User login
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7
Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
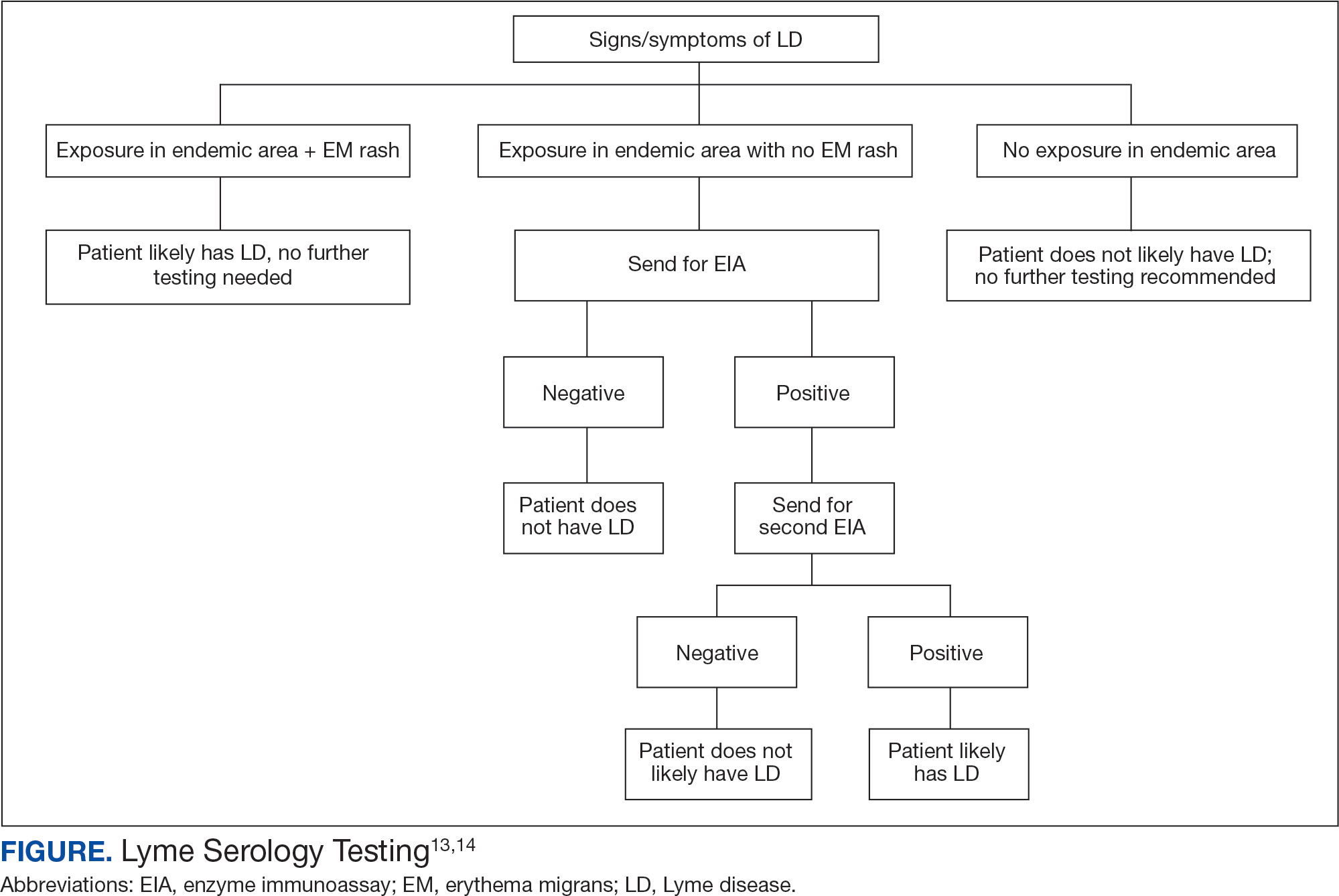
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.
Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
THE DIAGNOSIS: Cutaneous Leishmaniasis
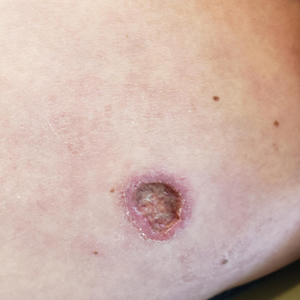
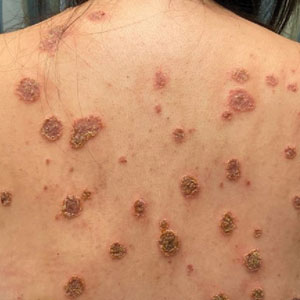
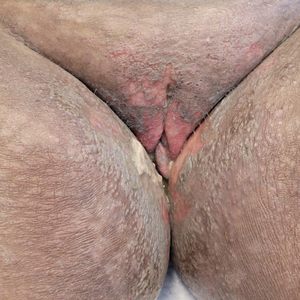
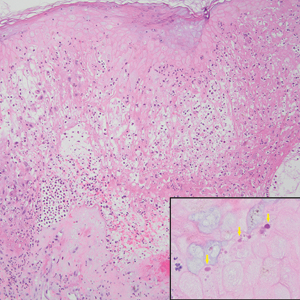
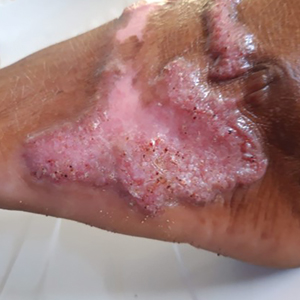
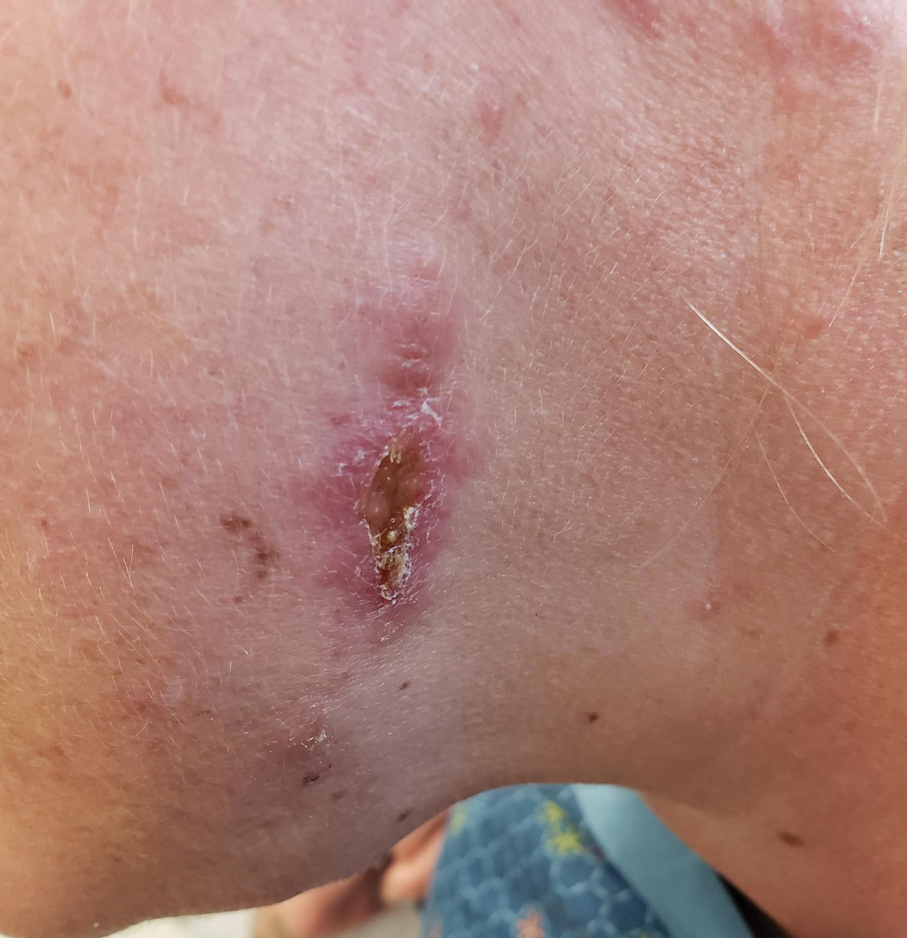
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.
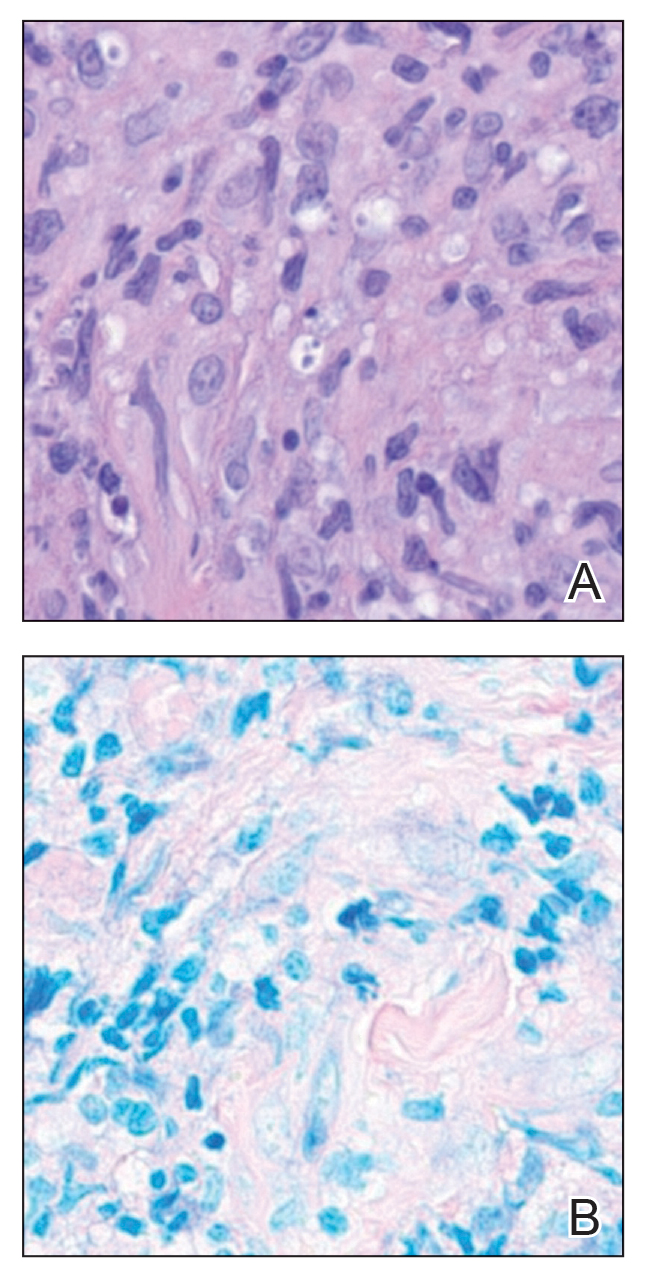
Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).
Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
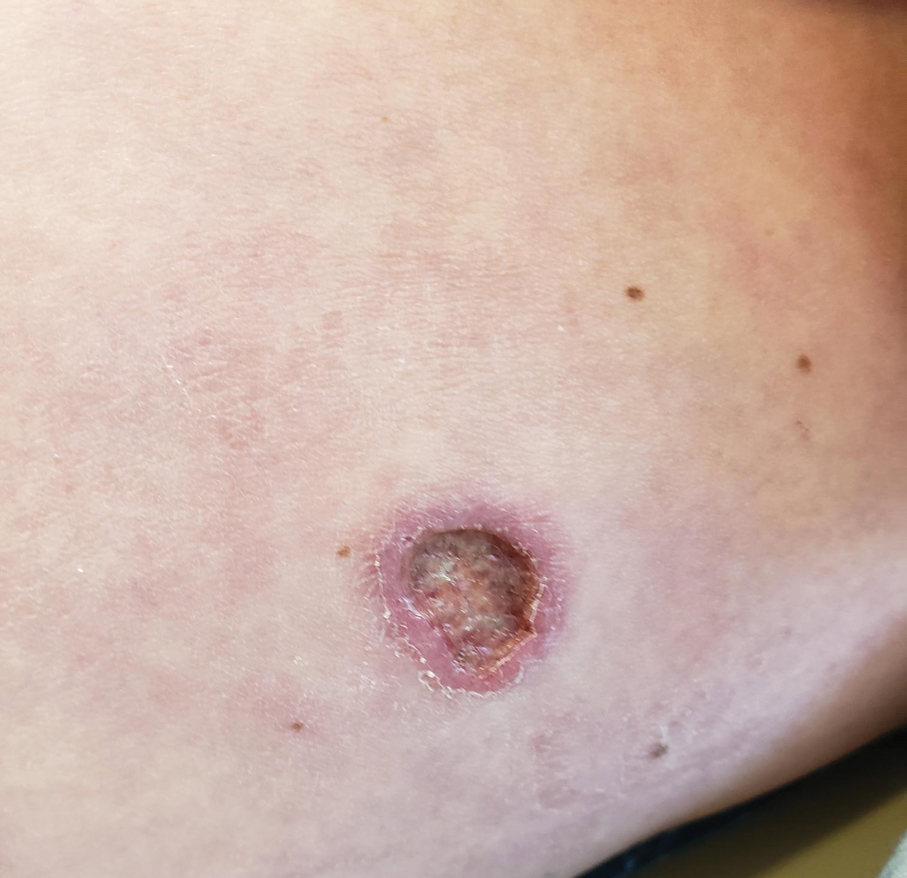
A 43-year-old woman presented to the dermatology clinic with widespread scaly plaques and ulcerations of 2 months’ duration. Her medical history was otherwise unremarkable. The patient reported that the eruption began after returning from a vacation to Costa Rica, during which she spent time on the beach and white-water rafting. She noted that she had been exposed to numerous insects during her trip, and that her roommate, who had accompanied her, had similar exposure history and lesions. The plaques were refractory to multiple oral antibiotics previously prescribed by primary care. Physical examination revealed submental lymphadenopathy and painless ulcerations with indurated borders without purulent drainage alongside scattered scaly papules and plaques on the face, neck, arms, and legs. A biopsy was taken from an ulceration edge on the left thigh.
Military-Backed French Biotech Brings Ricin Antidote
Military-Backed French Biotech Brings Ricin Antidote
France has authorized Ricimed, the first antibody-based treatment specifically indicated for acute ricin intoxication, providing clinicians with a targeted option beyond supportive care for exposure to one of the most lethal naturally occurring toxins.
Fabentech is a French biopharmaceutical company specializing in medical countermeasures against biological threats and infectious diseases.
The polyclonal antibody technology used in the development of Ricimed has received marketing authorization in France as a treatment for ricin poisoning. Ricin is a highly toxic natural substance that can cause death within hours to a few days of exposure.
Supported by the Ministry of Armed Forces and Veterans Affairs (Directorate General of Armaments [DGA] and Armed Forces Health Service) in France, Ricimed is the first approved antidote for ricin poisoning, a condition for which treatment was previously limited to supportive measures alone.
Historical Incident
One incident, in particular, remains etched in espionage history. On September 7, 1978 in London during the Cold War, Bulgarian dissident writer Georgi Markov, living in exile, was struck by the umbrella of a passer-by while waiting at a bus stop. He felt a slight sting. Four days later, he died in the hospital due to a sudden and unexplained illness. An autopsy revealed that he had been poisoned by a tiny metal pellet implanted at the tip of an umbrella containing ricin, a lethal toxin. The legend of the “Bulgarian umbrella,” later invoked in other assassination attempts, was born.
Since then, although Markov remains the only known individual to have been killed by ricin poisoning, this theoretically extremely toxic substance, which can be manufactured relatively easily from castor beans, a widely available plant, has continued to fascinate authors of thrillers and spy novels.
Numerous works of fiction depict characters who succumb to ricin poisoning. The toxin is notably portrayed as a favored weapon of the main character in the hit television series Breaking Bad.
However, ricin is not confined to the realm of science fiction. For several years, authorities in various countries have feared that extremist groups could carry out attacks using ricin. The threat has been taken particularly seriously since 2018, when a clandestine ricin laboratory operated by members of the Islamic State was dismantled in Germany. Since then, several similar attack plots have been thwarted.
This context triggered a race among major powers to develop an effective antidote as quickly as possible. In this effort, Fabentech has risen to a challenge.
“Having demonstrated its ability to target and then neutralize ricin before it causes irreparable damage, Ricimed is a treatment that works based on polyclonal antibodies and compensates for the absence of a vaccine or specific treatment,” Fabentech said in a press release.
The polyclonal antibody technology used by Fabentech offers potential for the development of antidotes against bioterrorist attacks and for the treatment of many infectious diseases.
Ricimed contributed to the deployment of a European health shield against intentional biological threats in France.
Military Backing
Speaking to Le Figaro, France’s oldest national newspaper, Fabentech CEO Sébastien Iva explained that ricin disrupts the body by halting cell function, while noting several other drug candidates in development at the firm.
Typically, the lungs sustain fatal damage. Our treatment interrupts this toxic process. In animals administered the antidote, we observed pulmonary function recovery, allowing survival.
Given that the possibility of terrorist attacks using ricin is considered a national security issue, Fabentech benefited from the support by the Ministry of the Armed Forces and the DGA and lasted nearly a decade of research and development work.
The granting of marketing authorisation was also supported by the French Armed Forces and welcomed by the French Minister of the Armed Forces, Catherine Vautrin, who previously served as France’s Minister of Labour, Health, and Solidarity.
“Supporting the development of companies in France capable of manufacturing antidotes against certain biological agents helps guarantee the operational superiority of our armed forces. Developing and producing such drugs when they do not yet exist on the market is also serving the nation and the public interest,” she said.
Although the threat posed by ricin remains hypothetical, Fabentech reports a strong interest from potential clients, with many countries seeking protection against possible bioterrorist attacks.
The DGA had already placed an order for several doses of Ricimed for deployment in France. For optimal effectiveness, the antidote must be administered within 6 hours of poisoning. Iva confirmed that multiple countries had already expressed interest in acquiring the antidote.
This story was translated from JIM, part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
France has authorized Ricimed, the first antibody-based treatment specifically indicated for acute ricin intoxication, providing clinicians with a targeted option beyond supportive care for exposure to one of the most lethal naturally occurring toxins.
Fabentech is a French biopharmaceutical company specializing in medical countermeasures against biological threats and infectious diseases.
The polyclonal antibody technology used in the development of Ricimed has received marketing authorization in France as a treatment for ricin poisoning. Ricin is a highly toxic natural substance that can cause death within hours to a few days of exposure.
Supported by the Ministry of Armed Forces and Veterans Affairs (Directorate General of Armaments [DGA] and Armed Forces Health Service) in France, Ricimed is the first approved antidote for ricin poisoning, a condition for which treatment was previously limited to supportive measures alone.
Historical Incident
One incident, in particular, remains etched in espionage history. On September 7, 1978 in London during the Cold War, Bulgarian dissident writer Georgi Markov, living in exile, was struck by the umbrella of a passer-by while waiting at a bus stop. He felt a slight sting. Four days later, he died in the hospital due to a sudden and unexplained illness. An autopsy revealed that he had been poisoned by a tiny metal pellet implanted at the tip of an umbrella containing ricin, a lethal toxin. The legend of the “Bulgarian umbrella,” later invoked in other assassination attempts, was born.
Since then, although Markov remains the only known individual to have been killed by ricin poisoning, this theoretically extremely toxic substance, which can be manufactured relatively easily from castor beans, a widely available plant, has continued to fascinate authors of thrillers and spy novels.
Numerous works of fiction depict characters who succumb to ricin poisoning. The toxin is notably portrayed as a favored weapon of the main character in the hit television series Breaking Bad.
However, ricin is not confined to the realm of science fiction. For several years, authorities in various countries have feared that extremist groups could carry out attacks using ricin. The threat has been taken particularly seriously since 2018, when a clandestine ricin laboratory operated by members of the Islamic State was dismantled in Germany. Since then, several similar attack plots have been thwarted.
This context triggered a race among major powers to develop an effective antidote as quickly as possible. In this effort, Fabentech has risen to a challenge.
“Having demonstrated its ability to target and then neutralize ricin before it causes irreparable damage, Ricimed is a treatment that works based on polyclonal antibodies and compensates for the absence of a vaccine or specific treatment,” Fabentech said in a press release.
The polyclonal antibody technology used by Fabentech offers potential for the development of antidotes against bioterrorist attacks and for the treatment of many infectious diseases.
Ricimed contributed to the deployment of a European health shield against intentional biological threats in France.
Military Backing
Speaking to Le Figaro, France’s oldest national newspaper, Fabentech CEO Sébastien Iva explained that ricin disrupts the body by halting cell function, while noting several other drug candidates in development at the firm.
Typically, the lungs sustain fatal damage. Our treatment interrupts this toxic process. In animals administered the antidote, we observed pulmonary function recovery, allowing survival.
Given that the possibility of terrorist attacks using ricin is considered a national security issue, Fabentech benefited from the support by the Ministry of the Armed Forces and the DGA and lasted nearly a decade of research and development work.
The granting of marketing authorisation was also supported by the French Armed Forces and welcomed by the French Minister of the Armed Forces, Catherine Vautrin, who previously served as France’s Minister of Labour, Health, and Solidarity.
“Supporting the development of companies in France capable of manufacturing antidotes against certain biological agents helps guarantee the operational superiority of our armed forces. Developing and producing such drugs when they do not yet exist on the market is also serving the nation and the public interest,” she said.
Although the threat posed by ricin remains hypothetical, Fabentech reports a strong interest from potential clients, with many countries seeking protection against possible bioterrorist attacks.
The DGA had already placed an order for several doses of Ricimed for deployment in France. For optimal effectiveness, the antidote must be administered within 6 hours of poisoning. Iva confirmed that multiple countries had already expressed interest in acquiring the antidote.
This story was translated from JIM, part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
France has authorized Ricimed, the first antibody-based treatment specifically indicated for acute ricin intoxication, providing clinicians with a targeted option beyond supportive care for exposure to one of the most lethal naturally occurring toxins.
Fabentech is a French biopharmaceutical company specializing in medical countermeasures against biological threats and infectious diseases.
The polyclonal antibody technology used in the development of Ricimed has received marketing authorization in France as a treatment for ricin poisoning. Ricin is a highly toxic natural substance that can cause death within hours to a few days of exposure.
Supported by the Ministry of Armed Forces and Veterans Affairs (Directorate General of Armaments [DGA] and Armed Forces Health Service) in France, Ricimed is the first approved antidote for ricin poisoning, a condition for which treatment was previously limited to supportive measures alone.
Historical Incident
One incident, in particular, remains etched in espionage history. On September 7, 1978 in London during the Cold War, Bulgarian dissident writer Georgi Markov, living in exile, was struck by the umbrella of a passer-by while waiting at a bus stop. He felt a slight sting. Four days later, he died in the hospital due to a sudden and unexplained illness. An autopsy revealed that he had been poisoned by a tiny metal pellet implanted at the tip of an umbrella containing ricin, a lethal toxin. The legend of the “Bulgarian umbrella,” later invoked in other assassination attempts, was born.
Since then, although Markov remains the only known individual to have been killed by ricin poisoning, this theoretically extremely toxic substance, which can be manufactured relatively easily from castor beans, a widely available plant, has continued to fascinate authors of thrillers and spy novels.
Numerous works of fiction depict characters who succumb to ricin poisoning. The toxin is notably portrayed as a favored weapon of the main character in the hit television series Breaking Bad.
However, ricin is not confined to the realm of science fiction. For several years, authorities in various countries have feared that extremist groups could carry out attacks using ricin. The threat has been taken particularly seriously since 2018, when a clandestine ricin laboratory operated by members of the Islamic State was dismantled in Germany. Since then, several similar attack plots have been thwarted.
This context triggered a race among major powers to develop an effective antidote as quickly as possible. In this effort, Fabentech has risen to a challenge.
“Having demonstrated its ability to target and then neutralize ricin before it causes irreparable damage, Ricimed is a treatment that works based on polyclonal antibodies and compensates for the absence of a vaccine or specific treatment,” Fabentech said in a press release.
The polyclonal antibody technology used by Fabentech offers potential for the development of antidotes against bioterrorist attacks and for the treatment of many infectious diseases.
Ricimed contributed to the deployment of a European health shield against intentional biological threats in France.
Military Backing
Speaking to Le Figaro, France’s oldest national newspaper, Fabentech CEO Sébastien Iva explained that ricin disrupts the body by halting cell function, while noting several other drug candidates in development at the firm.
Typically, the lungs sustain fatal damage. Our treatment interrupts this toxic process. In animals administered the antidote, we observed pulmonary function recovery, allowing survival.
Given that the possibility of terrorist attacks using ricin is considered a national security issue, Fabentech benefited from the support by the Ministry of the Armed Forces and the DGA and lasted nearly a decade of research and development work.
The granting of marketing authorisation was also supported by the French Armed Forces and welcomed by the French Minister of the Armed Forces, Catherine Vautrin, who previously served as France’s Minister of Labour, Health, and Solidarity.
“Supporting the development of companies in France capable of manufacturing antidotes against certain biological agents helps guarantee the operational superiority of our armed forces. Developing and producing such drugs when they do not yet exist on the market is also serving the nation and the public interest,” she said.
Although the threat posed by ricin remains hypothetical, Fabentech reports a strong interest from potential clients, with many countries seeking protection against possible bioterrorist attacks.
The DGA had already placed an order for several doses of Ricimed for deployment in France. For optimal effectiveness, the antidote must be administered within 6 hours of poisoning. Iva confirmed that multiple countries had already expressed interest in acquiring the antidote.
This story was translated from JIM, part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
Military-Backed French Biotech Brings Ricin Antidote
Military-Backed French Biotech Brings Ricin Antidote
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
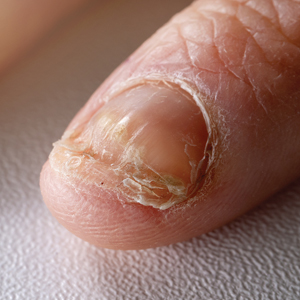
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
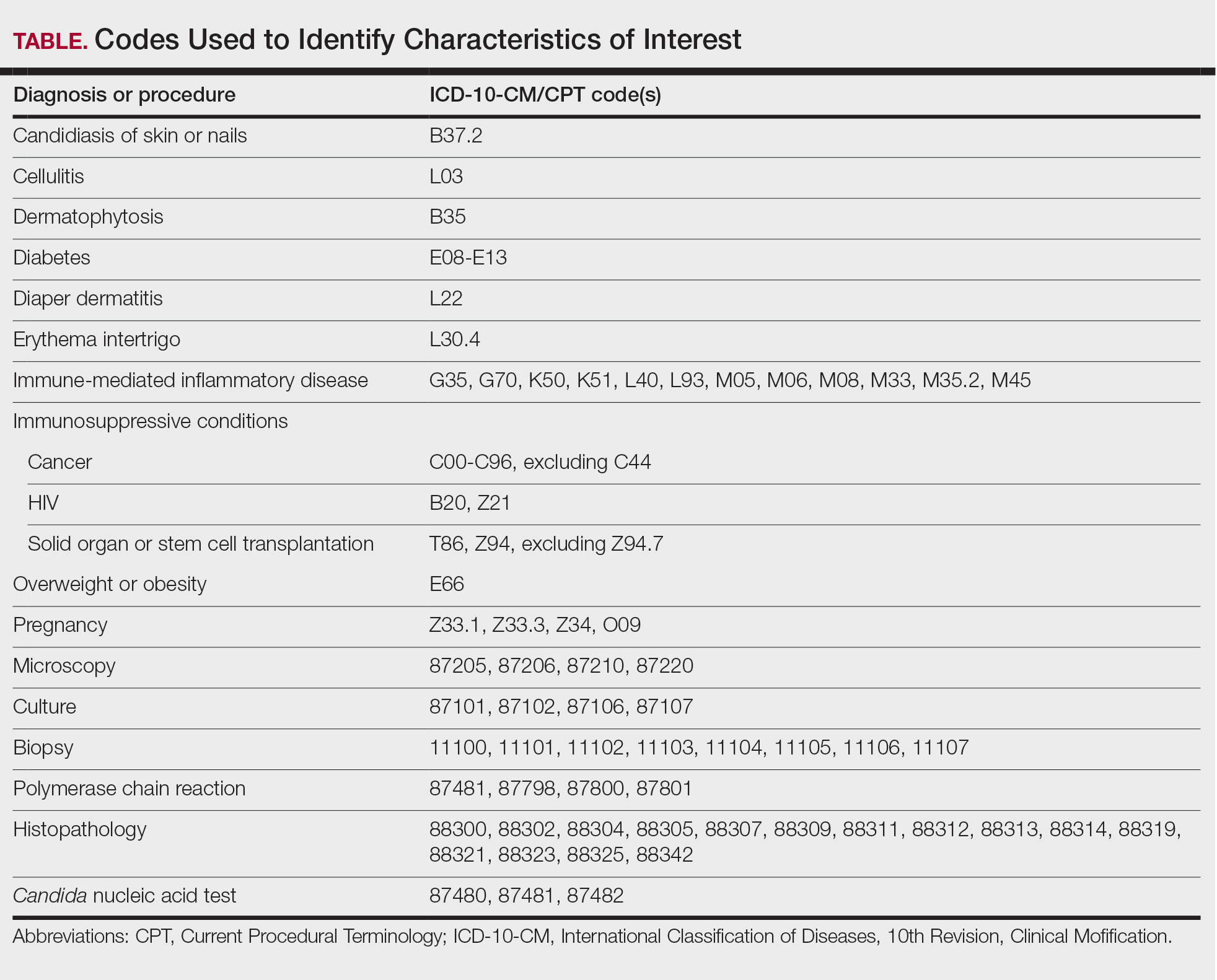
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.
Results
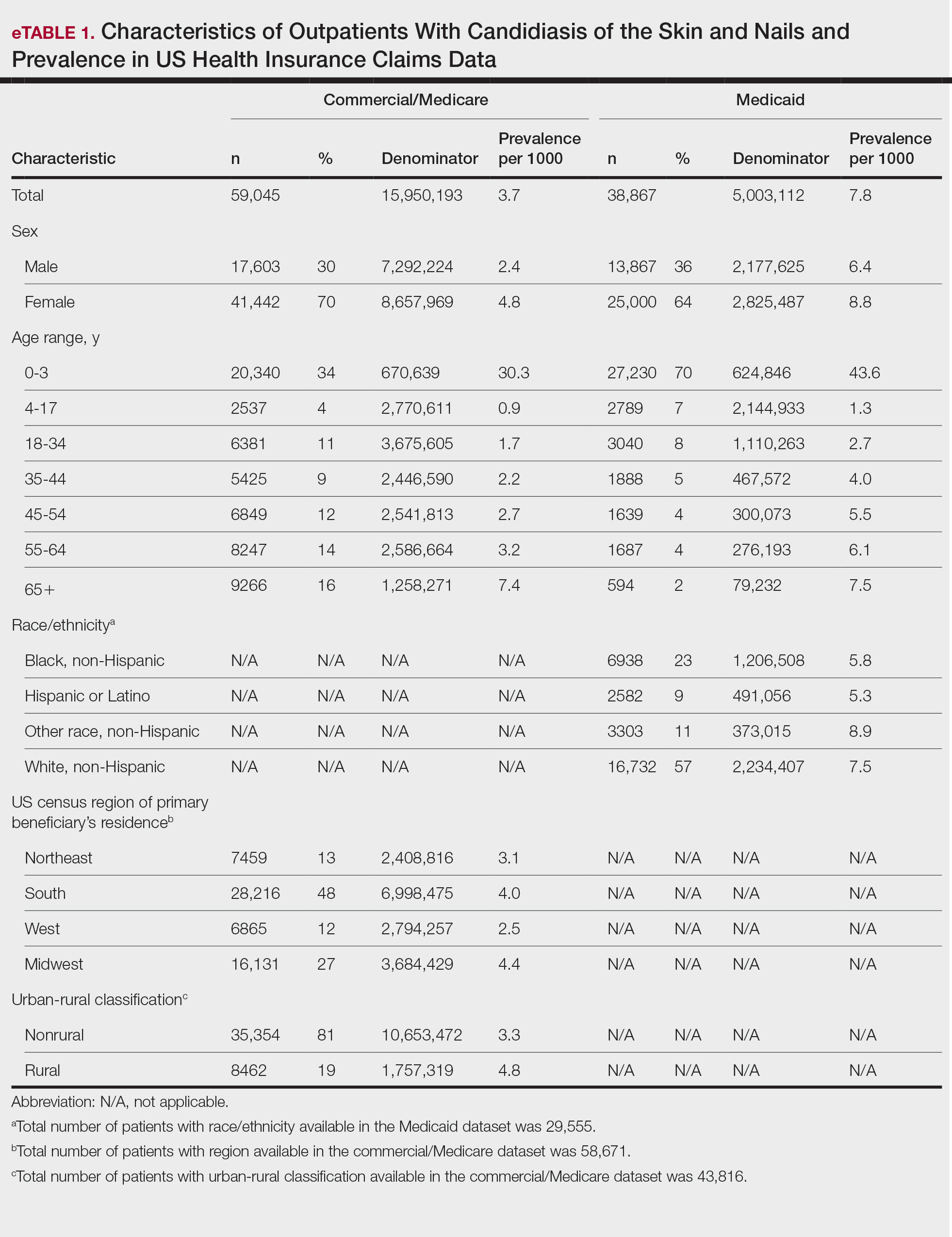
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).
Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).

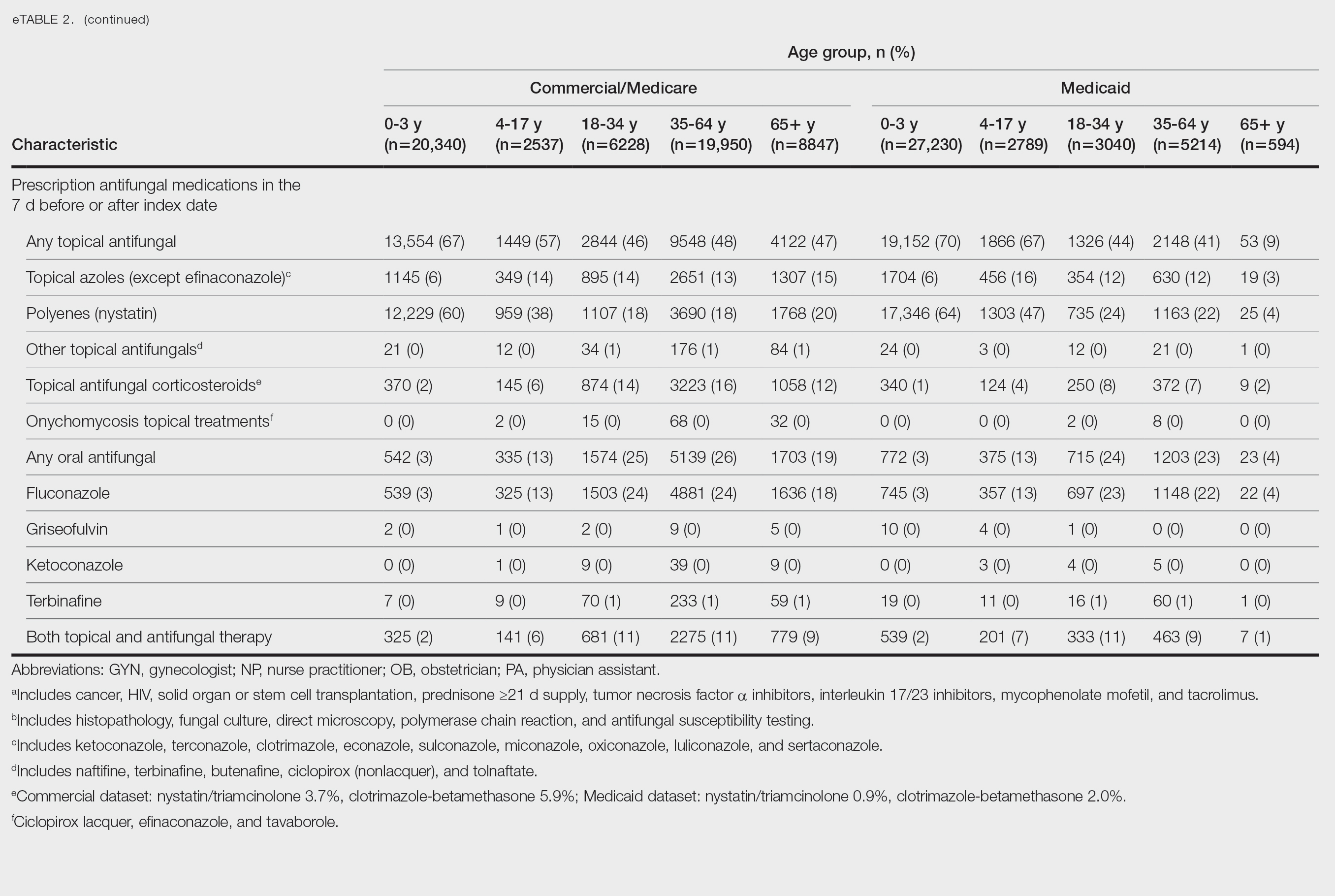
Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Practice Points
- Candidiasis of the skin or nails is a common outpatient condition that is most frequently diagnosed in infants, toddlers, and adults aged 65 years or older.
- Most cases are diagnosed clinically without diagnostic testing and treated with topical antifungals, but increased attention to formal diagnosis and treatment may be warranted given the emergence of antifungal-resistant Candida species.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.
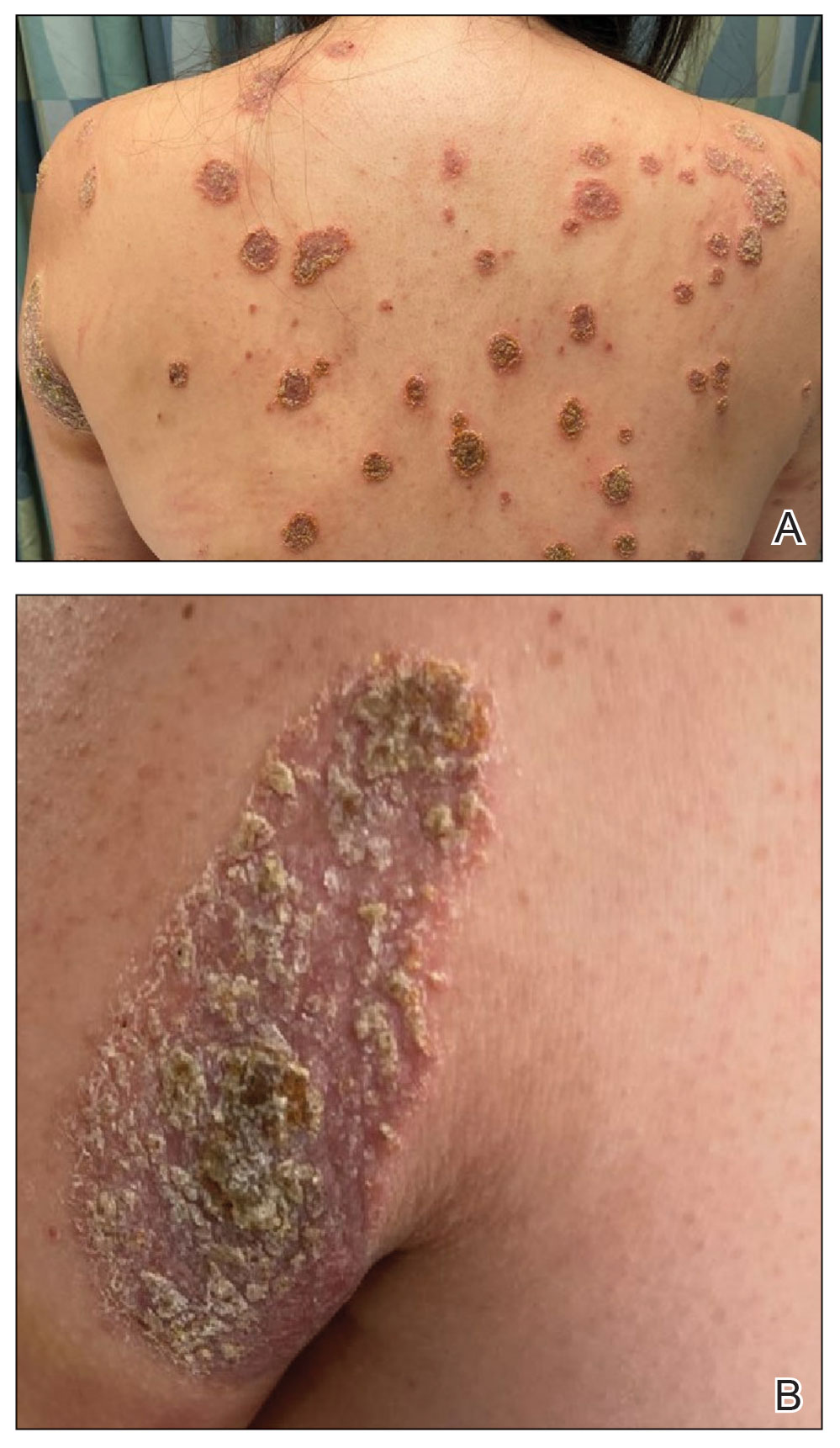
Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
Practice Points
- Consider a rupioid id reaction when a patient presents with lesions featuring scale that is dirty appearing and resembles an oyster shell.
- Recognize that exuberant id reactions can manifest with peripheral eosinophilia; its presence should not lead you to automatically rule out an id reaction in favor of other eosinophilic eruptions.
- Focus on uncovering the source of an id reaction (eg, contactants, infections, bites); resolving the primary insult is essential for rapid clearance of even dramatic rupioid eruptions.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48
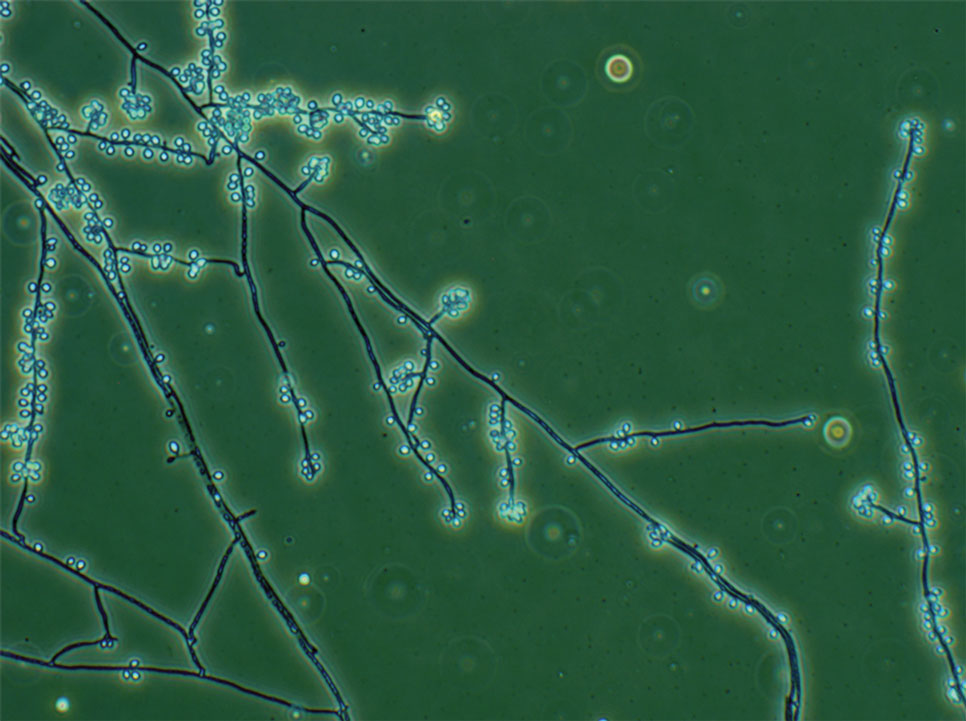
Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.
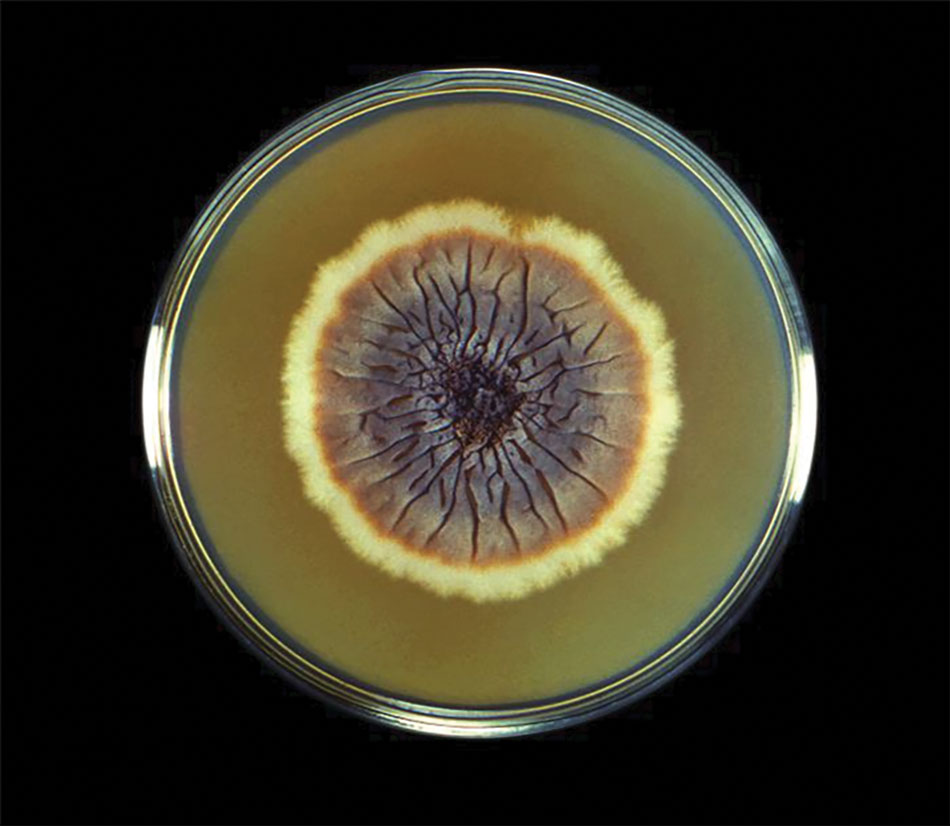
A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Practice Points
- Sporotrichosis is an implantation mycosis that is considered a neglected tropical disease warranting global advocacy to prevent infections and improve patient outcomes.
- Common diagnostic methods such as microscopy may have a low sensitivity for confirming sporotrichosis. Culture from lesional tissue or pus is considered the gold standard for diagnosis.
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2
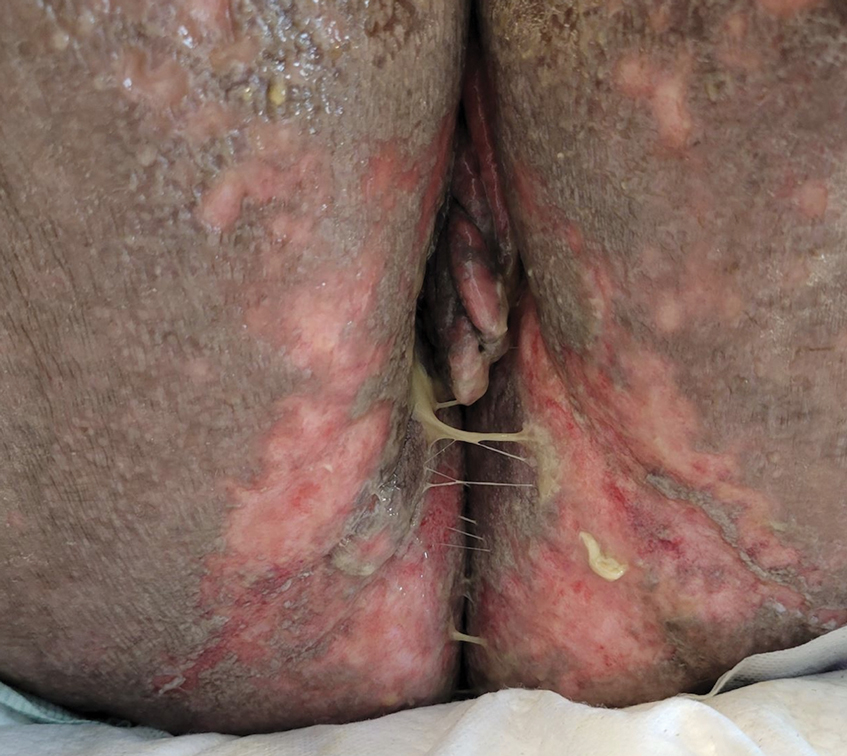
Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
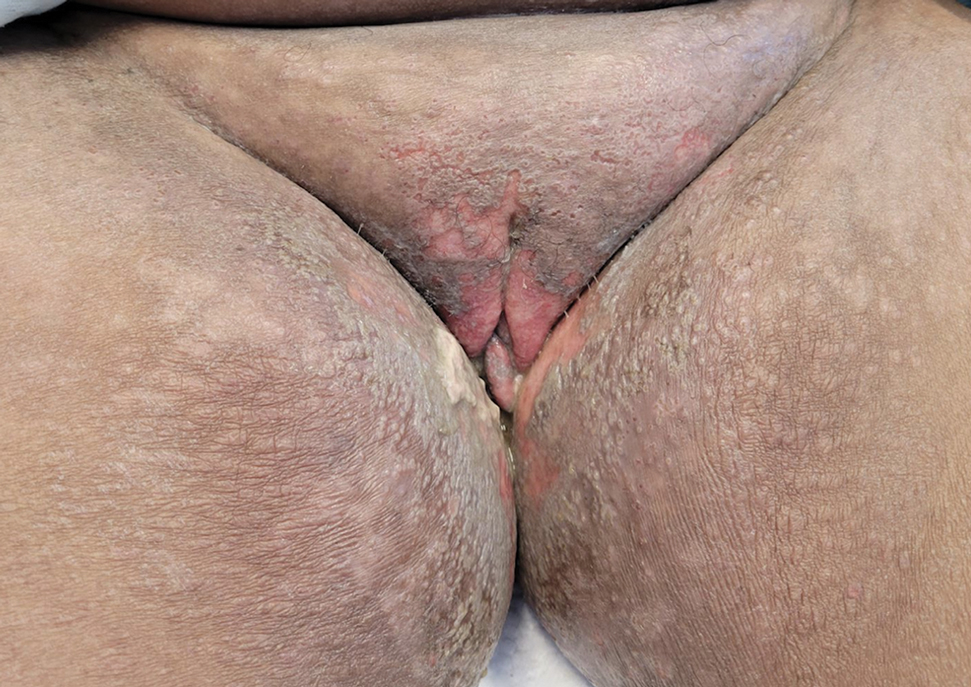
A 40-year-old woman presented to the emergency department with painful, well-defined, edematous labial erosions of several weeks’ duration. The patient reported a medical history of stage IIIA (T4N0M0B0) mycosis fungoides. She had been hospitalized 2 months prior for sepsis that was attributed to a polymicrobial bacteremia involving Acinetobacter baumannii and Staphylococcus epidermidis. During that admission, a polymerase chain reaction test conducted on a skin swab from a lesion on the labia majora confirmed the presence of herpes simplex virus type 2. At the current presentation, physical examination by dermatology also revealed discrete, coalescing, erythematous erosions on the buttocks, groin, and proximal thighs with a punched-out appearance. These areas also exhibited skin sloughing and were covered with a gray-brown exudate. No other mucosal surfaces were involved.
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.
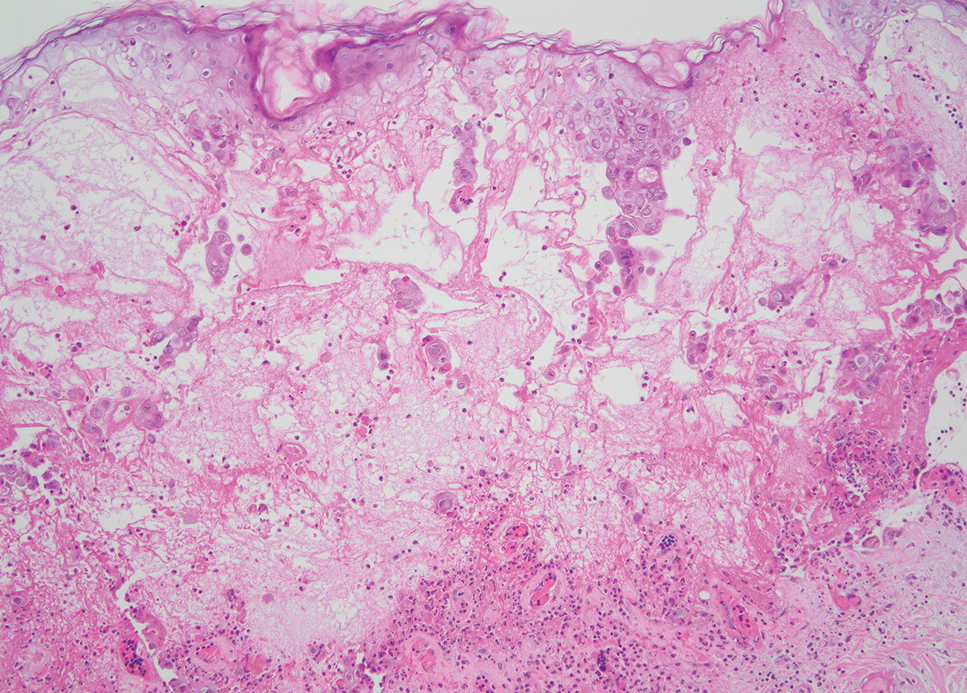
Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8
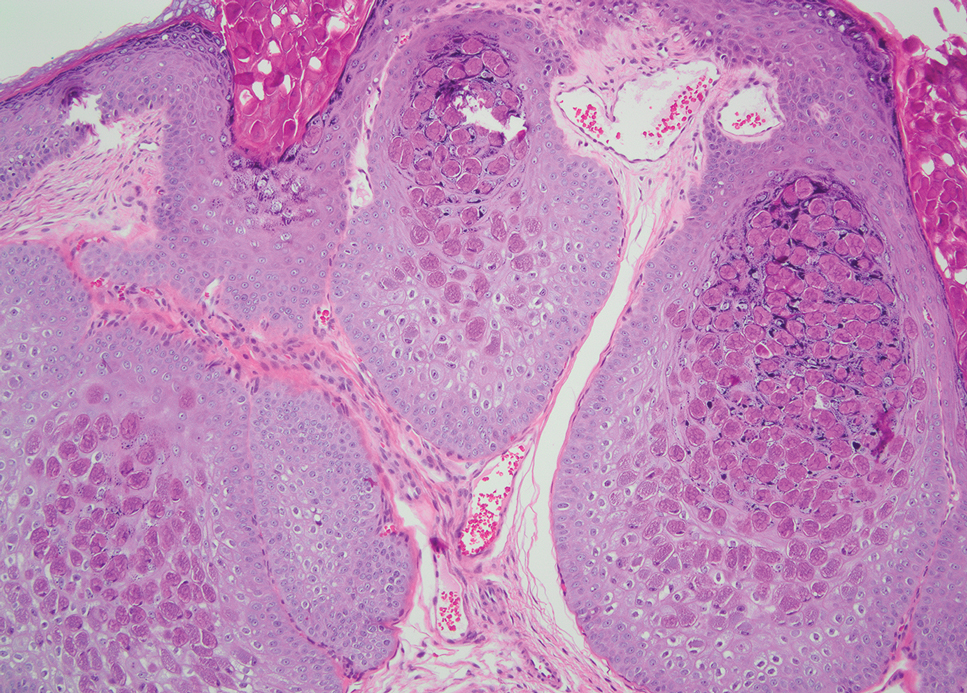
Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).
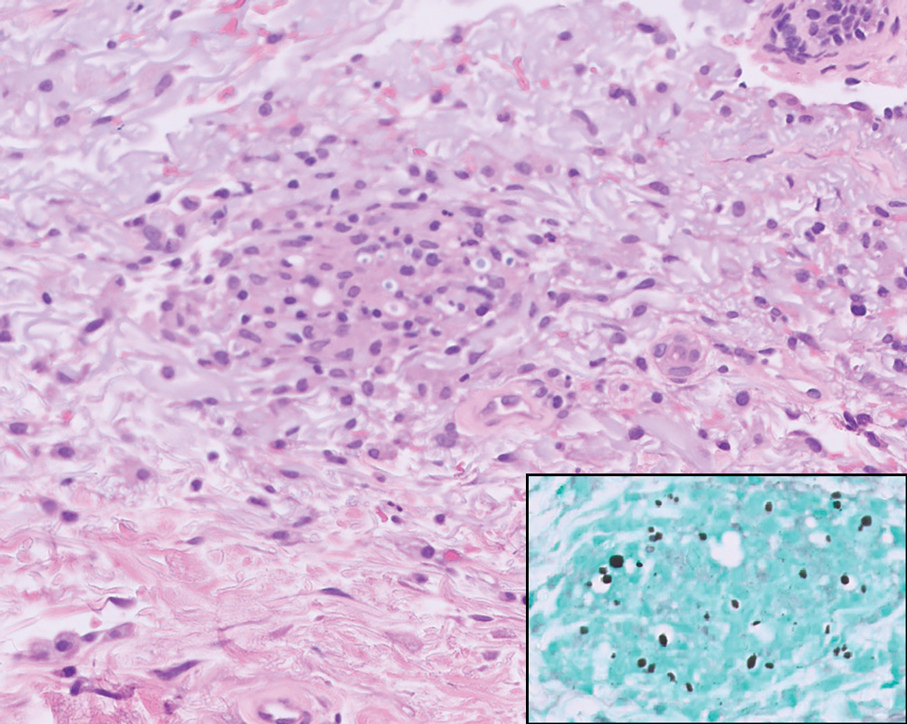
Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).
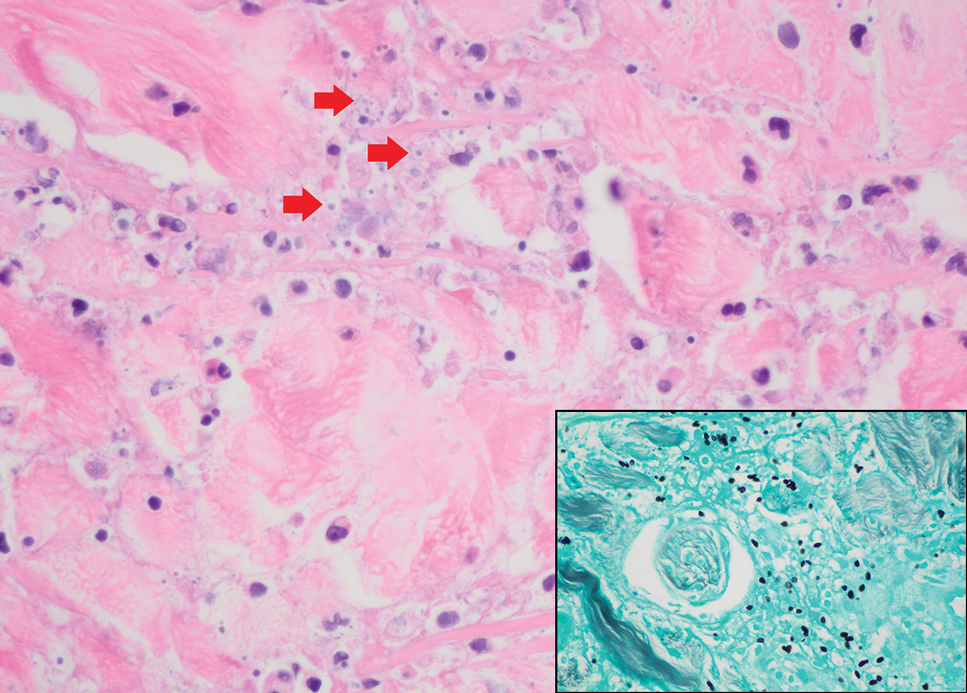
×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
A 42-year-old man with a history of multidrug-resistant HIV/AIDS presented to the emergency department for evaluation of pruritic, scattered, umbilicated papules on the left cheek, neck, and arms of 3 days’ duration. The patient’s most recent CD4+ T-cell count 6 weeks prior to the development of the rash was 1 cell/mm3. He was noncompliant with antiretroviral therapy. He reported that the lesions had progressed rapidly, starting on the face and extending down the neck and arms. Physical examination revealed scattered umbilicated and centrally crusted papules and plaques on the left cheek, neck, and arms. Erosions involving the oral mucosa also were noted, which the patient reported had been present for several weeks. An oral swab was positive for herpes simplex virus 2 on polymerase chain reaction. A shave biopsy of a lesion from the left cheek was performed.
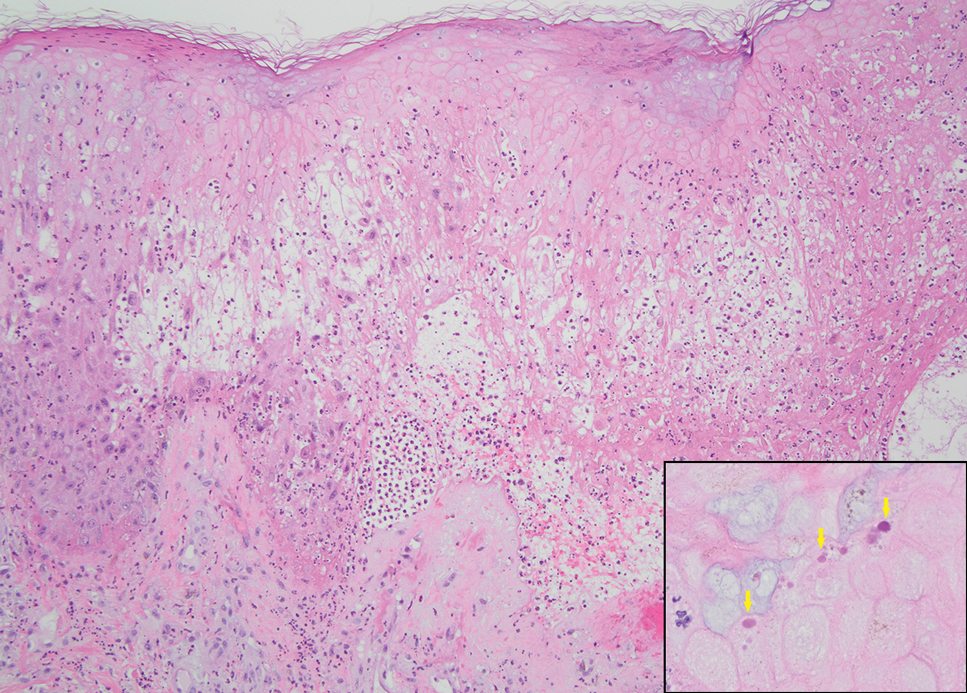
Approach to Diagnosing and Managing Implantation Mycoses
Approach to Diagnosing and Managing Implantation Mycoses
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.
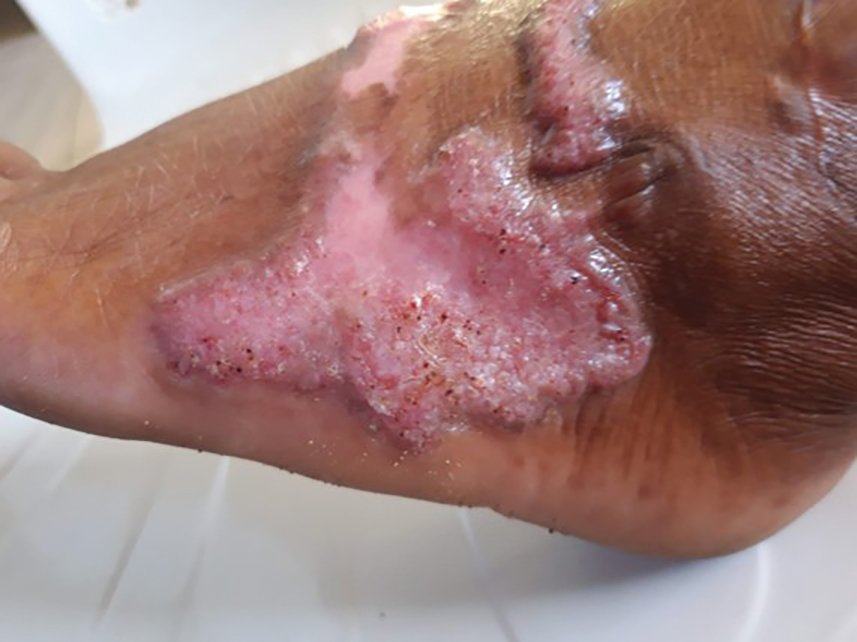
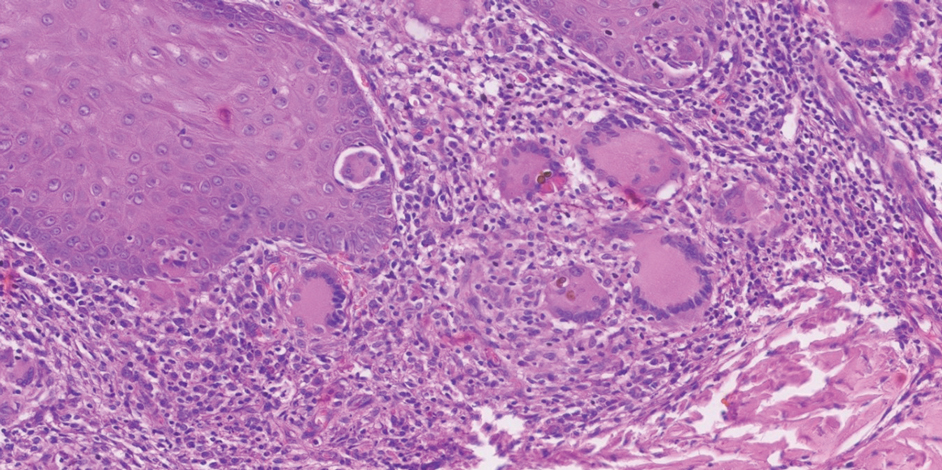
Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26
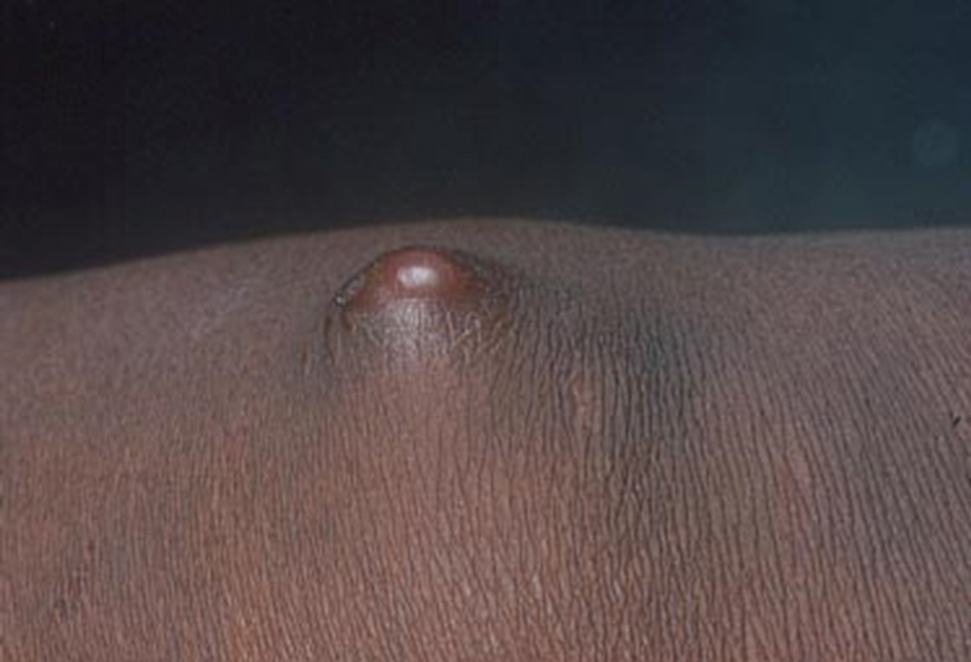
Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32
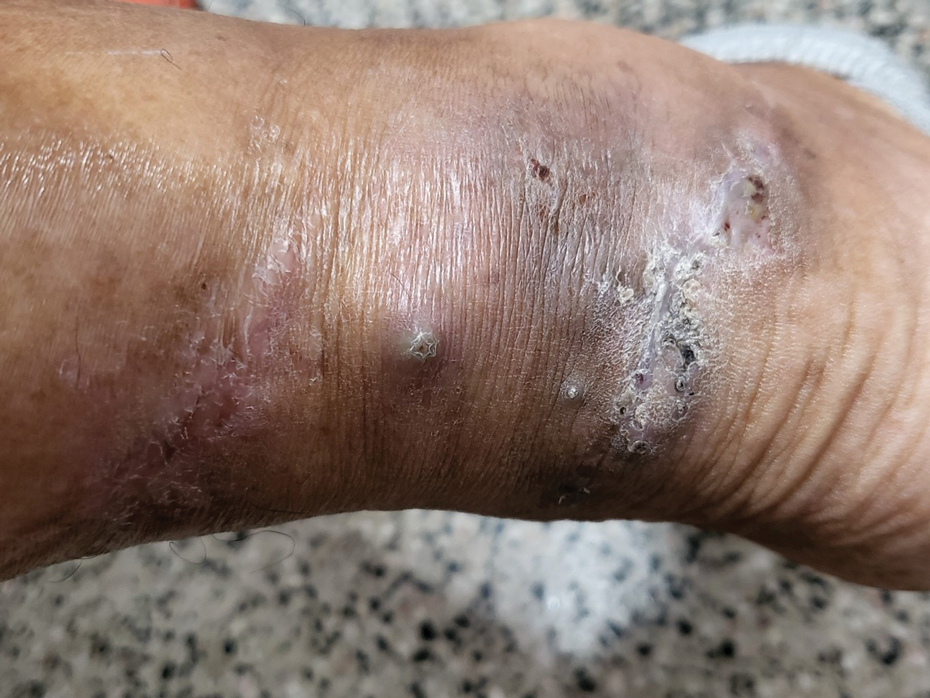
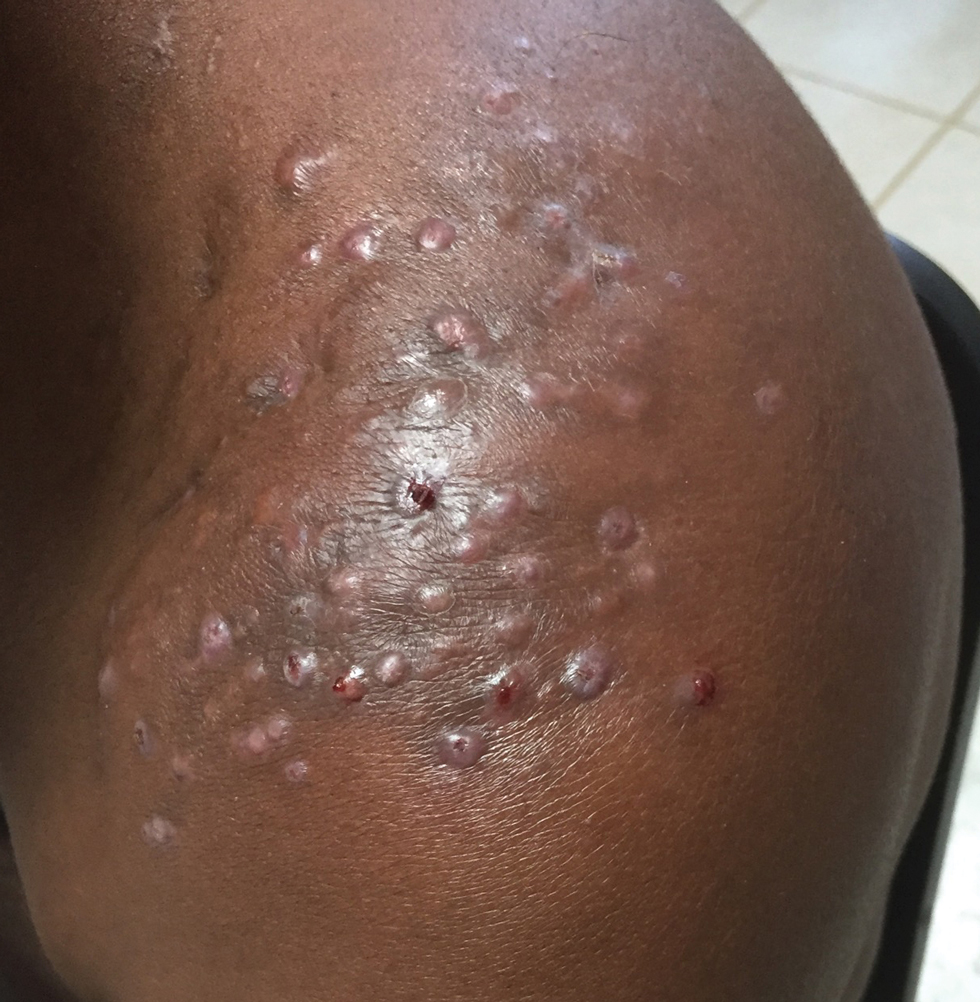
Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36
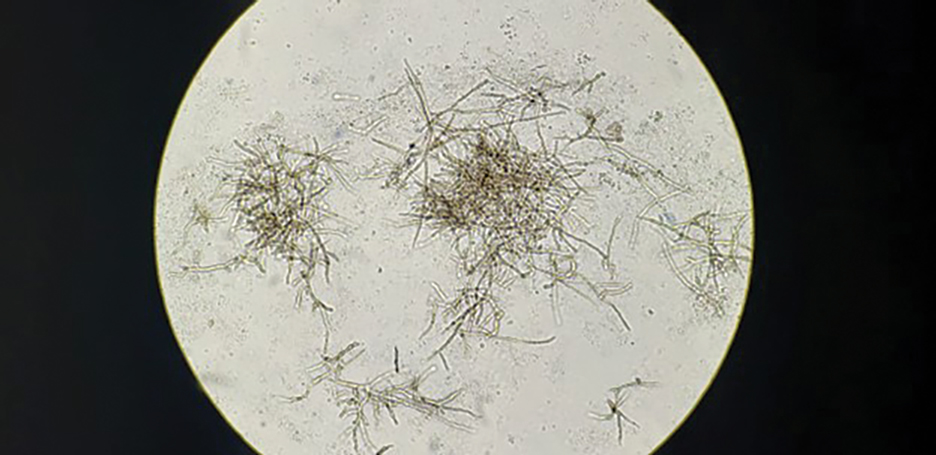
Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.


Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26

Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32


Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36

Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.


Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26

Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32


Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36

Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
Approach to Diagnosing and Managing Implantation Mycoses
Approach to Diagnosing and Managing Implantation Mycoses
Practice Points
- Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are implantation mycoses that cause substantial morbidity, decreased quality of life, and social stigma.
- Consider obtaining a biopsy of suspected chromoblastomycosis and subcutaneous phaeohyphomycosis to confirm infection while sending half of the sample for culture for organism identification.
- Distinguishing between actinomycetoma (caused by filamentous bacteria) and eumycetoma (caused by fungi) is critical for appropriate mycetoma treatment.
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.
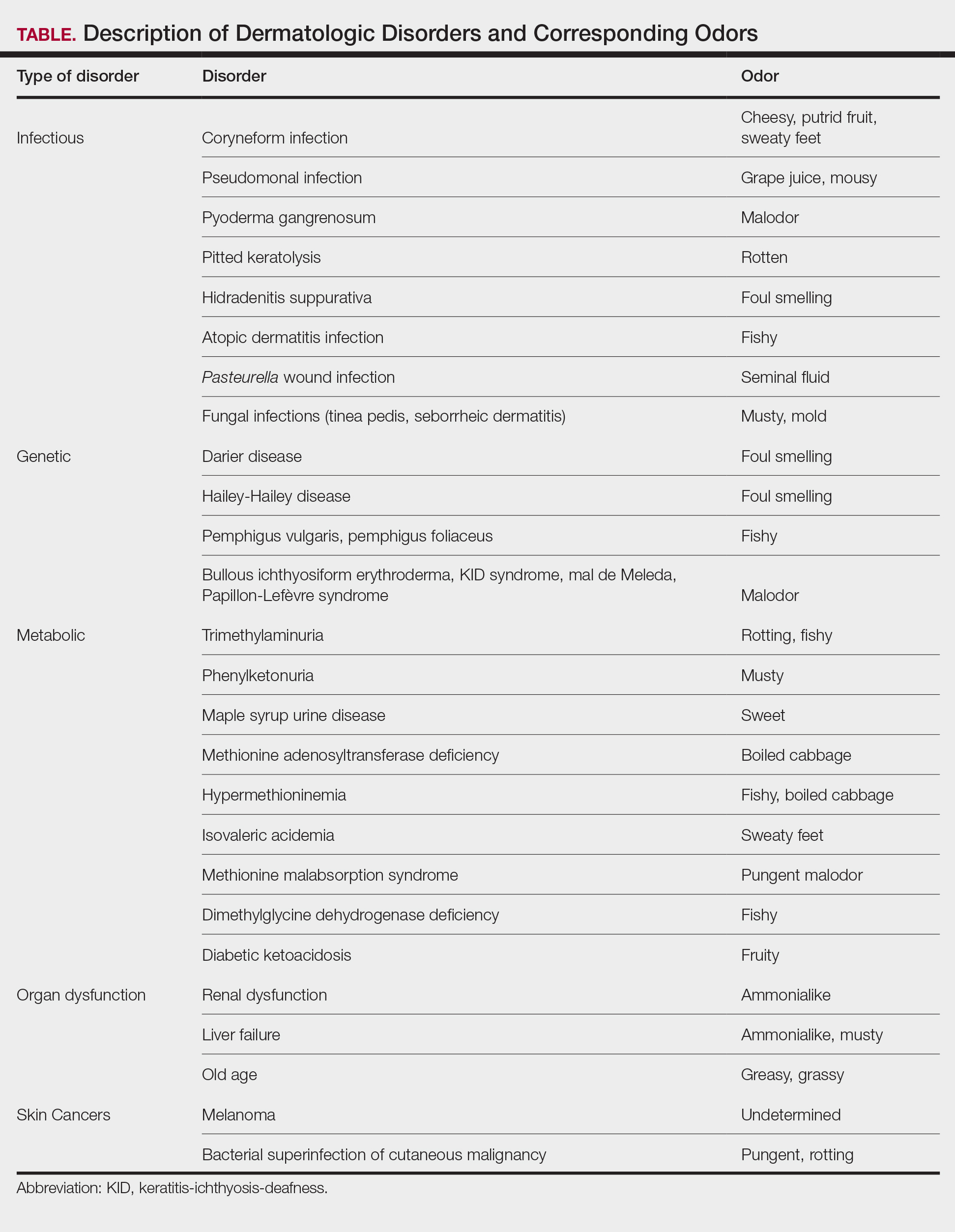
Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.

Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.

Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
PRACTICE POINTS
- Olfaction may be underutilized in making dermatologic diagnoses. Clinicians should include smell in their physical examination, as characteristic odors are associated with infectious disorders, disorders of keratinization and acantholysis, and metabolic disorders.
- Recognizing distinctive smells can help narrow the differential diagnosis and prompt targeted testing in dermatology.
- Canines and electronic noses have demonstrated the potential to detect certain malignancies, including melanoma, based on unique volatile organic compound profiles.