User login
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
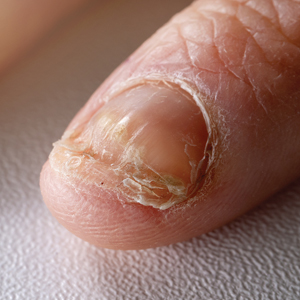
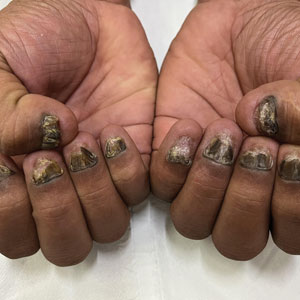
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
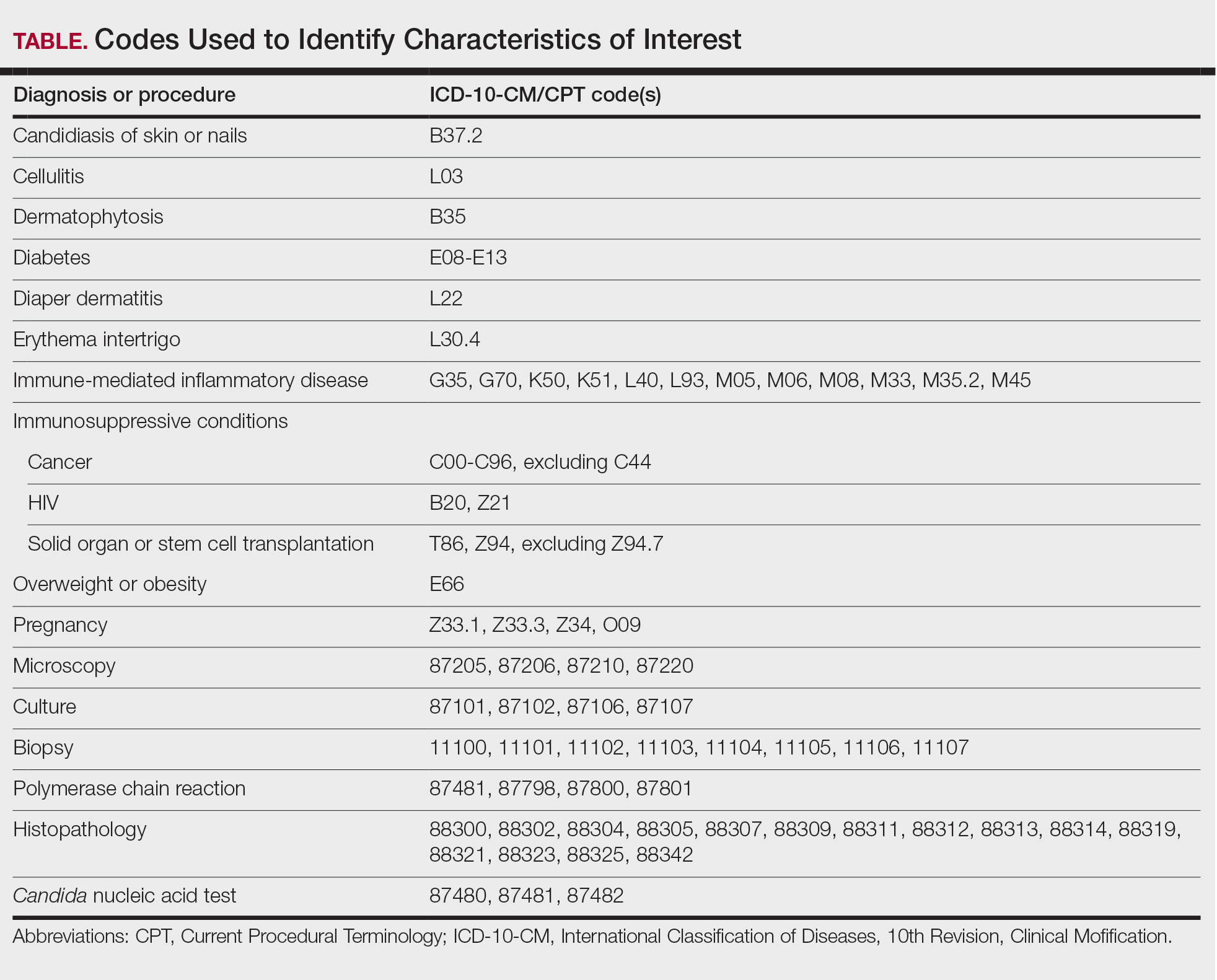
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.
Results
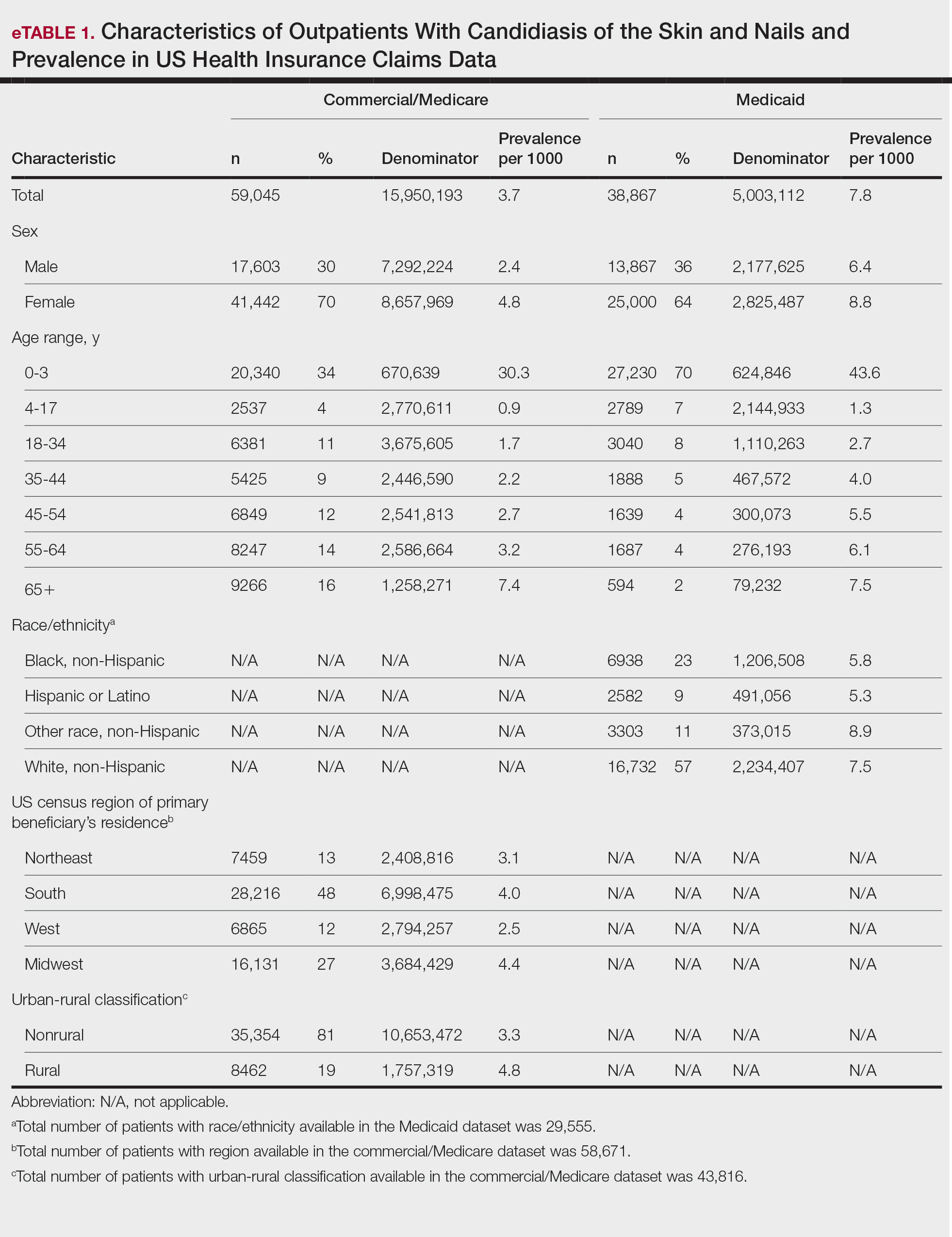
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).
Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).

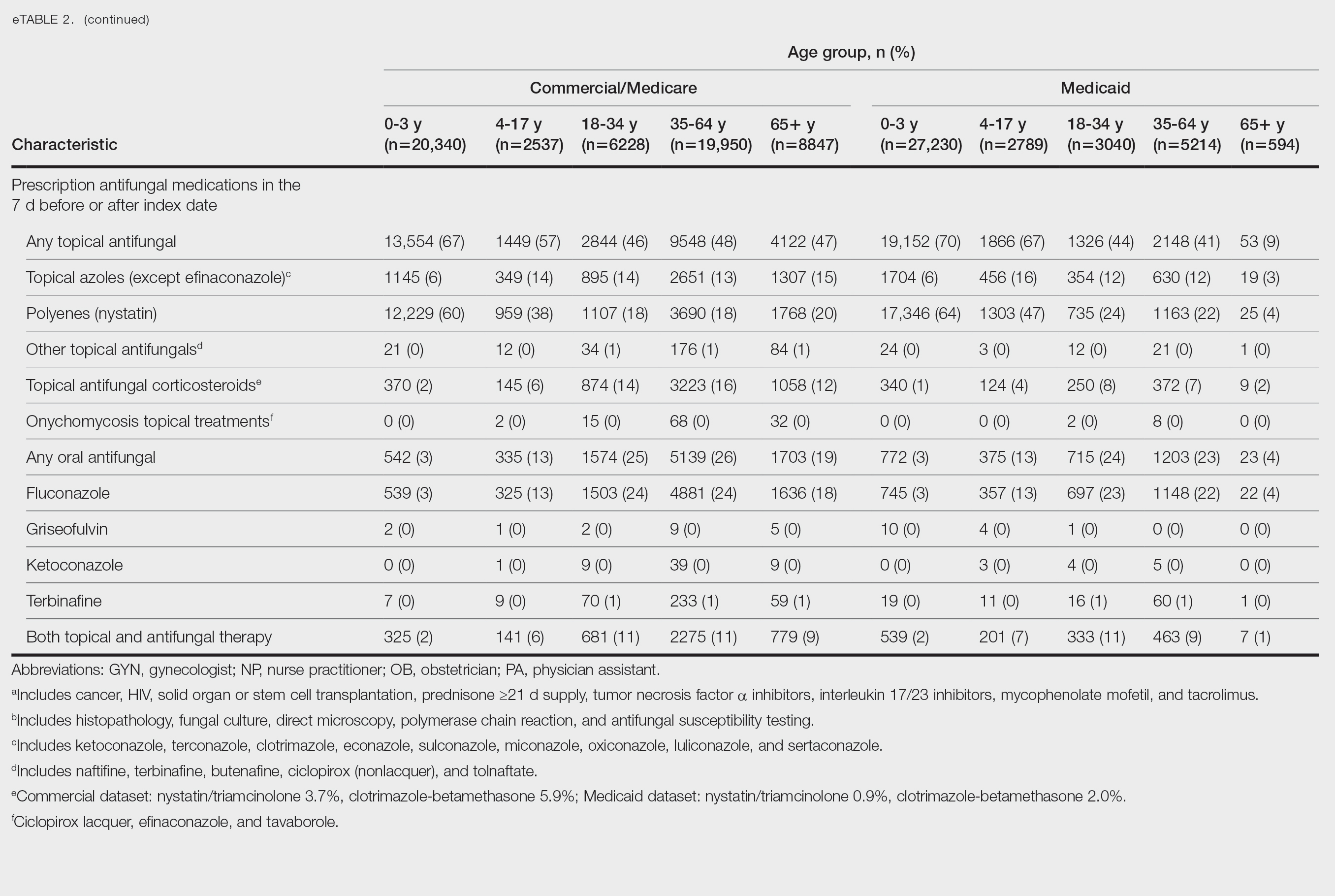
Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Practice Points
- Candidiasis of the skin or nails is a common outpatient condition that is most frequently diagnosed in infants, toddlers, and adults aged 65 years or older.
- Most cases are diagnosed clinically without diagnostic testing and treated with topical antifungals, but increased attention to formal diagnosis and treatment may be warranted given the emergence of antifungal-resistant Candida species.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
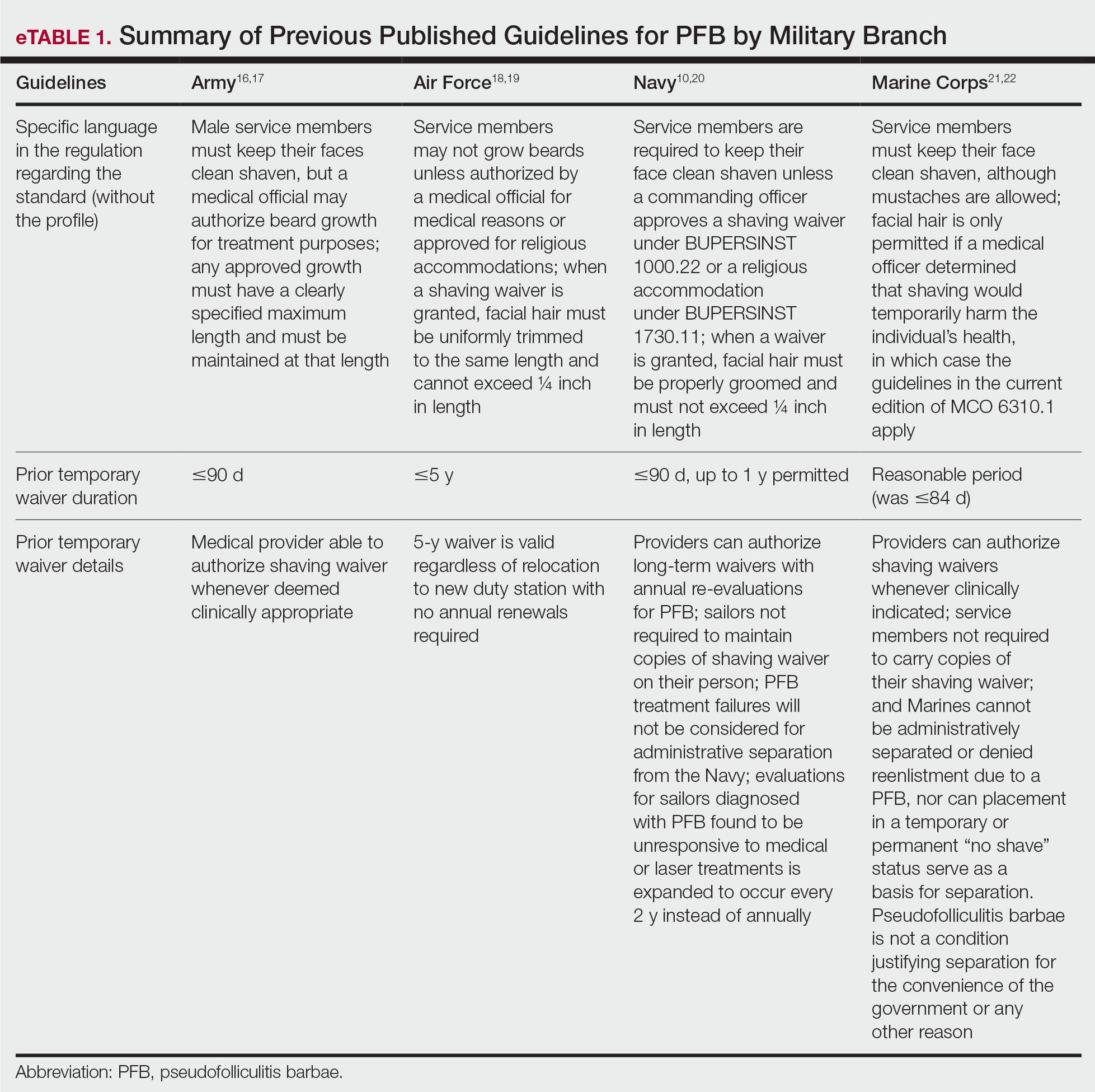
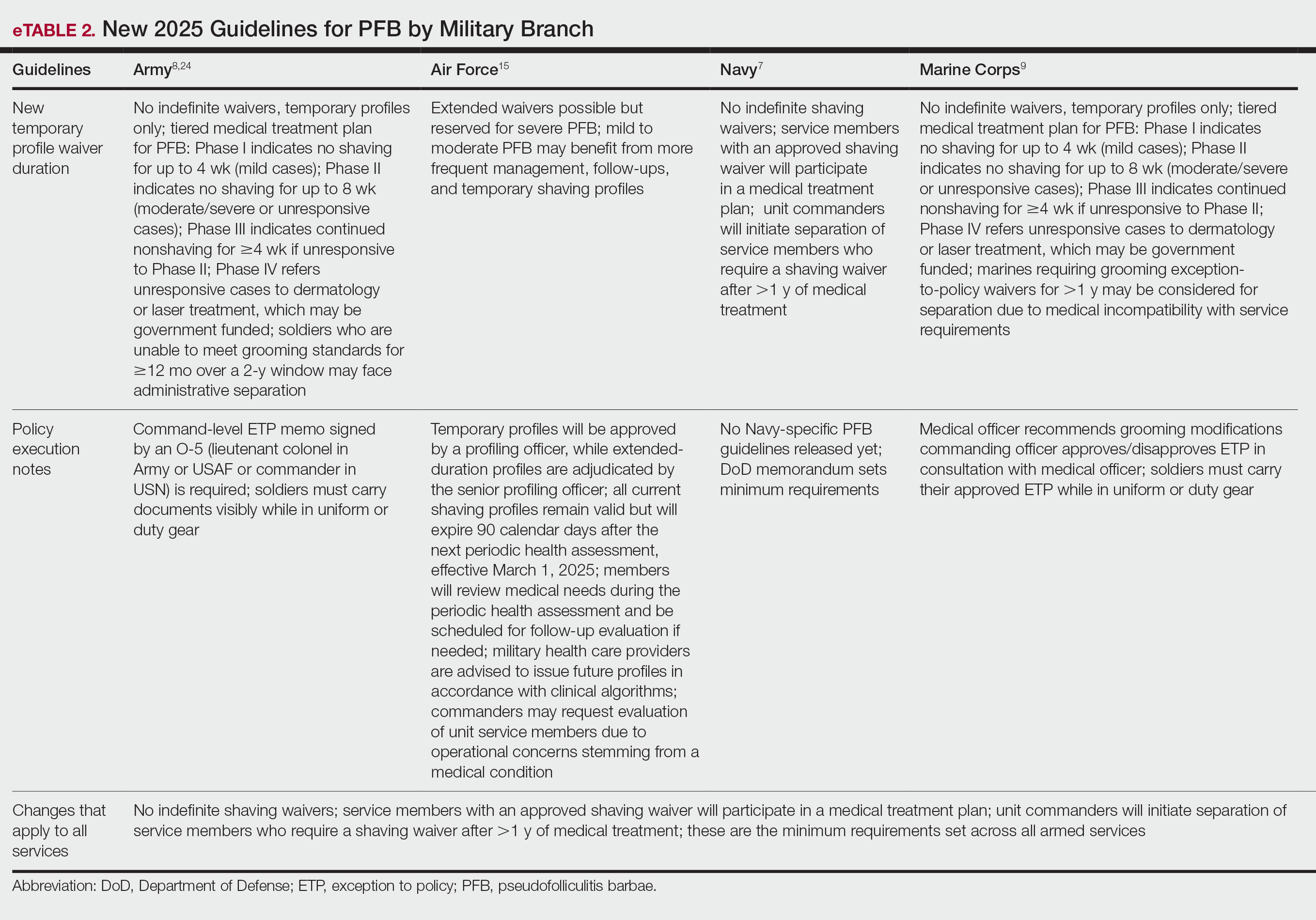
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24
The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Practice Points
- Revised US Department of Defense grooming policies eliminate permanent shaving waivers and limit medical waivers for pseudofolliculitis barbae (PFB) to no more than 1 year, after which administrative separation may be initiated if grooming standards cannot be met.
- These changes impose increased administrative and clinical demands on service members, military medical personnel, and commanders, requiring recurrent evaluation, documentation, and approvals for temporary shaving waivers.
- Civilian dermatologists should be aware of these policy changes and their potential career implications to appropriately counsel active-duty personnel and prospective military recruits.
- Laser hair removal may see increased utilization as a treatment option for service members for whom conservative management fails.
Progressive Dystrophy of the Fingernails and Toenails
Progressive Dystrophy of the Fingernails and Toenails
THE DIAGNOSIS: Nail Lichen Planus
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3
Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.
Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7
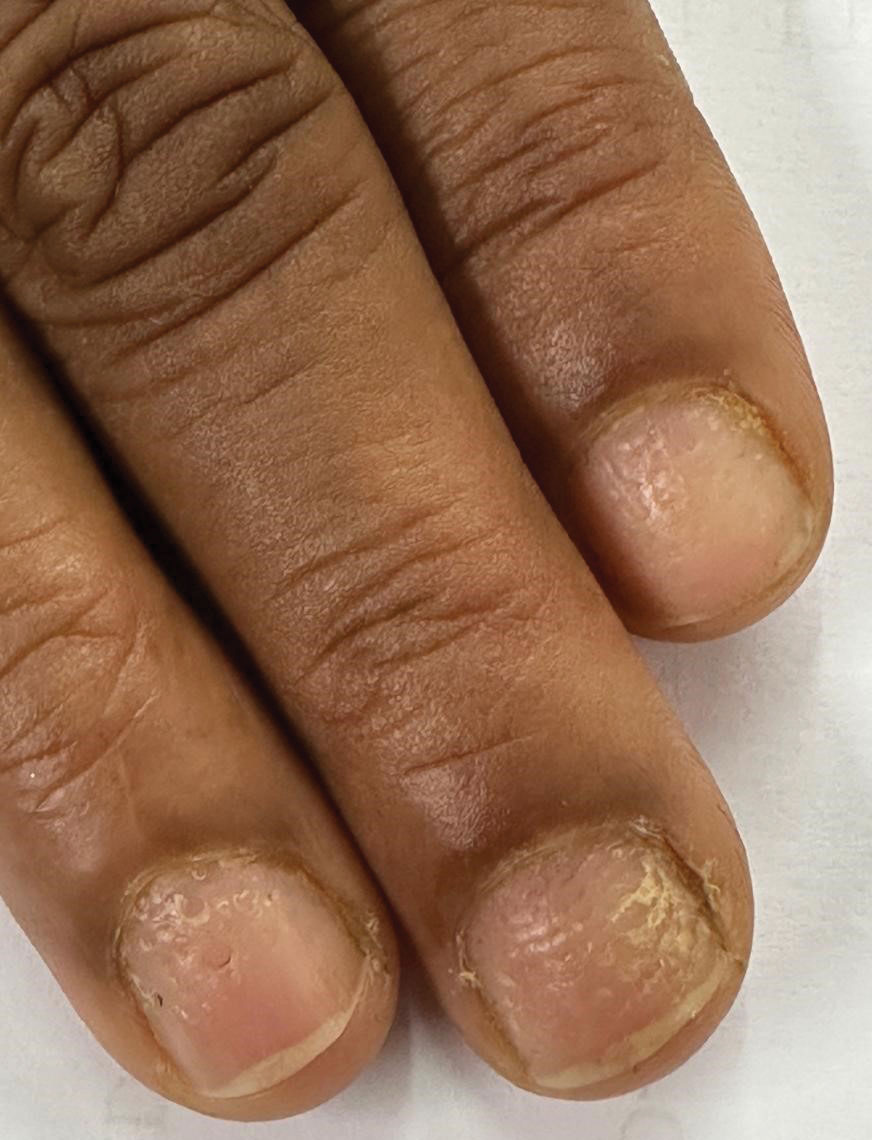
Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
THE DIAGNOSIS: Nail Lichen Planus
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3

Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.

Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7

Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
THE DIAGNOSIS: Nail Lichen Planus
The biopsy results showed features of hypergranulosis of the matricial epithelium, irregular acanthosis, apoptotic keratinocytes along the basal layer, and a lichenoid infiltrate consistent with nail lichen planus. The patient was started on topical clobetasol propionate 0.05% applied once daily under overnight occlusion. Additionally, intramatricial triamcinolone acetonide (2.5 mg/mL; 0.1 mL per injection) was administered into the affected nail matrix at 4-week intervals for a total of 2 sessions. At the 2-month follow-up visit, the patient reported improvement in longitudinal ridging; however, he subsequently was lost to follow-up.
Nail lichen planus is a chronic inflammatory disorder that occurs in 10% to 15% of patients with lichen planus worldwide and is more common in adults than children.1 It can manifest independently or concurrently with cutaneous and/or oral mucosal involvement. The fingernails are more commonly affected than the toenails.2 The clinical features of nail lichen planus can be classified based on involvement of the nail matrix (longitudinal ridging, red lunula, thinning of the nail plate, koilonychia, trachyonychia, pterygium, and anonychia) or nail bed (onycholysis, subungual hyperkeratosis, and splinter hemorrhages).1
In our patient, who presented with chronic progressive nail dystrophy affecting all 20 nails, onychomycosis, nail psoriasis, onychotillomania, and idiopathic trachyonychia were included in the differential.1
Onychomycosis manifests as white or yellow-brown discoloration of the nail, onycholysis, subungual hyperkeratosis, and thickening of the nail plate. Diagnosis is confirmed by the presence of septate hyphae (dermatophytes) or budding yeast cells (Candida species) on a potassium hydroxide mount. Other diagnostic modalities include dermoscopy, fungal culture, and histopathology of nail clippings, with demonstration of fungal elements identified on periodic acid-Schiff staining (eFigure 1).3

Nail psoriasis characteristically manifests as deep irregular pitting of the nails. Other features favoring psoriasis include involvement of the nail matrix manifesting as leukonychia, red lunula, and crumbling, as well as involvement of the nail bed manifesting as onycholysis, subungual hyperkeratosis, salmon patches/oil spots, and splinter hemorrhages (eFigure 2).4 Diagnosis primarily is clinical, supported by histopathology when uncertainty exists.

Onychotillomania is a behavioral disorder characterized by an irresistible urge or impulse in patients to either pick or pull at their fingernails and/or toenails. Clinicopathologic features of the involved nails are nonspecific and atypical, with possible involvement of periungual and digital skin. Diagnosis of onychotillomania is challenging.5 Dermoscopic features including anonychia with multiple obliquely arranged nail bed hemorrhages, gray pigmentation of the nail bed, and wavy lines, has been proposed to aid the diagnosis of onychotillomania.6
Idiopathic trachyonychia is isolated nail involvement characterized by rough, ridged, and thin nails affecting multiple or all of the fingernails and toenails without an underlying systemic or dermatologic condition (eFigure 3). The terms trachyonychia and 20-nail dystrophy have been used interchangeably in the literature; however, trachyonychia does not always involve all 20 nails. Other conditions causing widespread dystrophy of all 20 nails cannot be diagnosed as 20-nail dystrophy or trachyonychia without the distinct morphologic features of thin brittle nails with pronounced longitudinal ridging.7

Prompt diagnosis and early intervention in nail lichen planus is crucial due to the potential for irreversible scarring. First-line treatment options include intramatricial and intramuscular triamcinolone acetonide for 3 to 6 months.4 Second-line therapies include oral retinoids such as acitretin and alitretinoin and immunosuppressive agents such as azathioprine, mycophenolate mofetil, and cyclosporine. Other reported treatment options include clobetasol propionate, tacrolimus, dapsone, griseofulvin, etanercept, hydroxychloroquine, methotrexate, and UV therapy.4
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:32-45. doi:10.2174/1872213X13666191026090713
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Sidiropoulou P, Sgouros D, Theodoropoulos K, et al. Onychotillomania: a chameleon-like disorder: case report and review of literature. Skin Appendage Disord. 2019;5:104-107. doi:10.1159/000489941
- Maddy AJ, Tosti A. Dermoscopic features of onychotillomania: a study of 36 cases. J Am Acad Dermatol. 2018;79:702-705. doi:10.1016 /j.jaad.2018.04.015
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
Progressive Dystrophy of the Fingernails and Toenails
Progressive Dystrophy of the Fingernails and Toenails
A 35-year-old man presented to the dermatology department with gradually progressive dystrophy of the fingernails and toenails of 20 years’ duration. The patient reported no history of other dermatologic conditions. Physical examination revealed longitudinal ridging of all 20 nails and discoloration of the nail plates, as well as a few nails showing pterygium and anonychia; the skin and mucosal surfaces were otherwise normal, and nail plate thinning was not observed. A potassium hydroxide mount was negative. A biopsy of the nail matrix on the left thumbnail was performed.
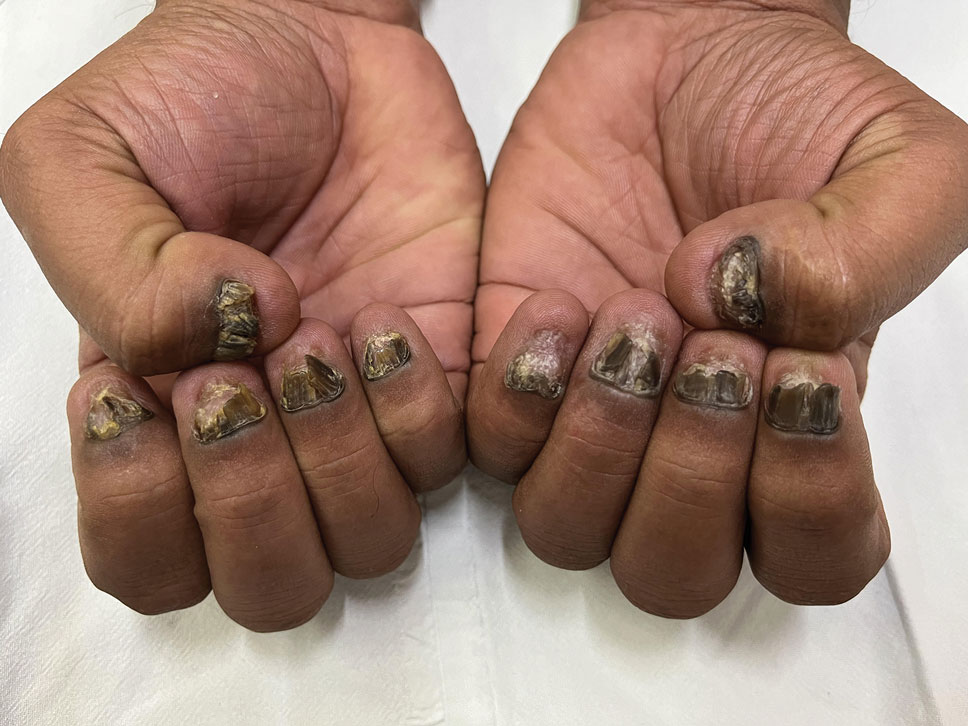
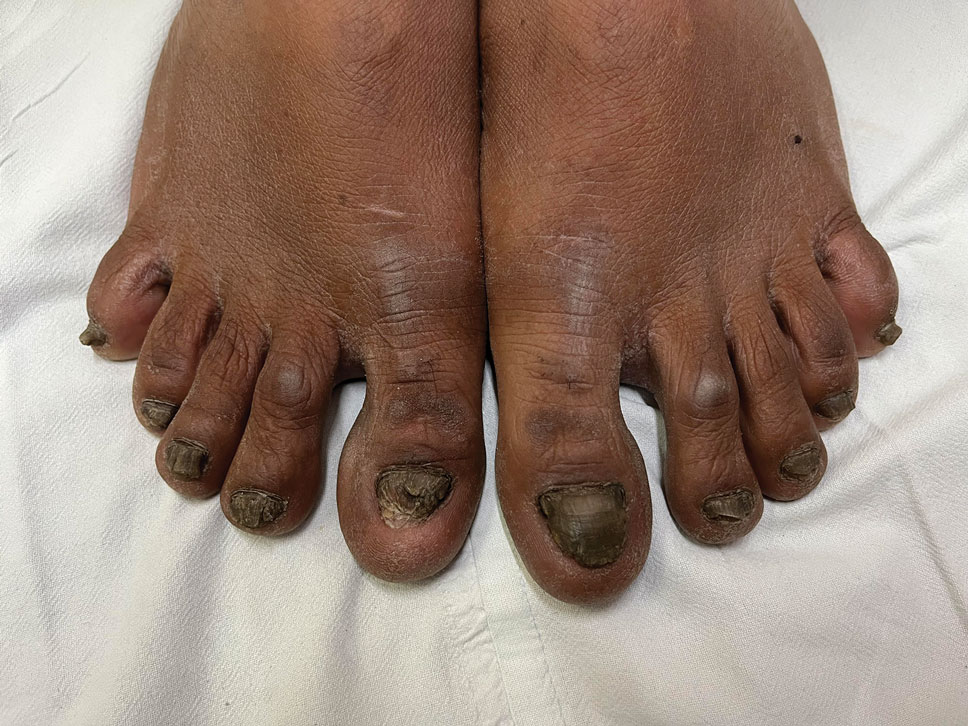
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.
Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10
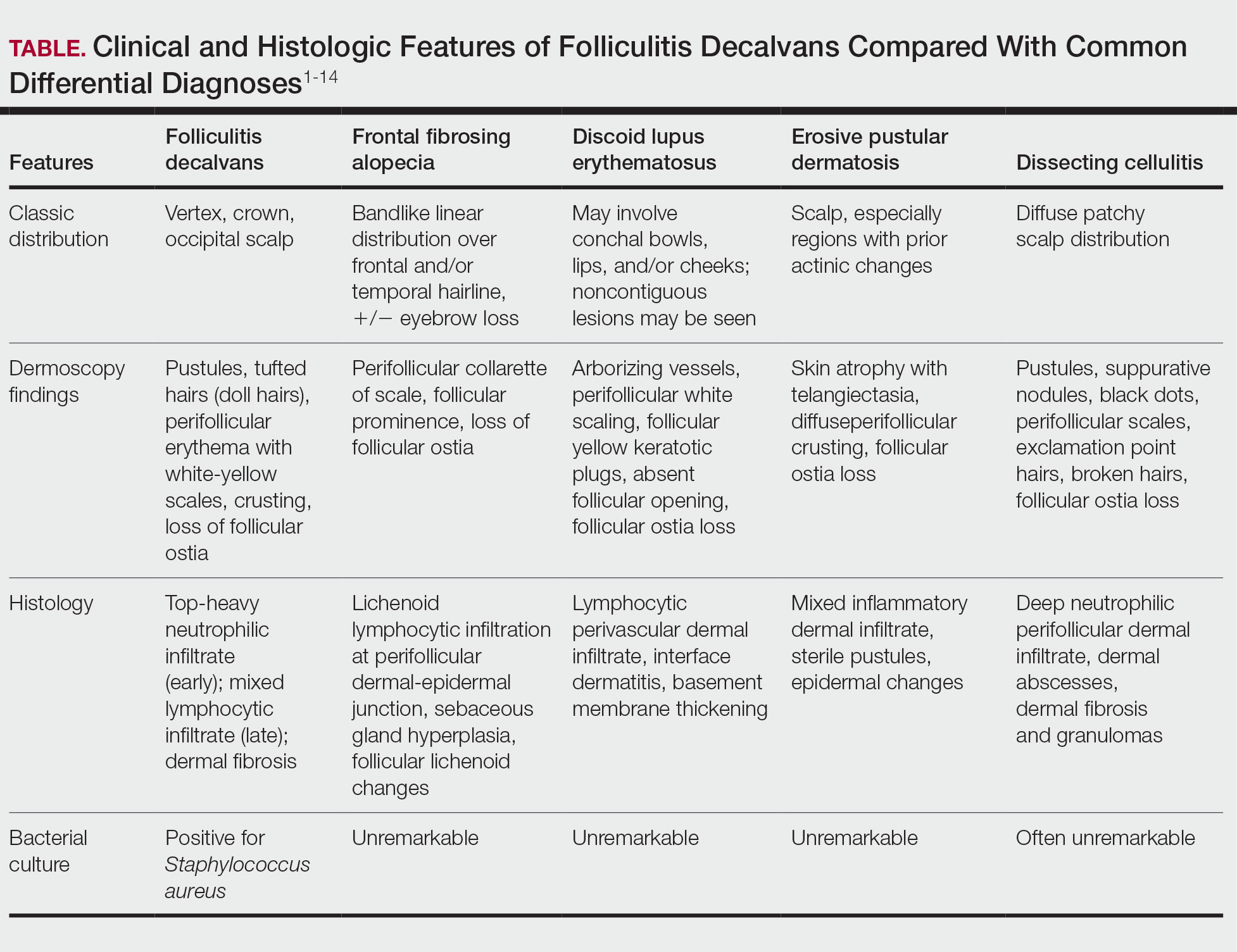
While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
A 52-year-old woman presented to the dermatology department with an intermittently pruritic rash in a bandlike distribution on the left upper forehead and the frontal and temporal scalp of 4 years’ duration. The rash initially was diagnosed as psoriasis at an outside facility. Treatment over the year prior to presentation included tildrakizumab-asmn; topical crisaborole 2%; and excimer laser, which was complicated by blistering. The patient reported no history of topical or injected steroid use in the involved areas. Physical examination at the current presentation revealed arcuate erythematous plaques with follicular prominence, perifollicular scaling, pustules, and lone hairs. There also were porcelain-white atrophic plaques with loss of follicular ostia that were most prominent over the temporal scalp. A biopsy of the left lateral forehead was performed.
Management of Facial Hair in Women
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Facial hair growth in women is complex and multifaceted. It is not a disease but rather a part of normal anatomy or a symptom influenced by an underlying condition such as hypertrichosis, a hormonal imbalance (eg, hirsutism due to polycystic ovary syndrome [PCOS]), mechanical factors such as pseudofolliculitis barbae (PFB) from shaving, and perimenopausal and postmenopausal hormonal shifts. Additionally, normal facial hair patterns can vary substantially based on genetics, ethnicity, and cultural background. Some populations may naturally have more visible vellus or terminal hairs on the face, which are entirely physiologic rather than indicative of an underlying disorder. Despite this, societal expectations and beauty standards across many cultures dictate that facial hair in women is undesirable, often associating hair-free skin with femininity and attractiveness. This perception drives many women to seek treatment—not necessarily for medical reasons, but due to social pressure and aesthetic preferences.
Hypertrichosis, whether congenital or acquired, refers to excessive hair growth that is not androgen dependent and can appear on any site of the body. Causes include genetic predisposition, porphyria, thyroid disorders, internal malignancies, malnutrition, anorexia nervosa, or use of medications such as cyclosporine, prednisolone, and phenytoin.1 Hirsutism, by contrast, is characterized by the growth of terminal hairs in women at androgen-dependent sites such as the face, neck, and upper chest, where coarse hair typically grows in men.2 This condition often is associated with excess androgens produced by the ovaries or adrenal glands, most commonly due to PCOS although genetic factors may contribute.
Before initiating treatment, a thorough history and physical examination are essential to determine the underlying cause of conditions associated with facial hair growth in women. Clinicians should assess for signs of hyperandrogenism, menstrual irregularities, virilization, medication use, and family history. In cases of a suspected endocrine disorder, further laboratory evaluation may be warranted to guide appropriate management. While each cause of facial hair growth in women has unique management considerations, the shared impact on psychosocial well-being and adherence to grooming standards in the US military warrants an all-encompassing yet targeted approach. This comprehensive review discusses management options for women with facial hair in the military based on a review of PubMed articles indexed for MEDLINE conducted in November 2024 using combinations of the following search terms: hirsutism, facial hair, pseudofolliculitis barbae, women, female, military, grooming standards, hyperandrogenism, and hair removal.
Treatment Modalities
The available treatment modalities, including their mechanisms, potential risks, and considerations are summarized in the eTable.

Mechanical—Shaving remains one of the most widely utilized methods of hair removal in women due to its accessibility and ease of use. It does not disrupt the anagen phase of the hair growth cycle, making it a temporary method that requires frequent repetition (often daily), particularly for individuals with rapid hair growth. The belief that shaving causes hair to grow back thicker or faster is a common misconception. Shaving does not alter the thickness or growth rate of hair; instead, it leaves a blunt tip, making the hair feel coarser or appear thicker than uncut hair.3 Despite its relative convenience, shaving can lead to skin irritation due to mechanical trauma. Potential complications include PFB, superficial abrasions known more broadly as shaving irritation, and an increased risk for infections such as bacterial or fungal folliculitis.4
Chemical depilation, which uses thioglycolates mixed with alkali compounds, disrupts disulfide bonds in the hair, effectively breaking down the shaft without affecting the bulb. The depilatory requires application to the skin for approximately 3 to 15 minutes depending on the specific formulation and the thickness or texture of the hair. While it is a cost-effective option that easily can be done at home, the chemicals involved may trigger irritant contact dermatitis or folliculitis and produce an unpleasant odor from hydrogen disulfide gas.5 They also can lead to PFB.
Epilation removes the entire hair shaft and bulb, with results lasting approximately 6 weeks.6 Methods range from using tweezers to pluck single hairs and devices that simultaneously remove multiple hairs to hot or cold waxing, which use resin to grip and remove hair. Threading is a technique that uses twisted thread to remove the hair at the follicle level; this method may not alter hair growth unless performed during the anagen phase, during which repeated plucking can damage the matrix and potentially lead to permanent hair reduction.5 Common adverse effects include pain during removal, burns from waxing, folliculitis, PFB, postinflammatory hyperpigmentation, and scarring, particularly when multiple hairs are removed at once.
Pharmacologic—Pharmacologic therapy commonly is used to manage hirsutism and typically begins with a trial of combined oral contraceptives (COCs) containing estrogen and progestin, which are considered the first-line option unless contraindicated.7 If response to COC monotherapy is inadequate, an antiandrogen such as spironolactone may be added. Combination therapy with a COC and an antiandrogen generally is reserved for severe cases or patients who previously have shown suboptimal response to COCs alone.7 Patients should be counseled to discontinue antiandrogen therapy if they become pregnant due to the risk for fetal undervirilization observed in animal studies.8,9 Typical dosing of spironolactone, a competitive inhibitor of 5-α-reductase and androgen receptors, ranges from 100 mg to 200 mg daily.10 Reported adverse effects include polyuria, postural hypotension, menstrual irregularities, hyperkalemia, and potential liver dysfunction. Although spironolactone has demonstrated tumorigenic effects in animal studies, no such effects have been observed in humans.11
Eflornithine hydrochloride cream 13.9% is the first topical prescription medication approved by the US Food and Drug Administration for reduction of unwanted facial hair in women.12 It works by irreversibly blocking the activity of ornithine decarboxylase, an enzyme involved in the rate-limiting step of polyamine synthesis, which is essential for hair growth. In a randomized, double-blind clinical trial evaluating its effectiveness and safety, twice-daily application for 24 weeks resulted in a clinically meaningful reduction in hair length and density (measured as surface area) compared with the control group.13 When eflornithine hydrochloride cream 13.9% is discontinued, hair growth gradually returns to baseline. Studies have shown that hair regrowth typically begins within 8 weeks after treatment is stopped; within several months, hair returns to pretreatment levels.14 Adverse effects of eflornithine hydrochloride cream generally are mild and may include local irritation and acneform eruptions. In a randomized bilateral vehicle-controlled trial of 31 women, both eflornithine and vehicle creams were well tolerated, with 1 patient reporting mild tingling with eflornithine that resolved with continued use for 7 days.15
Procedural—Photoepilation therapies widely are considered by dermatologists to be among the most effective methods for reducing unwanted hair.16 Laser hair removal employs selective photothermolysis, a principle by which specific wavelengths of light target melanin in hair follicles. This method results in localized thermal damage, destroying hair follicles and reducing regrowth. Wavelengths between 600 and 1100 nm are most effective for hair removal; widely used devices include the ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and long-pulsed Nd:YAG lasers (1064 nm). Cooling mechanisms such as cryogen spray or contact cooling often are employed to minimize epidermal damage and lessen patient discomfort.
The hair matrix is most responsive to laser treatment during the anagen phase, necessitating multiple sessions to ensure all hairs are treated during this optimal growth stage. Generally, 4 to 6 sessions spaced at intervals of 4 to 6 weeks are required to achieve satisfactory results.17 Matching the laser wavelength to the absorption properties of melanin—the target chromophore—enables selective destruction of melanin-rich hair follicles while minimizing damage to surrounding skin.
The ideal laser wavelength primarily affects melanin concentrated in the hair bulb, leading to follicular destruction while reducing the risk for unintended depigmentation of the epidermis; however, competing structures in the skin (eg, epidermal pigment) also can absorb laser energy, diminishing treatment efficacy and increasing the risk for adverse effects. Shorter wavelengths are effective for lighter skin types, while longer wavelengths such as the Nd:YAG laser are safer for individuals with darker skin types as they bypass melanin in the epidermis.
It is important to note that laser hair removal is ineffective for white and gray hairs due to the lack of melanin. As a result, alternative methods such as electrolysis, which does not rely on pigment, may be more appropriate for permanent hair removal in individuals with nonpigmented hairs. Research indicates that combining topical eflornithine with alexandrite or Nd:YAG lasers improves outcomes for reducing unwanted facial hair.18
In military settings, laser hair removal is utilized for specific conditions such as PFB in male service members to assist with the reduction of hair and mitigation of symptoms.19 The majority of military dermatology clinics have devices for laser hair removal; however, dermatology services are not available at many military treatment facilities, and dermatologic care may be provided by the local civilian dermatologists. That said, laser therapy is covered in the civilian sector for active-duty service members with PFB of the face and neck under certain criteria. These include a documented safety risk in environments requiring respiratory protection, failure of conservative treatments, and evaluation by a military dermatologist who confirms the necessity of civilian-provided laser therapy when it is unavailable at a military facility.20 While such policies demonstrate the military’s recognition of laser therapy as a viable solution for certain grooming-related conditions, many are unaware that the existing laser hair removal policy also applies to women. Increasing awareness of this coverage could help female service members access treatment options that align with both medical and professional grooming needs.
Intense pulsed light (IPL) systems are nonlaser devices that emit broad-spectrum light in the 590- to 1200-nm range. They utilize a flash lamp to achieve thermal damage. Filters are used to narrow the wavelength range based on the specific target. Intense pulsed light devices are less precise than lasers but remain effective for hair reduction. In addition to hair removal, IPL devices are employed in the treatment of pigmented and vascular lesions. Common adverse effects of both laser and IPL hair removal include transient erythema, perifollicular edema, and pigmentary changes, especially in patients with darker skin types. Rare complications include blistering, scarring, and paradoxical hair stimulation in which untreated areas develop increased hair growth.
Electrolysis is recognized as the only method of truly permanent hair removal and is effective for all hair colors.21 However, the variability in technique among practitioners often leads to inconsistent results, with some patients experiencing hair regrowth. Galvanic electrolysis involves inserting a fine needle into the hair follicle and applying an electrical current to destroy the it and the rapidly dividing cells of the matrix.22 The introduction of thermolytic electrolysis, which uses a high-frequency alternating current (commonly 13.56 MHz or 27.12 MHz), has enhanced efficiency by creating heat at the needle tip to destroy the follicle. This approach is faster and now is commonly combined with galvanic electrolysis.23 While no controlled clinical trials directly compare these methods, many patients experience permanent hair removal, with approximately 15% to 25% regrowth within 6 months.22,24
Alternative Options—Home-use laser and light-based devices have become increasingly popular for managing unwanted hair due to their affordability and convenience, with most devices priced less than $1000.25 These devices utilize various technologies, including lasers (808 nm), IPL, or combinations of IPL and radiofrequency.26 Despite their accessibility, peer-reviewed research on their safety profile and effectiveness is limited, as existing data primarily come from industry-funded, uncontrolled studies with short follow-up durations—making it difficult to assess long-term outcomes.25
Psychosocial Impact
A 2023 study of active-duty female service members with PCOS highlighted the unique challenges they face while managing symptoms such as facial hair within the constraints of military service.27 Although the study focused on PCOS, the findings shed light on how facial hair specifically impacts the psychological well-being of servicewomen. Participants described facial hair as one of the most visible and stigmatizing symptoms, often leading to feelings of embarrassment and diminished confidence. Participants also highlighted the professional implications of facial hair, with some describing feelings of scrutiny and judgment from peers and leadership in public. These challenges can be more pronounced in deployments or field exercises where hygiene resources are limited. The lack of access not only affects self-perception but also can hinder the ability of servicewomen to meet implicit expectations for grooming and appearance.27 There is a notable gap in research examining the impact of facial hair on military servicewomen. Given the unique environmental challenges and professional expectations, further investigation is warranted to better understand how facial hair affects women and to optimize treatment approaches in this population.
Final Thoughts
Limited awareness and understanding of facial hair in woman contribute to stigma, often leaving affected individuals to navigate challenges in isolation. Given the impact on confidence, professional appearance, and adherence to military grooming standards, it is essential for health care practitioners to recognize and address facial hair in women. Importantly, laser hair removal is covered by TRICARE for active-duty female service members with PFB, yet many remain unaware of this benefit. Increased awareness of available mechanical, pharmacologic, and procedural treatment options allows for tailored management, ensuring that women receive appropriate medical care.
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48:161-181. doi:10.1067/mjd.2003.100
Blume-Peytavi U, Hahn S. Medical treatment of hirsutism. Dermatol Ther. 2008;21:329-339. doi:10.1111/j.1529-8019.2008.00215.x
Kang CN, Shah M, Lynde C, et al. Hair removal practices: a literature review. Skin Therapy Lett. 2021;26:6-11.
Matheson E, Bain J. Hirsutism in women. Am Fam Physician. 2019;100:168-175.
Shenenberger DW, Utecht LM. Removal of unwanted facial hair. Am Fam Physician. 2002;66:1907-1911.
Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964;12:465-474.
Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257. doi:10.1210/jc.2018-00241
Barrionuevo P, Nabhan M, Altayar O, et al. Treatment options for hirsutism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2018;103:1258-1264. doi:10.1210/jc.2017-02052
Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. Published online August 9, 2023. doi:10.1016/j.eclinm.2023.102162
Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement. Hum Reprod Update. 2012;18:146-170.
Hussein RS, Abdelbasset WK. Updates on hirsutism: a narrative review. Int J Biomedicine. 2022;12:193-198. doi:10.21103/Article12(2)_RA4
Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:1-5.
Wolf JE Jr, Shander D, Huber F, et al. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46:94-98. doi:10.1111/j.1365-4632.2006.03079.x
Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol. 2001;2:197-202. doi:10.2165/00128071-200102030-00009
Hamzavi I, Tan E, Shapiro J, et al. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57:54-59. doi:10.1016/j.jaad.2006.09.025
Goldberg DJ. Laser hair removal. In: Goldberg DJ, ed. Laser Dermatology: Pearls and Problems. Blackwell; 2008.
Hussain M, Polnikorn N, Goldberg DJ. Laser-assisted hair removal in Asian skin: efficacy, complications, and the effect of single versus multiple treatments. Dermatol Surg. 2003;29:249-254. doi:10.1046/j.1524-4725.2003.29059.x
Smith SR, Piacquadio DJ, Beger B, et al. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: a randomized trial. Dermatol Surg. 2006;32:1237-1243. doi:10.1111/j.1524-4725.2006.32282.x
Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
TRICARE Operations Manual 6010.59-M. Supplemental Health Care Program (SHCP)—Chapter 17. Contractor Responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed February 13, 2024. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
Yanes DA, Smith P, Avram MM. A review of best practices for gender-affirming laser hair removal. Dermatol Surg. 2024;50:S201-S204. doi:10.1097/DSS.0000000000004441
Wagner RF Jr, Tomich JM, Grande DJ. Electrolysis and thermolysis for permanent hair removal. J Am Acad Dermatol. 1985;12:441-449. doi:10.1016/s0190-9622(85)70062-x
Olsen EA. Methods of hair removal. J Am Acad Dermatol. 1999;40:143-157. doi:10.1016/s0190-9622(99)70181-7
Kligman AM, Peters L. Histologic changes of human hair follicles after electrolysis: a comparison of two methods. Cutis. 1984;34:169-176.
Hession MT, Markova A, Graber EM. A review of hand-held, home-use cosmetic laser and light devices. Dermatol Surg. 2015;41:307-320. doi:10.1097/DSS.0000000000000283
Wheeland RG. Permanent hair reduction with a home-use diode laser: safety and effectiveness 1 year after eight treatments. Lasers Surg Med. 2012;44:550-557. doi:10.1002/lsm.22051
Hopkins D, Walker SC, Wilson C, et al. The experience of living with polycystic ovary syndrome in the military. Mil Med. 2024;189:E188-E197. doi:10.1093/milmed/usad241
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).
Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Practice Points
- Synthetic hair extensions are made from materials that can expose patients to high levels of carcinogens beginning in early childhood.
- The apple cider vinegar rinse method is an anecdotal remedy lacking data validating its ability to mitigate adverse reactions and complications associated with synthetic hair extensions, including carcinogenic exposure to materials they comprise.
- Dermatologists should inform patients of the potential exposure risks when using synthetic hair extensions to help patients make informed decisions regarding future styling habits and hair care choices.
Longitudinal Erythronychia Manifesting With Pain and Cold Sensitivity
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.