User login
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
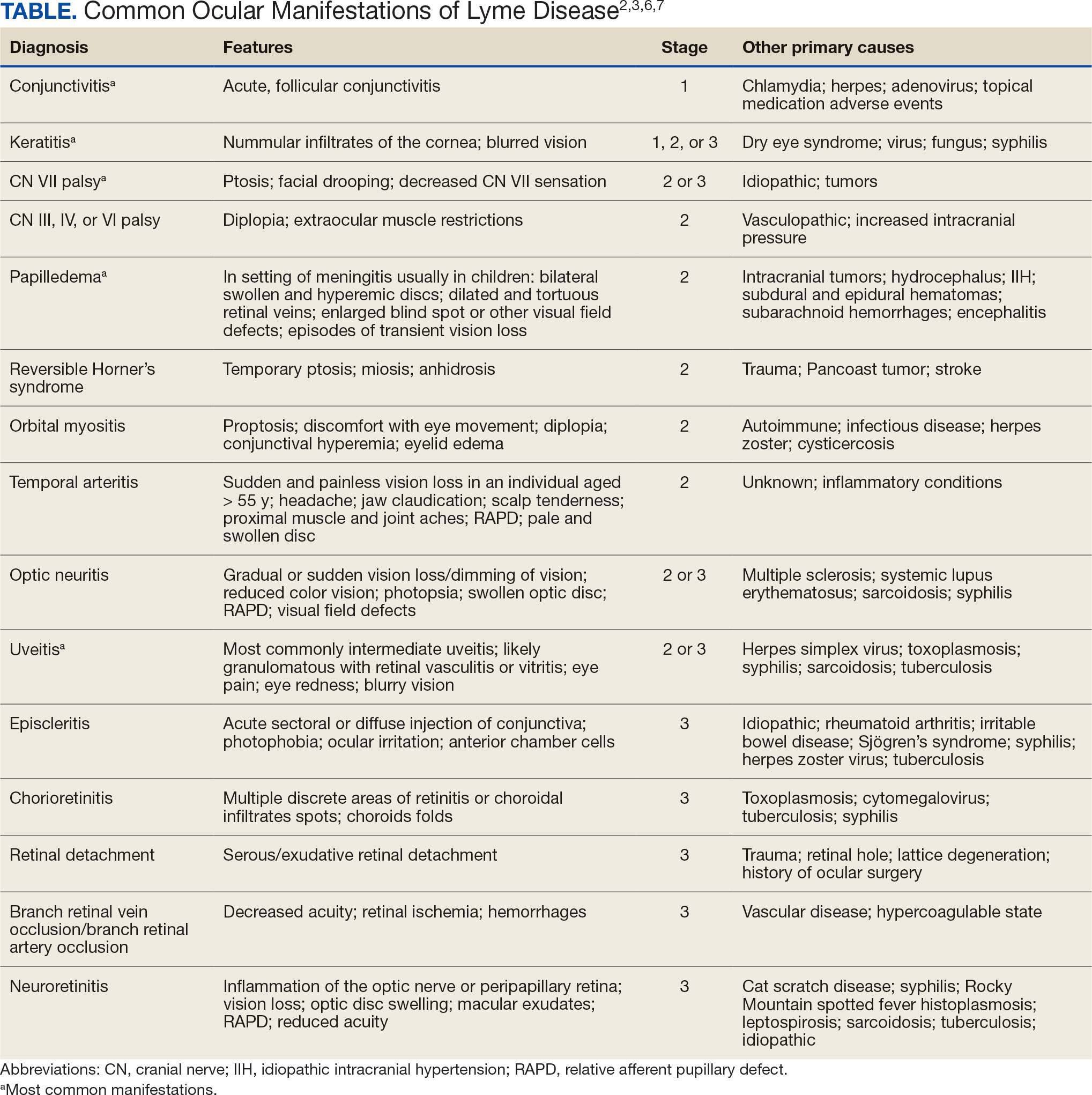
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7
Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
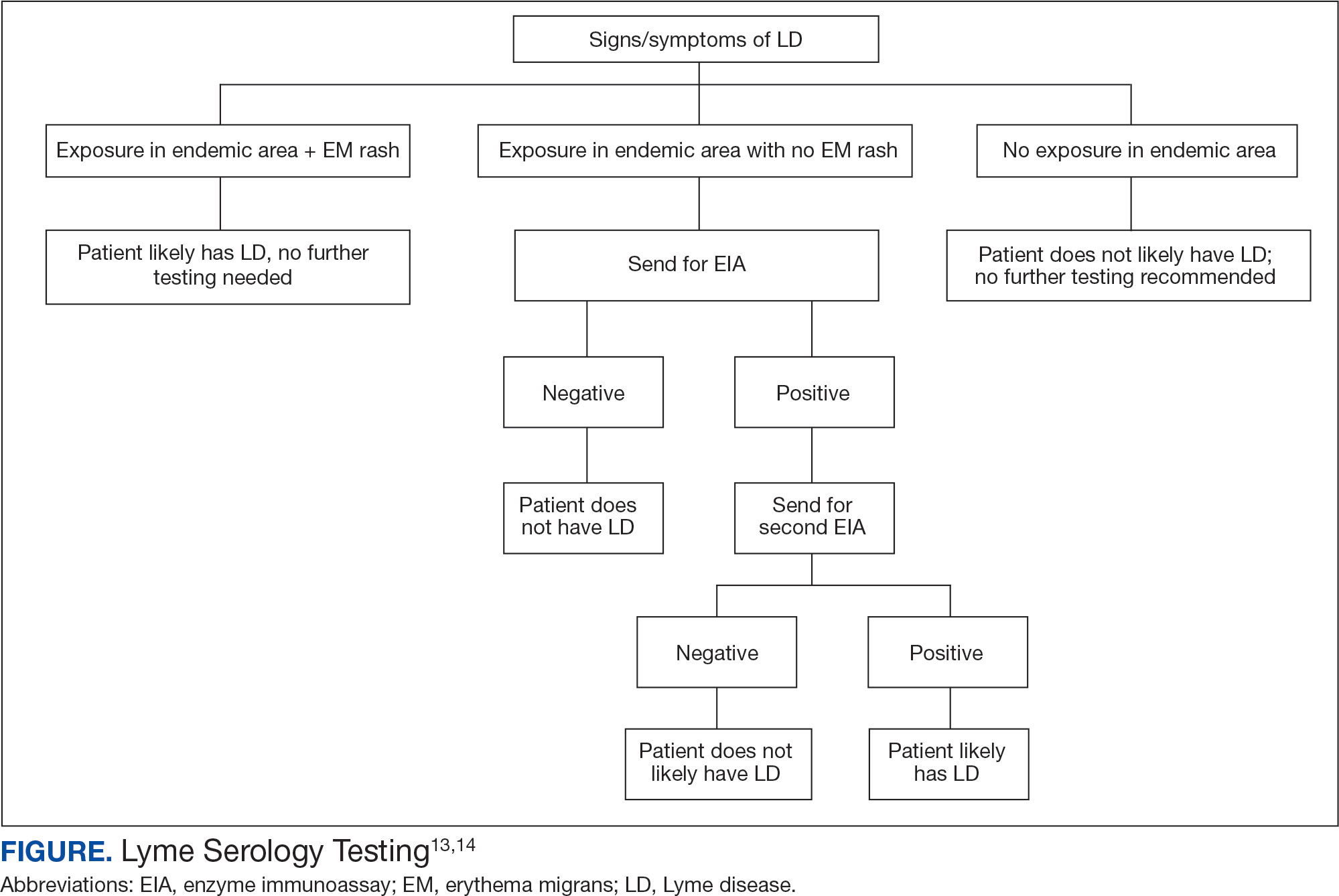
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.
Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
