User login
Continuous Testing Method for Contact Allergy to Topical Therapies in the Management of Chronic and Postoperative Wounds
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
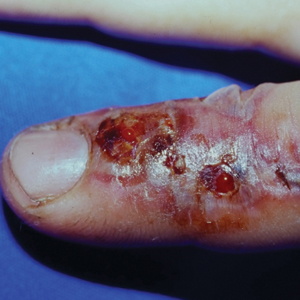
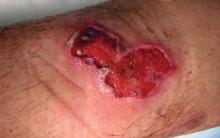
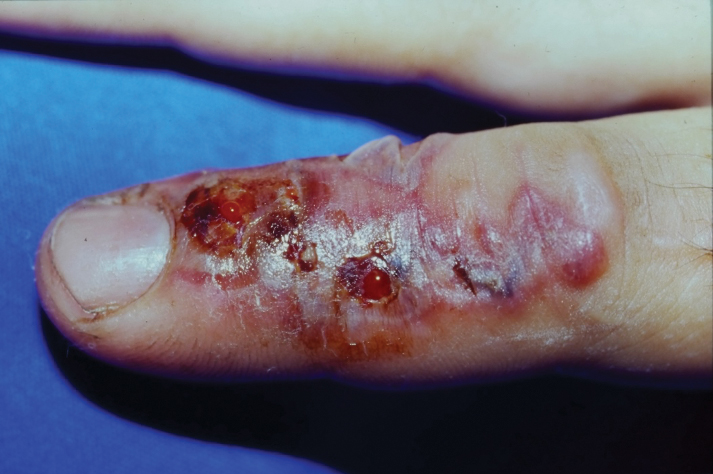
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
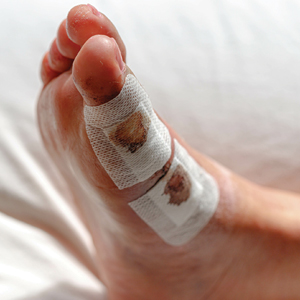
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Key Features of North American Venomous Snake Bites
Key Features of North American Venomous Snake Bites
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19

Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19


Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
North American venomous snakes traditionally are classified as members of either the Viperidae (eg, rattlesnakes, copperheads, cottonmouths) or Elapidae (eg, coral snakes) families and account for roughly 5000 to 10,000 reported envenomations annually.1,2 In 2021, America’s Poison Centers reported 2287 calls related to copperheads, 71 related to coral snakes, 229 related to cottonmouths, 1184 related to rattlesnakes, and 524 related to unknown snakes.3 The majority of calls related to snake bites were for adult patients, resulting in absent to minor outcomes. Only 1 death due to a rattlesnake bite was reported.3 Death by envenomation from a North American snake species is considered rare and typically is attributed to a lapse in medical attention; however, rattlesnakes are the most common reported cause of death by snake envenomation (Figure 1).1,3 A study comparing snake bites and hospital stays in the southeast vs southwest United States found that the southeast had the highest incidence of copperhead bites (37%), while the southwest had a higher incidence of rattlesnake bites (70%); those who were bitten by a rattlesnake were reported to have more severe symptoms and greater need for medical attention and antivenin.4 Some reports have linked pediatric and elderly patients to worse outcomes.5 However, one study examining 24,388 emergency department visits for snake bites from 2006 through 2014 found that the majority of pediatric cases were handled by non– trauma centers in the southern United States,6 supporting evidence found by Campbell et al7 indicating that most snake bites in children can be managed with conservative care. Though reported complications—including weakness, paralysis, hypovolemic shock, thrombocytopenia, and death—from North American venomous snake bites are low, they are still considered a medical emergency.8 It is essential for physicians to understand the clinical manifestations and treatment of North American venomous snake bites and to educate patients on how to protect themselves against and avoid provoking snakes, particularly in rural areas.2 In this article, we review the characteristics of common North American venomous snakes and the clinical manifestations of their bites. We also discuss the appropriate measures for staging, evaluating, and treating snake envenomation to improve patient management and care.

Features of North American Venomous Snakes
Individual snakes within the Viperidae family vary in size, markings and coloration, activity, and region, and physicians should consult their local health departments regarding snakes that are common in their area.2 Cottonmouth snakes are semiaquatic and traditionally are found within the southern and central United States. With a spade-shaped head and distinct two-tone coloration, cottonmouths may be mistaken for other nonvenomous water snakes in these regions (Figure 2).2 Copperheads, true to their name, are red in color; they inhabit a large portion of the southeastern United States and eastern Texas regions and are the cause of the majority of venomous snake bites in North America (Figure 3). Both cottonmouths and copperheads are believed to bite and envenomate as a defensive mechanism when provoked.


Coral snakes, found in the eastern United States and Texas regions, are the only subspecies of the Elapidae family (Figure 4).2,9 They can be distinguished from the nonvenomous milk snake by their characteristic banding, as coral snakes are patterned in a red-yellow-black band sequence and milk snakes are patterned in a red-black-yellow or white sequence. The differences in appearance of these snakes often is remembered by the phrase “red on yellow kills a fellow.”

Anatomic differences between the Viperidae and Elapidae families, including fang size, placement, and type, as well as venom composition, are directly linked to clinical manifestations of the bites. Viperidae fangs extend from the maxillary bones and are mobile, long, and hollow, making it easy for the snake to control fang movement and envenomation.9 Viperidae snakes are uniquely capable of inflicting puncture wounds without the injection of venom, known as dry bites. In contrast, Elapidae snakes have short, hollow, and fixed fangs, and thus patients can protect themselves by wearing appropriate clothing and covered footwear.9 Currently, identifying the type of snake responsible for the bite relies on visualization of the snake and/or the identification of clinical symptoms of envenomation by a dermatologist.
Clinical Manifestations of Venomous Snake Bites
Clinical manifestations and cutaneous findings often are used to grade the severity of venomous snake bites as well as to dictate treatment procedures. Grade 0 indicates a bite has occurred without envenomation, while grades I to V describe the progression and severity of envenomation.10 Grade I describes minimal erythema and edema around the site (fang marks may or may not be present) and no systemic symptoms. Grade II describes erythema and edema extending up the extremity to the first joint (eg, hand to wrist), pain, some systemic symptoms if there is rapid progression, and potential bleeding at the site. Grade III describes erythema and edema spreading to the second joint in the extremity, pain, and systemic symptoms, including coagulation defects. Grade IV describes erythema and edema of the whole extremity, a rapid reaction and progression following the bite, and risk for compartment syndrome. Grade V includes erythema and edema beyond the extremity and increasing systemic symptoms.10
Local pain and edema, usually on easily accessible or exposed extremities, are the most common clinical symptoms reported following a Viperidae snake bite.11 Due to their capability of producing a dry bite, puncture markings alone do not indicate envenomation. Patients will need to be monitored for several hours for signs of envenomation, which may include swelling, pain, ecchymosis, and indications of systemic manifestation (eg, weakness, dizziness, nausea, severe hypotension, thrombocytopenia).11 Viperidae venom hemorrhagic metalloproteinases act on capillary blood vessels by cleaving basement membrane proteins and allowing for extravasation of fluid into local tissue.12 The inflammatory response produced at the site of envenomation likely is due to the release of tumor necrosis factor á and endogenous matrix metalloprotein.12 There is a higher risk for death associated with bites from rattlesnakes within the Viperidae family because their venom contains a unique neurotoxin that works by blocking presynaptic junctions and causing a range of paralytic symptoms from ptosis to respiratory failure.13
The severity of Elapidae bites is thought to be related to the amount of venom injected, the size of the victim, and the length of the snake. Though clothing may offer protection, envenomation occurs in 75% of coral snake bites and can produce devastating consequences due to the venom content.14 In a retrospective study between 2002 and 2004, 90% of Elapidae snake bite patients (n=82) reported local pain, redness, and paresthesia, while around 7% developed systemic symptoms.15 Elapidae venom primarily is neurotoxic and is thought to spread via lymphatics.16 Delayed reactions are common and may take up to 12 hours to develop. Patients should be monitored, as local reactions may progress to weakness, fasciculations, extremity paralysis, and lastly, respiratory paralysis. Due to the risk for progression, all patients with likely coral snake bites should be given antivenin.8,15,17
Much like the North American coral snake, the venomous snake species Gloydius blomhoffii—referred to as the salmosa or mamushi snake depending on the region of origin (ie, Korea or Japan)—is a frequent source of devastating rural snake bites due to neurotoxins (Figure 5). The species’ slender fangs are thought to directly inject the snake’s potent venom, which contains hemorrhagic toxins and α-neurotoxins and Β-neurotoxins, into the bloodstream; however, the salmosa is considered a viper like the North American cottonmouth and copperhead because of its triangular head shape and hollow fangs, which allow for the accommodation of venom-containing glands and mechanism of venom injection. Salmosa venom shares both Viperidae and Elapidae characteristics. Cutaneous findings such as progressive edema, erythema, and bleeding frequently are reported and are attributed to the proteases and hemorrhagic toxins characteristic of vipers (Figure 6). α-Neurotoxins and Β-neurotoxins, similar to the proteolytic venom of the Elapidae family, are responsible for the unique visual disturbances (binocular diplopia) caused by the salmosa.12,18,19


Treatment
Treating snake bites begins with assessing the patient’s airway, breathing, and circulation, followed by a thorough medical and encounter history (including description of how the bite occurred). Due to the range of Viperidae symptoms, it generally is recommended that patients remove any restrictive clothing or jewelry near the bite and/or over the affected limb or body part, place the affected body part at the level of the heart, and go to the nearest medical facility for prompt care. Historically, empiric antibiotics often were used to prevent wound infections; however, studies have since demonstrated that antibiotics are not necessary and lack efficacy in uncomplicated snake bites.16,20 In a study of 114 pediatric cases from 1995 to 2005, it was determined that most patients could be managed with conservative treatment directed at pain management and swelling reduction via elevation of the affected extremity.6 While conservative management may be all that is needed to care for the majority of cases, one retrospective study from Texas indicated that 70% of pediatric venomous snake bites were treated with either intravenous antibiotics and/or antivenin, highlighting the variability in management and opportunity for improvement.21
Antivenin, specifically antivenin (Crotalidae) polyvalent, is the indicated treatment for Viperidae hemorrhagic or coagulopathic envenomation.13,22 Per guidelines from the World Health Organization, physical examination will yield a grading of the snake bite based on cutaneous findings. Grades III to V are considered moderate to severe and should be given antivenin.23 Physicians should look for signs of progressive injury and coagulopathy, such as increased swelling, bruising, hypotension, or altered mental status.22 Due to the major neurotoxic risks associated with Elapidae venom, all coral snake bites should be treated with antivenin; early intubation and ventilation may be considered.13 Similarly, patients who report a salmosa snake bite require prompt treatment with antivenin and/or cepharanthine, an additive agent to reduce swelling and pain.18 Due to the nature of the neurotoxins contained in the salmosa venom (α-neurotoxin causing postsynaptic inhibition of the neuromuscular junction and Β-neurotoxin inhibiting neurotransmitter release from the presynaptic terminal), anticholinesterases, which work by blocking the enzymatic breakdown of the neurotransmitter acetylcholine, should not be used.19 While bleeding and skin and systemic changes may be reversed by antivenin, visual changes are unlikely to resolve with antivenin administration due to the presynaptic binding of Β-neurotoxin and the blockade of neuromuscular signaling.19
Antivenin should be administered intravenously for the fastest onset of action in a setting suitable for the management of anaphylaxis.24 In situations when the benefits may outweigh the risks (eg, if the patient has had a prior allergic reaction or is not in an environment where they can be watched for at least 8 hours for progression of envenomation or adverse reactions), premedication with an antihistamine or epinephrine may be considered.17 Per the World Allergy Organization and World Health Organization, adverse reactions should be treated with crystalloid solutions and antihistamines, corticosteroids, or epinephrine as indicated.25 In a qualitative analysis of emergency physicians’ attitudes toward antivenin, most expressed treatment hesitancy due to lack of knowledge and experience using the medication.26 When possible, snake bites should thus be managed in consultation with a toxicologist.2
Conclusion
Snake bites and envenomation occur commonly in the United States due to exposure to a variety of venomous snakes in the North American Viperidae and Elapidae families. Appropriate and successful management of snake bites by physicians requires general knowledge of regional snakes, the cutaneous and systemic manifestations of snake bites and envenomation, and current treatment methods.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
- Greene SC, Folt J, Wyatt K, et al. Epidemiology of fatal snakebites in the United States 1981-2018. Am J Emerg Med. 2021;45:309-316.
- Wozniak EJ, Wisser J, Schwartz M. Venomous adversaries: a reference to snake identification, field safety, and bite-victim first aid for disaster-response personnel deploying into the hurricaneprone regions of North America. Wilderness Environ Med. 2006; 17:246-266.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 annual report of National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin Toxicol (Phila). 2022;60:1381-1643.
- Chotai PN, Watlington J, Lewis S, et al. Pediatric snakebites: comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297-302.
- Johnson PN, McGoodwin L, Banner W Jr. Utilisation of Crotalidae polyvalent immune fab (ovine) for Viperidae envenomations in children. Emerg Med J. 2008;25:793-798.
- Tadros A, Sharon M, Davis S, et al. Emergency department visits by pediatric patients for snakebites. Pediatr Emerg Care. 2022; 38:279-282.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snake bites: lessons learned from 114 cases. J Pediatr Surg. 2008;43:1338-1341.
- Peterson ME. Snake bites: coral snakes. Clin Tech Small Anim Pract. 2006;21:183-186.
- Porter KR. Herpetology. WB Saunders Company; 1972.
- Rana A, Kheora S. Grading and envenomation of the snake bite among the emergency cases in a medical college in rural India. Hmlyn Jr Appl Med Sci Res. 2021;2:33-36.
- Peterson ME. Snake bite: pit vipers. Clin Tech Small Anim Pract. 2006;21:174-182.
- Gutierrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-850.
- Weinstein SA, Dart RC, Staples A, et al. Envenomations: an overview of clinical toxicology for the primary care physician. Am Fam Physician. 2009;80:793-802.
- Kitchens CS, Van Mierop LH. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): a study of 39 victims. JAMA. 1987;258:1615-1618.
- Morgan DL, Borys DJ, Stanford R, et al. Texas coral snake (Micrurus tener) bites. South Med J. 2007;100:152-156.
- Clark RF, Delden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993; 11:583-586.
- Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347-356.
- Hifumi T, Sakai A, Kondo Y, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3:16.
- Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snake bite (Agkistrodon blomhoffi “mamushi”) case report. Brain Nerve. 2010;62:273-277.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-372.
- Correa JA, Fallon SC, Cruz AT, et al. Management of pediatric snake bites: are we doing too much? J Pediatr Surg. 2014;49:1009-1015.
- Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med. 2001;47:181-188.
- World Health Organization Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed. World Health Organization; 2016.
- Clark RF, McKinney PE, Chase PB, et al. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann Emerg Med. 2002;39:671-676.
- World Health Organization. WHO Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. Accessed November 25, 2024. https://extranet.who.int/prequal/vaccines/guidelines-production-control-and-regulation-snake-antivenom-immunoglobulins
- Tupetz A, Barcenas LK, Phillips AJ, et al. Bites study: a qualitive analysis among emergency medicine physicians on snake envenomation management practices. PloS One. 2022;17:E0262215.
Key Features of North American Venomous Snake Bites
Key Features of North American Venomous Snake Bites
PRACTICE POINTS
- Venomous snake bites require prompt medical attention and assessment of symptoms to determine the optimal course of management and need for antivenin.
- Envenomation may cause may cause discoloration and swelling of the skin as well as thrombotic or paralytic changes.
Central Line Skin Reactions in Children: Survey Addresses Treatment Protocols in Use
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Axolotl Salamander Holds Potential for Cosmeceuticals, Wound Healing
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Wound Healing: Dermatologist’s Toolbox Requires Frequent Updates
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024
Probiotics, Prebiotics, and Provocative Claims About Bacillus Lysate
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
PRACTICE POINTS
- Buruli ulcer (BU) is a necrotizing cutaneous disease caused by Mycobacterium ulcerans with possible transmission from aquatic insects and mosquitoes.
- Buruli ulcer often manifests in children as painless induration that gradually progresses to painless or mildly painful irregular skin ulceration.
- Treatment options for BU include rifampin and streptomycin, but larger lesions may require surgical debridement.
- No vaccine currently exists for M ulcerans, but clinical trials targeting mycolyl transferase are underway.
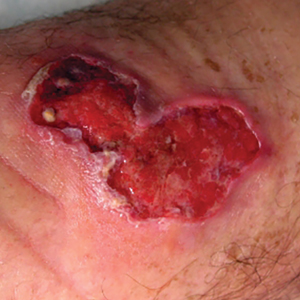
Case Series Highlight Necrotic Wounds Associated with Xylazine-Tainted Fentanyl
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Emerging Insights in Keloid Pathogenesis and Therapeutics
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Sea Buckthorn
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.