer Drugs: Do Patients Prefer Faster Access or Clinical Benefit?
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
New Cancer Vaccines on the Horizon: Renewed Hope or Hype?
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vorasidenib for Certain IDH-Mutant Gliomas: Is It Worth It?
After years with limited treatment options, experts hailed vorasidenib “a promising breakthrough,” “a paradigm shift,” a “new hope,” and “probably the most important advance in the treatment of low-grade gliomas in the last decade.”
Promising results from vorasidenib’s pivotal INDIGO trial fueled petitions and patient advocacy circles to push for the drug’s approval. And, in August 2024, the Food and Drug Administration (FDA) approved vorasidenib for grade 2 astrocytomas or oligodendrogliomas with an IDH1 or IDH2 mutation.
But following the approval, some experts expressed concerns and doubts about the drug and the INDIGO trial, bringing a host of unanswered questions into sharper focus.
In an editorial, Stanislav Lazarev, MD, and Kunal K. Sindhu, MD, both radiation oncologists from Icahn School of Medicine at Mount Sinai, New York City, suggest that the FDA approval “might be premature given the high cost of this drug and lack of clear benefit over standard treatments.”
Another recent critique also pointed to the lack of clear evidence that vorasidenib is superior to the prevailing standard of care, despite the drug’s high cost. These authors noted that “patients want to live longer, and if not, at least live better,” but “based on the INDIGO study, it is impossible to say whether vorasidenib can provide either.”
Vorasidenib is now one of the most expensive cancer therapies, with an annual cost of nearly $500,000, but the INDIGO trial did not explore whether the drug led to improved overall survival or better quality of life. Among the trial’s design flaws, experts called out the use of progression-free survival as the primary outcome, instead of overall survival, and the use of an inappropriate comparator group.
INDIGO was a phase 3 trial that included 331 adult patients (median age, 40.5 years) with grade-2 IDH-mutant recurrent or residual glioma after surgery. To be eligible, patients had to be followed for at least 1 year, and up to 5 years, post surgery and had to be considered appropriate candidates for a watch-and-wait approach.
Participants were randomly assigned to receive either 40 mg of vorasidenib or a matching placebo orally, once daily, in continuous 28-day cycles until imaging-confirmed tumor disease progression or unacceptable toxicity, at which point crossover to vorasidenib from placebo was permitted. Over one third (n = 58) of patients in the placebo group crossed over and 90% of them (n = 52) received vorasidenib.
Median progression-free survival was significantly better in the vorasidenib group at 27.7 months vs 11.1 months in the placebo group (hazard ratio [HR], 0.39).
A key secondary endpoint — time to next intervention — was also significant; the likelihood of being alive and not receiving further treatment at 18 months was 85.6% in the vorasidenib group and 47.4% in the placebo group (HR, 0.26). This finding indicates that most patients receiving vorasidenib could delay chemoradiation for 18 months or longer.
Despite these impressive outcomes, some experts noted that using progression-free survival as the primary endpoint was a major flaw of the INDIGO trial because, currently, there is no evidence that progression-free survival is a reliable surrogate endpoint for overall survival in this setting.
The high rate of crossover to vorasidenib is another issue because it may limit a longer-term analysis of overall survival. If, for instance, overall survival is the same between the groups, it could signal that the drug is effective in both groups or, alternatively, that the drug has no effect on survival in either group.
“That is a legitimate concern,” Seema Nagpal, MD, a neuro-oncologist at Stanford University in California, and a site principal investigator for the INDIGO trial, said in an interview. “We don’t know that this drug changes overall survival, and I think we’re not going to get a super clean answer on that.”
Another major issue centers on the standard of care assigned to control patients in the INDIGO trial.
In the trial, vorasidenib was compared with placebo — an appropriate standard-of-care comparison for patients with low-risk gliomas. These patients often initially undergo watch-and-wait to delay chemoradiation. But Lazarev and Sindhu argue that the patients in INDIGO were really high risk, which means the control group should have received the standard of care for these patients: Chemoradiation following surgery.
This question about the appropriate standard of care stems from ongoing uncertainty about the distinction between high- and low-risk gliomas.
The classification for gliomas falls into either low risk or high risk for early disease progression. The RTOG 9802 criteria, often used for glioma risk stratification, defines low-risk patients as those younger than 40 years with gross total resection and high-risk patients as those aged 40 years or older with any extent of resection or those younger than 40 years with subtotal or biopsy resection.
But an evolving understanding of genetic anomalies that affect prognoses in this tumor type has muddied the current high- and low-risk distinctions.
“People haven’t totally figured out what high and low risk means,” Nagpal acknowledged.
This uncertainty has spilled over into the INDIGO trial.
While the trial excluded patients who had any features indicating high risk, such as brain stem involvement or neurocognitive deficits, the researchers also did not explicitly define patients as low risk. However, the inclusion criteria specified that patients had to be observed for at least 1 year after surgery and be considered appropriate for a watch-and-wait protocol, which does suggest patients were considered low risk, said Nagpal.
Still, some experts argue that the patients in INDIGO were not low risk.
Patients had residual or recurrent disease so “wouldn’t be classified as low risk,” said Sindhu in an interview. The standard of care for these patients is chemoradiation, Lazarev added.
“The definition of a phase 3 clinical trial is that you compare the novel intervention to the standard of care,” said Lazarev. “Level 1 evidence clearly shows that omitting chemoradiation leads to worse outcomes, with patients literally dying sooner. For the investigators to knowingly exclude this proven treatment raises serious ethical and methodological questions about the study’s design.”
In a recent opinion piece, Nagpal agreed that most patients selected for INDIGO would not have been considered low risk by many providers. All patients selected for INDIGO had postoperative residual/recurrent disease and many were older than 40 years.
But, Nagpal explained, the risk stratification of the INDIGO patients was still lower than what is commonly considered high risk. The patients had all been observed for a year or more already, “so by definition, the clinician treating them already decided they were not high risk,” she said.
In another recent opinion piece, oncologists suggested that, because patients in the INDIGO trial do not squarely fall into either category, instead representing a “grey area,” it’s time to create a new risk category.
“Perhaps the time has come to abandon the old binary risk stratification (“low risk” vs “high risk”), which still contains arbitrary elements (such as the age cutoff), proving impractical in real-world clinical decision-making, and to adopt a new one, also taking into account many emerging prognostic biomarkers,” the authors wrote.
Despite the uncertainty surrounding risk categories, the INDIGO authors justified their study design.
A watch-and-wait period for patients in the trial, which “represents the earliest clinical phase in tumorigenesis of IDH-mutant WHO grade 2 glioma,” is “an opportunity to detect a clear signal of antitumor activity for new therapies in placebo-controlled trials” and “postpone the use of radiation therapy and chemotherapy,” the authors explained.
Lazarev, however, questioned the premise that chemoradiation should be delayed.
Oncologists’ desire to delay chemoradiation for their patients reflects “a limited understanding of modern irradiation therapy,” Lazarev said. “Modern technology has improved dramatically. We’re more precise, our understanding about late side effects is better. So, the big picture is that the absolute risk of late neurocognitive affects that actually will affect patients’ quality of life, their ability to work, go to school, succeed on a personal or professional level is exceedingly low.”
Nagpal strongly disagreed.
“Please come to my clinic and ask an actual patient,” said Nagpal. “Once a radiation oncologist has irradiated the patient, they almost never seen them again. People who are on the medical side, who follow these patients from beginning to end, recognize that delaying radiation is a huge deal.”
Although vorasidenib isn’t a cure, Nagpal said, it is a less toxic way to delay radiation “because that is a real and disabling thing” for patients and is why neuro-oncologists are excited about alternative treatment options.
Another issue surrounding the vorasidenib approval lies in the FDA’s vague prescribing information. The prescribing information does not specify that patients should be followed for at least 1 year post surgery or that patients need to be lower risk. Prescribing physicians may, therefore, think vorasidenib is appropriate for any patient with a grade-2 IDH mutant glioma at any time and defer or not offer chemoradiation to high-risk patients.
Amid lingering questions about the INDIGO trial design and ongoing uncertainties about how to define and treat this patient population, experts remain divided on whether vorasidenib is worth it.
“If vorasidenib is truly transformative, it should be feasible to demonstrate its superiority over chemoradiotherapy,” Lazarev and Sindhu wrote. “For a drug with such a staggering price tag, an imperative should be placed on the investigators and manufacturer to provide clear evidence of efficacy, whether in terms of improved [overall survival] or quality of life, before vorasidenib is recommended for the treatment of IDH-mutant low-grade gliomas.”
The INDIGO trial was supported by Servier, the manufacturer of vorasidenib. Many of the study authors reported employment or support from the company. Nagpal reported consulting fees from Servier and AnHeart Therapeutics. Lazarev and Sindhu reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
After years with limited treatment options, experts hailed vorasidenib “a promising breakthrough,” “a paradigm shift,” a “new hope,” and “probably the most important advance in the treatment of low-grade gliomas in the last decade.”
Promising results from vorasidenib’s pivotal INDIGO trial fueled petitions and patient advocacy circles to push for the drug’s approval. And, in August 2024, the Food and Drug Administration (FDA) approved vorasidenib for grade 2 astrocytomas or oligodendrogliomas with an IDH1 or IDH2 mutation.
But following the approval, some experts expressed concerns and doubts about the drug and the INDIGO trial, bringing a host of unanswered questions into sharper focus.
In an editorial, Stanislav Lazarev, MD, and Kunal K. Sindhu, MD, both radiation oncologists from Icahn School of Medicine at Mount Sinai, New York City, suggest that the FDA approval “might be premature given the high cost of this drug and lack of clear benefit over standard treatments.”
Another recent critique also pointed to the lack of clear evidence that vorasidenib is superior to the prevailing standard of care, despite the drug’s high cost. These authors noted that “patients want to live longer, and if not, at least live better,” but “based on the INDIGO study, it is impossible to say whether vorasidenib can provide either.”
Vorasidenib is now one of the most expensive cancer therapies, with an annual cost of nearly $500,000, but the INDIGO trial did not explore whether the drug led to improved overall survival or better quality of life. Among the trial’s design flaws, experts called out the use of progression-free survival as the primary outcome, instead of overall survival, and the use of an inappropriate comparator group.
INDIGO was a phase 3 trial that included 331 adult patients (median age, 40.5 years) with grade-2 IDH-mutant recurrent or residual glioma after surgery. To be eligible, patients had to be followed for at least 1 year, and up to 5 years, post surgery and had to be considered appropriate candidates for a watch-and-wait approach.
Participants were randomly assigned to receive either 40 mg of vorasidenib or a matching placebo orally, once daily, in continuous 28-day cycles until imaging-confirmed tumor disease progression or unacceptable toxicity, at which point crossover to vorasidenib from placebo was permitted. Over one third (n = 58) of patients in the placebo group crossed over and 90% of them (n = 52) received vorasidenib.
Median progression-free survival was significantly better in the vorasidenib group at 27.7 months vs 11.1 months in the placebo group (hazard ratio [HR], 0.39).
A key secondary endpoint — time to next intervention — was also significant; the likelihood of being alive and not receiving further treatment at 18 months was 85.6% in the vorasidenib group and 47.4% in the placebo group (HR, 0.26). This finding indicates that most patients receiving vorasidenib could delay chemoradiation for 18 months or longer.
Despite these impressive outcomes, some experts noted that using progression-free survival as the primary endpoint was a major flaw of the INDIGO trial because, currently, there is no evidence that progression-free survival is a reliable surrogate endpoint for overall survival in this setting.
The high rate of crossover to vorasidenib is another issue because it may limit a longer-term analysis of overall survival. If, for instance, overall survival is the same between the groups, it could signal that the drug is effective in both groups or, alternatively, that the drug has no effect on survival in either group.
“That is a legitimate concern,” Seema Nagpal, MD, a neuro-oncologist at Stanford University in California, and a site principal investigator for the INDIGO trial, said in an interview. “We don’t know that this drug changes overall survival, and I think we’re not going to get a super clean answer on that.”
Another major issue centers on the standard of care assigned to control patients in the INDIGO trial.
In the trial, vorasidenib was compared with placebo — an appropriate standard-of-care comparison for patients with low-risk gliomas. These patients often initially undergo watch-and-wait to delay chemoradiation. But Lazarev and Sindhu argue that the patients in INDIGO were really high risk, which means the control group should have received the standard of care for these patients: Chemoradiation following surgery.
This question about the appropriate standard of care stems from ongoing uncertainty about the distinction between high- and low-risk gliomas.
The classification for gliomas falls into either low risk or high risk for early disease progression. The RTOG 9802 criteria, often used for glioma risk stratification, defines low-risk patients as those younger than 40 years with gross total resection and high-risk patients as those aged 40 years or older with any extent of resection or those younger than 40 years with subtotal or biopsy resection.
But an evolving understanding of genetic anomalies that affect prognoses in this tumor type has muddied the current high- and low-risk distinctions.
“People haven’t totally figured out what high and low risk means,” Nagpal acknowledged.
This uncertainty has spilled over into the INDIGO trial.
While the trial excluded patients who had any features indicating high risk, such as brain stem involvement or neurocognitive deficits, the researchers also did not explicitly define patients as low risk. However, the inclusion criteria specified that patients had to be observed for at least 1 year after surgery and be considered appropriate for a watch-and-wait protocol, which does suggest patients were considered low risk, said Nagpal.
Still, some experts argue that the patients in INDIGO were not low risk.
Patients had residual or recurrent disease so “wouldn’t be classified as low risk,” said Sindhu in an interview. The standard of care for these patients is chemoradiation, Lazarev added.
“The definition of a phase 3 clinical trial is that you compare the novel intervention to the standard of care,” said Lazarev. “Level 1 evidence clearly shows that omitting chemoradiation leads to worse outcomes, with patients literally dying sooner. For the investigators to knowingly exclude this proven treatment raises serious ethical and methodological questions about the study’s design.”
In a recent opinion piece, Nagpal agreed that most patients selected for INDIGO would not have been considered low risk by many providers. All patients selected for INDIGO had postoperative residual/recurrent disease and many were older than 40 years.
But, Nagpal explained, the risk stratification of the INDIGO patients was still lower than what is commonly considered high risk. The patients had all been observed for a year or more already, “so by definition, the clinician treating them already decided they were not high risk,” she said.
In another recent opinion piece, oncologists suggested that, because patients in the INDIGO trial do not squarely fall into either category, instead representing a “grey area,” it’s time to create a new risk category.
“Perhaps the time has come to abandon the old binary risk stratification (“low risk” vs “high risk”), which still contains arbitrary elements (such as the age cutoff), proving impractical in real-world clinical decision-making, and to adopt a new one, also taking into account many emerging prognostic biomarkers,” the authors wrote.
Despite the uncertainty surrounding risk categories, the INDIGO authors justified their study design.
A watch-and-wait period for patients in the trial, which “represents the earliest clinical phase in tumorigenesis of IDH-mutant WHO grade 2 glioma,” is “an opportunity to detect a clear signal of antitumor activity for new therapies in placebo-controlled trials” and “postpone the use of radiation therapy and chemotherapy,” the authors explained.
Lazarev, however, questioned the premise that chemoradiation should be delayed.
Oncologists’ desire to delay chemoradiation for their patients reflects “a limited understanding of modern irradiation therapy,” Lazarev said. “Modern technology has improved dramatically. We’re more precise, our understanding about late side effects is better. So, the big picture is that the absolute risk of late neurocognitive affects that actually will affect patients’ quality of life, their ability to work, go to school, succeed on a personal or professional level is exceedingly low.”
Nagpal strongly disagreed.
“Please come to my clinic and ask an actual patient,” said Nagpal. “Once a radiation oncologist has irradiated the patient, they almost never seen them again. People who are on the medical side, who follow these patients from beginning to end, recognize that delaying radiation is a huge deal.”
Although vorasidenib isn’t a cure, Nagpal said, it is a less toxic way to delay radiation “because that is a real and disabling thing” for patients and is why neuro-oncologists are excited about alternative treatment options.
Another issue surrounding the vorasidenib approval lies in the FDA’s vague prescribing information. The prescribing information does not specify that patients should be followed for at least 1 year post surgery or that patients need to be lower risk. Prescribing physicians may, therefore, think vorasidenib is appropriate for any patient with a grade-2 IDH mutant glioma at any time and defer or not offer chemoradiation to high-risk patients.
Amid lingering questions about the INDIGO trial design and ongoing uncertainties about how to define and treat this patient population, experts remain divided on whether vorasidenib is worth it.
“If vorasidenib is truly transformative, it should be feasible to demonstrate its superiority over chemoradiotherapy,” Lazarev and Sindhu wrote. “For a drug with such a staggering price tag, an imperative should be placed on the investigators and manufacturer to provide clear evidence of efficacy, whether in terms of improved [overall survival] or quality of life, before vorasidenib is recommended for the treatment of IDH-mutant low-grade gliomas.”
The INDIGO trial was supported by Servier, the manufacturer of vorasidenib. Many of the study authors reported employment or support from the company. Nagpal reported consulting fees from Servier and AnHeart Therapeutics. Lazarev and Sindhu reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
After years with limited treatment options, experts hailed vorasidenib “a promising breakthrough,” “a paradigm shift,” a “new hope,” and “probably the most important advance in the treatment of low-grade gliomas in the last decade.”
Promising results from vorasidenib’s pivotal INDIGO trial fueled petitions and patient advocacy circles to push for the drug’s approval. And, in August 2024, the Food and Drug Administration (FDA) approved vorasidenib for grade 2 astrocytomas or oligodendrogliomas with an IDH1 or IDH2 mutation.
But following the approval, some experts expressed concerns and doubts about the drug and the INDIGO trial, bringing a host of unanswered questions into sharper focus.
In an editorial, Stanislav Lazarev, MD, and Kunal K. Sindhu, MD, both radiation oncologists from Icahn School of Medicine at Mount Sinai, New York City, suggest that the FDA approval “might be premature given the high cost of this drug and lack of clear benefit over standard treatments.”
Another recent critique also pointed to the lack of clear evidence that vorasidenib is superior to the prevailing standard of care, despite the drug’s high cost. These authors noted that “patients want to live longer, and if not, at least live better,” but “based on the INDIGO study, it is impossible to say whether vorasidenib can provide either.”
Vorasidenib is now one of the most expensive cancer therapies, with an annual cost of nearly $500,000, but the INDIGO trial did not explore whether the drug led to improved overall survival or better quality of life. Among the trial’s design flaws, experts called out the use of progression-free survival as the primary outcome, instead of overall survival, and the use of an inappropriate comparator group.
INDIGO was a phase 3 trial that included 331 adult patients (median age, 40.5 years) with grade-2 IDH-mutant recurrent or residual glioma after surgery. To be eligible, patients had to be followed for at least 1 year, and up to 5 years, post surgery and had to be considered appropriate candidates for a watch-and-wait approach.
Participants were randomly assigned to receive either 40 mg of vorasidenib or a matching placebo orally, once daily, in continuous 28-day cycles until imaging-confirmed tumor disease progression or unacceptable toxicity, at which point crossover to vorasidenib from placebo was permitted. Over one third (n = 58) of patients in the placebo group crossed over and 90% of them (n = 52) received vorasidenib.
Median progression-free survival was significantly better in the vorasidenib group at 27.7 months vs 11.1 months in the placebo group (hazard ratio [HR], 0.39).
A key secondary endpoint — time to next intervention — was also significant; the likelihood of being alive and not receiving further treatment at 18 months was 85.6% in the vorasidenib group and 47.4% in the placebo group (HR, 0.26). This finding indicates that most patients receiving vorasidenib could delay chemoradiation for 18 months or longer.
Despite these impressive outcomes, some experts noted that using progression-free survival as the primary endpoint was a major flaw of the INDIGO trial because, currently, there is no evidence that progression-free survival is a reliable surrogate endpoint for overall survival in this setting.
The high rate of crossover to vorasidenib is another issue because it may limit a longer-term analysis of overall survival. If, for instance, overall survival is the same between the groups, it could signal that the drug is effective in both groups or, alternatively, that the drug has no effect on survival in either group.
“That is a legitimate concern,” Seema Nagpal, MD, a neuro-oncologist at Stanford University in California, and a site principal investigator for the INDIGO trial, said in an interview. “We don’t know that this drug changes overall survival, and I think we’re not going to get a super clean answer on that.”
Another major issue centers on the standard of care assigned to control patients in the INDIGO trial.
In the trial, vorasidenib was compared with placebo — an appropriate standard-of-care comparison for patients with low-risk gliomas. These patients often initially undergo watch-and-wait to delay chemoradiation. But Lazarev and Sindhu argue that the patients in INDIGO were really high risk, which means the control group should have received the standard of care for these patients: Chemoradiation following surgery.
This question about the appropriate standard of care stems from ongoing uncertainty about the distinction between high- and low-risk gliomas.
The classification for gliomas falls into either low risk or high risk for early disease progression. The RTOG 9802 criteria, often used for glioma risk stratification, defines low-risk patients as those younger than 40 years with gross total resection and high-risk patients as those aged 40 years or older with any extent of resection or those younger than 40 years with subtotal or biopsy resection.
But an evolving understanding of genetic anomalies that affect prognoses in this tumor type has muddied the current high- and low-risk distinctions.
“People haven’t totally figured out what high and low risk means,” Nagpal acknowledged.
This uncertainty has spilled over into the INDIGO trial.
While the trial excluded patients who had any features indicating high risk, such as brain stem involvement or neurocognitive deficits, the researchers also did not explicitly define patients as low risk. However, the inclusion criteria specified that patients had to be observed for at least 1 year after surgery and be considered appropriate for a watch-and-wait protocol, which does suggest patients were considered low risk, said Nagpal.
Still, some experts argue that the patients in INDIGO were not low risk.
Patients had residual or recurrent disease so “wouldn’t be classified as low risk,” said Sindhu in an interview. The standard of care for these patients is chemoradiation, Lazarev added.
“The definition of a phase 3 clinical trial is that you compare the novel intervention to the standard of care,” said Lazarev. “Level 1 evidence clearly shows that omitting chemoradiation leads to worse outcomes, with patients literally dying sooner. For the investigators to knowingly exclude this proven treatment raises serious ethical and methodological questions about the study’s design.”
In a recent opinion piece, Nagpal agreed that most patients selected for INDIGO would not have been considered low risk by many providers. All patients selected for INDIGO had postoperative residual/recurrent disease and many were older than 40 years.
But, Nagpal explained, the risk stratification of the INDIGO patients was still lower than what is commonly considered high risk. The patients had all been observed for a year or more already, “so by definition, the clinician treating them already decided they were not high risk,” she said.
In another recent opinion piece, oncologists suggested that, because patients in the INDIGO trial do not squarely fall into either category, instead representing a “grey area,” it’s time to create a new risk category.
“Perhaps the time has come to abandon the old binary risk stratification (“low risk” vs “high risk”), which still contains arbitrary elements (such as the age cutoff), proving impractical in real-world clinical decision-making, and to adopt a new one, also taking into account many emerging prognostic biomarkers,” the authors wrote.
Despite the uncertainty surrounding risk categories, the INDIGO authors justified their study design.
A watch-and-wait period for patients in the trial, which “represents the earliest clinical phase in tumorigenesis of IDH-mutant WHO grade 2 glioma,” is “an opportunity to detect a clear signal of antitumor activity for new therapies in placebo-controlled trials” and “postpone the use of radiation therapy and chemotherapy,” the authors explained.
Lazarev, however, questioned the premise that chemoradiation should be delayed.
Oncologists’ desire to delay chemoradiation for their patients reflects “a limited understanding of modern irradiation therapy,” Lazarev said. “Modern technology has improved dramatically. We’re more precise, our understanding about late side effects is better. So, the big picture is that the absolute risk of late neurocognitive affects that actually will affect patients’ quality of life, their ability to work, go to school, succeed on a personal or professional level is exceedingly low.”
Nagpal strongly disagreed.
“Please come to my clinic and ask an actual patient,” said Nagpal. “Once a radiation oncologist has irradiated the patient, they almost never seen them again. People who are on the medical side, who follow these patients from beginning to end, recognize that delaying radiation is a huge deal.”
Although vorasidenib isn’t a cure, Nagpal said, it is a less toxic way to delay radiation “because that is a real and disabling thing” for patients and is why neuro-oncologists are excited about alternative treatment options.
Another issue surrounding the vorasidenib approval lies in the FDA’s vague prescribing information. The prescribing information does not specify that patients should be followed for at least 1 year post surgery or that patients need to be lower risk. Prescribing physicians may, therefore, think vorasidenib is appropriate for any patient with a grade-2 IDH mutant glioma at any time and defer or not offer chemoradiation to high-risk patients.
Amid lingering questions about the INDIGO trial design and ongoing uncertainties about how to define and treat this patient population, experts remain divided on whether vorasidenib is worth it.
“If vorasidenib is truly transformative, it should be feasible to demonstrate its superiority over chemoradiotherapy,” Lazarev and Sindhu wrote. “For a drug with such a staggering price tag, an imperative should be placed on the investigators and manufacturer to provide clear evidence of efficacy, whether in terms of improved [overall survival] or quality of life, before vorasidenib is recommended for the treatment of IDH-mutant low-grade gliomas.”
The INDIGO trial was supported by Servier, the manufacturer of vorasidenib. Many of the study authors reported employment or support from the company. Nagpal reported consulting fees from Servier and AnHeart Therapeutics. Lazarev and Sindhu reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Inside the Patient-Oncologist Bond: Why It’s Often So Strong
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
For Radiation ‘Downwinders,’ Cancer Compensation Is On Hold
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.