User login
Alopecia Induced by Poly-L-Lactic Acid Injection
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.
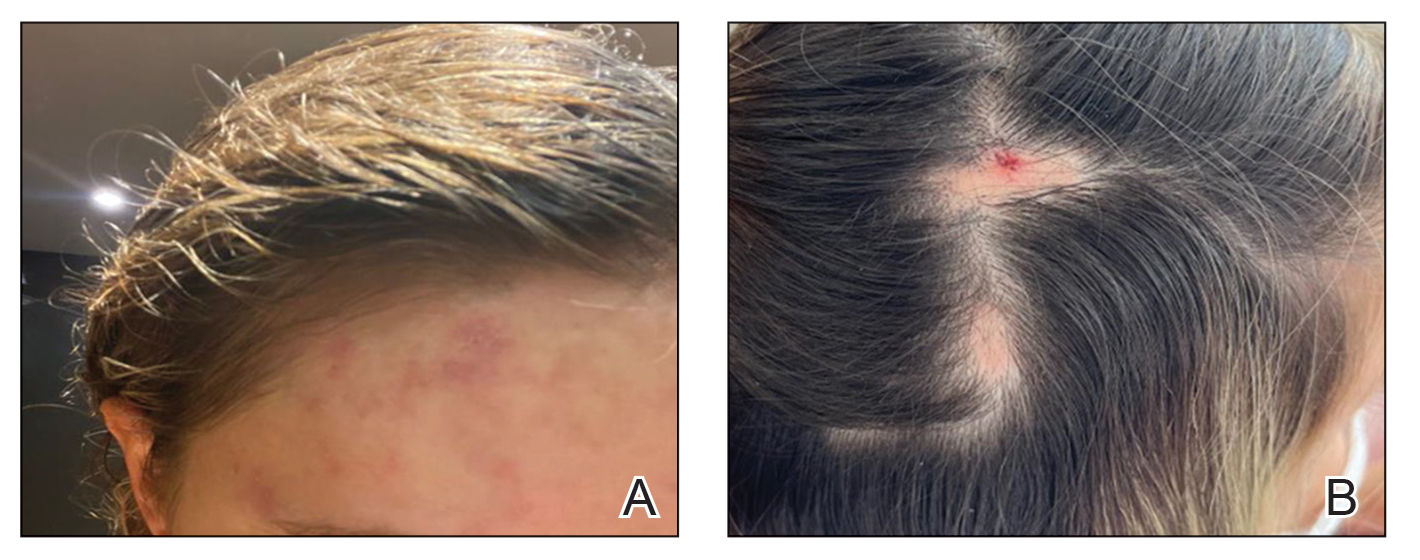
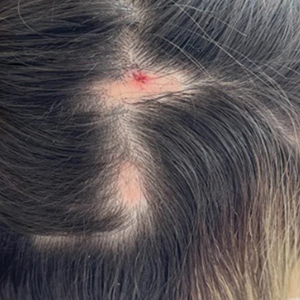
The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
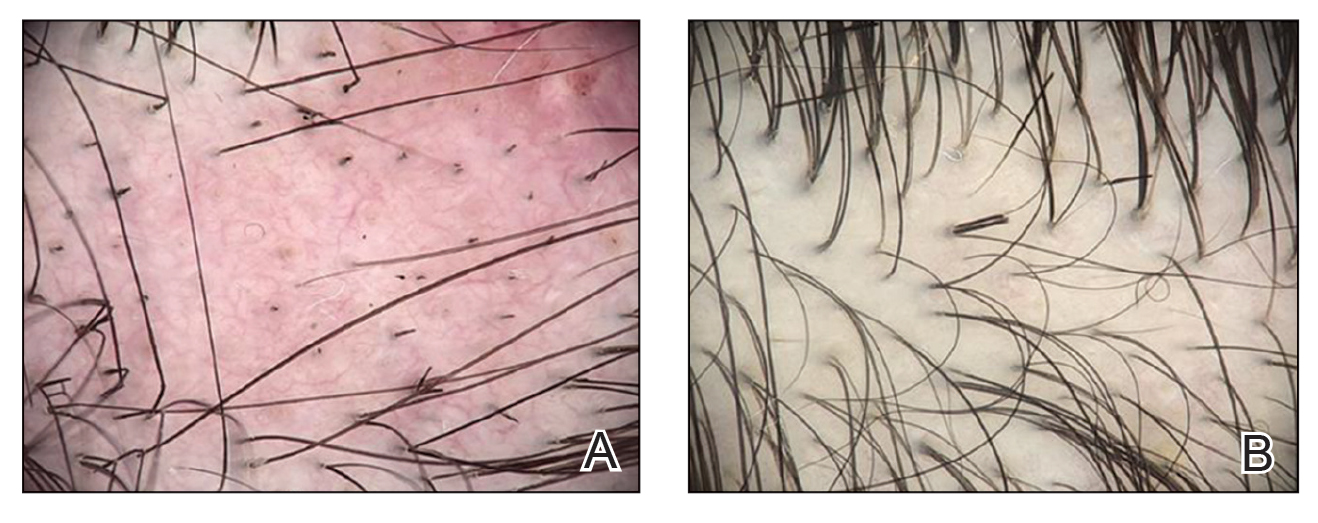
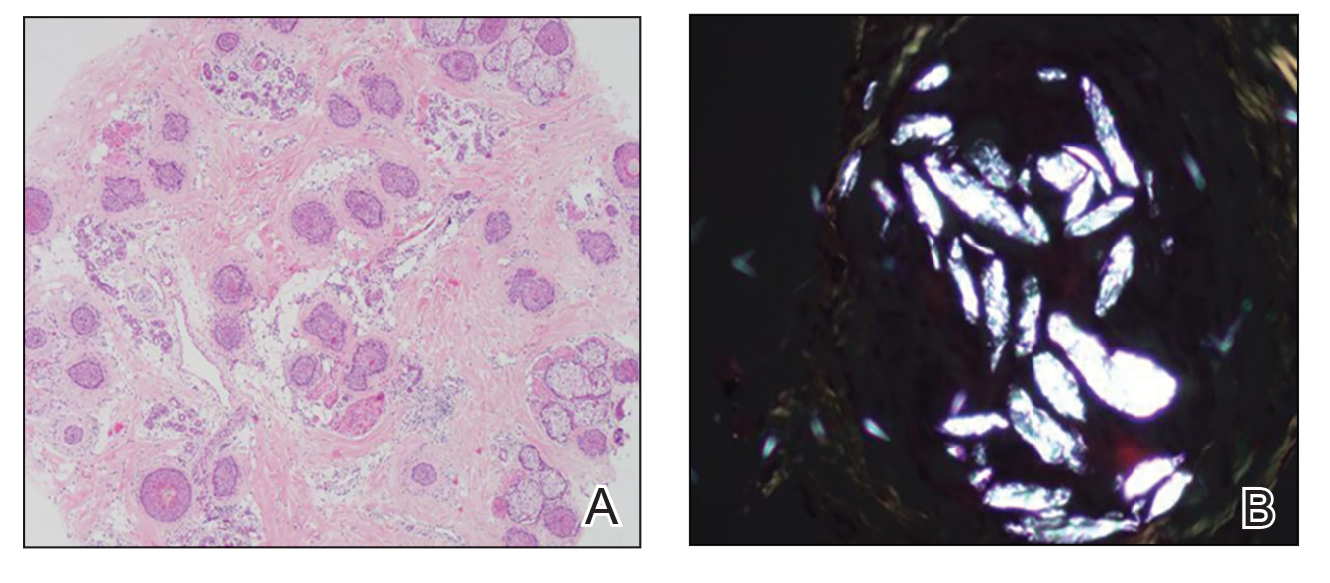
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.
Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Practice Points
- Alopecia is a potential adverse event of poly-L-lactic acid (PLLA) injection, and prior reports of embolization and retinal ischemia with PLLA use raise the concern of its occlusive potential.
- The combination of extravascular compression due to the presence of the filler material in the subcutaneous tissue as well as intravascular PLLA embolism may contribute to tissue ischemia–induced alopecia in the affected areas.
- Poly-L-lactic acid also may cause a local inflammatory reaction that is alopecia areata–like, which would explain its similar trichoscopy findings.