User login
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
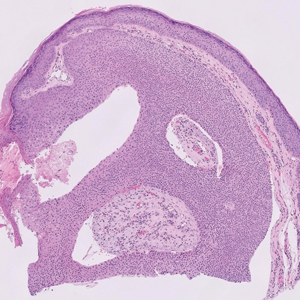
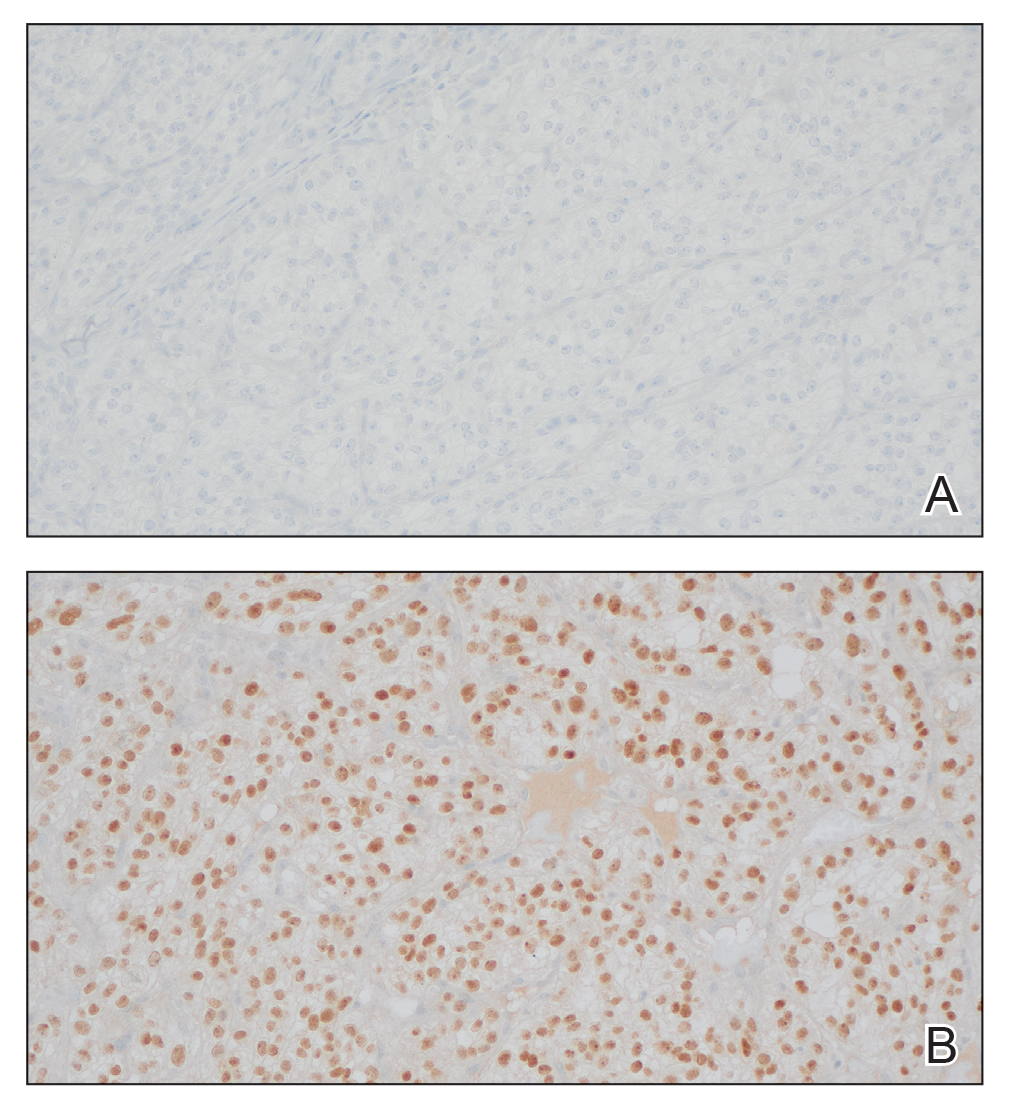
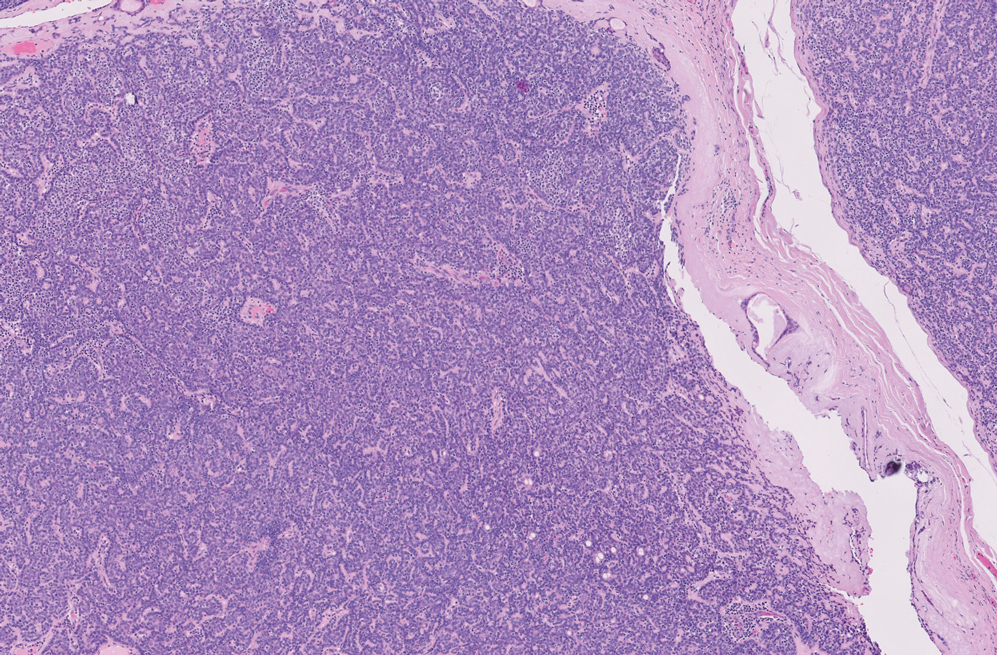
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.
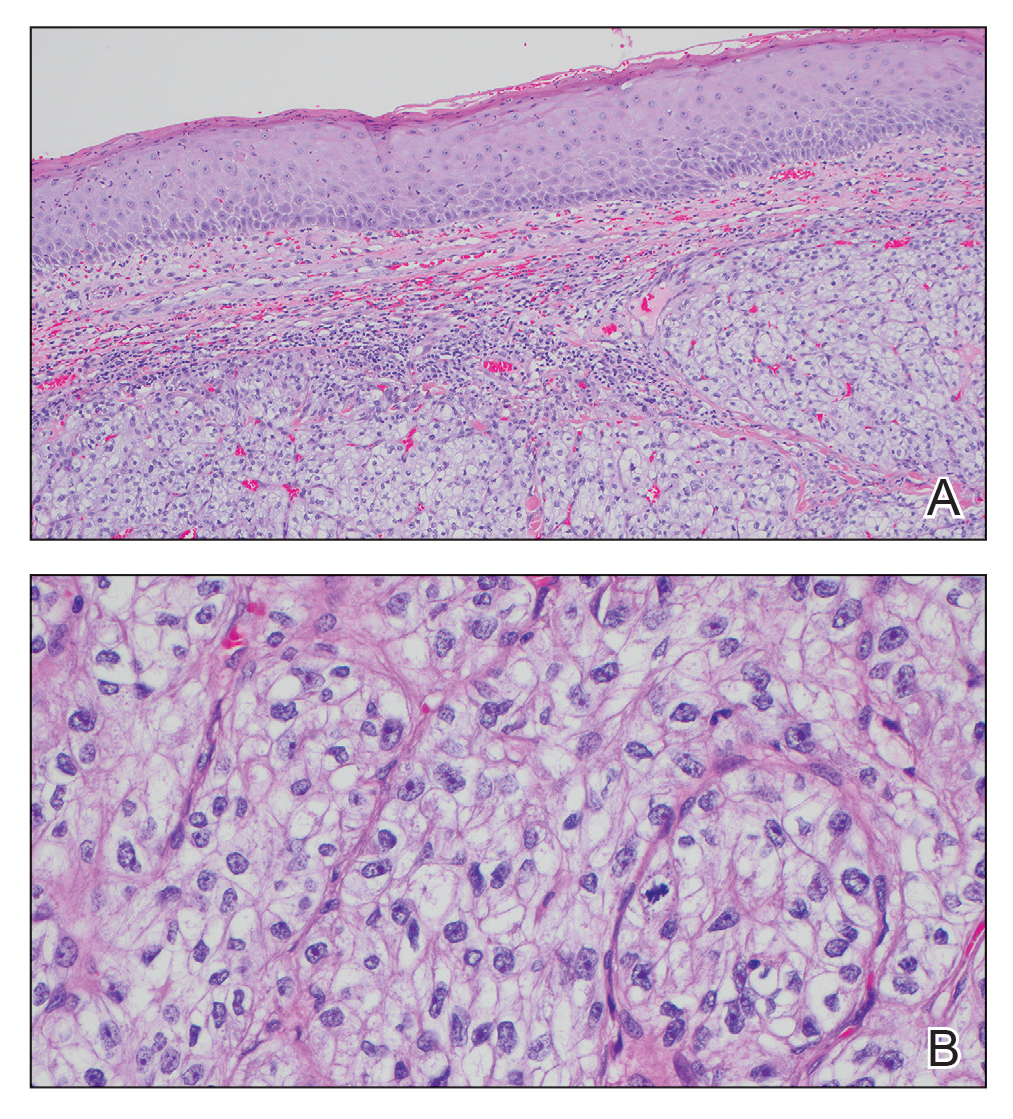
Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
THE DIAGNOSIS: Metastatic Renal Cell Carcinoma
The shave biopsy revealed large cells with prominent nucleoli, clear cytoplasm, and thin cell borders in a nestlike arrangement (Figure 1). Immunohistochemical examination was negative for cytokeratin 5/6 and positive for PAX8 (Figure 2), which finalized the diagnosis of metastatic renal cell carcinoma (RCC). Later, our patient had a core biopsy-proven metastasis to the C6 spinous process, with concern for additional metastasis to the liver and lungs on positron emission tomography. Our patient’s treatment plan included pembrolizumab and axitinib to manage further cutaneous metastasis and radiation therapy for the C6 spinous process metastasis.


Renal cell carcinoma denotes cancer originating from the renal epithelium and is the most common kidney tumor in adults.1 Renal cell carcinoma accounts for more than 90% of kidney malignancies in the United States and has 3 main subtypes: clear cell RCC, papillary RCC, and chromophobe RCC.2 About 25% of cases metastasize, commonly to the lungs, liver, bones, lymph nodes, contralateral kidney, and adrenal glands.3
Cutaneous metastasis of RCC is rare, with an incidence of approximately 3.3%.4 Notably, 80% to 90% of patients with metastatic skin lesions had a prior diagnosis of RCC.2 Skin metastases associated with RCC predominantly are found on the face and scalp, appearing as nodular, swiftly expanding, circular, or oval-shaped growths. The robust vascular element of these lesions can lead to confusion with regard to the proper diagnosis, as they often resemble hemangiomas, pyogenic granulomas, or Kaposi sarcomas.4
Many cutaneous metastases linked to RCC exhibit a histomorphologic pattern consistent with clear cell adenocarcinoma.2 The malignant cells are large and possess transparent cytoplasm, round to oval nuclei, and prominent nucleoli. The cells can form glandular, acinar, or papillary arrangements; extravasated red blood cells frequently are found within the surrounding fibrovascular tissue.5 The presence of cytoplasmic glycogen can be revealed through periodic acid–Schiff staining. Other immunohistochemical markers commonly used to identify skin metastasis of RCC include epithelioid membrane antigen, carcinoembryonic antigen, and CD-10.1
Various mechanisms are involved in the cutaneous metastases of RCC. The most common pathway involves infiltration of the skin directly overlying the malignant renal mass; additional potential mechanisms include the introduction of abnormal cells into the skin during surgical or diagnostic interventions and their dissemination through the lymphatic system or bloodstream.1 Among urogenital malignancies other than RCC, skin metastases predominantly manifest in the abdominal region.2 Conversely, the head and neck region are more frequently impacted in RCC. The vascular composition of these tumors plays a role in facilitating the extension of cancer cells through the bloodstream, fostering the emergence of distant metastases.6
The development of cutaneous metastasis in RCC is associated with a poor prognosis, as most patients die within 6 months of detection.3 Treatment options thus are limited and palliative. Although local excision is an alternative treatment for localized cutaneous metastasis, it often provides little benefit in the presence of extensive metastasis; radiotherapy also has been shown to have a limited effect on primary RCC, though its devascularization of the lesion may be effective in metastatic cases.5 Immune checkpoint inhibitors such as nivolumab and ipilimumab have improved progression-free survival in patients with metastatic RCC, though uncertainty remains regarding their efficacy in attenuating cutaneous metastasis.5,6
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
- Kanwal R. Metastasis in renal cell carcinoma: biology and treatment. Adv Cancer Biol Metastasis. 2023;7:100094. doi:10.1016 /j.adcanc.2023.100094
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10:E3614. doi:10.7759/cureus.3614
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. doi:10.1093/annonc/mdr362
- Lorenzo-Rios D, Cruzval-O’Reilly E, Rabelo-Cartagena J. Facial cutaneous metastasis in renal cell carcinoma. Cureus. 2020;12:E12093. doi:10.7759/cureus.12093
- Iliescu CA, Beiu C, Racovit·a¢ A, et al. Atypical presentation of rapidly progressive cutaneous metastases of clear cell renal carcinoma: a case report. Medicina. 2024;60:1797. doi:10.3390/medicina60111797
- Joyce MJ. Management of skeletal metastases in renal cell carcinoma patients. In: Bukowski RM, Novick AC, eds. Clinical Management of Renal Tumors. Springer; 2008: 421-459.
Vascular Nodule on the Upper Chest
Vascular Nodule on the Upper Chest
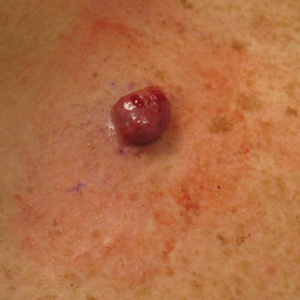
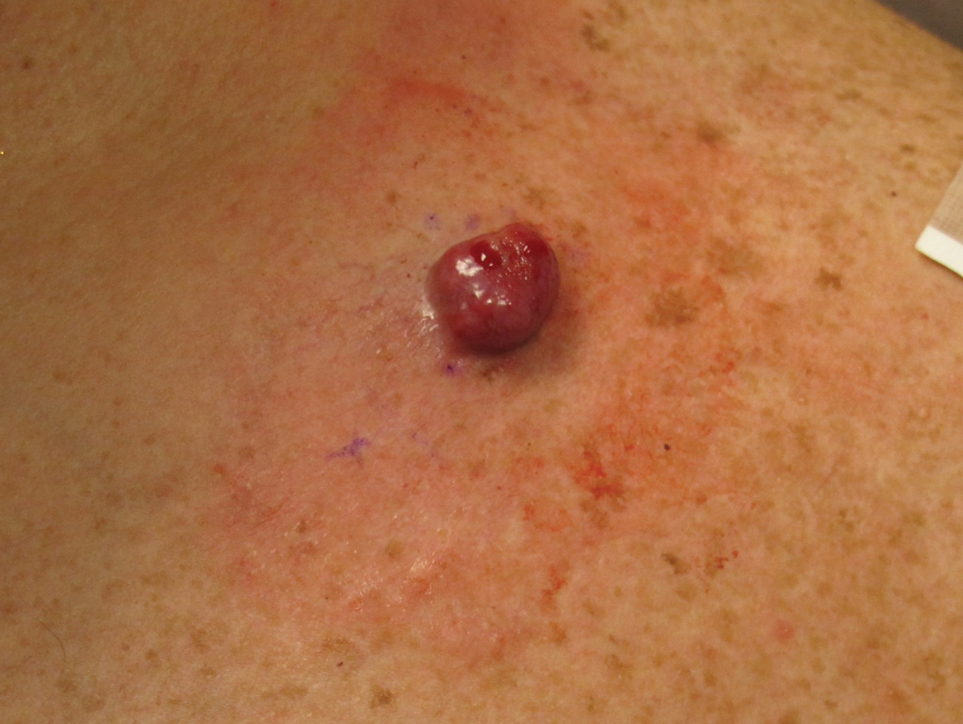
A 45-year-old man presented to the dermatology clinic with a bleeding nodule on the upper chest of 2 months’ duration. He had a history of a low-grade mucoepidermoid carcinoma of the left parotid gland that was diagnosed 14 years prior and was treated via parotidectomy with 1 positive lymph node removed. Two months prior to the current presentation, the patient presented to the emergency department with unintentional weight loss and fatigue and subsequently was diagnosed with clear cell renal cell carcinoma that was treated via radical nephrectomy.
At the current presentation, the patient denied any recent fatigue, fever, weight loss, shortness of breath, or abdominal pain but reported neck stiffness. Physical examination revealed a solitary, smooth, vascular, 1.5×1.5 cm nodule on the left upper chest with no overlying skin changes. The remainder of the skin examination was unremarkable. A shave biopsy of the nodule was performed.
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
THE DIAGNOSIS: Poroma
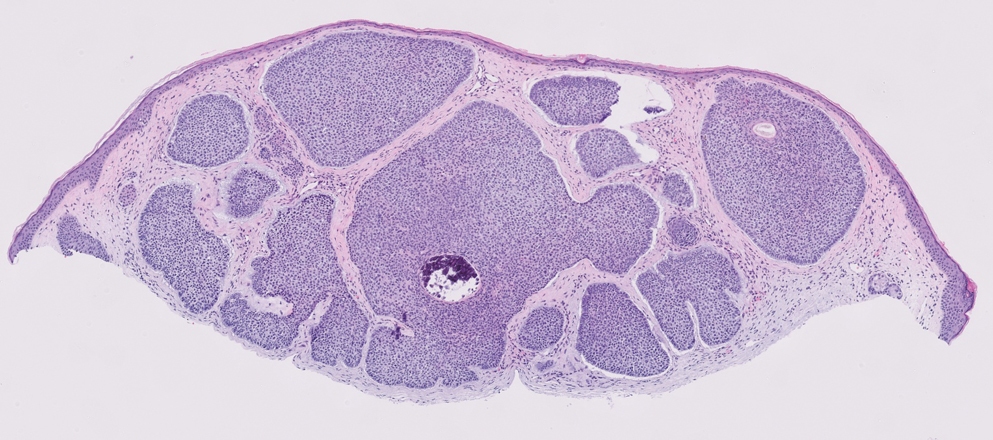
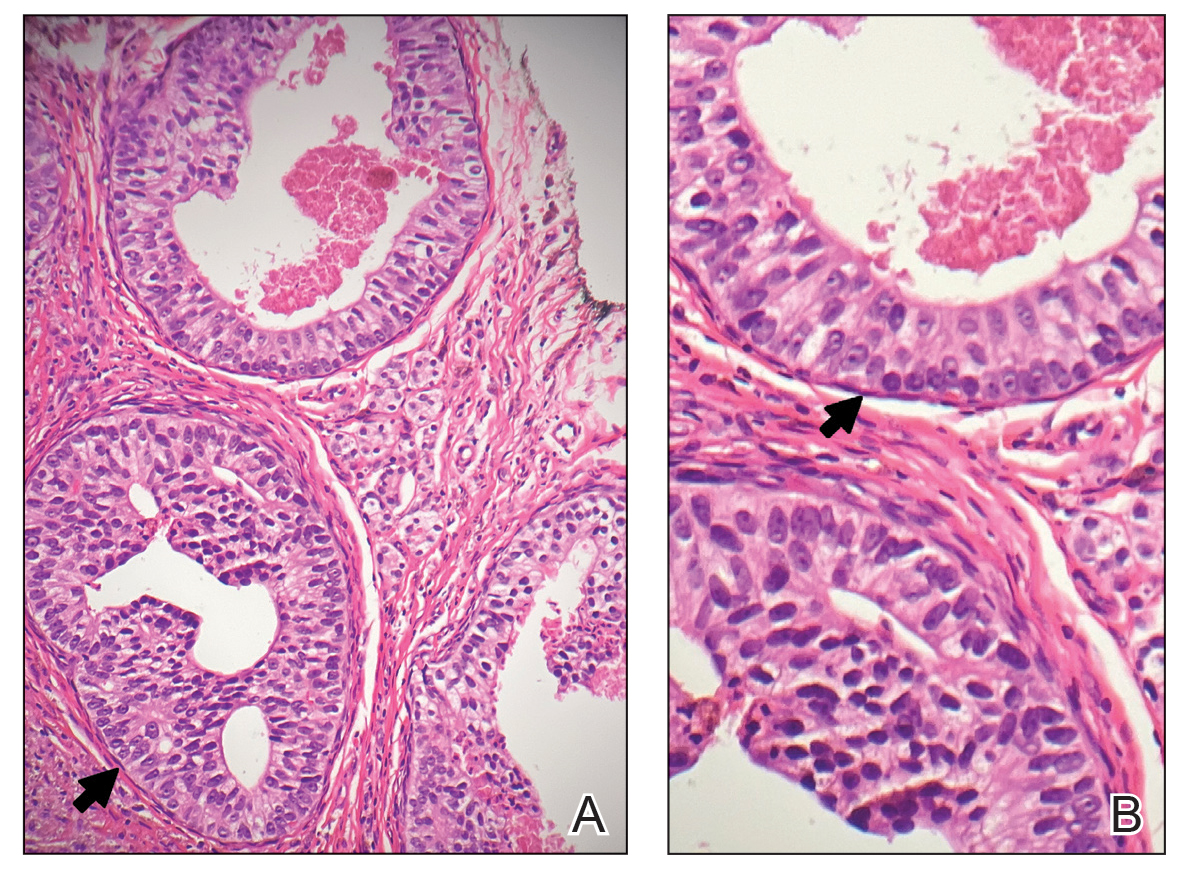
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11
Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5
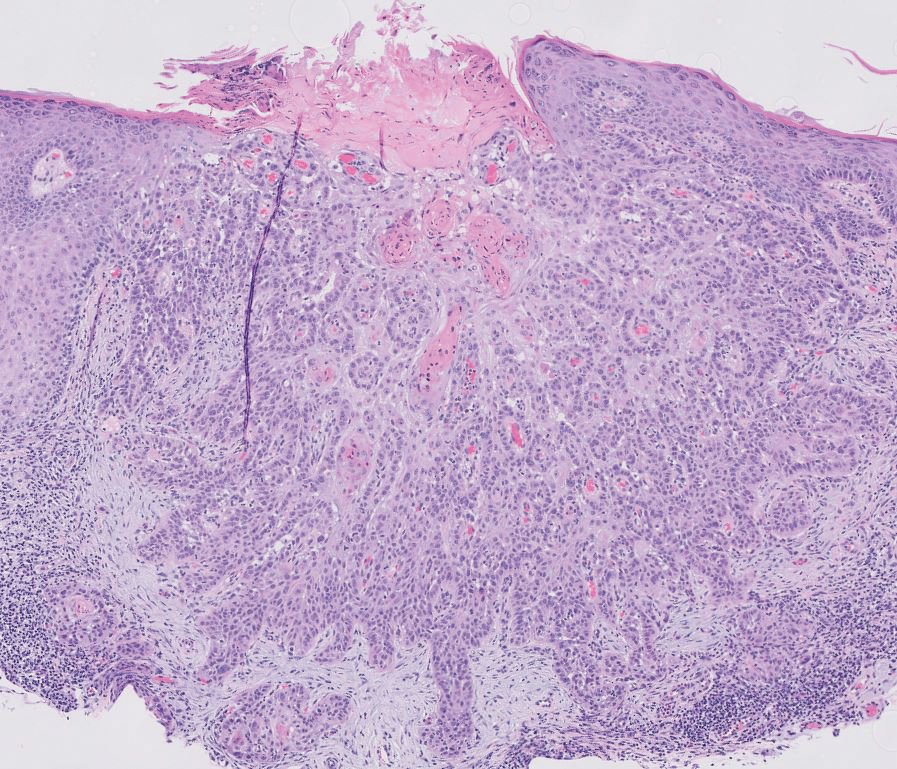
Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13
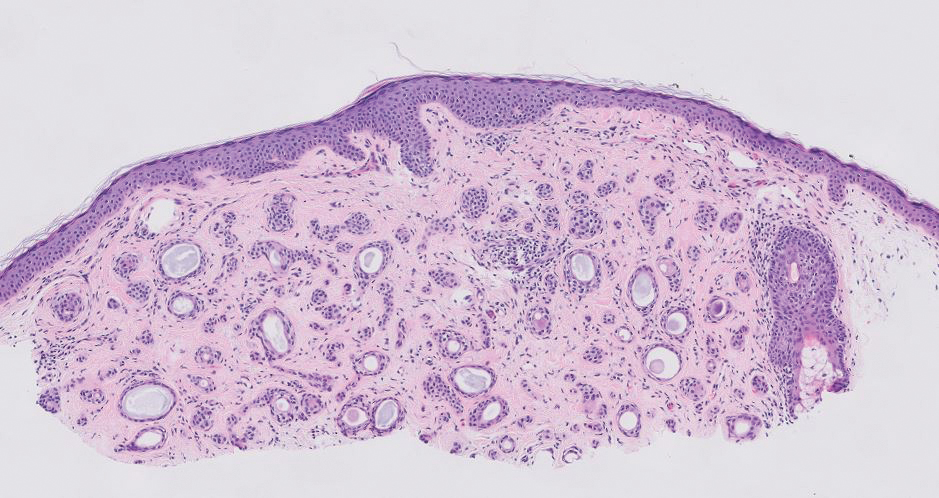
Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4
Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
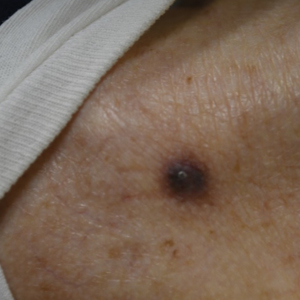
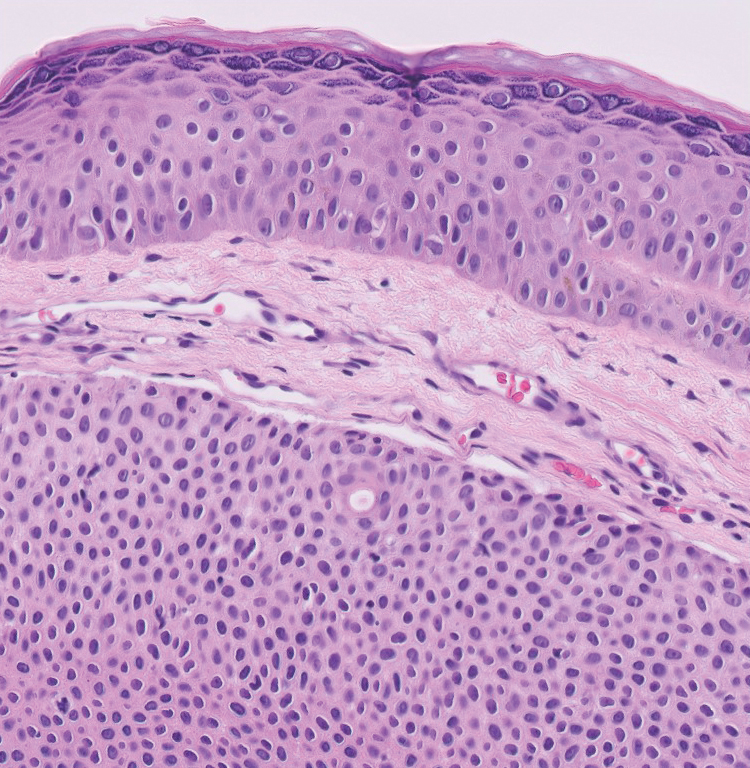
A 57-year-old man with no notable medical history presented to the dermatology clinic for evaluation of an asymptomatic papule on the left lower eyelid. The patient reported that the lesion seemed to wax and wane in size over time. Physical examination revealed a small, pink, verrucous papule on the left lower eyelid. A shave biopsy of the lesion revealed a well-circumscribed collection of small, monomorphic, cuboidal cells with basophilic round nuclei, inconspicuous nucleoli, and compact eosinophilic cytoplasm (top) with focal areas of duct formation (bottom) that was sharply demarcated from normal keratinocytes.
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
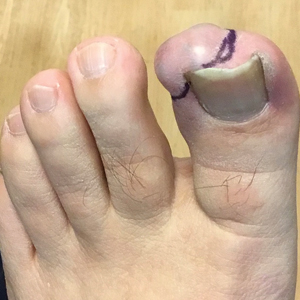
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
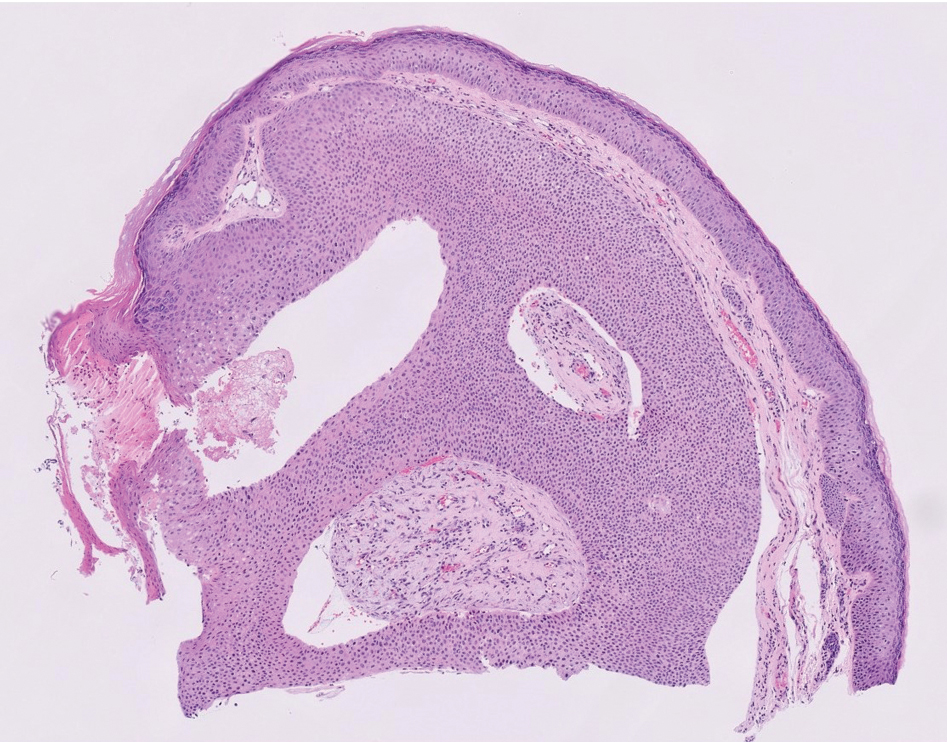
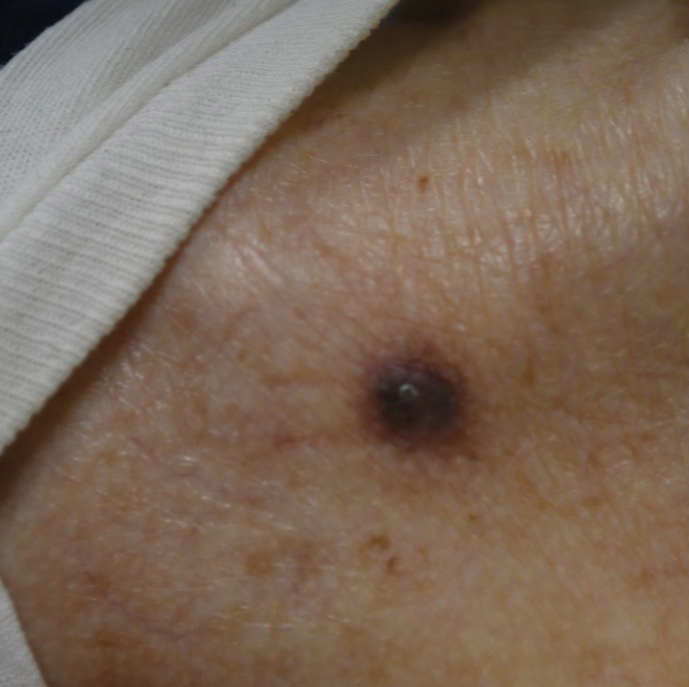
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.
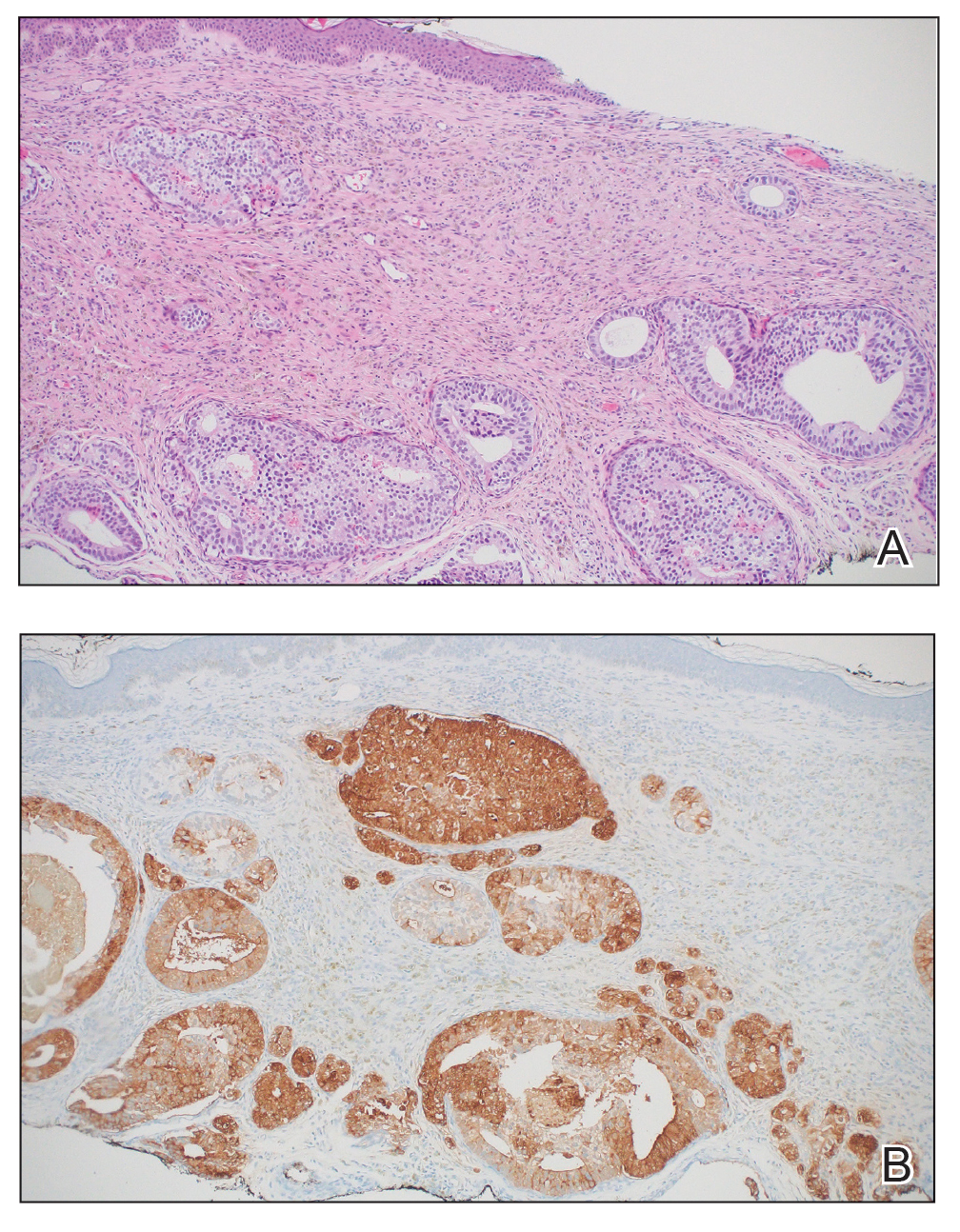
Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
PRACTICE POINTS
- Cutaneous metastasis of prostate cancer can have various manifestations and portends a poor prognosis.
- New skin lesions that develop in patients with a high clinical suspicion for prostate cancer warrant consideration of cutaneous metastasis.
Merkel Cell Carcinoma Less Common, With higher Mortality Than Melanoma
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
MRI-Invisible Prostate Lesions: Are They Dangerous?
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.
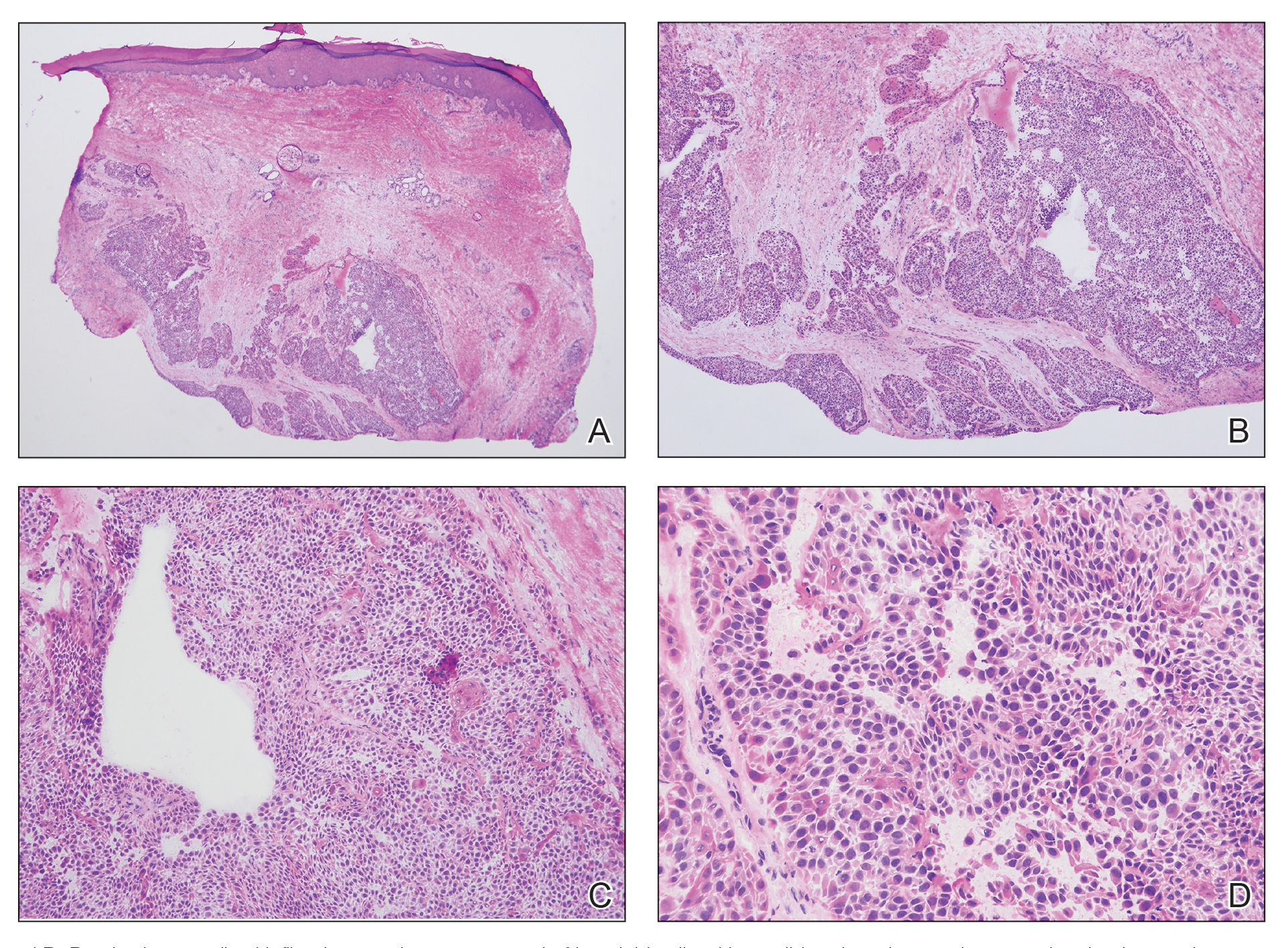
Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
A 56-year-old man was referred to the dermatology clinic for treatment of a recurrent nodule on the left first toe. The lesion first appeared 12 years prior and was resected by an outside dermatologist, who diagnosed the lesion as benign based on biopsy results. Approximately 10 years later, the lesion began to grow back with a similar appearance to the original nodule; it again was diagnosed as benign based on another biopsy and excised by the outside dermatologist. Two years later, the patient had a second recurrence of the lesion, which was excised by his dermatologist. The biopsy report at that time identified the lesion as a low-grade adnexal neoplasm. The patient had a rapid recurrence of the tumor after 6 months and was referred to our clinic for Mohs micrographic surgery. Physical examination revealed a tender, 2.5×1.8-cm, firm, exophytic, subcutaneous nodule on the left first toe with no associated lymphadenopathy.
Skin Cancer Risk Elevated Among Blood, Marrow Transplant Survivors
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves Cosibelimab for Cutaneous SCC
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
Skin Cancer Screening: Biopsy-Free Technology Advancing
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024
New Cancer Drugs: Do Patients Prefer Faster Access or Clinical Benefit?
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY