User login
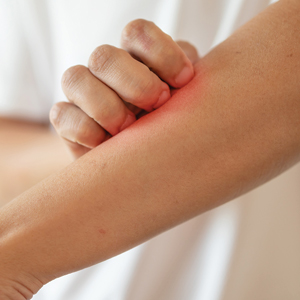
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
THE DIAGNOSIS: Cutaneous Cryptococcosis
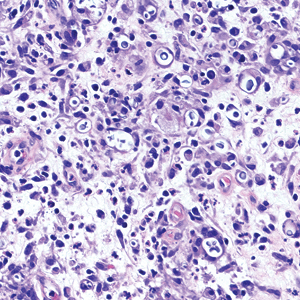
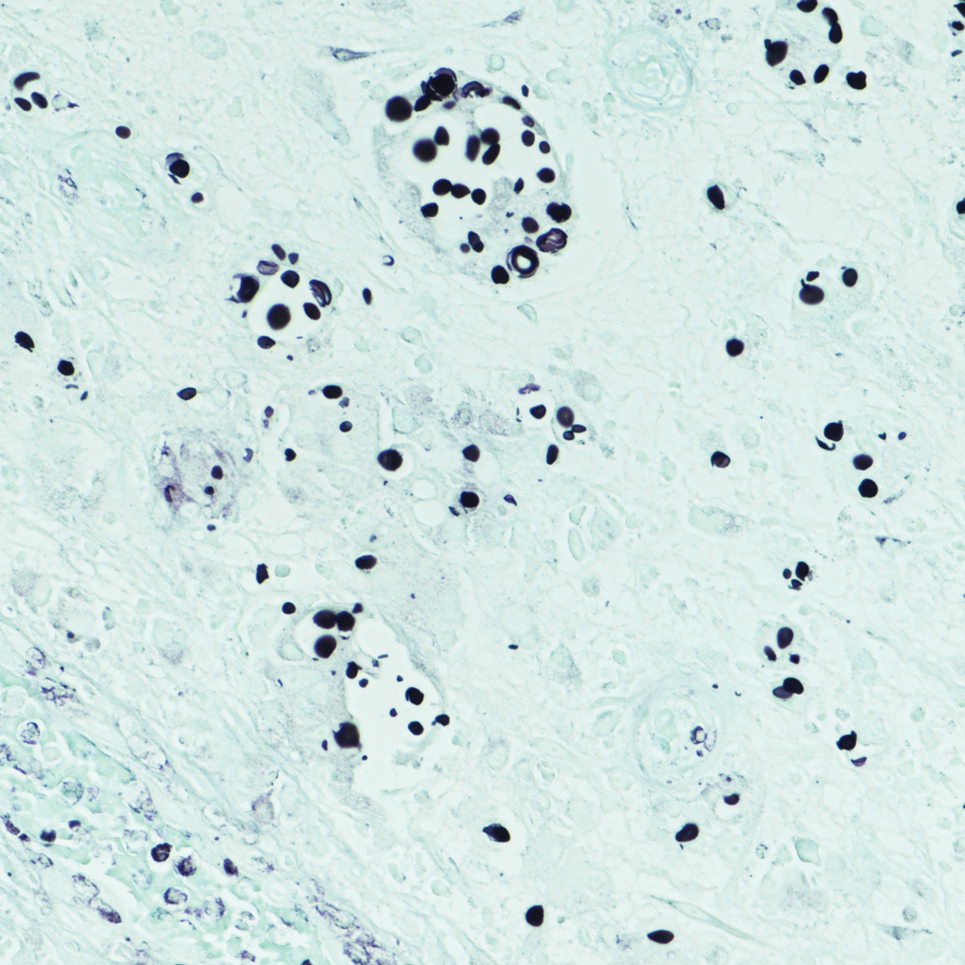
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
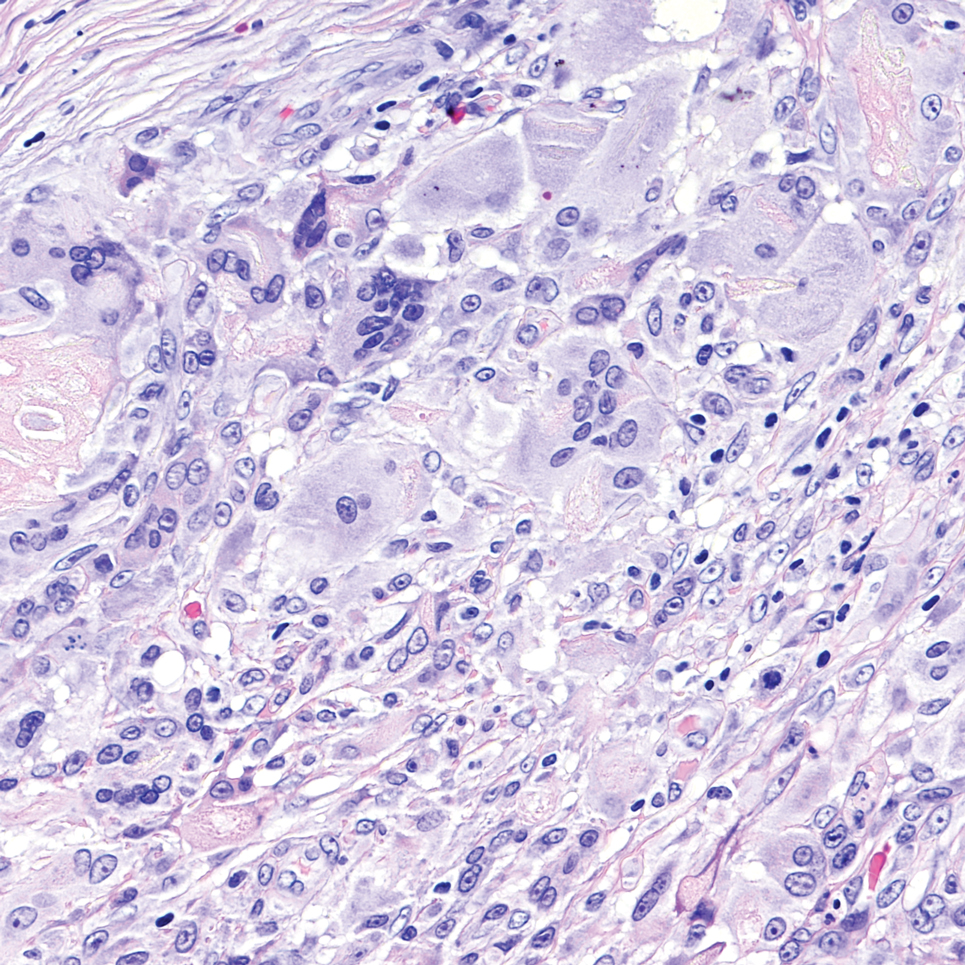
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
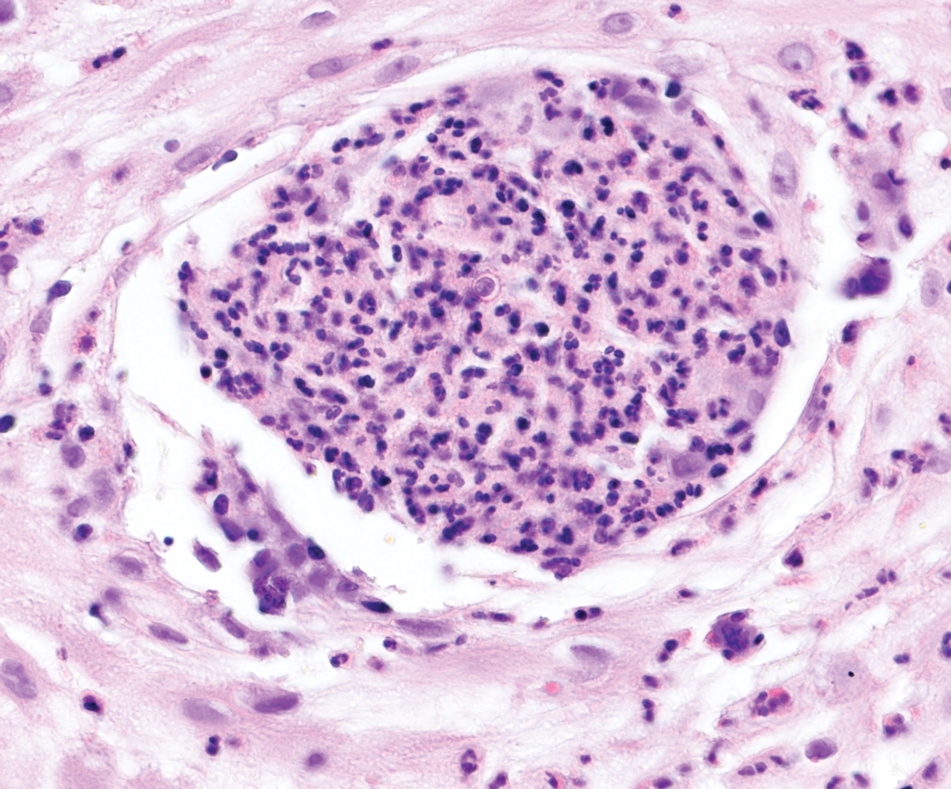
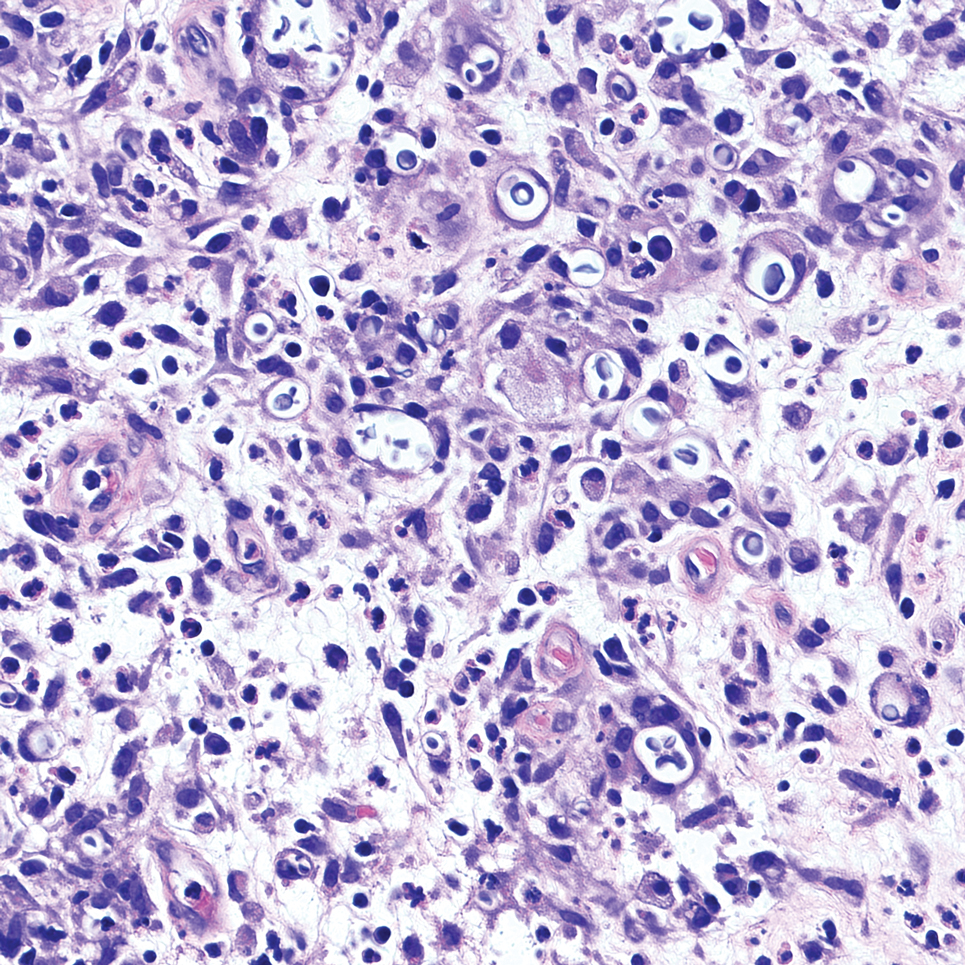
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13
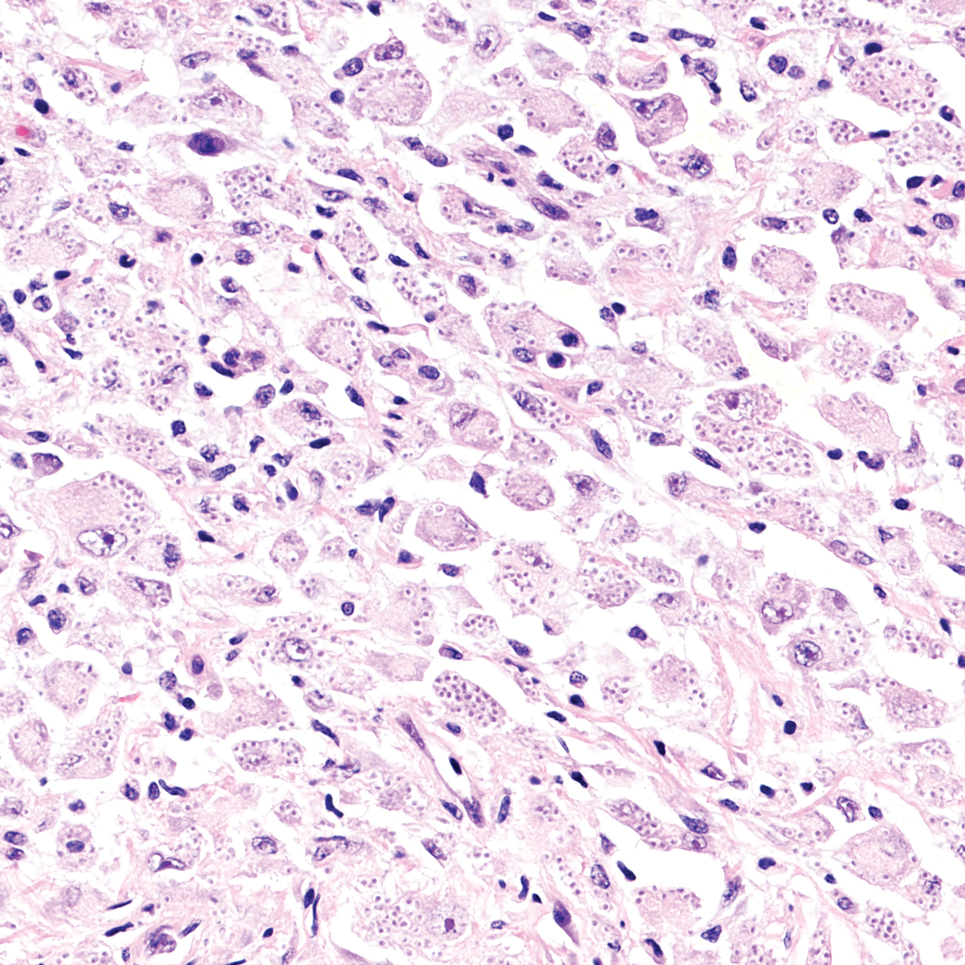
Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10
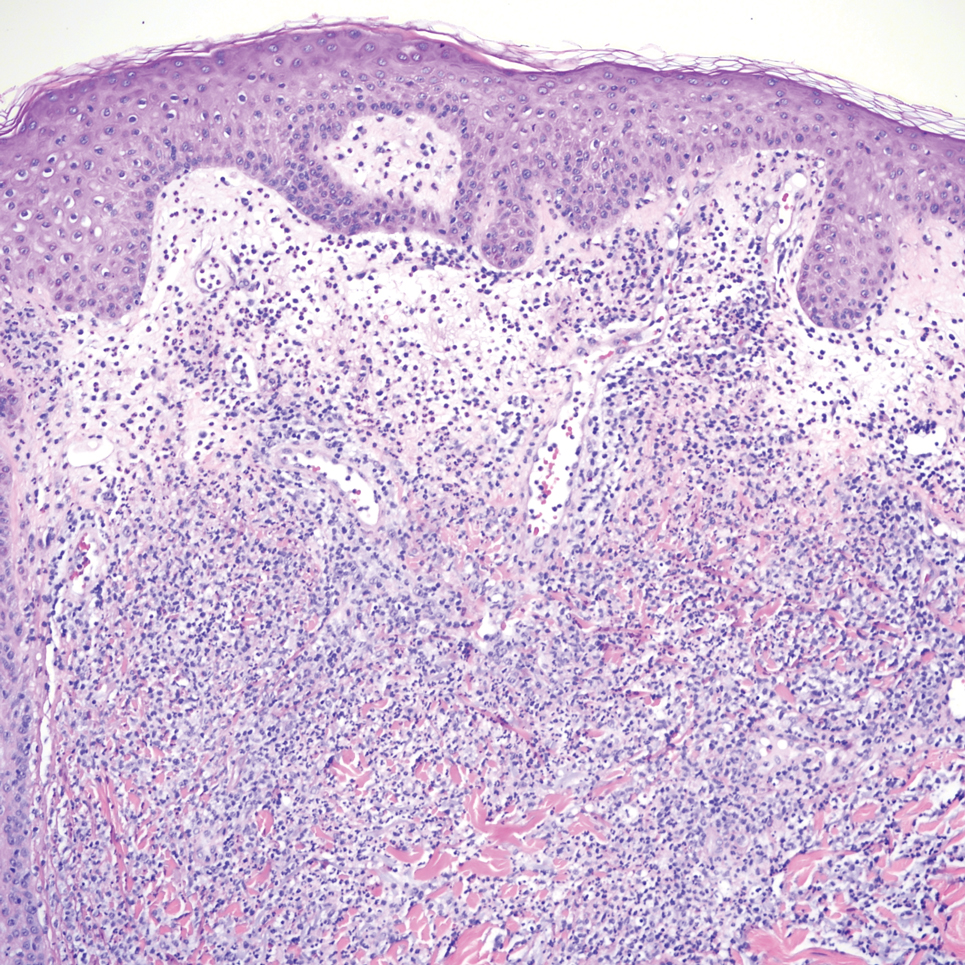
Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
A 51-year-old man with a history of multiple sclerosis treated with fingolimod presented to the dermatology department with an ulcerated lesion on the left forearm of 2 to 3 months’ duration. The patient reported that he recently presented to the emergency department for drainage of the lesion, which was unsuccessful. Shortly after, he traumatized the lesion at his construction job. At the current presentation, physical examination revealed a 1-cm, flesh-colored to faintly pink, ulcerated nodule on the left forearm. A biopsy was performed.
New RSV Vaccine Shows Strong Protection in Veterans
TOPLINE:
A single dose of the recombinant respiratory syncytial virus (RSV) vaccine demonstrates effectiveness against infections and associated hospitalizations in veterans aged 60 years or older during the 2023-2024 respiratory illness season. This protection extends across age groups and immunocompromised individuals.
METHODOLOGY:
Researchers conducted a target trial emulation study to evaluate the real-world effectiveness of a single dose of recombinant RSV vaccine (RSVPreF3 or RSVpreF) among veterans enrolled in the Veterans Health Administration in the United States between September 1 and December 31, 2023.
They analyzed 146,852 vaccinated veterans (69.2%, RSVPreF; 29.9%, RSVPreF3) propensity matched with 582,936 unvaccinated ones (median age, ~76 years; ~94% men; immunocompromised individuals, 11.2%) who were followed up for a median of 124 days.
The primary outcome was any positive RSV test result obtained from day 14 after vaccination.
The secondary outcomes were RSV-associated emergency department or urgent care visits, hospitalizations, intensive care unit (ICU) admissions, and death.
TAKEAWAY:
Vaccine effectiveness against documented RSV infections was 78.1% (95% CI, 72.6-83.5), with incidence rates of infections lower in the vaccinated group than in the unvaccinated group (1.7 vs 7.3 per 1000 person-years).
Likewise, vaccine effectiveness against RSV-associated emergency department or urgent care visits was 78.7% (95% CI, 72.2-84.8), with rates of infections lower in the vaccinated group than in the unvaccinated group (1.3 vs 5.7 per 1000 person-years).
Immunocompromised veterans demonstrated a lower vaccine effectiveness of 71.6% (95% CI, 55.4-85.2); however, infection rates remained lower in the vaccinated group than in the unvaccinated group (5.8 vs 19.9 per 1000 person-years).
Hospitalizations, ICU admission rates, and mortality rates were also lower in the vaccinated group than in the unvaccinated group.
IN PRACTICE:
“These results give confidence that an RSV vaccine for older adults is likely to provide protection against RSV infection and RSV disease, at least in the first season following vaccination,” wrote the author of an accompanying comment.
SOURCE:
The study was funded by the US Department of Veterans Affairs Cooperative Studies Program. It was published online on January 20, 2025, in The Lancet Infectious Diseases (2025 Jan 20. doi:10.1016/S1473-3099(24)00796-5)
LIMITATIONS:
This study did not account for veterans who sought care outside of the Veterans Health Administration. While the study employed rigorous matching to ensure the similarity of demographic, geographic, and clinical characteristics, there could still have been residual confounding. Also, the study was not designed to estimate the protective effect of the vaccine against mild RSV illness.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs Cooperative Studies Program and funded in part by the US Department of Health and Human Services Biomedical Advanced Research and Development Authority and US Food and Drug Administration. One of the authors reported receiving consulting support from Van-Breemen & Hynes and having a subcontract at Oregon State University for a Patient-Centered Outcomes Research Institute grant. Others reported no conflicts of interest.■
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A single dose of the recombinant respiratory syncytial virus (RSV) vaccine demonstrates effectiveness against infections and associated hospitalizations in veterans aged 60 years or older during the 2023-2024 respiratory illness season. This protection extends across age groups and immunocompromised individuals.
METHODOLOGY:
Researchers conducted a target trial emulation study to evaluate the real-world effectiveness of a single dose of recombinant RSV vaccine (RSVPreF3 or RSVpreF) among veterans enrolled in the Veterans Health Administration in the United States between September 1 and December 31, 2023.
They analyzed 146,852 vaccinated veterans (69.2%, RSVPreF; 29.9%, RSVPreF3) propensity matched with 582,936 unvaccinated ones (median age, ~76 years; ~94% men; immunocompromised individuals, 11.2%) who were followed up for a median of 124 days.
The primary outcome was any positive RSV test result obtained from day 14 after vaccination.
The secondary outcomes were RSV-associated emergency department or urgent care visits, hospitalizations, intensive care unit (ICU) admissions, and death.
TAKEAWAY:
Vaccine effectiveness against documented RSV infections was 78.1% (95% CI, 72.6-83.5), with incidence rates of infections lower in the vaccinated group than in the unvaccinated group (1.7 vs 7.3 per 1000 person-years).
Likewise, vaccine effectiveness against RSV-associated emergency department or urgent care visits was 78.7% (95% CI, 72.2-84.8), with rates of infections lower in the vaccinated group than in the unvaccinated group (1.3 vs 5.7 per 1000 person-years).
Immunocompromised veterans demonstrated a lower vaccine effectiveness of 71.6% (95% CI, 55.4-85.2); however, infection rates remained lower in the vaccinated group than in the unvaccinated group (5.8 vs 19.9 per 1000 person-years).
Hospitalizations, ICU admission rates, and mortality rates were also lower in the vaccinated group than in the unvaccinated group.
IN PRACTICE:
“These results give confidence that an RSV vaccine for older adults is likely to provide protection against RSV infection and RSV disease, at least in the first season following vaccination,” wrote the author of an accompanying comment.
SOURCE:
The study was funded by the US Department of Veterans Affairs Cooperative Studies Program. It was published online on January 20, 2025, in The Lancet Infectious Diseases (2025 Jan 20. doi:10.1016/S1473-3099(24)00796-5)
LIMITATIONS:
This study did not account for veterans who sought care outside of the Veterans Health Administration. While the study employed rigorous matching to ensure the similarity of demographic, geographic, and clinical characteristics, there could still have been residual confounding. Also, the study was not designed to estimate the protective effect of the vaccine against mild RSV illness.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs Cooperative Studies Program and funded in part by the US Department of Health and Human Services Biomedical Advanced Research and Development Authority and US Food and Drug Administration. One of the authors reported receiving consulting support from Van-Breemen & Hynes and having a subcontract at Oregon State University for a Patient-Centered Outcomes Research Institute grant. Others reported no conflicts of interest.■
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A single dose of the recombinant respiratory syncytial virus (RSV) vaccine demonstrates effectiveness against infections and associated hospitalizations in veterans aged 60 years or older during the 2023-2024 respiratory illness season. This protection extends across age groups and immunocompromised individuals.
METHODOLOGY:
Researchers conducted a target trial emulation study to evaluate the real-world effectiveness of a single dose of recombinant RSV vaccine (RSVPreF3 or RSVpreF) among veterans enrolled in the Veterans Health Administration in the United States between September 1 and December 31, 2023.
They analyzed 146,852 vaccinated veterans (69.2%, RSVPreF; 29.9%, RSVPreF3) propensity matched with 582,936 unvaccinated ones (median age, ~76 years; ~94% men; immunocompromised individuals, 11.2%) who were followed up for a median of 124 days.
The primary outcome was any positive RSV test result obtained from day 14 after vaccination.
The secondary outcomes were RSV-associated emergency department or urgent care visits, hospitalizations, intensive care unit (ICU) admissions, and death.
TAKEAWAY:
Vaccine effectiveness against documented RSV infections was 78.1% (95% CI, 72.6-83.5), with incidence rates of infections lower in the vaccinated group than in the unvaccinated group (1.7 vs 7.3 per 1000 person-years).
Likewise, vaccine effectiveness against RSV-associated emergency department or urgent care visits was 78.7% (95% CI, 72.2-84.8), with rates of infections lower in the vaccinated group than in the unvaccinated group (1.3 vs 5.7 per 1000 person-years).
Immunocompromised veterans demonstrated a lower vaccine effectiveness of 71.6% (95% CI, 55.4-85.2); however, infection rates remained lower in the vaccinated group than in the unvaccinated group (5.8 vs 19.9 per 1000 person-years).
Hospitalizations, ICU admission rates, and mortality rates were also lower in the vaccinated group than in the unvaccinated group.
IN PRACTICE:
“These results give confidence that an RSV vaccine for older adults is likely to provide protection against RSV infection and RSV disease, at least in the first season following vaccination,” wrote the author of an accompanying comment.
SOURCE:
The study was funded by the US Department of Veterans Affairs Cooperative Studies Program. It was published online on January 20, 2025, in The Lancet Infectious Diseases (2025 Jan 20. doi:10.1016/S1473-3099(24)00796-5)
LIMITATIONS:
This study did not account for veterans who sought care outside of the Veterans Health Administration. While the study employed rigorous matching to ensure the similarity of demographic, geographic, and clinical characteristics, there could still have been residual confounding. Also, the study was not designed to estimate the protective effect of the vaccine against mild RSV illness.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs Cooperative Studies Program and funded in part by the US Department of Health and Human Services Biomedical Advanced Research and Development Authority and US Food and Drug Administration. One of the authors reported receiving consulting support from Van-Breemen & Hynes and having a subcontract at Oregon State University for a Patient-Centered Outcomes Research Institute grant. Others reported no conflicts of interest.■
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
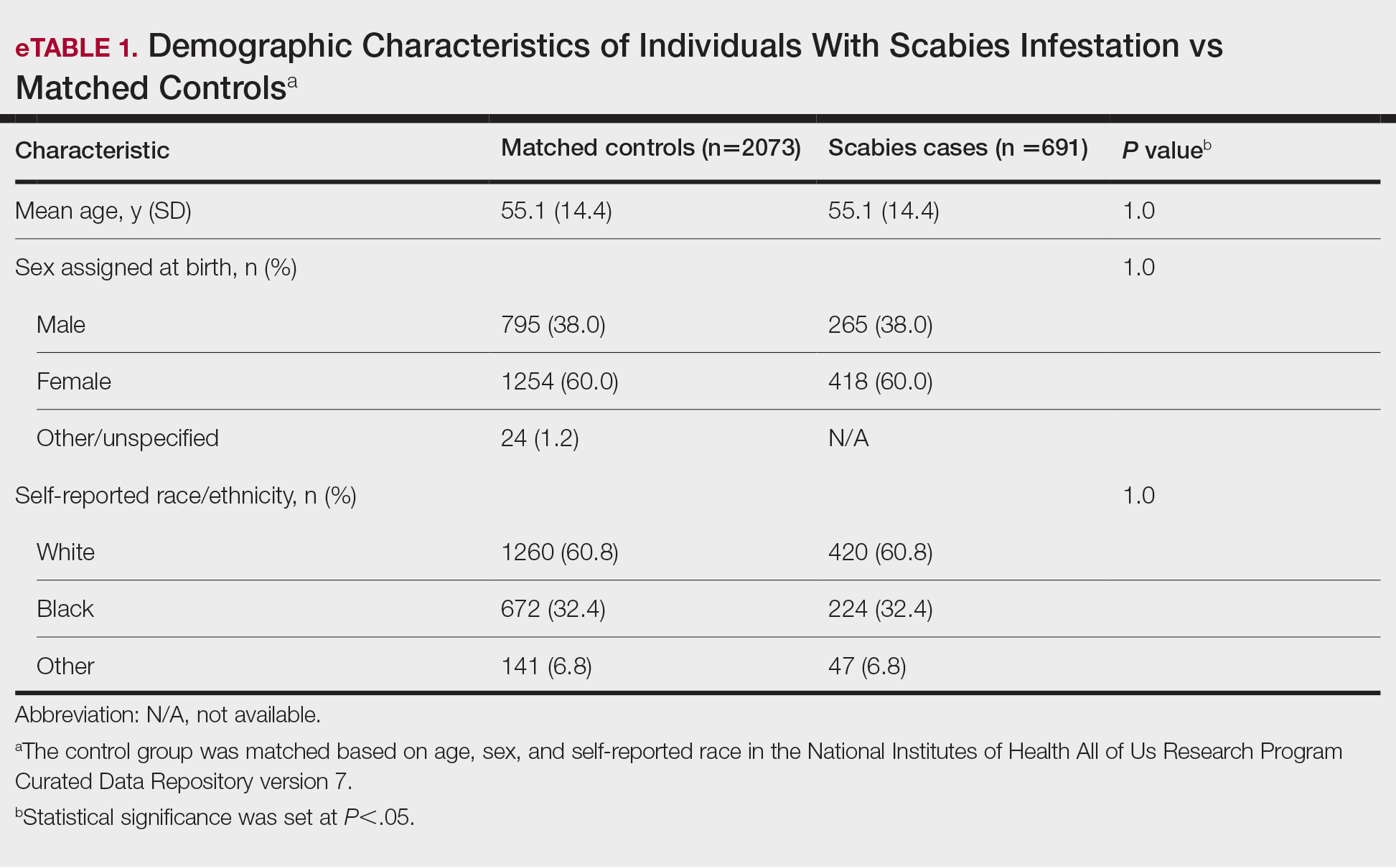
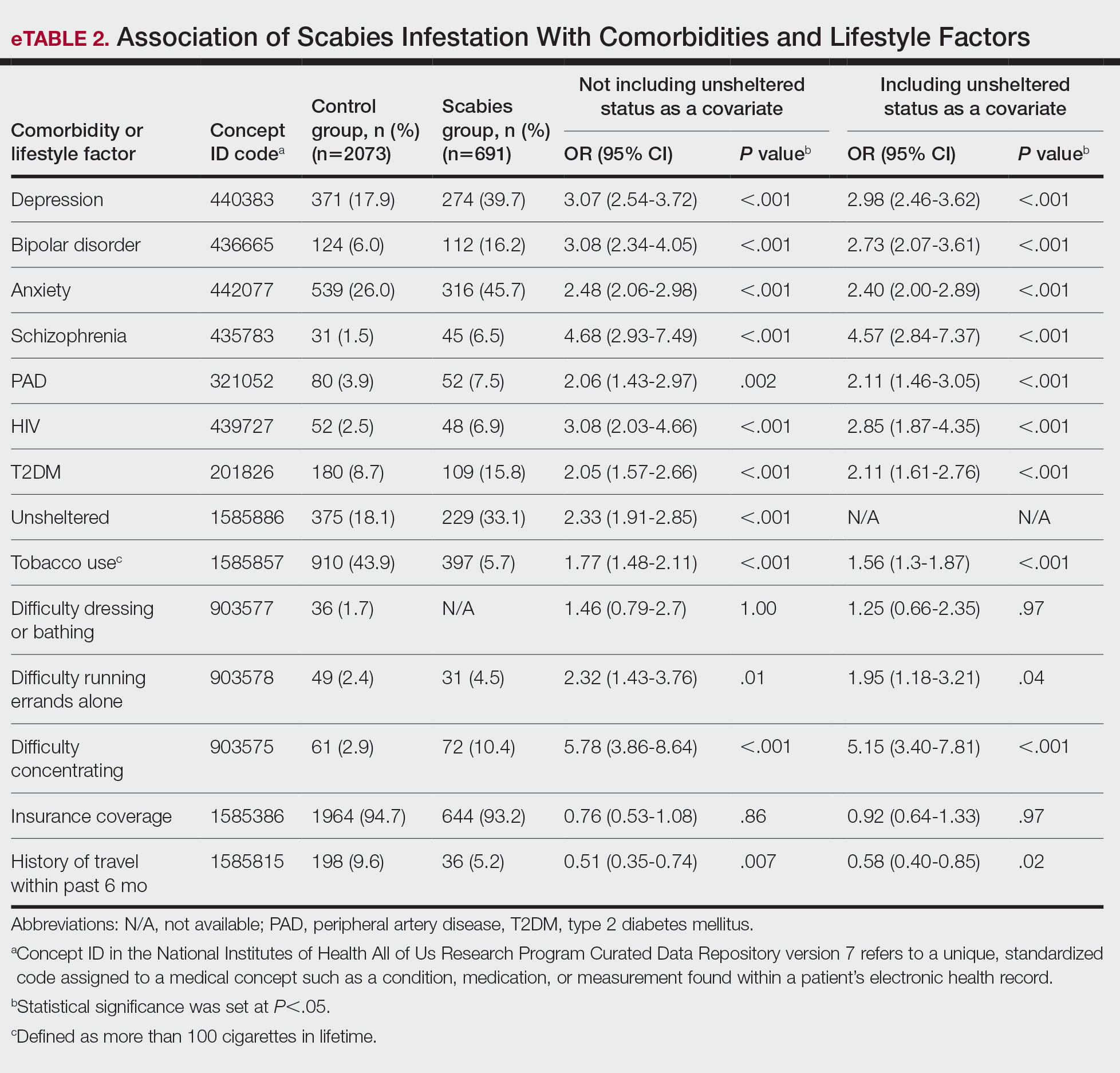
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).
This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
PRACTICE POINTS
- Scabies infestation is caused by the human itch mite (Sarcoptes scabiei var hominis) and can be spread via sexual contact in adults.
- Crowded living conditions are associated with scabies infestation in countries with high human development indices, such as the United States.
- Patients with certain comorbid conditions or lifestyle risk factors should be screened for scabies infestation when presenting with pruritus and other characteristic clinical findings.
Most Kids With COVID-Linked MIS-C Recover by 6 Months
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Valaciclovir Shows Promise in Preventing Herpes Zoster During Anifrolumab Treatment for Lupus
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Highly Contagious Norovirus Cases Spike This Season
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Do Antibiotics Before Conception Affect Fertility?
Is there a connection between antibiotics taken before conception and adverse outcomes, such as reduced fertility, miscarriages, and congenital malformations?
A meta-analysis published in the journal eClinicalMedicine suggests a potential link between antibiotics taken before conception and negative outcomes, such as reduced fertility, miscarriages, and congenital malformations. However, a German expert in reproductive toxicology warned against drawing false conclusions.
“It would be fatal if women who want to have children refused necessary antibiotic treatment because they are afraid of infertility, miscarriages, and malformations,” said Wolfgang Paulus, MD, from the Reproductive Toxicology Advisory Center at the University Women’s Hospital in Ulm, Germany. In an interview, the expert criticized not only the authors’ conclusions but also the selection of studies included in the meta-analysis.
Confusion Over Use and Exposure
The meta-analysis, conducted by Bekalu Kassie Alemu, PhD, and colleagues from the Department of Obstetrics and Gynecology at The Chinese University of Hong Kong included 15 studies involving over 1.2 million women to examine how preconception antibiotic use affects fertility and pregnancy outcomes. In most studies (n = 11) that were included in the meta-analysis, fertility was examined as an endpoint, primarily in infertile women. One study involved Danish pharmacy employees who handled antibiotics at work.
“Not only was the therapeutic use of antibiotics not examined in this study, but the biological plausibility is completely lacking in this context,” Paulus noted.
The possible effects of preconception antibiotics on miscarriages were investigated in four studies, while two studies focused on congenital malformations as an endpoint.
Mixed Findings on Infertility
Regarding infertility, the authors reported abnormalities in macrolides and sulfonamides. Women who had received macrolide antibiotics, such as azithromycin, before conception showed a 35% reduction in fertility rates.
However, Paulus questioned whether this was solely because of macrolides. “Macrolide antibiotics are typically used for chlamydia, and chlamydia infection is a significant factor in women with unmet fertility desires,” he explained. Often, the chlamydia has already caused damage, such as inflammatory processes in the fallopian tubes, contributing to infertility that cannot be resolved by administering antibiotics.
The meta-analysis also showed that women who received sulfonamide before conception had a 2.35-fold increased likelihood of infertility. However, this association is not always one-sided. The results for tetracyclines were heterogeneous; while chlortetracycline appeared to increase the risk for infertility, exposure to oxytetracycline appeared to decrease it.
Treatment with oxytetracycline and beta-lactam antibiotics (except penicillin G) was associated with a 64% lower likelihood of infertility. The authors also found that fluoroquinolone antibiotics were associated with a 13% lower likelihood of infertility.
Miscarriage and Malformation Risks
Alemu and colleagues found a significant association between the use of antibiotics before conception and adverse pregnancy outcomes, showing a 34% increased risk for miscarriages and an 85% higher risk for congenital malformations with the use of trimethoprim during preconception. These findings highlight the need for caution regarding antibiotic use in women who are planning to conceive.
“Most antibiotics have half-lives of only a few hours. Therefore, antibiotics administered before conception can hardly have a direct effect on embryonic development,” Paulus noted. He pointed out that extensive data exist on most antibiotic classes included in this meta-analysis regarding childhood anomalies when used during the sensitive phase of organ development. These data do not indicate an increased risk for malformation. Therefore, the increased risk for malformations due to exposure before conception seems less plausible.
Alemu and colleagues assumed that antibiotics might negatively affect female reproductive health by disrupting the gut microbiome. The reasons for the reduced risk for infertility associated with beta-lactams and fluoroquinolones require further investigation. They reach a significant conclusion: “Preconception antibiotics exposure in females increases the risk of infertility, miscarriage, and congenital anomalies.” However, differences exist between the antibiotic classes. While the risk for infertility, spontaneous miscarriages, and congenital malformations increases with the use of macrolide antibiotics, sulfonamides, and trimethoprim, it decreases with the use of beta-lactams and fluoroquinolone antibiotics.
Expert Disagreement
“It is conceivable that the use of antibiotics damages the physiological environment, such as in the vaginal area. This may allow unwanted microbes to establish themselves, leading to more adverse outcomes such as infertility and miscarriages,” Paulus acknowledged.
Disruption of the microbiome due to antibiotic therapy could also result in a deficiency in relevant vitamins and trace elements (eg, folic acid), which could contribute to organogenesis disorders. Therefore, it may be beneficial to stabilize the gut and vaginal flora using probiotics after antibiotic treatment.
However, Paulus disagrees with the study conclusions. First, the studies included in the meta-analysis, which were largely observational, did not allow for the direct effect of antibiotics on the examined outcomes. Second, “quinolone antibiotics are highlighted as positive here, as if they were less problematic for patients trying to have children.”
Quinolone antibiotics are generally “frowned upon,” regardless of whether the patient wants to have children, as they can cause damage to the tendons, muscles, joints, and nervous system. They are currently used only as reserve medications.
“Quinolone antibiotics should not be administered during pregnancy, as they have already caused problems in animal studies, and they should not be used before pregnancy because of their side-effect profile,” Paulus stressed.
Serious Consequences
Paulus clarified:
In these cases, antibiotic treatment is appropriate, and there should be no fear of adverse effects on fertility or pregnancy outcomes. “If antibiotics are not given and the infection worsens, the patient will be even less likely to conceive successfully.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is there a connection between antibiotics taken before conception and adverse outcomes, such as reduced fertility, miscarriages, and congenital malformations?
A meta-analysis published in the journal eClinicalMedicine suggests a potential link between antibiotics taken before conception and negative outcomes, such as reduced fertility, miscarriages, and congenital malformations. However, a German expert in reproductive toxicology warned against drawing false conclusions.
“It would be fatal if women who want to have children refused necessary antibiotic treatment because they are afraid of infertility, miscarriages, and malformations,” said Wolfgang Paulus, MD, from the Reproductive Toxicology Advisory Center at the University Women’s Hospital in Ulm, Germany. In an interview, the expert criticized not only the authors’ conclusions but also the selection of studies included in the meta-analysis.
Confusion Over Use and Exposure
The meta-analysis, conducted by Bekalu Kassie Alemu, PhD, and colleagues from the Department of Obstetrics and Gynecology at The Chinese University of Hong Kong included 15 studies involving over 1.2 million women to examine how preconception antibiotic use affects fertility and pregnancy outcomes. In most studies (n = 11) that were included in the meta-analysis, fertility was examined as an endpoint, primarily in infertile women. One study involved Danish pharmacy employees who handled antibiotics at work.
“Not only was the therapeutic use of antibiotics not examined in this study, but the biological plausibility is completely lacking in this context,” Paulus noted.
The possible effects of preconception antibiotics on miscarriages were investigated in four studies, while two studies focused on congenital malformations as an endpoint.
Mixed Findings on Infertility
Regarding infertility, the authors reported abnormalities in macrolides and sulfonamides. Women who had received macrolide antibiotics, such as azithromycin, before conception showed a 35% reduction in fertility rates.
However, Paulus questioned whether this was solely because of macrolides. “Macrolide antibiotics are typically used for chlamydia, and chlamydia infection is a significant factor in women with unmet fertility desires,” he explained. Often, the chlamydia has already caused damage, such as inflammatory processes in the fallopian tubes, contributing to infertility that cannot be resolved by administering antibiotics.
The meta-analysis also showed that women who received sulfonamide before conception had a 2.35-fold increased likelihood of infertility. However, this association is not always one-sided. The results for tetracyclines were heterogeneous; while chlortetracycline appeared to increase the risk for infertility, exposure to oxytetracycline appeared to decrease it.
Treatment with oxytetracycline and beta-lactam antibiotics (except penicillin G) was associated with a 64% lower likelihood of infertility. The authors also found that fluoroquinolone antibiotics were associated with a 13% lower likelihood of infertility.
Miscarriage and Malformation Risks
Alemu and colleagues found a significant association between the use of antibiotics before conception and adverse pregnancy outcomes, showing a 34% increased risk for miscarriages and an 85% higher risk for congenital malformations with the use of trimethoprim during preconception. These findings highlight the need for caution regarding antibiotic use in women who are planning to conceive.
“Most antibiotics have half-lives of only a few hours. Therefore, antibiotics administered before conception can hardly have a direct effect on embryonic development,” Paulus noted. He pointed out that extensive data exist on most antibiotic classes included in this meta-analysis regarding childhood anomalies when used during the sensitive phase of organ development. These data do not indicate an increased risk for malformation. Therefore, the increased risk for malformations due to exposure before conception seems less plausible.
Alemu and colleagues assumed that antibiotics might negatively affect female reproductive health by disrupting the gut microbiome. The reasons for the reduced risk for infertility associated with beta-lactams and fluoroquinolones require further investigation. They reach a significant conclusion: “Preconception antibiotics exposure in females increases the risk of infertility, miscarriage, and congenital anomalies.” However, differences exist between the antibiotic classes. While the risk for infertility, spontaneous miscarriages, and congenital malformations increases with the use of macrolide antibiotics, sulfonamides, and trimethoprim, it decreases with the use of beta-lactams and fluoroquinolone antibiotics.
Expert Disagreement
“It is conceivable that the use of antibiotics damages the physiological environment, such as in the vaginal area. This may allow unwanted microbes to establish themselves, leading to more adverse outcomes such as infertility and miscarriages,” Paulus acknowledged.
Disruption of the microbiome due to antibiotic therapy could also result in a deficiency in relevant vitamins and trace elements (eg, folic acid), which could contribute to organogenesis disorders. Therefore, it may be beneficial to stabilize the gut and vaginal flora using probiotics after antibiotic treatment.
However, Paulus disagrees with the study conclusions. First, the studies included in the meta-analysis, which were largely observational, did not allow for the direct effect of antibiotics on the examined outcomes. Second, “quinolone antibiotics are highlighted as positive here, as if they were less problematic for patients trying to have children.”
Quinolone antibiotics are generally “frowned upon,” regardless of whether the patient wants to have children, as they can cause damage to the tendons, muscles, joints, and nervous system. They are currently used only as reserve medications.
“Quinolone antibiotics should not be administered during pregnancy, as they have already caused problems in animal studies, and they should not be used before pregnancy because of their side-effect profile,” Paulus stressed.
Serious Consequences
Paulus clarified:
In these cases, antibiotic treatment is appropriate, and there should be no fear of adverse effects on fertility or pregnancy outcomes. “If antibiotics are not given and the infection worsens, the patient will be even less likely to conceive successfully.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is there a connection between antibiotics taken before conception and adverse outcomes, such as reduced fertility, miscarriages, and congenital malformations?
A meta-analysis published in the journal eClinicalMedicine suggests a potential link between antibiotics taken before conception and negative outcomes, such as reduced fertility, miscarriages, and congenital malformations. However, a German expert in reproductive toxicology warned against drawing false conclusions.
“It would be fatal if women who want to have children refused necessary antibiotic treatment because they are afraid of infertility, miscarriages, and malformations,” said Wolfgang Paulus, MD, from the Reproductive Toxicology Advisory Center at the University Women’s Hospital in Ulm, Germany. In an interview, the expert criticized not only the authors’ conclusions but also the selection of studies included in the meta-analysis.
Confusion Over Use and Exposure
The meta-analysis, conducted by Bekalu Kassie Alemu, PhD, and colleagues from the Department of Obstetrics and Gynecology at The Chinese University of Hong Kong included 15 studies involving over 1.2 million women to examine how preconception antibiotic use affects fertility and pregnancy outcomes. In most studies (n = 11) that were included in the meta-analysis, fertility was examined as an endpoint, primarily in infertile women. One study involved Danish pharmacy employees who handled antibiotics at work.
“Not only was the therapeutic use of antibiotics not examined in this study, but the biological plausibility is completely lacking in this context,” Paulus noted.
The possible effects of preconception antibiotics on miscarriages were investigated in four studies, while two studies focused on congenital malformations as an endpoint.
Mixed Findings on Infertility
Regarding infertility, the authors reported abnormalities in macrolides and sulfonamides. Women who had received macrolide antibiotics, such as azithromycin, before conception showed a 35% reduction in fertility rates.
However, Paulus questioned whether this was solely because of macrolides. “Macrolide antibiotics are typically used for chlamydia, and chlamydia infection is a significant factor in women with unmet fertility desires,” he explained. Often, the chlamydia has already caused damage, such as inflammatory processes in the fallopian tubes, contributing to infertility that cannot be resolved by administering antibiotics.
The meta-analysis also showed that women who received sulfonamide before conception had a 2.35-fold increased likelihood of infertility. However, this association is not always one-sided. The results for tetracyclines were heterogeneous; while chlortetracycline appeared to increase the risk for infertility, exposure to oxytetracycline appeared to decrease it.
Treatment with oxytetracycline and beta-lactam antibiotics (except penicillin G) was associated with a 64% lower likelihood of infertility. The authors also found that fluoroquinolone antibiotics were associated with a 13% lower likelihood of infertility.
Miscarriage and Malformation Risks
Alemu and colleagues found a significant association between the use of antibiotics before conception and adverse pregnancy outcomes, showing a 34% increased risk for miscarriages and an 85% higher risk for congenital malformations with the use of trimethoprim during preconception. These findings highlight the need for caution regarding antibiotic use in women who are planning to conceive.
“Most antibiotics have half-lives of only a few hours. Therefore, antibiotics administered before conception can hardly have a direct effect on embryonic development,” Paulus noted. He pointed out that extensive data exist on most antibiotic classes included in this meta-analysis regarding childhood anomalies when used during the sensitive phase of organ development. These data do not indicate an increased risk for malformation. Therefore, the increased risk for malformations due to exposure before conception seems less plausible.
Alemu and colleagues assumed that antibiotics might negatively affect female reproductive health by disrupting the gut microbiome. The reasons for the reduced risk for infertility associated with beta-lactams and fluoroquinolones require further investigation. They reach a significant conclusion: “Preconception antibiotics exposure in females increases the risk of infertility, miscarriage, and congenital anomalies.” However, differences exist between the antibiotic classes. While the risk for infertility, spontaneous miscarriages, and congenital malformations increases with the use of macrolide antibiotics, sulfonamides, and trimethoprim, it decreases with the use of beta-lactams and fluoroquinolone antibiotics.
Expert Disagreement
“It is conceivable that the use of antibiotics damages the physiological environment, such as in the vaginal area. This may allow unwanted microbes to establish themselves, leading to more adverse outcomes such as infertility and miscarriages,” Paulus acknowledged.
Disruption of the microbiome due to antibiotic therapy could also result in a deficiency in relevant vitamins and trace elements (eg, folic acid), which could contribute to organogenesis disorders. Therefore, it may be beneficial to stabilize the gut and vaginal flora using probiotics after antibiotic treatment.
However, Paulus disagrees with the study conclusions. First, the studies included in the meta-analysis, which were largely observational, did not allow for the direct effect of antibiotics on the examined outcomes. Second, “quinolone antibiotics are highlighted as positive here, as if they were less problematic for patients trying to have children.”
Quinolone antibiotics are generally “frowned upon,” regardless of whether the patient wants to have children, as they can cause damage to the tendons, muscles, joints, and nervous system. They are currently used only as reserve medications.
“Quinolone antibiotics should not be administered during pregnancy, as they have already caused problems in animal studies, and they should not be used before pregnancy because of their side-effect profile,” Paulus stressed.
Serious Consequences
Paulus clarified:
In these cases, antibiotic treatment is appropriate, and there should be no fear of adverse effects on fertility or pregnancy outcomes. “If antibiotics are not given and the infection worsens, the patient will be even less likely to conceive successfully.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM ECLINICALMEDICINE
Reducing Risk, One Mask at a Time: What the Science Says
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Tularemia: A Rare But Nationally Notifiable Disease
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
The pediatrician’s first patient of the day was an 8-year-old boy, accompanied by both of his parents. It was the boy’s third visit in just over a week for fever and left-sided neck swelling, and the family was understandably anxious for answers.
“The antibiotics don’t seem to be working,” the mother explained. “He still has fever every day, as high as 104, and his neck looks just as swollen.”
A quick review of the chart revealed the boy’s initial diagnosis had been bacterial lymphadenitis, for which amoxicillin-clavulanate had been prescribed. Three days later, given lack of clinical improvement, therapy was transitioned to clindamycin. On examination, the boy was febrile and ill-appearing with a 3-cm by 5-cm tender, non-fluctuant swelling over the left sternocleidomastoid muscle.
The pediatrician ran through a quick mental checklist of diagnostic possibilities for his patient’s continued symptoms. Staphylococcal lymphadenitis still seemed possible. Could the boy be infected with methicillin-resistant Staphylococcus aureus that was also clindamycin resistant? Alternately, perhaps the problem was “source control” and the boy had developed an occult neck abscess that needed to be drained. An ultrasound could help sort that out. Finally, the pediatrician considered less common bacterial causes of lymph node swelling and fever. He placed Bartonella henselae, the cause of cat scratch disease, near the top of his list. “I’ve never seen it,” he told the parents, “But we could also consider tularemia.”
On average, 200 cases of tularemia are reported in the United States each year, and the incidence of disease is increasing, according to a surveillance report released by the Centers for Disease Control and Prevention in December 2023.1
Between 2011 and 2022, 2462 tularemia cases were reported in the United States. That translated to an average annual incidence of 0.064 per 100,000 population, an increase of 56% compared with 2001-2010. Forty-seven states reported at least one case of tularemia, although half of all reported cases came from four states — Arkansas (18%), Kansas (11%), Missouri (11%), and Oklahoma (10%). The incidence of tularemia was highest in children ages 5-9 years old, older men, and American Indian or Alaska Natives individuals. Although cases occurred year-round, 78% had symptom onset May through September.
In the United States, most human cases of tularemia have been arthropod borne, transmitted by the bite of an infected tick or deer fly. Infection also can be spread through contact with infected animals or animal tissue, particularly rabbits, hares, muskrats, prairie dogs, and other rodents, including hamsters. Outbreaks of tularemia have occurred among pet store hamsters, and at least one child in the United States developed tularemia after being bitten by a pet hamster.
Tularemia is almost always associated with fever but other clinical manifestations vary by the type of exposure. Ulceroglandular disease occurs after a tick or deer fly bite or after handling an infected animal. An ulcer develops at the site where the bacteria entered the body, along with enlargement of regional lymph nodes. Less commonly, lymph node swelling can occur without the development of an ulcer. If the bacteria enter through the eye, symptoms include conjunctivitis and swelling of pre-auricular lymph nodes. Eating or drinking contaminated food or water is associated with sore throat, mouth ulcers, tonsillitis, and swelling of lymph glands in the neck. Pneumonic tularemia, the most serious form of the disease, typically happens after inhaling bacteria-containing dust or aerosols and is associated with cough, chest pain, and difficulty breathing. Pneumonic tularemia can develop if other forms of tularemia are untreated, and the bacteria spread to the lung.
Back in the exam room, the pediatrician carefully re-examined the boy’s scalp. A 1-cm poorly healing ulcer on the left occiput added support for the diagnosis of ulceroglandular tularemia, the most common form of the disease in children. Serologic testing ultimately confirmed the diagnosis and the boy’s symptoms resolved with treatment.
Gentamicin administered intravenously or intramuscularly is the drug of choice for the treatment of tularemia in children. Ciprofloxacin is considered an alternative but is not approved by the U.S. Food and Drug Administration for this indication.
The pediatrician reported the case of tularemia to his local health department. Tularemia is a nationally notifiable disease in the United States; state health departments report to the CDC through the National Notifiable Diseases Surveillance System. In turn, public health authorities shared information to prevent tularemia. Steps to prevent tick and deer fly bites include the use of an Environmental Protection Agency–registered insect repellent. Individuals who hunt, trap, or skin animals are encouraged to wear gloves when handling animals —especially rabbits, muskrats, and prairie dogs — and cook game meat thoroughly. Tularemia can be inadvertently aerosolized if an infected animal or carcass is run over with a tractor or lawnmower. Checking for carcasses before mowing may reduce the risk.
Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Reference
1. Rich SN et al. Tularemia—United States, 2011-2022. MMWR Morb Mortal Wkly Rep 2025;73:1152–1156. doi:
Traumatic Brain Injury May Reactivate Herpes Virus Leading to Neurodegeneration
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
a new study suggested.
Using a three-dimensional (3D) human brain tissue model, researchers observed that quiescent HSV-1 can be reactivated by a mechanical jolt mimicking concussion, leading to signature markers of Alzheimer’s disease, including neuroinflammation and production of amyloid beta and phosphorylated tau (p-tau) and gliosis — a phenotype made worse by repeated head injury.
“This opens the question as to whether antiviral drugs or anti-inflammatory agents might be useful as early preventive treatments after head trauma to stop HSV-1 activation in its tracks and lower the risk of Alzheimer’s disease,” lead investigator Dana Cairns, PhD, with the Department of Biomedical Engineering at Tufts University, Medford, Massachusetts, said in a statement.
But outside experts urged caution in drawing any firm conclusions, pending further study.
The study was published online in the journal Science Signaling.
HSV-1: A Major Alzheimer’s Disease Risk Factor?
TBI is a major risk factor for Alzheimer’s disease and dementia, but the pathways in the brain leading from TBI to dementia are unknown.
HSV-1 is found in over 80% of people; varicella zoster virus (VZV) is found in about 95%. Both viruses are known to enter the brain and lay dormant in neurons and glial cells. Prior evidence indicates that HSV-1 in the brain of APOE-ε4 carriers confers a strong risk for Alzheimer’s disease.
A number of years ago, the team created a 3D model of human brain tissue to study the link between TBI, the viruses, and dementia. The model is 6 mm wide, shaped like a donut, and made of a spongy material of silk protein and collagen saturated with neural stem cells. The cells mature into neurons, communicate with each other, and form a network that mimics the brain environment.
In an earlier study using the model quiescently infected with HSV-1, Cairns and colleagues found that subsequent exposure to VZV created the inflammatory conditions that led to reactivation of HSV-1.
This led them to wonder what would happen if they subjected the brain tissue model to a physical disruption akin to a concussion. Would HSV-1 wake up and start the process of neurodegeneration?
To investigate, they examined the effects of one or more controlled blows to the 3D human brain tissue model in the absence or presence of quiescent HSV-1 infection.
After repeated, mild controlled blows, researchers found that the latently infected 3D brain tissue displayed reactivated HSV-1 and the production and accumulation of amyloid beta and p-tau — which promotes neurodegeneration. The blows also activated gliosis, which is associated with destructive neuroinflammation.
These effects are collectively associated with Alzheimer’s disease, dementia, and chronic traumatic encephalopathy, they pointed out, and were increased with additional injury but were absent in tissue not infected with HSV-1.
“These data suggest that HSV-1 in the brain is pivotal in increasing the risk of Alzheimer’s disease, as other recent studies using cerebral organoids have suggested,” the researchers wrote.
They propose that following brain injury, “whether by infection or mechanical damage, the resulting inflammation induces HSV-1 reactivation in the brain leading to the development of Alzheimer’s disease/dementia and that HSV-1 is a major cause of the disease, especially in APOE4 carriers.”
Future studies should investigate “possible ways of mitigating or stopping the damage caused by head injury, thereby reducing subsequent development of Alzheimer’s disease by implementing efforts to prevent the reactivation of virus in brain such as anti-inflammatory and/or antiviral treatment post-injury,” researchers suggested.
Outside Experts Weigh in
Several outside experts offered perspective on the study in a statement from the UK nonprofit Science Media Centre.
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, London, England, said that, while the study is interesting, there are limitations.
“The increase in Alzheimer’s-like brain changes in these latent virus-containing cells subjected to injury does not resemble the pathology that is found in the brain of people with Alzheimer’s disease,” Spires-Jones noted.
“These experiments were also in cells grown in artificial conditions without important Alzheimer’s-related factors such as age and blood vessel changes. Finally, these experiments were repeated in a small number of experimental replicates (three times per experiment), so these results will need to be confirmed in more relevant biological systems with larger studies to be sure there is a biological link between latent herpes simplex virus type 1, brain injury, and Alzheimer’s pathology,” Spires-Jones cautioned.
Robert Howard, MD, MRCPsych, University College London (UCL) Division of Psychiatry, said the study suggests a possible mechanism for the association between HSV-1, brain injury, and Alzheimer’s disease.
“However, as so often in science, it is very important to bear in mind that association does not mean causation. Much more research will be needed before this can be seriously considered a plausible mechanism for the development of dementia,” Howard cautioned.
“Avoidance of brain injuries, such as those encountered in some contact sports, is already known to be an important way to prevent dementia, and I’m unconvinced that this reflects anything more complicated than mechanical damage causing death of brain cells,” he added.
Jennifer Pocock, PhD, with UCL Queen Square Institute of Neurology, noted the role of microglia, which are activated by mild and repetitive TBI, isn’t addressed in the study.
“This paper seems to suggest that only astrocytes contribute to the reported neuroinflammation in brain tissue. Also, the inclusion of APOE3/4 is not clearly defined. Because of this, the findings are likely to represent an over interpretation for the ‘real world’ as the inclusion of microglia may negate or accentuate them, depending on the severity of the TBI,” Pocock said.
The study was funded by the US Army Research Office and Department of Defense. The authors have declared no relevant conflicts of interest. Spires-Jones and Howard had no relevant disclosures related to this study. Pocock has received research funding from AstraZeneca and Daiichi Sankyo.
A version of this article appeared on Medscape.com.
FROM SCIENCE SIGNALING