User login
Nonmelanoma Skin Cancer Risk May Be Reduced in Patients on PCSK9 Inhibitors
TOPLINE:
Proprotein convertase subtilisin/kexin type 9 ( those older than 65 years, and those with immunosuppression.
METHODOLOGY:
- To evaluate the risk for NMSC — basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) — in patients with ASCVD on PCSK9 inhibitors, researchers analyzed data from the US Collaborative Network in the TriNetX database of adults aged ≥ 40 years with ASCVD who received statin therapy between 2016 and 2022.
- A total of 73,636 patients were included, divided equally between those receiving a PCSK9 inhibitor (evolocumab, alirocumab, or inclisiran) plus statin therapy and the control group (those on statin therapy only).
- The analysis used propensity score matching for head-to-head comparisons, with hazard ratios (HRs) estimated using Cox proportional hazard models.
- Stratified analyses examined outcomes by age, sex, Fitzpatrick skin type, and immune status. (Immunosuppressed patients were those treated with immunosuppressants for more than 90 days in the year before the index date — the date when exposed patients were first prescribed a PCSK9 inhibitor, which was also index date for matched patients in the statin-only group.)
TAKEAWAY:
- Patients with ASCVD in the PCSK9 group showed significantly lower risks for NMSC (HR, 0.78; 95% CI, 0.71-0.87), BCC (HR, 0.78; 95% CI, 0.69-0.89), and SCC (HR, 0.79; 95% CI, 0.67-0.93) than control individuals on a statin only (P < .001 for all three).
- Both evolocumab and alirocumab demonstrated similar protective effects against the development of NMSC.
- The reduced risk for NMSC was particularly notable among patients aged 65-79 years (HR, 0.75; 95% CI, 0.66-0.86) and those aged ≥ 80 years (HR, 0.74; 95% CI, 0.60-0.91).
- Men showed a more pronounced reduction in the risk for NMSC (HR, 0.73; 95% CI, 0.64-0.83) than women (HR, 0.93; 95% CI, 0.78-1.11). The effect on lowering NMSC risk was also evident among immunosuppressed patients in the PCSK9 group (HR, 0.68; 95% CI, 0.60-0.75).
IN PRACTICE:
“The findings suggest the promising pleiotropic effect of PCSK9 inhibitors on the chemoprevention of NMSC,” the study authors wrote. Referring to previous studies that “provided mechanistic clues to our findings,” they added that “further studies are required to investigate the underlying mechanisms and establish causality.”
SOURCE:
The study was led by Cheng-Yuan Li, Taipei Veterans General Hospital, Taipei, Taiwan, and was published online in The British Journal of Dermatology.
LIMITATIONS:
Electronic health records lack information on sun protection habits, family history of skin cancer, diet, body mass index, and air pollution exposure, risk factors for NMSC. The study also lacked detailed information on enrollees’ lipid profiles and was focused mostly on patients in the United States, limiting the generalizability of the findings to other regions.
DISCLOSURES:
The study was supported by grants from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Proprotein convertase subtilisin/kexin type 9 ( those older than 65 years, and those with immunosuppression.
METHODOLOGY:
- To evaluate the risk for NMSC — basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) — in patients with ASCVD on PCSK9 inhibitors, researchers analyzed data from the US Collaborative Network in the TriNetX database of adults aged ≥ 40 years with ASCVD who received statin therapy between 2016 and 2022.
- A total of 73,636 patients were included, divided equally between those receiving a PCSK9 inhibitor (evolocumab, alirocumab, or inclisiran) plus statin therapy and the control group (those on statin therapy only).
- The analysis used propensity score matching for head-to-head comparisons, with hazard ratios (HRs) estimated using Cox proportional hazard models.
- Stratified analyses examined outcomes by age, sex, Fitzpatrick skin type, and immune status. (Immunosuppressed patients were those treated with immunosuppressants for more than 90 days in the year before the index date — the date when exposed patients were first prescribed a PCSK9 inhibitor, which was also index date for matched patients in the statin-only group.)
TAKEAWAY:
- Patients with ASCVD in the PCSK9 group showed significantly lower risks for NMSC (HR, 0.78; 95% CI, 0.71-0.87), BCC (HR, 0.78; 95% CI, 0.69-0.89), and SCC (HR, 0.79; 95% CI, 0.67-0.93) than control individuals on a statin only (P < .001 for all three).
- Both evolocumab and alirocumab demonstrated similar protective effects against the development of NMSC.
- The reduced risk for NMSC was particularly notable among patients aged 65-79 years (HR, 0.75; 95% CI, 0.66-0.86) and those aged ≥ 80 years (HR, 0.74; 95% CI, 0.60-0.91).
- Men showed a more pronounced reduction in the risk for NMSC (HR, 0.73; 95% CI, 0.64-0.83) than women (HR, 0.93; 95% CI, 0.78-1.11). The effect on lowering NMSC risk was also evident among immunosuppressed patients in the PCSK9 group (HR, 0.68; 95% CI, 0.60-0.75).
IN PRACTICE:
“The findings suggest the promising pleiotropic effect of PCSK9 inhibitors on the chemoprevention of NMSC,” the study authors wrote. Referring to previous studies that “provided mechanistic clues to our findings,” they added that “further studies are required to investigate the underlying mechanisms and establish causality.”
SOURCE:
The study was led by Cheng-Yuan Li, Taipei Veterans General Hospital, Taipei, Taiwan, and was published online in The British Journal of Dermatology.
LIMITATIONS:
Electronic health records lack information on sun protection habits, family history of skin cancer, diet, body mass index, and air pollution exposure, risk factors for NMSC. The study also lacked detailed information on enrollees’ lipid profiles and was focused mostly on patients in the United States, limiting the generalizability of the findings to other regions.
DISCLOSURES:
The study was supported by grants from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Proprotein convertase subtilisin/kexin type 9 ( those older than 65 years, and those with immunosuppression.
METHODOLOGY:
- To evaluate the risk for NMSC — basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) — in patients with ASCVD on PCSK9 inhibitors, researchers analyzed data from the US Collaborative Network in the TriNetX database of adults aged ≥ 40 years with ASCVD who received statin therapy between 2016 and 2022.
- A total of 73,636 patients were included, divided equally between those receiving a PCSK9 inhibitor (evolocumab, alirocumab, or inclisiran) plus statin therapy and the control group (those on statin therapy only).
- The analysis used propensity score matching for head-to-head comparisons, with hazard ratios (HRs) estimated using Cox proportional hazard models.
- Stratified analyses examined outcomes by age, sex, Fitzpatrick skin type, and immune status. (Immunosuppressed patients were those treated with immunosuppressants for more than 90 days in the year before the index date — the date when exposed patients were first prescribed a PCSK9 inhibitor, which was also index date for matched patients in the statin-only group.)
TAKEAWAY:
- Patients with ASCVD in the PCSK9 group showed significantly lower risks for NMSC (HR, 0.78; 95% CI, 0.71-0.87), BCC (HR, 0.78; 95% CI, 0.69-0.89), and SCC (HR, 0.79; 95% CI, 0.67-0.93) than control individuals on a statin only (P < .001 for all three).
- Both evolocumab and alirocumab demonstrated similar protective effects against the development of NMSC.
- The reduced risk for NMSC was particularly notable among patients aged 65-79 years (HR, 0.75; 95% CI, 0.66-0.86) and those aged ≥ 80 years (HR, 0.74; 95% CI, 0.60-0.91).
- Men showed a more pronounced reduction in the risk for NMSC (HR, 0.73; 95% CI, 0.64-0.83) than women (HR, 0.93; 95% CI, 0.78-1.11). The effect on lowering NMSC risk was also evident among immunosuppressed patients in the PCSK9 group (HR, 0.68; 95% CI, 0.60-0.75).
IN PRACTICE:
“The findings suggest the promising pleiotropic effect of PCSK9 inhibitors on the chemoprevention of NMSC,” the study authors wrote. Referring to previous studies that “provided mechanistic clues to our findings,” they added that “further studies are required to investigate the underlying mechanisms and establish causality.”
SOURCE:
The study was led by Cheng-Yuan Li, Taipei Veterans General Hospital, Taipei, Taiwan, and was published online in The British Journal of Dermatology.
LIMITATIONS:
Electronic health records lack information on sun protection habits, family history of skin cancer, diet, body mass index, and air pollution exposure, risk factors for NMSC. The study also lacked detailed information on enrollees’ lipid profiles and was focused mostly on patients in the United States, limiting the generalizability of the findings to other regions.
DISCLOSURES:
The study was supported by grants from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New Cancer Vaccines on the Horizon: Renewed Hope or Hype?
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
US Study Pinpoints Merkel Cell Risk Factors
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Inside the Patient-Oncologist Bond: Why It’s Often So Strong
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
Rose Gerber was 39, mother to a third grader and a kindergartener, when the diagnosis came: Advanced HER2-positive breast cancer.
“On one of my first or second appointments, I took in a little picture of Alexander and Isabella,” Gerber said. Gerber showed her oncologist the picture and told her: “I’ll do anything. I just want to be there for them.”
That was 21 years ago. Today, her current cancer status is “no evidence of disease.”
Over the past 2 decades, Gerber has gotten to be there for her children. Her youngest is now a television producer and her oldest, a CPA.
In that time,
“I’ve seen multiple physicians over my 21 years, but my oncologist has always been the focal point, guiding me in the right direction,” Gerber said in an interview.
Over the years, Jaga guided Gerber through a range of treatment decisions, including a Herceptin clinical trial that the mom of two views as lifesaving. Jaga often took on the role of both doctor and therapist, even providing comfort in the smaller moments when Gerber would fret about her weight gain.
The oncologist-patient “bond is very, very, very special,” said Gerber, who now works as director of patient advocacy and education at the Community Oncology Alliance.
Gerber isn’t alone in calling out the depth of the oncologist-patient bond.
Over years, sometimes decades, patients and oncologists can experience a whole world together: The treatment successes, relapses, uncertainties, and tough calls. As a result, a deep therapeutic alliance often develops. And with each new hurdle or decision, that collaborative, human connection between doctor and patient continues to form new layers.
“It’s like a shared bonding experience over trauma, like strangers trapped on a subway and then we get out, and we’re now on the other side, celebrating together,” said Saad Khan, MD, an associate professor of medicine (oncology) at Stanford University in California.
Connecting Through Stress
Although studies exploring the oncologist-patient bond are limited, some research suggests that a strong therapeutic alliance between patients and oncologists not only provides a foundation for quality care but can also help improve patients’ quality of life, protect against suicidal ideation, and increase treatment adherence.
Because of how stressful and frightening a cancer diagnosis can be, creating “a trusting, uninterrupted, almost sacred environment for them” is paramount for Khan. “I have no doubt that the most important part of their treatment is that they find an oncologist in whom they have total confidence,” Khan wrote in a blog.
The stress that patients with cancer experience is well documented, but oncologists take on a lot themselves and can also experience intense stress (.
“I consider my patient’s battles to be my battles,” Khan wrote.
The stress can start with the daily schedule. Oncologists often have a high volume of patients and tend to spend more time with each individual than most.
According to a 2023 survey, oncologists see about 68 patients a week, on average, but some oncologists, like Khan, have many more. Khan typically sees 20-30 patients a day and continues to care for many over years.
The survey also found that oncologists tend to spend a lot of time with their patients. Compared with other physicians, oncologists are two times more likely to spend at least 25 minutes with each patient.
With this kind of patient volume and time, Khan said, “you’re going to be exhausted.”
What can compound the exhaustion are the occasions oncologists need to deliver bad news — this treatment isn’t working, your cancer has come roaring back and, perhaps the hardest, we have no therapeutic options left. The end-of-life conversations, in particular, can be heartbreaking, especially when a patient is young and not ready to stop trying.
“It can be hard for doctors to discuss the end of life,” Don Dizon, MD, director of the Pelvic Malignancies Program at Lifespan Cancer Institute and director of Medical Oncology at Rhode Island Hospital, Providence, wrote in a column in 2023. Instead, it can be tempting and is often easier to focus on the next treatment, “instilling hope that there’s more that can be done,” even if doing more will only do harm.
In the face of these challenging decisions, growing a personal connection with patients over time can help keep oncologists going.
“We’re not just chemotherapy salesmen,” Khan said in an interview. “We get to know their social support network, who’s going to be driving them [to and from appointments], where they go on vacation, their cat’s name, who their neighbors are.”
A ‘Special Relationship’
Ralph V. Boccia, MD, is often asked what he does.
The next question that often comes — “Why do I do what I do?” — is Boccia’s favorite.
“Someone needs to take these patients through their journey,” Boccia, the founder of The Center for Cancer and Blood Disorders, Bethesda, Maryland, typically responds. He also often notes that “it is a special relationship you develop with the patient and their families.”
Boccia thinks about one long-term patient who captures this bond.
Joan Pinson, 70, was diagnosed with multiple myeloma about 25 years ago, when patients’ average survival was about 4 years.
Over a quarter century, Pinson has pivoted to different treatments, amid multiple relapses and remissions. Throughout most of this cancer journey, Boccia has been her primary oncologist, performing a stem cell transplant in 2000 and steering her to six clinical trials.
Her last relapse was 2 years ago, and since then she has been doing well on oral chemotherapy.
“Every time I relapsed, by the next appointment, he’d say, ‘here is what we are going to do,’ ” Pinson recalled. “I never worried, I never panicked. I knew he would take care of me.”
Over the years, Pinson and Boccia have shared many personal moments, sometimes by accident. One special moment happened early on in Pinson’s cancer journey. During an appointment, Boccia had “one ear to the phone” as his wife was about to deliver their first baby, Pinson recalled.
Later, Pinson met that child as a young man working in Boccia’s lab. She has also met Boccia’s wife, a nurse, when she filled in one day in the chemotherapy room.
Boccia now also treats Pinson’s husband who has prostate cancer, and he ruled out cancer when Pinson’s son, now in his 40s, had some worrisome symptoms.
More than 2 decades ago, Pinson told Boccia her goal was to see her youngest child graduate from high school. Now, six grandsons later, she has lived far beyond that goal.
“He has kept me alive,” said Pinson.
The Dying Patient
Harsha Vyas, MD, FACP, remembers the first encounter his office had with a 29-year-old woman referred with a diagnosis of stage IV breast cancer.
After just 15 minutes in the waiting room, the woman announced she was leaving. Although office staff assured the woman that she was next, the patient walked out.
Several months later, Vyas was called for an inpatient consult. It was the same woman.
Her lungs were full of fluid, and she was struggling to breathe, said Vyas, president and CEO of the Cancer Center of Middle Georgia, Dublin, and assistant professor at Augusta University in Georgia.
The woman, a single mother, told Vyas about her three young kids at home and asked him, “Doc, do something, please help me,” he recalled.
“Absolutely,” Vyas told her. But he had to be brutally honest about her prognosis and firm that she needed to follow his instructions. “You have a breast cancer I cannot cure,” he said. “All I can do is control the disease.”
From that first day, until the day she died, she came to every appointment and followed the treatment plan Vyas laid out.
For about 2 years, she responded well to treatment. And as the time passed and the trust grew, she began to open up to him. She showed him pictures. She talked about her children and being a mother.
“I’ve got to get my kids in a better place. I’m going to be there for them,” he recalled her saying.
Vyas admired her resourcefulness. She held down a part-time job, working retail and at a local restaurant. She figured out childcare so she could get to her chemotherapy appointments every 3 weeks and manage the copays.
Several years later, when she knew she was approaching the end of her life, she asked Vyas a question that hit hard.
“Doc, I don’t want to die and my kids find me dead. What can we do about it?”
Vyas, who has three daughters, imagined how traumatic this would be for a child. She and Vyas made the shared decision to cease treatment and begin home hospice. When the end was approaching, a hospice worker took over, waiting for bodily functions to cease.
When news of a death comes, “I say a little prayer, it’s almost like a send-off for that soul. That helps me absorb the news ... and let it go.”
But when the bond grows strong over time, as with his patient with breast cancer, Vyas said, “a piece of her is still with me.”
Khan had no relevant disclosures. Boccia and Vyas had no disclosures.
A version of this article appeared on Medscape.com.
Many Patients With Cancer Visit EDs Before Diagnosis
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CMAJ
Plasma Omega-6 and Omega-3 Fatty Acids Inversely Associated With Cancer
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Disparities in Skin Cancer Outcomes in the Latine/Hispanic Population
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13
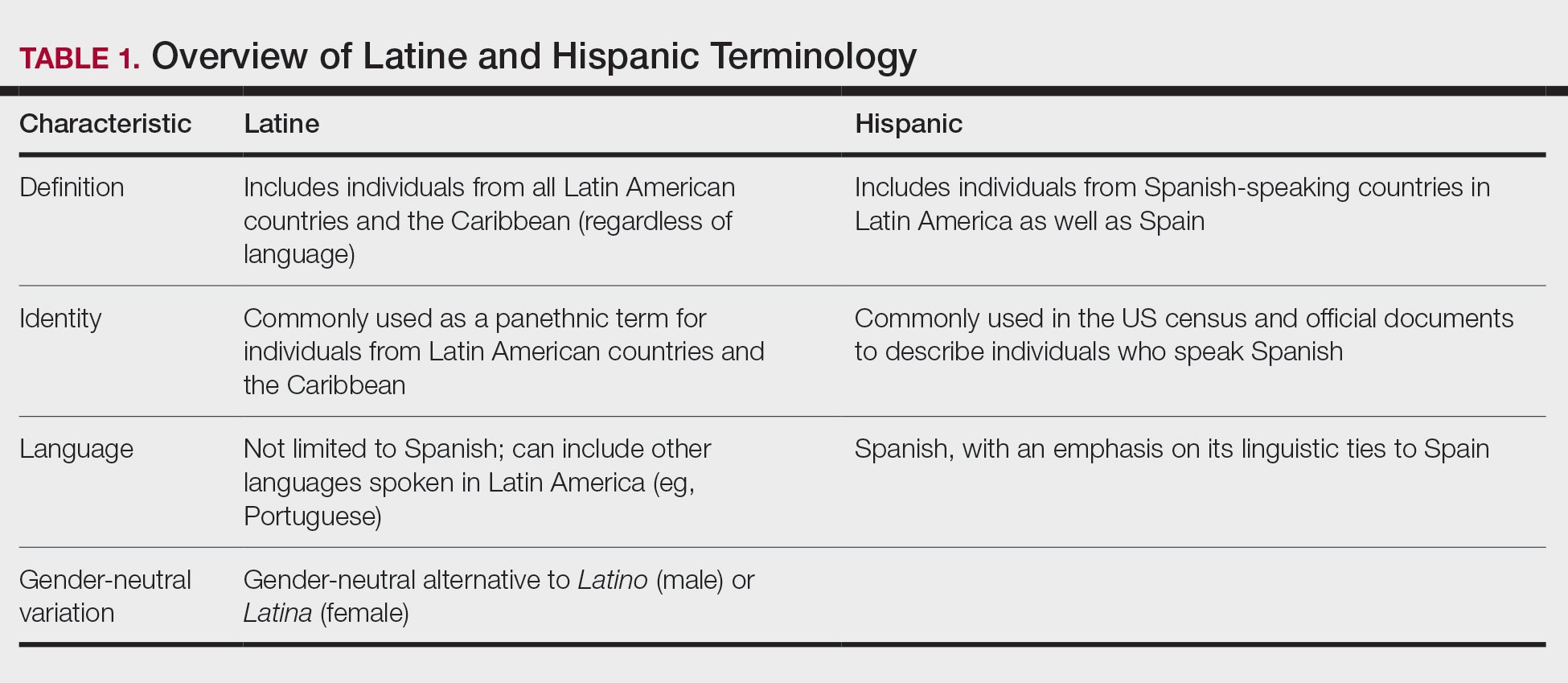
More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18
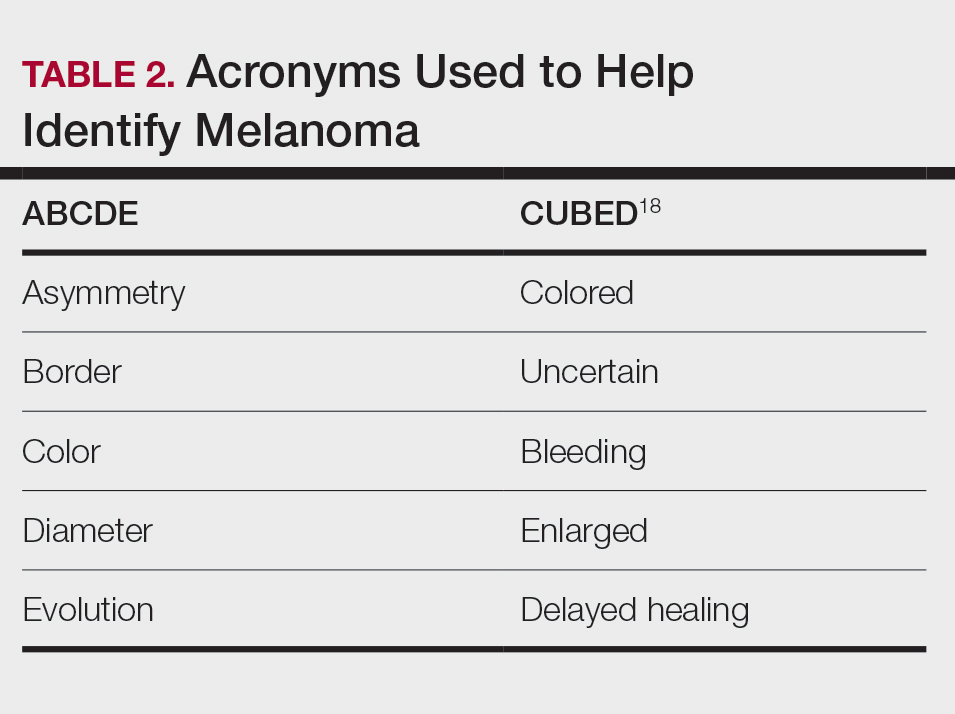
Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
Practice Points
- The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by disparities in skin cancer outcomes.
- Factors influencing skin cancer outcomes in Latine/Hispanic patients in the United States are complex and multidimensional, including lack of familiarity among dermatologists with skin cancer manifestation in this population compared to non-Hispanic White individuals as well as limited data elucidating risk factors for skin cancer in patients with skin of color and sociocultural factors.
Community Outreach Benefits Dermatology Residents and Their Patients
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
Resident Pearls
- Outreach initiatives can help residents feel more connected to their community and expand access to care.
- Skin cancer screenings, free clinics, and teledermatology are a few ways residents may get involved in their local communities.
Study Finds Elevated Skin Cancer Risk Among US Veterans
of recent national data.
“US veterans are known to have increased risk of cancers and cancer morbidity compared to the general US population,” one of the study authors, Sepideh Ashrafzadeh, MD, a third-year dermatology resident at Massachusetts General Hospital, Boston, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the results were presented. “There have been several studies that have shown that US veterans have an increased prevalence of melanoma compared to nonveterans,” she said, noting, however, that no study has investigated the prevalence of nonmelanoma skin cancers (NMSCs), which include basal cell carcinomas and squamous cell carcinomas, compared with the general population.
To address this knowledge gap, the researchers performed a national cross-sectional study of adults aged 18 years or older from the 2019-2023 National Health Interview Surveys to examine the prevalence of melanoma and NMSCs among veterans compared with the general US population. They aggregated and tabulated the data by veteran status, defined as having served at any point in the US armed forces, reserves, or national guard, and by demographic and socioeconomic status variables. Next, they performed multivariate logistic regression for skin cancer risk adjusted for age, sex, race, ethnicity, urbanicity, and disability status.
The study population consisted of 14,301 veterans and 209,936 nonveterans. Compared with nonveterans, veterans were more likely to have been diagnosed with skin cancer at some point in their lives (7% vs 2.4%; P < .001); had a higher mean age of skin cancer diagnosis (61.1 vs 55.8 years; P < .001); were more likely to have been diagnosed with melanoma (2.8% vs 0.9%; P < .001), and were more likely to have been diagnosed with NMSC (4.4% vs 1.6%; P < .001).
The researchers found that older age, White race, non-Hispanic ethnicity, and veteran status were all associated with higher odds of developing NMSCs, even after adjusting for relevant covariates. Specifically, veterans had 1.23 higher odds of developing NMSC than the general population, while two factors were protective for developing NMSCs: Living in a rural setting (adjusted odds ratio [aOR], 0.78) and receiving supplemental security income or disability income (aOR, 0.69).
In another part of the study, the researchers evaluated demographic and socioeconomic variables associated with developing melanoma among veterans. These included the following: Male (aOR, 1.16), older age (50-64 years: aOR, 6.82; 65-74 years: aOR, 12.55; and 75 years or older: aOR, 16.16), White race (aOR, 9.24), and non-Hispanic ethnicity (aOR, 7.15).
“Veterans may have occupational risks such as sun and chemical exposure, as well as behavioral habits for sun protection, that may contribute to their elevated risk of melanoma and NMSCs,” Ashrafzadeh said. “Therefore, US veterans would benefit from targeted and regular skin cancer screenings, sun protective preventative resources such as hats and sunscreen, and access to medical and surgical care for diagnosis and treatment of skin cancers.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the findings, said that a key strength of the study is that it drew from a nationally representative sample. “A limitation is that skin cancer was self-reported rather than based on documented medical histories,” Ko said. “The study confirms that skin cancer risk is higher in older individuals (> 75 as compared to < 50) and in individuals of self-reported white race and non-Hispanic ethnicity,” she added.
Neither the researchers nor Ko reported having relevant disclosures.
A version of this article first appeared on Medscape.com.
of recent national data.
“US veterans are known to have increased risk of cancers and cancer morbidity compared to the general US population,” one of the study authors, Sepideh Ashrafzadeh, MD, a third-year dermatology resident at Massachusetts General Hospital, Boston, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the results were presented. “There have been several studies that have shown that US veterans have an increased prevalence of melanoma compared to nonveterans,” she said, noting, however, that no study has investigated the prevalence of nonmelanoma skin cancers (NMSCs), which include basal cell carcinomas and squamous cell carcinomas, compared with the general population.
To address this knowledge gap, the researchers performed a national cross-sectional study of adults aged 18 years or older from the 2019-2023 National Health Interview Surveys to examine the prevalence of melanoma and NMSCs among veterans compared with the general US population. They aggregated and tabulated the data by veteran status, defined as having served at any point in the US armed forces, reserves, or national guard, and by demographic and socioeconomic status variables. Next, they performed multivariate logistic regression for skin cancer risk adjusted for age, sex, race, ethnicity, urbanicity, and disability status.
The study population consisted of 14,301 veterans and 209,936 nonveterans. Compared with nonveterans, veterans were more likely to have been diagnosed with skin cancer at some point in their lives (7% vs 2.4%; P < .001); had a higher mean age of skin cancer diagnosis (61.1 vs 55.8 years; P < .001); were more likely to have been diagnosed with melanoma (2.8% vs 0.9%; P < .001), and were more likely to have been diagnosed with NMSC (4.4% vs 1.6%; P < .001).
The researchers found that older age, White race, non-Hispanic ethnicity, and veteran status were all associated with higher odds of developing NMSCs, even after adjusting for relevant covariates. Specifically, veterans had 1.23 higher odds of developing NMSC than the general population, while two factors were protective for developing NMSCs: Living in a rural setting (adjusted odds ratio [aOR], 0.78) and receiving supplemental security income or disability income (aOR, 0.69).
In another part of the study, the researchers evaluated demographic and socioeconomic variables associated with developing melanoma among veterans. These included the following: Male (aOR, 1.16), older age (50-64 years: aOR, 6.82; 65-74 years: aOR, 12.55; and 75 years or older: aOR, 16.16), White race (aOR, 9.24), and non-Hispanic ethnicity (aOR, 7.15).
“Veterans may have occupational risks such as sun and chemical exposure, as well as behavioral habits for sun protection, that may contribute to their elevated risk of melanoma and NMSCs,” Ashrafzadeh said. “Therefore, US veterans would benefit from targeted and regular skin cancer screenings, sun protective preventative resources such as hats and sunscreen, and access to medical and surgical care for diagnosis and treatment of skin cancers.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the findings, said that a key strength of the study is that it drew from a nationally representative sample. “A limitation is that skin cancer was self-reported rather than based on documented medical histories,” Ko said. “The study confirms that skin cancer risk is higher in older individuals (> 75 as compared to < 50) and in individuals of self-reported white race and non-Hispanic ethnicity,” she added.
Neither the researchers nor Ko reported having relevant disclosures.
A version of this article first appeared on Medscape.com.
of recent national data.
“US veterans are known to have increased risk of cancers and cancer morbidity compared to the general US population,” one of the study authors, Sepideh Ashrafzadeh, MD, a third-year dermatology resident at Massachusetts General Hospital, Boston, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the results were presented. “There have been several studies that have shown that US veterans have an increased prevalence of melanoma compared to nonveterans,” she said, noting, however, that no study has investigated the prevalence of nonmelanoma skin cancers (NMSCs), which include basal cell carcinomas and squamous cell carcinomas, compared with the general population.
To address this knowledge gap, the researchers performed a national cross-sectional study of adults aged 18 years or older from the 2019-2023 National Health Interview Surveys to examine the prevalence of melanoma and NMSCs among veterans compared with the general US population. They aggregated and tabulated the data by veteran status, defined as having served at any point in the US armed forces, reserves, or national guard, and by demographic and socioeconomic status variables. Next, they performed multivariate logistic regression for skin cancer risk adjusted for age, sex, race, ethnicity, urbanicity, and disability status.
The study population consisted of 14,301 veterans and 209,936 nonveterans. Compared with nonveterans, veterans were more likely to have been diagnosed with skin cancer at some point in their lives (7% vs 2.4%; P < .001); had a higher mean age of skin cancer diagnosis (61.1 vs 55.8 years; P < .001); were more likely to have been diagnosed with melanoma (2.8% vs 0.9%; P < .001), and were more likely to have been diagnosed with NMSC (4.4% vs 1.6%; P < .001).
The researchers found that older age, White race, non-Hispanic ethnicity, and veteran status were all associated with higher odds of developing NMSCs, even after adjusting for relevant covariates. Specifically, veterans had 1.23 higher odds of developing NMSC than the general population, while two factors were protective for developing NMSCs: Living in a rural setting (adjusted odds ratio [aOR], 0.78) and receiving supplemental security income or disability income (aOR, 0.69).
In another part of the study, the researchers evaluated demographic and socioeconomic variables associated with developing melanoma among veterans. These included the following: Male (aOR, 1.16), older age (50-64 years: aOR, 6.82; 65-74 years: aOR, 12.55; and 75 years or older: aOR, 16.16), White race (aOR, 9.24), and non-Hispanic ethnicity (aOR, 7.15).
“Veterans may have occupational risks such as sun and chemical exposure, as well as behavioral habits for sun protection, that may contribute to their elevated risk of melanoma and NMSCs,” Ashrafzadeh said. “Therefore, US veterans would benefit from targeted and regular skin cancer screenings, sun protective preventative resources such as hats and sunscreen, and access to medical and surgical care for diagnosis and treatment of skin cancers.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the findings, said that a key strength of the study is that it drew from a nationally representative sample. “A limitation is that skin cancer was self-reported rather than based on documented medical histories,” Ko said. “The study confirms that skin cancer risk is higher in older individuals (> 75 as compared to < 50) and in individuals of self-reported white race and non-Hispanic ethnicity,” she added.
Neither the researchers nor Ko reported having relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ASDS 2024
Cancer’s Other Toll: Long-Term Financial Fallout for Survivors
Overall, patients with cancer tend to face higher rates of debt collection, medical collections, and bankruptcies, as well as lower credit scores, according to two new studies presented at the American College of Surgeons Clinical Congress 2024.
“These are the first studies to provide numerical evidence of financial toxicity among cancer survivors,” Benjamin C. James, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts, who worked on both studies, said in a statement. “Previous data on this topic largely relies on subjective survey reviews.”
In one study, researchers used the Massachusetts Cancer Registry to identify 99,175 patients diagnosed with cancer between 2010 and 2019 and matched them with 188,875 control individuals without cancer. Researchers then assessed financial toxicity using Experian credit bureau data for participants.
Overall, patients with cancer faced a range of financial challenges that often lasted years following their diagnosis.
Patients were nearly five times more likely to experience bankruptcy and had average credit scores nearly 80 points lower than control individuals without cancer. The drop in credit scores was more pronounced for survivors of bladder, liver, lung, and colorectal cancer (CRC) and persisted for up to 9.5 years.
For certain cancer types, in particular, “we are looking years after a diagnosis, and we see that the credit score goes down and it never comes back up,” James said.
The other study, which used a sample of 7227 patients with CRC from Massachusetts, identified several factors that correlated with lower credit scores.
Compared with patients who only had surgery, peers who underwent radiation only experienced a 62-point drop in their credit score after their diagnosis, while those who had chemotherapy alone had just over a 14-point drop in their credit score. Among patients who had combination treatments, those who underwent both surgery and radiation experienced a nearly 16-point drop in their credit score and those who had surgery and chemoradiation actually experienced a 2.59 bump, compared with those who had surgery alone.
Financial toxicity was worse for patients younger than 62 years, those identifying as Black or Hispanic individuals, unmarried individuals, those with an annual income below $52,000, and those living in deprived areas.
The studies add to findings from the 2015 North American Thyroid Cancer Survivorship Study, which reported that 50% of thyroid cancer survivors encountered financial toxicity because of their diagnosis.
James said the persistent financial strain of cancer care, even in a state like Massachusetts, which mandates universal healthcare, underscores the need for “broader policy changes and reforms, including reconsidering debt collection practices.”
“Financial security should be a priority in cancer care,” he added.
The studies had no specific funding. The authors have disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Overall, patients with cancer tend to face higher rates of debt collection, medical collections, and bankruptcies, as well as lower credit scores, according to two new studies presented at the American College of Surgeons Clinical Congress 2024.
“These are the first studies to provide numerical evidence of financial toxicity among cancer survivors,” Benjamin C. James, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts, who worked on both studies, said in a statement. “Previous data on this topic largely relies on subjective survey reviews.”
In one study, researchers used the Massachusetts Cancer Registry to identify 99,175 patients diagnosed with cancer between 2010 and 2019 and matched them with 188,875 control individuals without cancer. Researchers then assessed financial toxicity using Experian credit bureau data for participants.
Overall, patients with cancer faced a range of financial challenges that often lasted years following their diagnosis.
Patients were nearly five times more likely to experience bankruptcy and had average credit scores nearly 80 points lower than control individuals without cancer. The drop in credit scores was more pronounced for survivors of bladder, liver, lung, and colorectal cancer (CRC) and persisted for up to 9.5 years.
For certain cancer types, in particular, “we are looking years after a diagnosis, and we see that the credit score goes down and it never comes back up,” James said.
The other study, which used a sample of 7227 patients with CRC from Massachusetts, identified several factors that correlated with lower credit scores.
Compared with patients who only had surgery, peers who underwent radiation only experienced a 62-point drop in their credit score after their diagnosis, while those who had chemotherapy alone had just over a 14-point drop in their credit score. Among patients who had combination treatments, those who underwent both surgery and radiation experienced a nearly 16-point drop in their credit score and those who had surgery and chemoradiation actually experienced a 2.59 bump, compared with those who had surgery alone.
Financial toxicity was worse for patients younger than 62 years, those identifying as Black or Hispanic individuals, unmarried individuals, those with an annual income below $52,000, and those living in deprived areas.
The studies add to findings from the 2015 North American Thyroid Cancer Survivorship Study, which reported that 50% of thyroid cancer survivors encountered financial toxicity because of their diagnosis.
James said the persistent financial strain of cancer care, even in a state like Massachusetts, which mandates universal healthcare, underscores the need for “broader policy changes and reforms, including reconsidering debt collection practices.”
“Financial security should be a priority in cancer care,” he added.
The studies had no specific funding. The authors have disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Overall, patients with cancer tend to face higher rates of debt collection, medical collections, and bankruptcies, as well as lower credit scores, according to two new studies presented at the American College of Surgeons Clinical Congress 2024.
“These are the first studies to provide numerical evidence of financial toxicity among cancer survivors,” Benjamin C. James, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts, who worked on both studies, said in a statement. “Previous data on this topic largely relies on subjective survey reviews.”
In one study, researchers used the Massachusetts Cancer Registry to identify 99,175 patients diagnosed with cancer between 2010 and 2019 and matched them with 188,875 control individuals without cancer. Researchers then assessed financial toxicity using Experian credit bureau data for participants.
Overall, patients with cancer faced a range of financial challenges that often lasted years following their diagnosis.
Patients were nearly five times more likely to experience bankruptcy and had average credit scores nearly 80 points lower than control individuals without cancer. The drop in credit scores was more pronounced for survivors of bladder, liver, lung, and colorectal cancer (CRC) and persisted for up to 9.5 years.
For certain cancer types, in particular, “we are looking years after a diagnosis, and we see that the credit score goes down and it never comes back up,” James said.
The other study, which used a sample of 7227 patients with CRC from Massachusetts, identified several factors that correlated with lower credit scores.
Compared with patients who only had surgery, peers who underwent radiation only experienced a 62-point drop in their credit score after their diagnosis, while those who had chemotherapy alone had just over a 14-point drop in their credit score. Among patients who had combination treatments, those who underwent both surgery and radiation experienced a nearly 16-point drop in their credit score and those who had surgery and chemoradiation actually experienced a 2.59 bump, compared with those who had surgery alone.
Financial toxicity was worse for patients younger than 62 years, those identifying as Black or Hispanic individuals, unmarried individuals, those with an annual income below $52,000, and those living in deprived areas.
The studies add to findings from the 2015 North American Thyroid Cancer Survivorship Study, which reported that 50% of thyroid cancer survivors encountered financial toxicity because of their diagnosis.
James said the persistent financial strain of cancer care, even in a state like Massachusetts, which mandates universal healthcare, underscores the need for “broader policy changes and reforms, including reconsidering debt collection practices.”
“Financial security should be a priority in cancer care,” he added.
The studies had no specific funding. The authors have disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
FROM ACSCS 2024