User login
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Codes, Contracts, and Commitments: Who Defines What is a Profession?
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
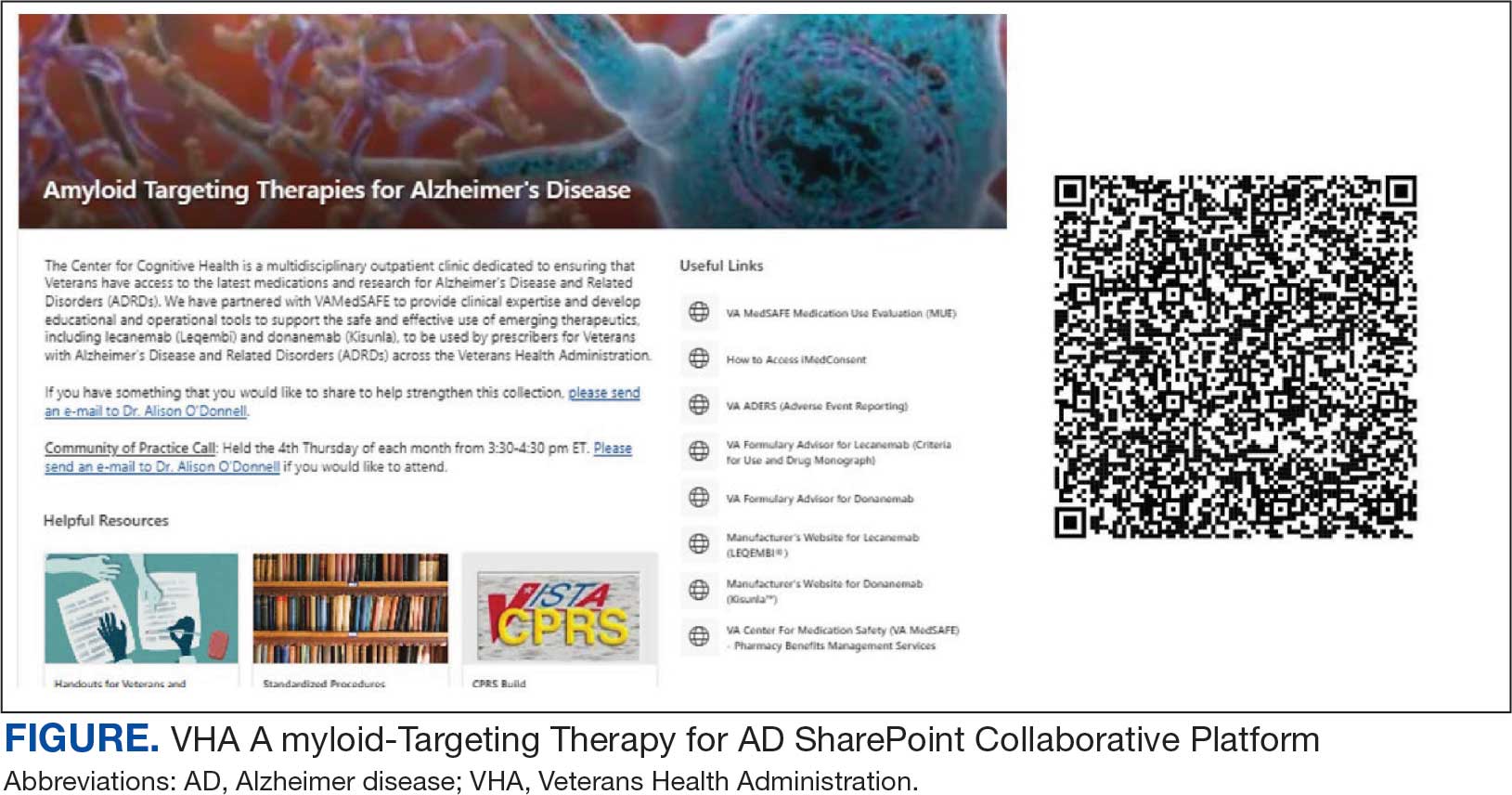
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.
Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.
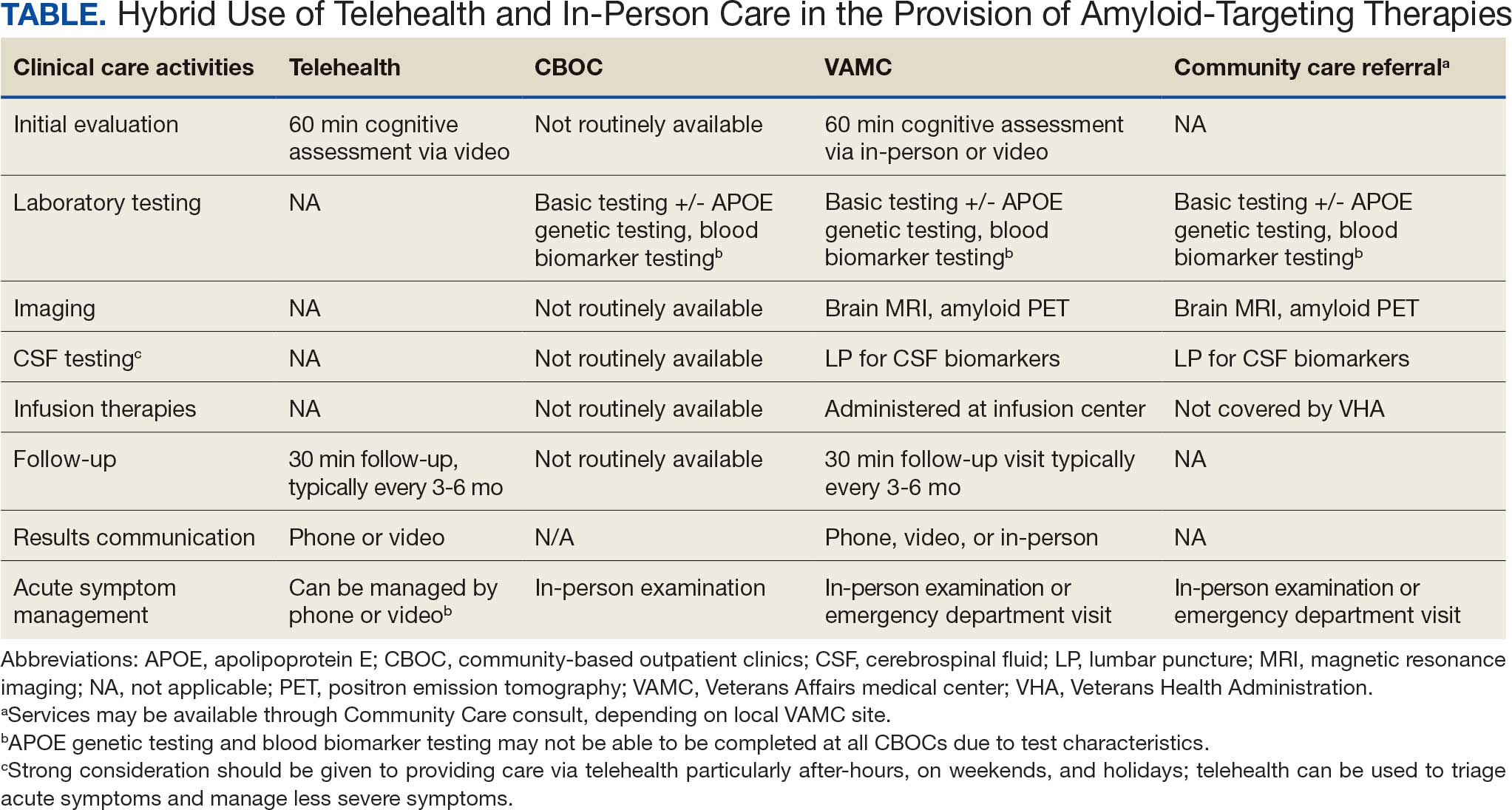
The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Once and Future Veterans Health Administration
The Once and Future Veterans Health Administration
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3
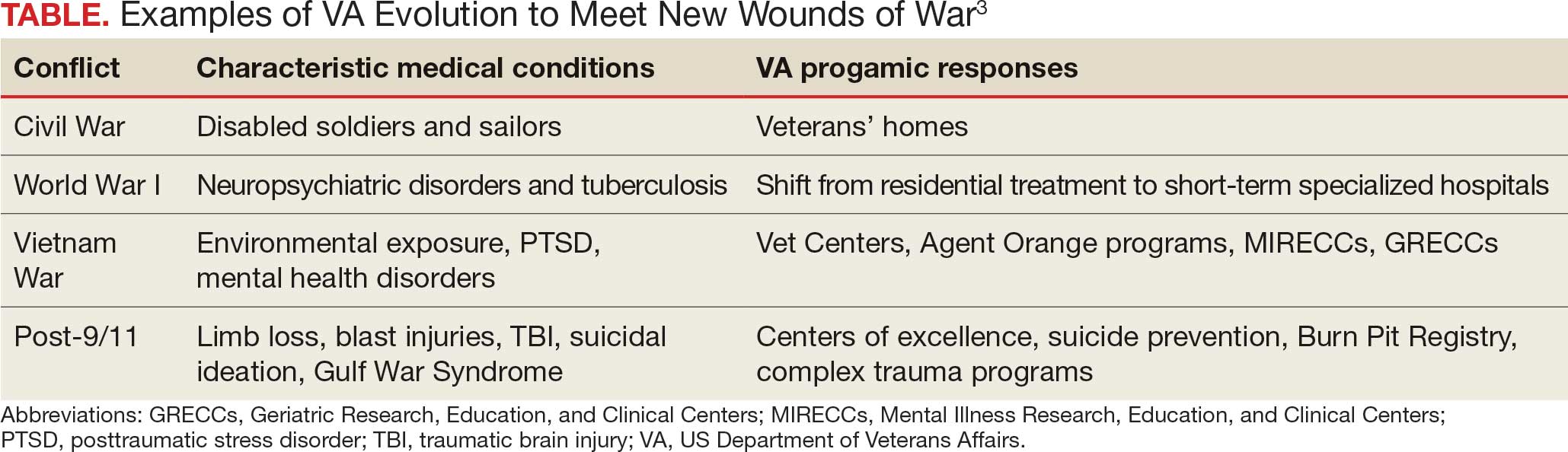
Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3

Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3

Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
The Once and Future Veterans Health Administration
The Once and Future Veterans Health Administration
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Transplantation palliative care: The time is ripe
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Antibiotic Stewardship in Acne: Practical Tips From Dr. Lorraine L. Rosamilia
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Introduction: Health Professions Education Evaluation and Research (HPEER) Advanced Fellowship Abstracts
The original four HPEER Advanced Fellowship sites were established by the Department of Veterans Affairs (VA) Office of Academic Affiliation in 2014, and expanded in 2020 to include 8 sites and a national coordinating center with leadership shared between VA facilities in Houston and White River Junction. The VA invests heavily in training the nation’s healthcare professionals. The mission of HPEER is to develop leaders who can educate, evaluate, and innovate in Health Professions Education for the VA and the nation. All HPEER sites take part in a nationally coordinated curriculum covering topics in curriculum design, learner assessment, leadership, interprofessional education, as well as scholarship and educational research.
As part of the national HPEER curriculum covering scholarship and educational research, and in concert with Wednesday, May 14, 2025 VA Research Week 2025, HPEER organized a joint conference with the Center for Health Professions Education at the Uniformed Services University of the Health Sciences (USUHS). This interagency online event included poster sessions and oral presentations from HPEER fellows and students in USUHS certificate and graduate degree programs.
Education scholarship is broad, ranging from descriptions of curricular innovations and works in progress to advanced research using techniques drawn from psychology, sociology, anthropology, economics, and other scientific disciplines. The abstracts presented here summarize some of the work being done by HPEER fellows. Dougherty et al (Boston) described a project to create a primer outlining methodology for conducting and interpreting cost-effectiveness evaluations in the context of proposed HPE innovations. Cohen et al (Cleveland) found reduction in potentially problematic orders in the context of life-sustaining treatment following a multifaceted intervention program. Sorenson (Dublin, Georgia) reported an expanded Tai Chi program that included modifications allowing seated positions for veterans with mobility limitations. Young et al (Dublin) described an interprofessional curriculum to strengthen communication between nurses and social workers in their conversations with women veterans living in rural settings. Misedah-Robinson et al (Houston) showed that a new training program strengthened coordinators’ self-reports of preparedness and confidence in their ability to support veterans who have experienced human trafficking. Tovar et al (Salt Lake City) describe a methodology for using data from the VHA Corporate Data Warehouse to optimize schedules of HPE students assigned to VA clinical rotations. Yanez et al (San Francisco) presented initial observations of learner-centered outcomes following participation in a new multidisciplinary integrative health elective. Resto et al (West Haven) reported that implementation of self-serve kiosks increased distribution of substance use harm reduction resources beyond usual clinical care.
A second joint conference between VA HPEER and USUHS is planned for VA Research Week 2026; we look forward to the abstracts that will be produced by this new cohort of fellows, as well as to the future scholarship and contributions to the field that will be made by alumni of the HPEER Advanced Fellowship.
The original four HPEER Advanced Fellowship sites were established by the Department of Veterans Affairs (VA) Office of Academic Affiliation in 2014, and expanded in 2020 to include 8 sites and a national coordinating center with leadership shared between VA facilities in Houston and White River Junction. The VA invests heavily in training the nation’s healthcare professionals. The mission of HPEER is to develop leaders who can educate, evaluate, and innovate in Health Professions Education for the VA and the nation. All HPEER sites take part in a nationally coordinated curriculum covering topics in curriculum design, learner assessment, leadership, interprofessional education, as well as scholarship and educational research.
As part of the national HPEER curriculum covering scholarship and educational research, and in concert with Wednesday, May 14, 2025 VA Research Week 2025, HPEER organized a joint conference with the Center for Health Professions Education at the Uniformed Services University of the Health Sciences (USUHS). This interagency online event included poster sessions and oral presentations from HPEER fellows and students in USUHS certificate and graduate degree programs.
Education scholarship is broad, ranging from descriptions of curricular innovations and works in progress to advanced research using techniques drawn from psychology, sociology, anthropology, economics, and other scientific disciplines. The abstracts presented here summarize some of the work being done by HPEER fellows. Dougherty et al (Boston) described a project to create a primer outlining methodology for conducting and interpreting cost-effectiveness evaluations in the context of proposed HPE innovations. Cohen et al (Cleveland) found reduction in potentially problematic orders in the context of life-sustaining treatment following a multifaceted intervention program. Sorenson (Dublin, Georgia) reported an expanded Tai Chi program that included modifications allowing seated positions for veterans with mobility limitations. Young et al (Dublin) described an interprofessional curriculum to strengthen communication between nurses and social workers in their conversations with women veterans living in rural settings. Misedah-Robinson et al (Houston) showed that a new training program strengthened coordinators’ self-reports of preparedness and confidence in their ability to support veterans who have experienced human trafficking. Tovar et al (Salt Lake City) describe a methodology for using data from the VHA Corporate Data Warehouse to optimize schedules of HPE students assigned to VA clinical rotations. Yanez et al (San Francisco) presented initial observations of learner-centered outcomes following participation in a new multidisciplinary integrative health elective. Resto et al (West Haven) reported that implementation of self-serve kiosks increased distribution of substance use harm reduction resources beyond usual clinical care.
A second joint conference between VA HPEER and USUHS is planned for VA Research Week 2026; we look forward to the abstracts that will be produced by this new cohort of fellows, as well as to the future scholarship and contributions to the field that will be made by alumni of the HPEER Advanced Fellowship.
The original four HPEER Advanced Fellowship sites were established by the Department of Veterans Affairs (VA) Office of Academic Affiliation in 2014, and expanded in 2020 to include 8 sites and a national coordinating center with leadership shared between VA facilities in Houston and White River Junction. The VA invests heavily in training the nation’s healthcare professionals. The mission of HPEER is to develop leaders who can educate, evaluate, and innovate in Health Professions Education for the VA and the nation. All HPEER sites take part in a nationally coordinated curriculum covering topics in curriculum design, learner assessment, leadership, interprofessional education, as well as scholarship and educational research.
As part of the national HPEER curriculum covering scholarship and educational research, and in concert with Wednesday, May 14, 2025 VA Research Week 2025, HPEER organized a joint conference with the Center for Health Professions Education at the Uniformed Services University of the Health Sciences (USUHS). This interagency online event included poster sessions and oral presentations from HPEER fellows and students in USUHS certificate and graduate degree programs.
Education scholarship is broad, ranging from descriptions of curricular innovations and works in progress to advanced research using techniques drawn from psychology, sociology, anthropology, economics, and other scientific disciplines. The abstracts presented here summarize some of the work being done by HPEER fellows. Dougherty et al (Boston) described a project to create a primer outlining methodology for conducting and interpreting cost-effectiveness evaluations in the context of proposed HPE innovations. Cohen et al (Cleveland) found reduction in potentially problematic orders in the context of life-sustaining treatment following a multifaceted intervention program. Sorenson (Dublin, Georgia) reported an expanded Tai Chi program that included modifications allowing seated positions for veterans with mobility limitations. Young et al (Dublin) described an interprofessional curriculum to strengthen communication between nurses and social workers in their conversations with women veterans living in rural settings. Misedah-Robinson et al (Houston) showed that a new training program strengthened coordinators’ self-reports of preparedness and confidence in their ability to support veterans who have experienced human trafficking. Tovar et al (Salt Lake City) describe a methodology for using data from the VHA Corporate Data Warehouse to optimize schedules of HPE students assigned to VA clinical rotations. Yanez et al (San Francisco) presented initial observations of learner-centered outcomes following participation in a new multidisciplinary integrative health elective. Resto et al (West Haven) reported that implementation of self-serve kiosks increased distribution of substance use harm reduction resources beyond usual clinical care.
A second joint conference between VA HPEER and USUHS is planned for VA Research Week 2026; we look forward to the abstracts that will be produced by this new cohort of fellows, as well as to the future scholarship and contributions to the field that will be made by alumni of the HPEER Advanced Fellowship.
Interview Tips for Dermatology Applicants From Dr. Scott Worswick
What qualities are dermatology programs looking for that may be different from 5 years ago?
DR. WORSWICK: Every dermatology residency program is different, and as a result, each program is looking for different qualities in its applicants. Overall, I don’t think there has been a huge change in what programs are generally looking for, though. While each program may have a particular trait it values more than another, in general, programs are looking to find residents who will be competent and caring doctors, who work well in teams, and who could be future leaders in our field.
What are common mistakes you see in dermatology residency interviews, and how can applicants avoid them?
DR. WORSWICK: Most dermatology applicants are highly accomplished and empathic soon-to-be physicians, so I haven’t found a lot of “mistakes” from this incredible group of people that we have the privilege of interviewing. From time to time, an applicant will lie in an interview, usually out of a desire to appear to be a certain way, and occasionally, they may be nervous and stumble over their words. The former is a really big problem when it happens, and I would recommend that applicants be honest in all their encounters. The latter is not a major problem, and in some cases, might be avoided by lots of practice in advance.
What types of questions do you recommend applicants ask their interviewers to demonstrate genuine interest in the program?
DR. WORSWICK: Because of the signaling system, I think that programs assume interest at baseline once an applicant has sent the signal. So, “demonstrating interest” is generally not something I would recommend to applicants during the interview day. It is important for applicants to determine on interview day if a program is a fit for them, so applicants should showcase their unique strengths and skills and find out about what makes any given program different from another. The match generally works well and gets applicants into a program that closely aligns with their strengths and interests. So, think of interview day as your time to figure out how good a fit a program is for you, and not the other way around.
How can applicants who feel they don't have standout research or leadership credentials differentiate themselves in the interview?
DR. WORSWICK: While leadership, and less so research experience, is a trait valued highly by most if not all dermatology programs, it is only a part of what an applicant can offer a program. Most programs employ holistic review and consider several factors, probably most commonly grades in medical school, leadership experience, mentorship, teaching, volunteering, Step 2 scores, and letters of recommendation. Any given applicant does not need to excel in all of these. If an applicant has not done a lot of research, they may not match into a research-heavy program, but it doesn’t mean they won’t match. They should determine in which areas they shine and signal the programs that align with those interests/strengths.
How should applicants discuss nontraditional experiences in a way that adds value rather than raising red flags?
DR. WORSWICK: In general, my recommendation would be to explain what happened leading up to the change or challenge so that someone reading the application clearly understands the circumstances of the experience, then add value to the description by explaining what was learned and how this might relate to the applicant being a dermatology resident. For example, if a resident took time off for financial reasons and had to work as a medical assitant for a year, a concise description that explains the need for the leave (financial) as well as what value was gained (a year of hands-on patient care experience that validated their choice of going into medicine) could be very helpful.
What qualities are dermatology programs looking for that may be different from 5 years ago?
DR. WORSWICK: Every dermatology residency program is different, and as a result, each program is looking for different qualities in its applicants. Overall, I don’t think there has been a huge change in what programs are generally looking for, though. While each program may have a particular trait it values more than another, in general, programs are looking to find residents who will be competent and caring doctors, who work well in teams, and who could be future leaders in our field.
What are common mistakes you see in dermatology residency interviews, and how can applicants avoid them?
DR. WORSWICK: Most dermatology applicants are highly accomplished and empathic soon-to-be physicians, so I haven’t found a lot of “mistakes” from this incredible group of people that we have the privilege of interviewing. From time to time, an applicant will lie in an interview, usually out of a desire to appear to be a certain way, and occasionally, they may be nervous and stumble over their words. The former is a really big problem when it happens, and I would recommend that applicants be honest in all their encounters. The latter is not a major problem, and in some cases, might be avoided by lots of practice in advance.
What types of questions do you recommend applicants ask their interviewers to demonstrate genuine interest in the program?
DR. WORSWICK: Because of the signaling system, I think that programs assume interest at baseline once an applicant has sent the signal. So, “demonstrating interest” is generally not something I would recommend to applicants during the interview day. It is important for applicants to determine on interview day if a program is a fit for them, so applicants should showcase their unique strengths and skills and find out about what makes any given program different from another. The match generally works well and gets applicants into a program that closely aligns with their strengths and interests. So, think of interview day as your time to figure out how good a fit a program is for you, and not the other way around.
How can applicants who feel they don't have standout research or leadership credentials differentiate themselves in the interview?
DR. WORSWICK: While leadership, and less so research experience, is a trait valued highly by most if not all dermatology programs, it is only a part of what an applicant can offer a program. Most programs employ holistic review and consider several factors, probably most commonly grades in medical school, leadership experience, mentorship, teaching, volunteering, Step 2 scores, and letters of recommendation. Any given applicant does not need to excel in all of these. If an applicant has not done a lot of research, they may not match into a research-heavy program, but it doesn’t mean they won’t match. They should determine in which areas they shine and signal the programs that align with those interests/strengths.
How should applicants discuss nontraditional experiences in a way that adds value rather than raising red flags?
DR. WORSWICK: In general, my recommendation would be to explain what happened leading up to the change or challenge so that someone reading the application clearly understands the circumstances of the experience, then add value to the description by explaining what was learned and how this might relate to the applicant being a dermatology resident. For example, if a resident took time off for financial reasons and had to work as a medical assitant for a year, a concise description that explains the need for the leave (financial) as well as what value was gained (a year of hands-on patient care experience that validated their choice of going into medicine) could be very helpful.
What qualities are dermatology programs looking for that may be different from 5 years ago?
DR. WORSWICK: Every dermatology residency program is different, and as a result, each program is looking for different qualities in its applicants. Overall, I don’t think there has been a huge change in what programs are generally looking for, though. While each program may have a particular trait it values more than another, in general, programs are looking to find residents who will be competent and caring doctors, who work well in teams, and who could be future leaders in our field.
What are common mistakes you see in dermatology residency interviews, and how can applicants avoid them?
DR. WORSWICK: Most dermatology applicants are highly accomplished and empathic soon-to-be physicians, so I haven’t found a lot of “mistakes” from this incredible group of people that we have the privilege of interviewing. From time to time, an applicant will lie in an interview, usually out of a desire to appear to be a certain way, and occasionally, they may be nervous and stumble over their words. The former is a really big problem when it happens, and I would recommend that applicants be honest in all their encounters. The latter is not a major problem, and in some cases, might be avoided by lots of practice in advance.
What types of questions do you recommend applicants ask their interviewers to demonstrate genuine interest in the program?
DR. WORSWICK: Because of the signaling system, I think that programs assume interest at baseline once an applicant has sent the signal. So, “demonstrating interest” is generally not something I would recommend to applicants during the interview day. It is important for applicants to determine on interview day if a program is a fit for them, so applicants should showcase their unique strengths and skills and find out about what makes any given program different from another. The match generally works well and gets applicants into a program that closely aligns with their strengths and interests. So, think of interview day as your time to figure out how good a fit a program is for you, and not the other way around.
How can applicants who feel they don't have standout research or leadership credentials differentiate themselves in the interview?
DR. WORSWICK: While leadership, and less so research experience, is a trait valued highly by most if not all dermatology programs, it is only a part of what an applicant can offer a program. Most programs employ holistic review and consider several factors, probably most commonly grades in medical school, leadership experience, mentorship, teaching, volunteering, Step 2 scores, and letters of recommendation. Any given applicant does not need to excel in all of these. If an applicant has not done a lot of research, they may not match into a research-heavy program, but it doesn’t mean they won’t match. They should determine in which areas they shine and signal the programs that align with those interests/strengths.
How should applicants discuss nontraditional experiences in a way that adds value rather than raising red flags?
DR. WORSWICK: In general, my recommendation would be to explain what happened leading up to the change or challenge so that someone reading the application clearly understands the circumstances of the experience, then add value to the description by explaining what was learned and how this might relate to the applicant being a dermatology resident. For example, if a resident took time off for financial reasons and had to work as a medical assitant for a year, a concise description that explains the need for the leave (financial) as well as what value was gained (a year of hands-on patient care experience that validated their choice of going into medicine) could be very helpful.
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Practice Point
- Oral antibiotics remain a cornerstone in the treatment of moderate to severe acne, but growing concerns about antibiotic resistance necessitate more intentional prescribing.