User login
Nail Alterations From Musical Instruments: Insights for Dermatologists Treating Musicians
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
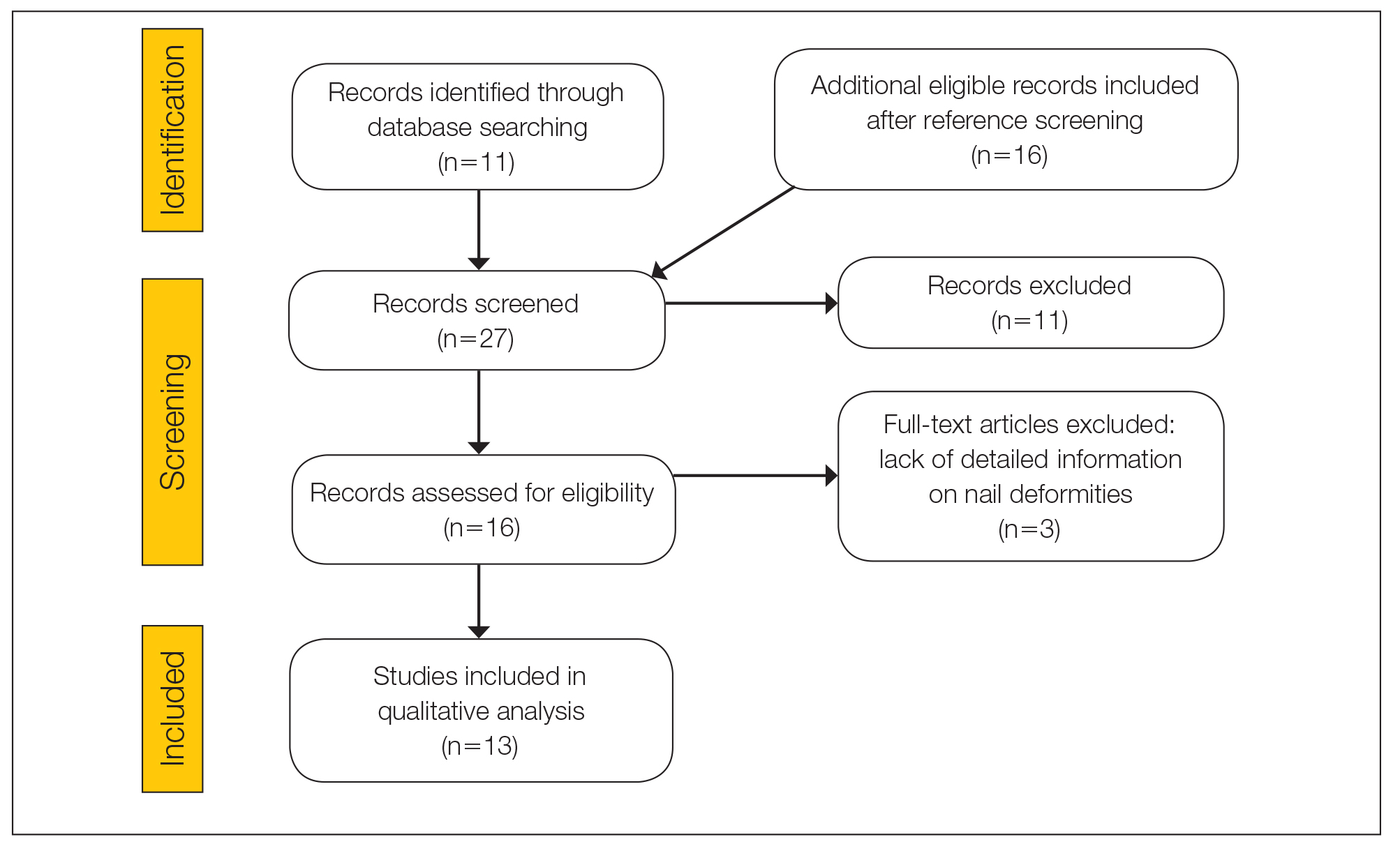
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).
Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
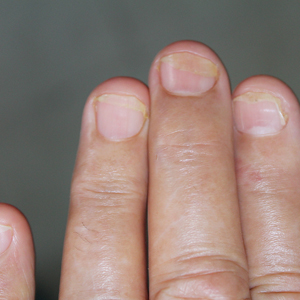
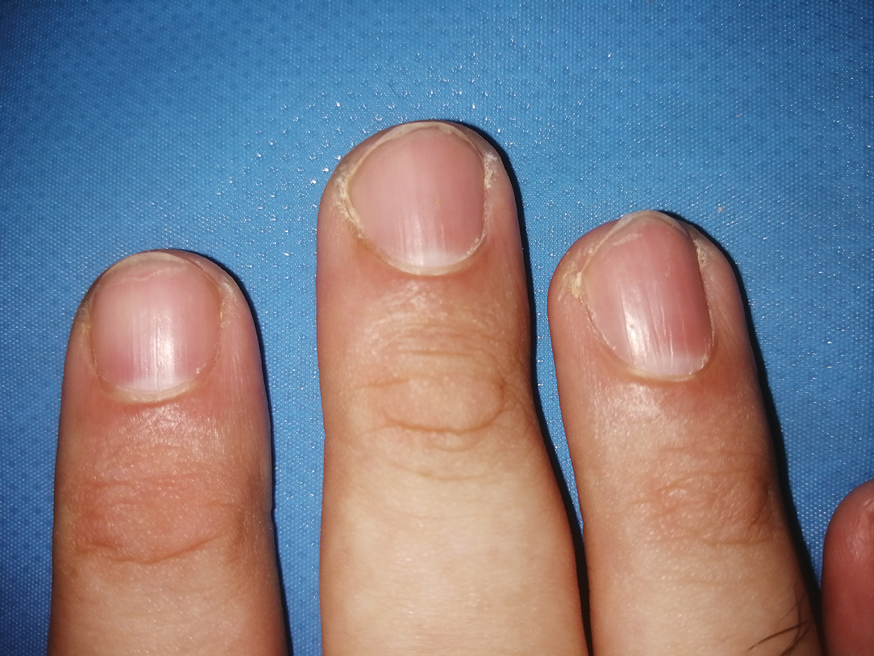
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8
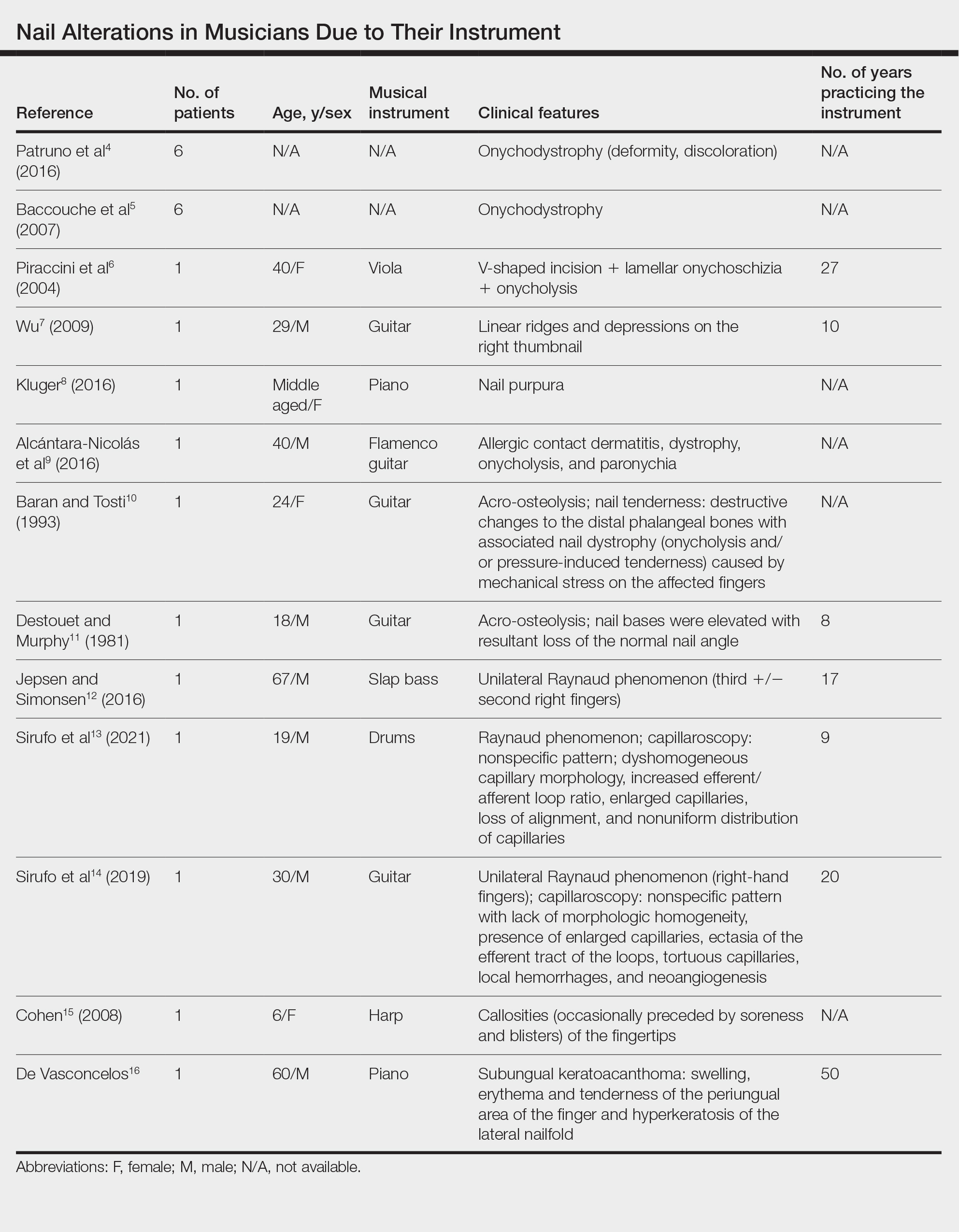
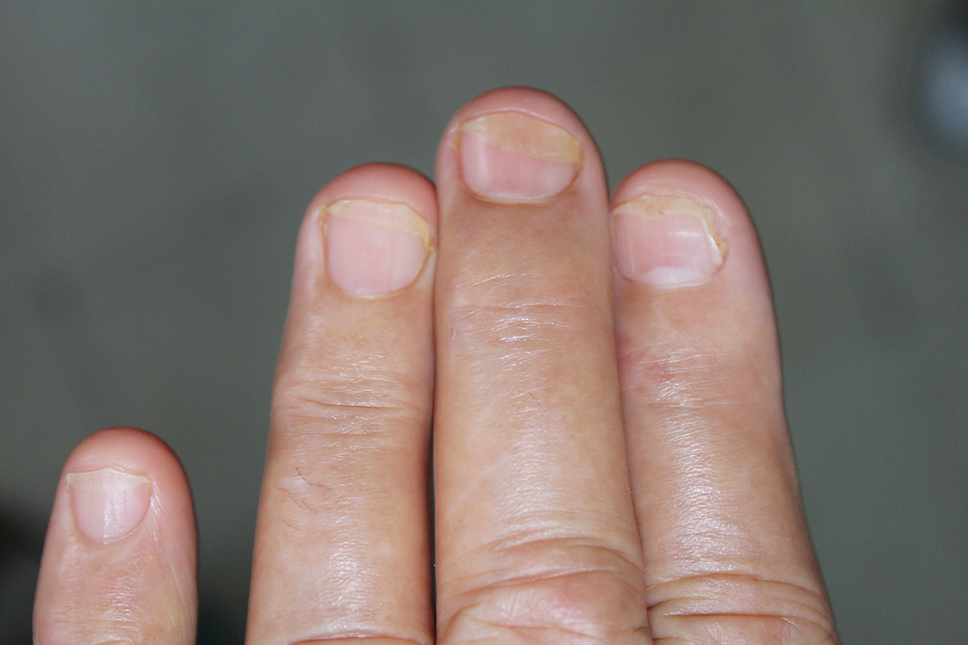
Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
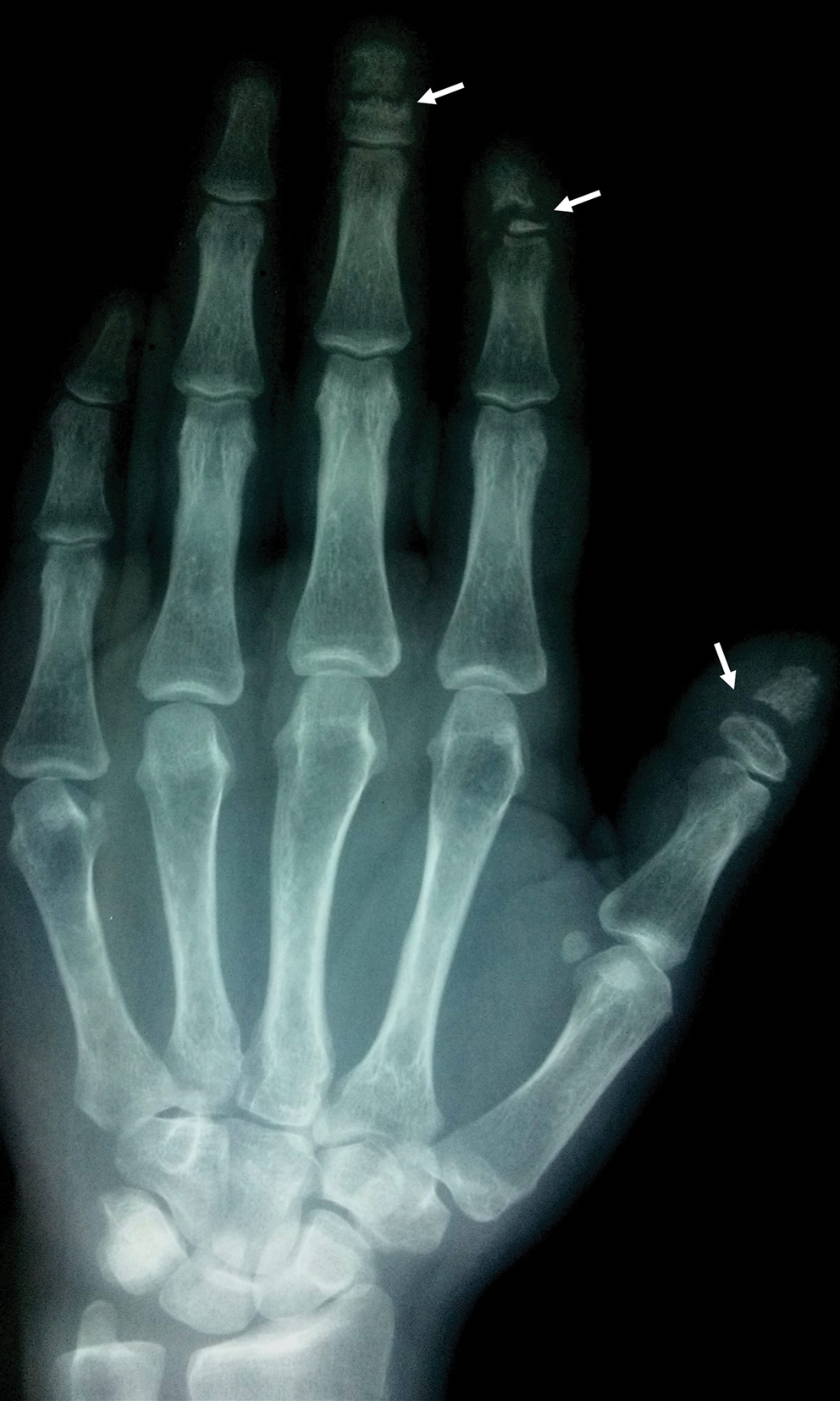
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14
A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).

Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8


Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14


A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).

Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8


Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14


A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
Practice Points
- Long-term practice and performance with a musical instrument predispose musicians to several skin conditions and nail disorders.
- Nail alterations in musicians include onychodystrophy, callosities of the fingertips, paronychia, distal onycholysis, lamellar onychoschizia, striations, subungual hemorrhage, and occupational Raynaud phenomenon.
- Nail lesions in musicians may be caused by localized pressure, friction-induced mechanical forces, allergic or irritant contact dermatitis, or infections.
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
The Comparison
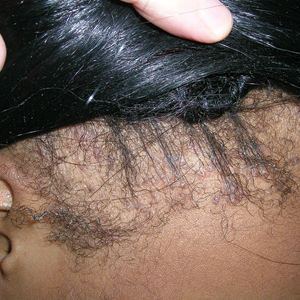
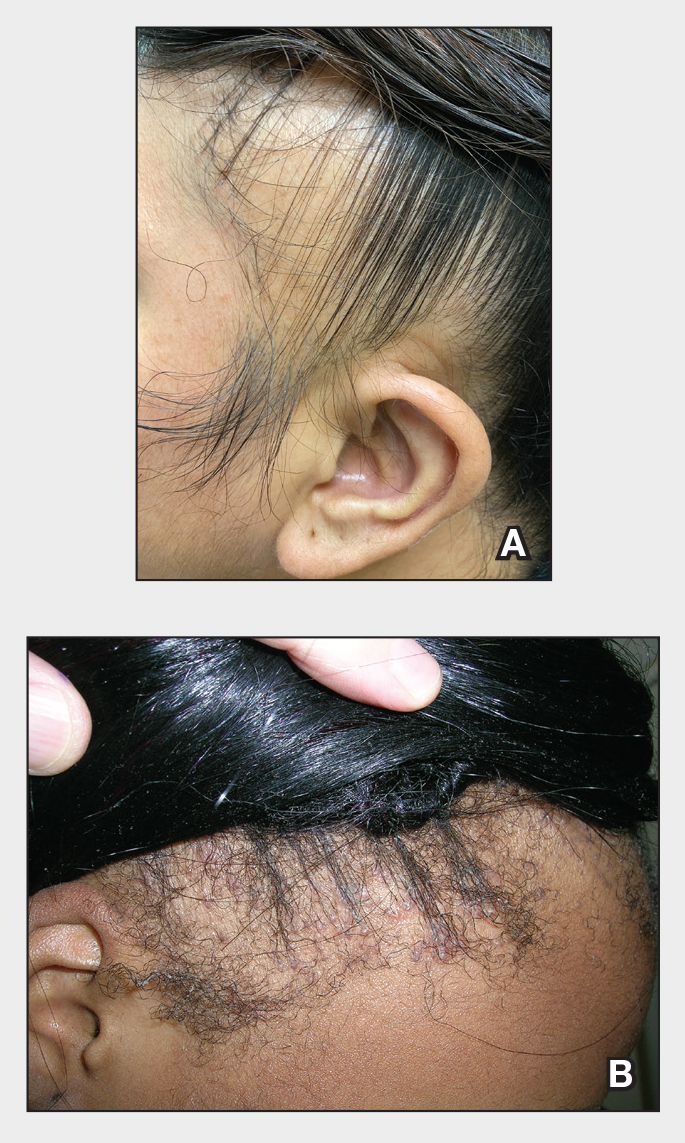
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Progressive Eyelash Loss and Scale of the Right Eyelid
The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
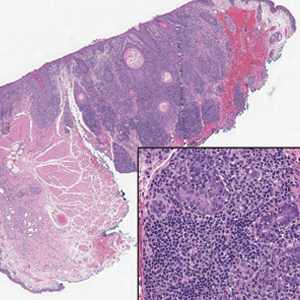
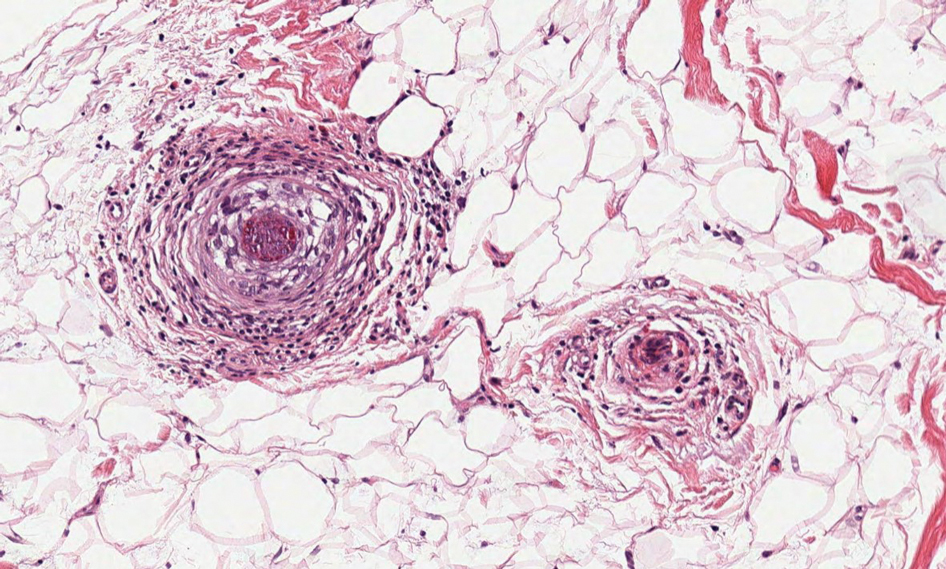
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
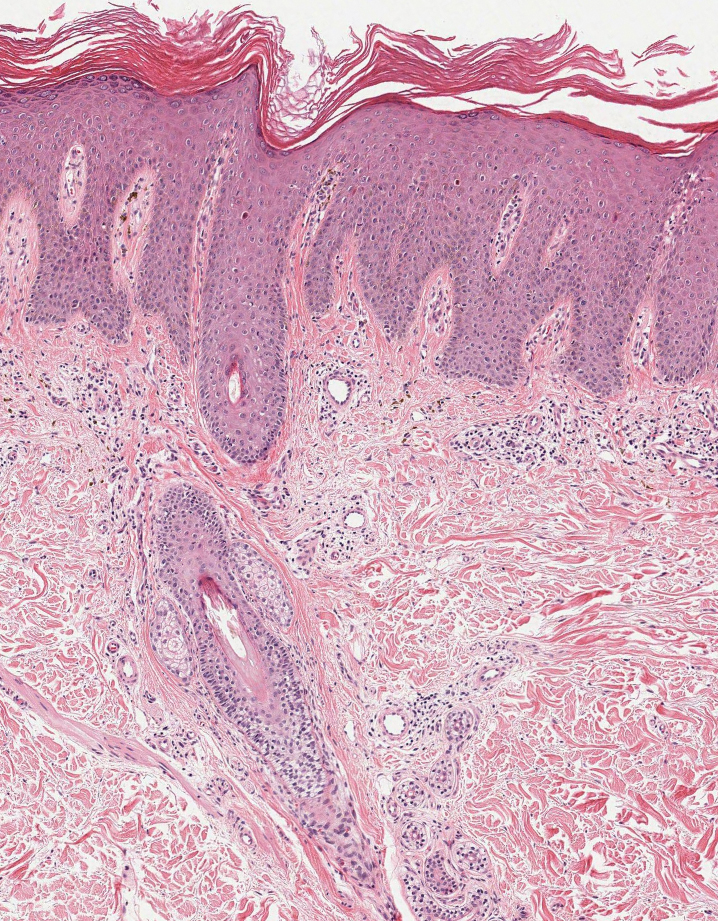
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.
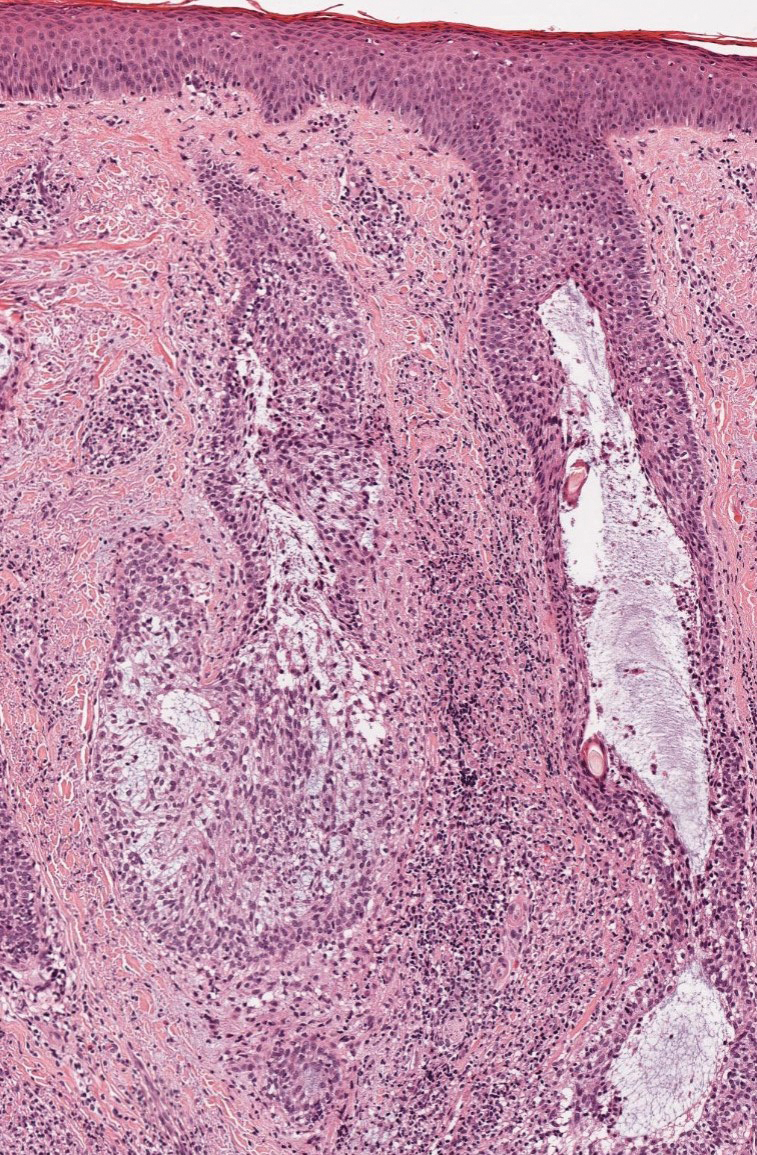
Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18
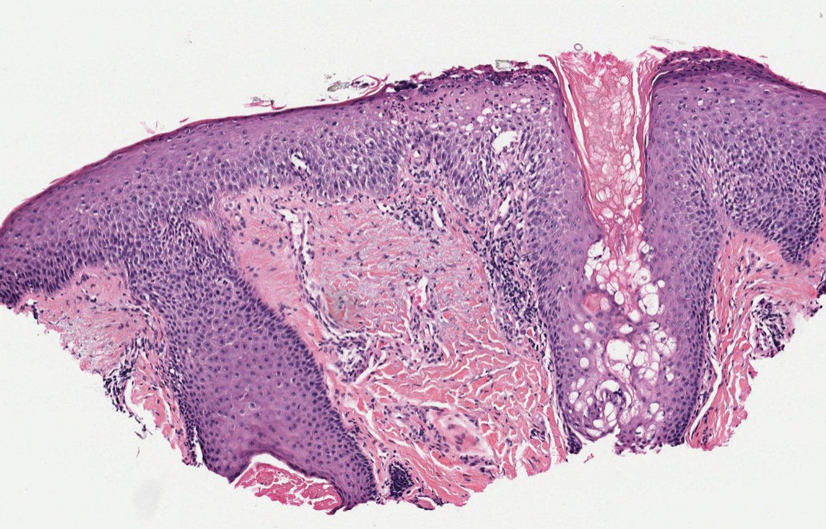
Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.

Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18

Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.


The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.

Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18

Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.


- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
An 88-year-old man presented with progressive eyelash loss and scale involving the right eyelids (top). Dermatopathologic examination was performed (bottom).
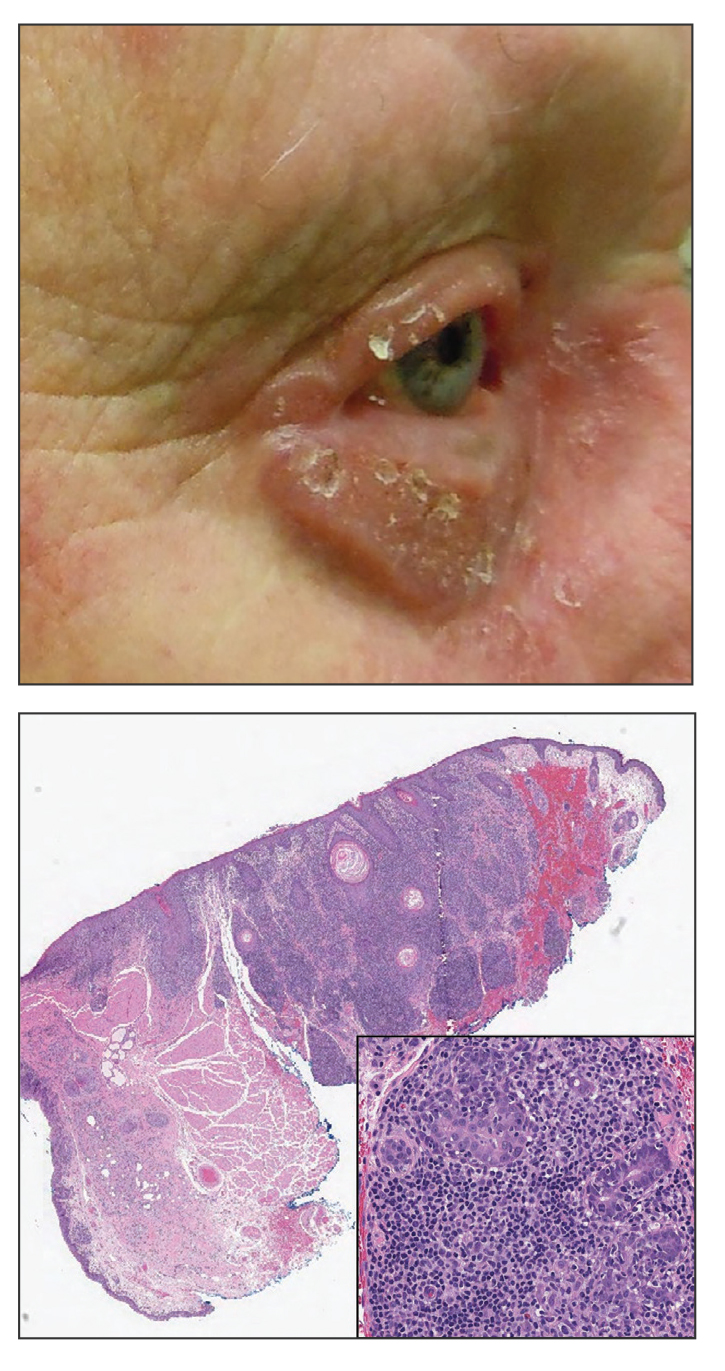
Pigmented Lesion on the Left Shoulder in an Older Woman
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.
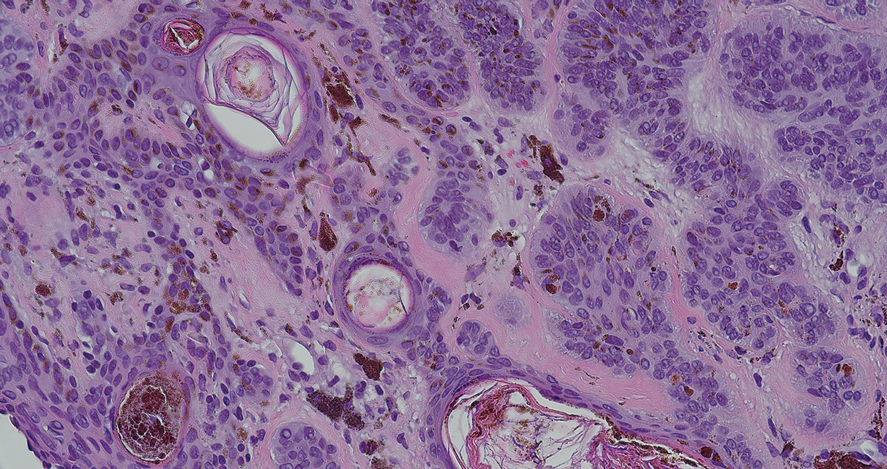
Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
A 92-year-old woman presented to dermatology as a new patient for a full-body skin examination. She had a history of sarcoidosis and a liposarcoma that had been excised more than 20 years prior. She had no history of skin cancer; however, her granddaughter recently was diagnosed with melanoma. Physical examination revealed a 5-mm, irregular, dark brown papule on the left shoulder (top) that was evaluated by dermoscopy (middle). A tangential biopsy was performed for histopathologic analysis (bottom).
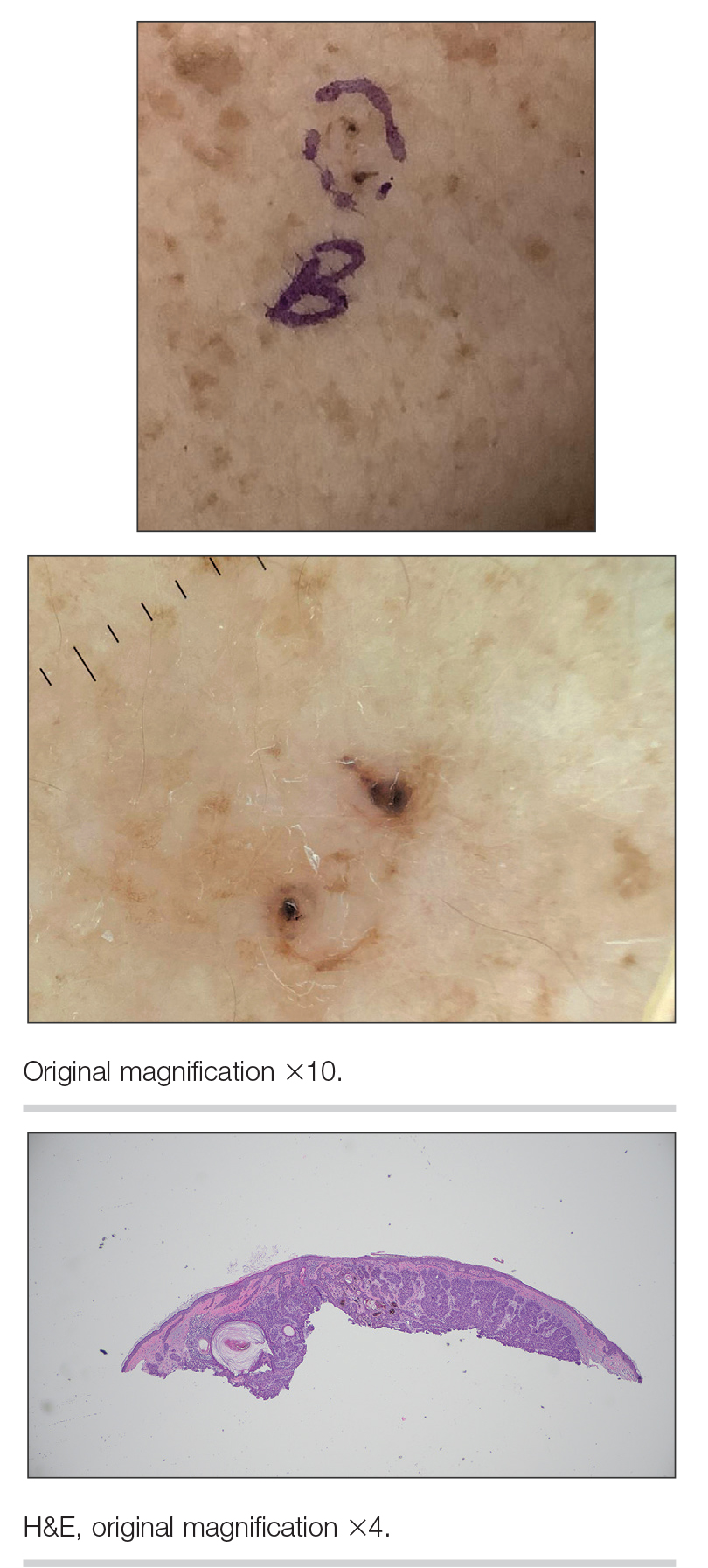
Trifluridine/tipiracil Plus Bevacizumab: A Game Changer in Late-Stage Refractory mCRC
An elderly gentleman was truly suffering, so his doctor decided to try something new.
“He’d had a number of cumulative side effects after almost two years of IV chemotherapy for his metastatic colon cancer,” said Anuj Patel, MD, a senior physician at Dana-Farber Cancer Institute in Boston, recalling his patient. “When we switched him to combination treatment with trifluridine/tipiracil and bevacizumab, he constantly remarked on how well he now felt. He described no side effects from this new regimen.”
Trifluridine/tipiracil (Lonsurf) had been used to treat advanced gastric cancer, while bevacizumab had been therapeutic for a wider range of diseases, including cervical, brain, liver, kidney, gynecological and lung cancers. Used together for treating refractory mCRC, well-known initial findings about their effectiveness have been proven true over time.
“Patients taking both drugs can experience, on average, a life extension of three months,” said Richard M. Goldberg, MD, professor emeritus of the West Virginia University Cancer Institute and director of Fight Colorectal Cancer.
The History of the Combined Therapy’s Approval
The FDA originally approved trifluridine/tipiracil in September 2015 for use in patients with metastatic colorectal cancer. Patients eligible to take it had to have been treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-vascular endothelial growth factor (VEGF) biological therapy, and—if RAS wild-type—an anti-epidermal growth factor receptor (EGFR) therapy, according to data published by the National Center for Biotechnology Information. The FDA’s August 2023 approval of the trifluridine/tipiracil and bevacizumab combination regimen is for patients meeting the same eligibility requirements.
Another drug, regorafenib, had already been approved by the FDA in September 2012 to treat mCRC. The drug has a wide range of potential side effects, however, including complications relating to the limbs.
“One of my patients tried regorafenib as his initial third- line treatment,” Dr. Goldberg said. “I checked in on him at his farm, and he was sitting in the barn near his tractor.
He had such severe hand-foot syndrome that he could barely walk.”
Trifluridine/tipiracil alone proved to be very helpful in this case. “We switched him to it, and he tolerated it well,” Dr. Goldberg continued. “He got his fields plowed and was on it for months before he passed away. We both felt it kept him going longer.”
A new research review confirms the regimen’s success, determining that trifluridine/tipiracil plus bevacizumab was associated with improved outcomes compared to therapy solely with trifluridine/tipiracil.
A True Practice Changer
Now that the regimen has been on the market for more than half a year, there are longer-term data available.
Patients on average live within the same timeframe as the patients in the SUNLIGHT study, and many feel physically better on the therapy. “The combination has very quickly shifted the standard of care,” Dr. Goldberg said.
The regimen can also provide significant psychological benefits to patients.
“As patients can maintain good performance status for longer with the combination, it increases the perception of quality of life,” said Jacobo Hincapie-Echeverri, MD, a GI and geriatric oncologist at Orlando Health Cancer Institute in Orlando, Florida.
The regimen is unique too, in that it can help doctors plan additional treatment strategies.
“This current approval, for the combination of trifluridine/tipiracil and bevacizumab, is practice-changing in that it helps clarify the sequence for later treatments for patients with mCRC,” said Dr. Patel, who is also clinical director of the Center for Esophageal and Gastric Cancer and assistant professor of medicine at Harvard Medical School, Boston. “Previously, it had been difficult to decide between trifluridine/tipiracil and regorafenib in this setting.”
The fact that the regimen has been shown to give time and improved quality of life to patients in ways regorafenib does not is clarifying. “Now, with the improved outcomes seen, I do think that trifluridine/tipiracil plus bevacizumab is the better option for most mCRC patients after IV chemotherapies,” Dr. Patel added.
When it comes to his specific experience with prescribing the regimen for his patients, Dr. Patel reported that it’s easier on his patients than other therapies.
“I find that it is generally well tolerated,” he elaborated. “As an oral agent, it is also usually somewhat easier to take (than other delivery methods of medication). These factors are critical for patients who have likely already had at least 2 or 3 prior lines of chemotherapy. I have had many patients with mCRC who, after disease progression on prior IV chemotherapy regimens, have had periods of meaningful disease control – often with fewer and manageable side effects.”
Dr. Goldberg mentioned another benefit.
“The nice thing about the combination of trifluridine/tipiracil and bevacizumab is that in terms of toxicity, there’s very little difference compared to the toxicity of trifluridine/tipiracil used alone.”
Are There Downsides to the Regimen?
The pros are obvious, but the regimen has some cons as well. Medically, patients should have a platelet count over 75,000/mm3 and absolute neutrophil count (ANC) over 1,500/mm3 prior to the start of each cycle, and their liver and renal function should be monitored.
Patients with metastatic colorectal cancer must be also carefully monitored for hematologic adverse events (AEs) , including chemotherapy-associated neutropenia. Biweekly treatments may reduce the risk of AEs as a whole, however, according to research.
The regimen is also expensive – an approximate cost of $8,191 for a 28-day supply. According to a new study, patients managing both AE expenses along with the cost of trifluridine/tipiracil-bevacizumab face a monthly bill of about $17,179.
Some very good news, though: 100% of Medicare drug plans cover trifluridine/tipiracil, with an average copay of $57-$292. Bevacizumab is also covered by Medicare, with a copay as low as $0-$25.
Private insurers do cover the drugs, depending on a patient’s specific plan. However, if a patient’s claim is denied, financial assistance for trifluridine/tipiracil through the drug’s manufacturers may be available for some patients, reducing prescriptions to a zero cost in some cases. Bevacizumab can be made available to patients who may not have health insurance at all, too. Patients can use a financial assistance tool through the drug’s manufacturer to receive up to $25,000 in yearly copay assistance.
What Does the Latest Research on the Regimen Indicate?
In May 2024, two abstracts were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) that explored expanded possible use of trifluridine/tipiracil plus bevacizumab as a treatment for metastatic colorectal cancer.
The first abstract studied trifluridine/tipiracil plus bevacizumab as upfront treatment for mCRC, adding capecitabine to the regimen.
“It’s a phase 1 study looking at dose findings for the three-drug combination, where the active drug is a chemotherapy agent classified as a fluoropyrimidine ... I would characterize this as a study combining two [fluoropyrimidines] with a single targeted therapy,” Dr. Goldberg said.
“Combining two fluoropyrimidines is an unusual approach, because they tend to have overlapping side effects, and the potential is there for either innate drug resistance to the class of drugs or that the combination of two agents that work by a similar mechanism of action could hasten the development of acquired drug resistance. There is apparently a signal that combining the two chemotherapy agents enhances each other’s activity in cell culture and animal models,” he added.
Ultimately, Dr. Goldberg said he thinks more evidence is needed to prove the regimen’s effectiveness.
“This is a very early study and really provides no information about its potential given that no response data was presented,” he added. “While this is an interesting idea, it is unclear if it will pan out until we see the data on the Phase II study in progress.”
The other abstract looked at the impact of colorectal liver metastases in patients with mCRC who in phase 3 of the SUNLIGHT trial received trifluridine/tipiracil with or without bevacizumab.
“There is not much that is novel here,” Dr. Goldberg said. “The retrospective analysis shows that trifluridine/tipiracil plus bevacizumab is better than trifluridine/tipiracil alone in the subset of patients with liver metastases, as it was shown to be in the entire patient population. While this is reassuring, it’s not unexpected, especially since the vast majority of people enrolled in the SUNLIGHT trial had liver metastases.”
The Bottom Line
In the future, the potential exists for trifluridine/tipiracil combined with bevacizumab to work in first-line and second-line patients.
“Seventy percent of colorectal cancer patients reach second line treatment right now, but only 30% reach third line treatment — either they become too sick to continue, or choose not to,” Dr. Goldberg said. “The hope is that using these drugs earlier can help more patients reach and prolong treatment.”
It’s also possible that the regimen can be applied in new ways.
“Further research combining trifluridine/tipiracil and bevacizumab with other targeted therapies could yield additional advances for refractory mCRC patients,” Dr. Hincapie-Echeverri said. “The survival benefit of this therapy reinforces the importance of continuing to develop new therapies to improve outcomes in the refractory mCRC setting.”
Dr. Patel’s patient felt lucky to simply live a longer life.
Because of the regimen, “his cancer remained stable for approximately 8 months. Upon its progression, he chose not to pursue any further chemotherapy. He instead expressed his gratitude at having been able to feel more like himself for nearly a year.”
Dr. Patel received research funding in 2017 from Taiho, which manufactures trifluridine/tipiracil. He receives no current funding from Taiho and has no additional conflicts of interest. Dr. Goldberg helped represent Taiho in a patent law dispute regarding Lonsurf for which he was paid, but he is no longer paid by the company. Dr. Hincapie-Echeverri is a speaker for Astellas Pharma, which does not manufacture trifluridine/tipiracil or bevacizumab, and he has no additional conflicts of interest.
An elderly gentleman was truly suffering, so his doctor decided to try something new.
“He’d had a number of cumulative side effects after almost two years of IV chemotherapy for his metastatic colon cancer,” said Anuj Patel, MD, a senior physician at Dana-Farber Cancer Institute in Boston, recalling his patient. “When we switched him to combination treatment with trifluridine/tipiracil and bevacizumab, he constantly remarked on how well he now felt. He described no side effects from this new regimen.”
Trifluridine/tipiracil (Lonsurf) had been used to treat advanced gastric cancer, while bevacizumab had been therapeutic for a wider range of diseases, including cervical, brain, liver, kidney, gynecological and lung cancers. Used together for treating refractory mCRC, well-known initial findings about their effectiveness have been proven true over time.
“Patients taking both drugs can experience, on average, a life extension of three months,” said Richard M. Goldberg, MD, professor emeritus of the West Virginia University Cancer Institute and director of Fight Colorectal Cancer.
The History of the Combined Therapy’s Approval
The FDA originally approved trifluridine/tipiracil in September 2015 for use in patients with metastatic colorectal cancer. Patients eligible to take it had to have been treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-vascular endothelial growth factor (VEGF) biological therapy, and—if RAS wild-type—an anti-epidermal growth factor receptor (EGFR) therapy, according to data published by the National Center for Biotechnology Information. The FDA’s August 2023 approval of the trifluridine/tipiracil and bevacizumab combination regimen is for patients meeting the same eligibility requirements.
Another drug, regorafenib, had already been approved by the FDA in September 2012 to treat mCRC. The drug has a wide range of potential side effects, however, including complications relating to the limbs.
“One of my patients tried regorafenib as his initial third- line treatment,” Dr. Goldberg said. “I checked in on him at his farm, and he was sitting in the barn near his tractor.
He had such severe hand-foot syndrome that he could barely walk.”
Trifluridine/tipiracil alone proved to be very helpful in this case. “We switched him to it, and he tolerated it well,” Dr. Goldberg continued. “He got his fields plowed and was on it for months before he passed away. We both felt it kept him going longer.”
A new research review confirms the regimen’s success, determining that trifluridine/tipiracil plus bevacizumab was associated with improved outcomes compared to therapy solely with trifluridine/tipiracil.
A True Practice Changer
Now that the regimen has been on the market for more than half a year, there are longer-term data available.
Patients on average live within the same timeframe as the patients in the SUNLIGHT study, and many feel physically better on the therapy. “The combination has very quickly shifted the standard of care,” Dr. Goldberg said.
The regimen can also provide significant psychological benefits to patients.
“As patients can maintain good performance status for longer with the combination, it increases the perception of quality of life,” said Jacobo Hincapie-Echeverri, MD, a GI and geriatric oncologist at Orlando Health Cancer Institute in Orlando, Florida.
The regimen is unique too, in that it can help doctors plan additional treatment strategies.
“This current approval, for the combination of trifluridine/tipiracil and bevacizumab, is practice-changing in that it helps clarify the sequence for later treatments for patients with mCRC,” said Dr. Patel, who is also clinical director of the Center for Esophageal and Gastric Cancer and assistant professor of medicine at Harvard Medical School, Boston. “Previously, it had been difficult to decide between trifluridine/tipiracil and regorafenib in this setting.”
The fact that the regimen has been shown to give time and improved quality of life to patients in ways regorafenib does not is clarifying. “Now, with the improved outcomes seen, I do think that trifluridine/tipiracil plus bevacizumab is the better option for most mCRC patients after IV chemotherapies,” Dr. Patel added.
When it comes to his specific experience with prescribing the regimen for his patients, Dr. Patel reported that it’s easier on his patients than other therapies.
“I find that it is generally well tolerated,” he elaborated. “As an oral agent, it is also usually somewhat easier to take (than other delivery methods of medication). These factors are critical for patients who have likely already had at least 2 or 3 prior lines of chemotherapy. I have had many patients with mCRC who, after disease progression on prior IV chemotherapy regimens, have had periods of meaningful disease control – often with fewer and manageable side effects.”
Dr. Goldberg mentioned another benefit.
“The nice thing about the combination of trifluridine/tipiracil and bevacizumab is that in terms of toxicity, there’s very little difference compared to the toxicity of trifluridine/tipiracil used alone.”
Are There Downsides to the Regimen?
The pros are obvious, but the regimen has some cons as well. Medically, patients should have a platelet count over 75,000/mm3 and absolute neutrophil count (ANC) over 1,500/mm3 prior to the start of each cycle, and their liver and renal function should be monitored.
Patients with metastatic colorectal cancer must be also carefully monitored for hematologic adverse events (AEs) , including chemotherapy-associated neutropenia. Biweekly treatments may reduce the risk of AEs as a whole, however, according to research.
The regimen is also expensive – an approximate cost of $8,191 for a 28-day supply. According to a new study, patients managing both AE expenses along with the cost of trifluridine/tipiracil-bevacizumab face a monthly bill of about $17,179.
Some very good news, though: 100% of Medicare drug plans cover trifluridine/tipiracil, with an average copay of $57-$292. Bevacizumab is also covered by Medicare, with a copay as low as $0-$25.
Private insurers do cover the drugs, depending on a patient’s specific plan. However, if a patient’s claim is denied, financial assistance for trifluridine/tipiracil through the drug’s manufacturers may be available for some patients, reducing prescriptions to a zero cost in some cases. Bevacizumab can be made available to patients who may not have health insurance at all, too. Patients can use a financial assistance tool through the drug’s manufacturer to receive up to $25,000 in yearly copay assistance.
What Does the Latest Research on the Regimen Indicate?
In May 2024, two abstracts were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) that explored expanded possible use of trifluridine/tipiracil plus bevacizumab as a treatment for metastatic colorectal cancer.
The first abstract studied trifluridine/tipiracil plus bevacizumab as upfront treatment for mCRC, adding capecitabine to the regimen.
“It’s a phase 1 study looking at dose findings for the three-drug combination, where the active drug is a chemotherapy agent classified as a fluoropyrimidine ... I would characterize this as a study combining two [fluoropyrimidines] with a single targeted therapy,” Dr. Goldberg said.
“Combining two fluoropyrimidines is an unusual approach, because they tend to have overlapping side effects, and the potential is there for either innate drug resistance to the class of drugs or that the combination of two agents that work by a similar mechanism of action could hasten the development of acquired drug resistance. There is apparently a signal that combining the two chemotherapy agents enhances each other’s activity in cell culture and animal models,” he added.
Ultimately, Dr. Goldberg said he thinks more evidence is needed to prove the regimen’s effectiveness.
“This is a very early study and really provides no information about its potential given that no response data was presented,” he added. “While this is an interesting idea, it is unclear if it will pan out until we see the data on the Phase II study in progress.”
The other abstract looked at the impact of colorectal liver metastases in patients with mCRC who in phase 3 of the SUNLIGHT trial received trifluridine/tipiracil with or without bevacizumab.
“There is not much that is novel here,” Dr. Goldberg said. “The retrospective analysis shows that trifluridine/tipiracil plus bevacizumab is better than trifluridine/tipiracil alone in the subset of patients with liver metastases, as it was shown to be in the entire patient population. While this is reassuring, it’s not unexpected, especially since the vast majority of people enrolled in the SUNLIGHT trial had liver metastases.”
The Bottom Line
In the future, the potential exists for trifluridine/tipiracil combined with bevacizumab to work in first-line and second-line patients.
“Seventy percent of colorectal cancer patients reach second line treatment right now, but only 30% reach third line treatment — either they become too sick to continue, or choose not to,” Dr. Goldberg said. “The hope is that using these drugs earlier can help more patients reach and prolong treatment.”
It’s also possible that the regimen can be applied in new ways.
“Further research combining trifluridine/tipiracil and bevacizumab with other targeted therapies could yield additional advances for refractory mCRC patients,” Dr. Hincapie-Echeverri said. “The survival benefit of this therapy reinforces the importance of continuing to develop new therapies to improve outcomes in the refractory mCRC setting.”
Dr. Patel’s patient felt lucky to simply live a longer life.
Because of the regimen, “his cancer remained stable for approximately 8 months. Upon its progression, he chose not to pursue any further chemotherapy. He instead expressed his gratitude at having been able to feel more like himself for nearly a year.”
Dr. Patel received research funding in 2017 from Taiho, which manufactures trifluridine/tipiracil. He receives no current funding from Taiho and has no additional conflicts of interest. Dr. Goldberg helped represent Taiho in a patent law dispute regarding Lonsurf for which he was paid, but he is no longer paid by the company. Dr. Hincapie-Echeverri is a speaker for Astellas Pharma, which does not manufacture trifluridine/tipiracil or bevacizumab, and he has no additional conflicts of interest.
An elderly gentleman was truly suffering, so his doctor decided to try something new.
“He’d had a number of cumulative side effects after almost two years of IV chemotherapy for his metastatic colon cancer,” said Anuj Patel, MD, a senior physician at Dana-Farber Cancer Institute in Boston, recalling his patient. “When we switched him to combination treatment with trifluridine/tipiracil and bevacizumab, he constantly remarked on how well he now felt. He described no side effects from this new regimen.”
Trifluridine/tipiracil (Lonsurf) had been used to treat advanced gastric cancer, while bevacizumab had been therapeutic for a wider range of diseases, including cervical, brain, liver, kidney, gynecological and lung cancers. Used together for treating refractory mCRC, well-known initial findings about their effectiveness have been proven true over time.
“Patients taking both drugs can experience, on average, a life extension of three months,” said Richard M. Goldberg, MD, professor emeritus of the West Virginia University Cancer Institute and director of Fight Colorectal Cancer.
The History of the Combined Therapy’s Approval
The FDA originally approved trifluridine/tipiracil in September 2015 for use in patients with metastatic colorectal cancer. Patients eligible to take it had to have been treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-vascular endothelial growth factor (VEGF) biological therapy, and—if RAS wild-type—an anti-epidermal growth factor receptor (EGFR) therapy, according to data published by the National Center for Biotechnology Information. The FDA’s August 2023 approval of the trifluridine/tipiracil and bevacizumab combination regimen is for patients meeting the same eligibility requirements.
Another drug, regorafenib, had already been approved by the FDA in September 2012 to treat mCRC. The drug has a wide range of potential side effects, however, including complications relating to the limbs.
“One of my patients tried regorafenib as his initial third- line treatment,” Dr. Goldberg said. “I checked in on him at his farm, and he was sitting in the barn near his tractor.
He had such severe hand-foot syndrome that he could barely walk.”
Trifluridine/tipiracil alone proved to be very helpful in this case. “We switched him to it, and he tolerated it well,” Dr. Goldberg continued. “He got his fields plowed and was on it for months before he passed away. We both felt it kept him going longer.”
A new research review confirms the regimen’s success, determining that trifluridine/tipiracil plus bevacizumab was associated with improved outcomes compared to therapy solely with trifluridine/tipiracil.
A True Practice Changer
Now that the regimen has been on the market for more than half a year, there are longer-term data available.
Patients on average live within the same timeframe as the patients in the SUNLIGHT study, and many feel physically better on the therapy. “The combination has very quickly shifted the standard of care,” Dr. Goldberg said.
The regimen can also provide significant psychological benefits to patients.
“As patients can maintain good performance status for longer with the combination, it increases the perception of quality of life,” said Jacobo Hincapie-Echeverri, MD, a GI and geriatric oncologist at Orlando Health Cancer Institute in Orlando, Florida.
The regimen is unique too, in that it can help doctors plan additional treatment strategies.
“This current approval, for the combination of trifluridine/tipiracil and bevacizumab, is practice-changing in that it helps clarify the sequence for later treatments for patients with mCRC,” said Dr. Patel, who is also clinical director of the Center for Esophageal and Gastric Cancer and assistant professor of medicine at Harvard Medical School, Boston. “Previously, it had been difficult to decide between trifluridine/tipiracil and regorafenib in this setting.”
The fact that the regimen has been shown to give time and improved quality of life to patients in ways regorafenib does not is clarifying. “Now, with the improved outcomes seen, I do think that trifluridine/tipiracil plus bevacizumab is the better option for most mCRC patients after IV chemotherapies,” Dr. Patel added.
When it comes to his specific experience with prescribing the regimen for his patients, Dr. Patel reported that it’s easier on his patients than other therapies.
“I find that it is generally well tolerated,” he elaborated. “As an oral agent, it is also usually somewhat easier to take (than other delivery methods of medication). These factors are critical for patients who have likely already had at least 2 or 3 prior lines of chemotherapy. I have had many patients with mCRC who, after disease progression on prior IV chemotherapy regimens, have had periods of meaningful disease control – often with fewer and manageable side effects.”
Dr. Goldberg mentioned another benefit.
“The nice thing about the combination of trifluridine/tipiracil and bevacizumab is that in terms of toxicity, there’s very little difference compared to the toxicity of trifluridine/tipiracil used alone.”
Are There Downsides to the Regimen?
The pros are obvious, but the regimen has some cons as well. Medically, patients should have a platelet count over 75,000/mm3 and absolute neutrophil count (ANC) over 1,500/mm3 prior to the start of each cycle, and their liver and renal function should be monitored.
Patients with metastatic colorectal cancer must be also carefully monitored for hematologic adverse events (AEs) , including chemotherapy-associated neutropenia. Biweekly treatments may reduce the risk of AEs as a whole, however, according to research.
The regimen is also expensive – an approximate cost of $8,191 for a 28-day supply. According to a new study, patients managing both AE expenses along with the cost of trifluridine/tipiracil-bevacizumab face a monthly bill of about $17,179.
Some very good news, though: 100% of Medicare drug plans cover trifluridine/tipiracil, with an average copay of $57-$292. Bevacizumab is also covered by Medicare, with a copay as low as $0-$25.
Private insurers do cover the drugs, depending on a patient’s specific plan. However, if a patient’s claim is denied, financial assistance for trifluridine/tipiracil through the drug’s manufacturers may be available for some patients, reducing prescriptions to a zero cost in some cases. Bevacizumab can be made available to patients who may not have health insurance at all, too. Patients can use a financial assistance tool through the drug’s manufacturer to receive up to $25,000 in yearly copay assistance.
What Does the Latest Research on the Regimen Indicate?
In May 2024, two abstracts were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) that explored expanded possible use of trifluridine/tipiracil plus bevacizumab as a treatment for metastatic colorectal cancer.
The first abstract studied trifluridine/tipiracil plus bevacizumab as upfront treatment for mCRC, adding capecitabine to the regimen.
“It’s a phase 1 study looking at dose findings for the three-drug combination, where the active drug is a chemotherapy agent classified as a fluoropyrimidine ... I would characterize this as a study combining two [fluoropyrimidines] with a single targeted therapy,” Dr. Goldberg said.
“Combining two fluoropyrimidines is an unusual approach, because they tend to have overlapping side effects, and the potential is there for either innate drug resistance to the class of drugs or that the combination of two agents that work by a similar mechanism of action could hasten the development of acquired drug resistance. There is apparently a signal that combining the two chemotherapy agents enhances each other’s activity in cell culture and animal models,” he added.
Ultimately, Dr. Goldberg said he thinks more evidence is needed to prove the regimen’s effectiveness.
“This is a very early study and really provides no information about its potential given that no response data was presented,” he added. “While this is an interesting idea, it is unclear if it will pan out until we see the data on the Phase II study in progress.”
The other abstract looked at the impact of colorectal liver metastases in patients with mCRC who in phase 3 of the SUNLIGHT trial received trifluridine/tipiracil with or without bevacizumab.
“There is not much that is novel here,” Dr. Goldberg said. “The retrospective analysis shows that trifluridine/tipiracil plus bevacizumab is better than trifluridine/tipiracil alone in the subset of patients with liver metastases, as it was shown to be in the entire patient population. While this is reassuring, it’s not unexpected, especially since the vast majority of people enrolled in the SUNLIGHT trial had liver metastases.”
The Bottom Line
In the future, the potential exists for trifluridine/tipiracil combined with bevacizumab to work in first-line and second-line patients.
“Seventy percent of colorectal cancer patients reach second line treatment right now, but only 30% reach third line treatment — either they become too sick to continue, or choose not to,” Dr. Goldberg said. “The hope is that using these drugs earlier can help more patients reach and prolong treatment.”
It’s also possible that the regimen can be applied in new ways.
“Further research combining trifluridine/tipiracil and bevacizumab with other targeted therapies could yield additional advances for refractory mCRC patients,” Dr. Hincapie-Echeverri said. “The survival benefit of this therapy reinforces the importance of continuing to develop new therapies to improve outcomes in the refractory mCRC setting.”
Dr. Patel’s patient felt lucky to simply live a longer life.
Because of the regimen, “his cancer remained stable for approximately 8 months. Upon its progression, he chose not to pursue any further chemotherapy. He instead expressed his gratitude at having been able to feel more like himself for nearly a year.”
Dr. Patel received research funding in 2017 from Taiho, which manufactures trifluridine/tipiracil. He receives no current funding from Taiho and has no additional conflicts of interest. Dr. Goldberg helped represent Taiho in a patent law dispute regarding Lonsurf for which he was paid, but he is no longer paid by the company. Dr. Hincapie-Echeverri is a speaker for Astellas Pharma, which does not manufacture trifluridine/tipiracil or bevacizumab, and he has no additional conflicts of interest.
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should South Park: The End of Obesity Be Required Viewing in Medical School?
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Postpartum Screening Critical for Urinary Symptoms and Related Mental Health
Bothersome urinary symptoms and incontinence at 12 months post partum are common and treatable, so screening for those symptoms as well as associated depression and anxiety is essential, write authors of a new study.
Sonia Bhandari Randhawa, MD, with the Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center in Dallas, led the study published in Urogynecology, which identified factors associated with persistent stress urinary incontinence (SUI), marked by leakage from sudden movements such as coughing or jumping; urgency UI (UUI), leakage after a sudden and intense need to urinate, even if the bladder isn’t full; and other overall bothersome urinary symptoms 1 year after delivery.
Associations by Subtype
Dr. Randhawa analyzed data provided by 419 patients (77% Hispanic White and 22% non-Hispanic Black). After multivariable analysis, SUI (n = 136, 32.5%) was significantly associated with greater body mass index (BMI) at the time of delivery and greater depression screening scores. Factors not associated included fetal birth weight, mode of delivery, degree of laceration, and breastfeeding status.
UUI (n = 69, 16.5%) was significantly associated with more births and higher anxiety screening scores. Women with overall urinary symptom bother also had significantly more births and higher anxiety screening scores.
“These findings support the [American College of Obstetricians and Gynecologists] recommendations for routine mental health and urinary incontinence screening in the postpartum period,” said Gena Dunivan, MD, director of the Division of Urogynecology and Pelvic Reconstructive Surgery at University of Alabama–Birmingham, who was not part of the study. “Routine screening for these issues will hopefully reduce the stigma, allowing more patients to receive the help they deserve.”
1 in 3 Postpartum Patients Affected by Urinary Incontinence
About one third of postpartum patients are affected by urinary incontinence, which is linked with poorer quality of life and mental health outcomes, the authors note.
Estimates of incontinence frequency post partum vary depending on the population studied, differences in subgroups, and definition of urinary incontinence. A strength of the study was its sizable population, made up almost entirely of Hispanic White and non-Hispanic Black women receiving care at a large safety-net hospital.
“This study has important clinical implications for postpartum patients,” the authors write. “Given an array of proven treatment options for both UUI and SUI, maternal health surveillance needs to include routine inquiry about UI to overcome patients’ reluctance for seeking care. Next, as elevated BMI was identified as a risk factor for persistent postpartum SUI, maintaining a healthy weight should be routinely encouraged during antenatal and postpartum clinic visits.”
Lauren Giugale, MD, director of UPMC’s Magee-Womens Hospital Postpartum Pelvic Floor Healing Clinic in Pittsburgh, Pennsylvania, says an important aspect of the study is that it measured urinary symptoms 1 year after delivery and shows that these symptoms persist. “A lot of studies look more short term,” she noted.
She also pointed to the study’s population of Black and Hispanic women, populations which “have been pretty hard to capture in urogynecology research. It’s important for us to understand these urinary symptoms are affecting those women as well as White women.”
Association With Anxiety
The association between postpartum depression scores and SUI is important, she says, but Dr. Randhawa’s team also “uniquely looked at anxiety scores in postpartum women. They showed an association between anxiety scores and UUI, so there’s certainly a potential impact of postpartum urinary symptoms on maternal mental health and maternal well-being.” The relationship between anxiety and depression and postpartum urinary symptoms is not well understood and warrants further research, she says.
In her role, Dr. Giugale says, she always asks about urinary symptoms, particularly in postpartum women. But she notes that some ob.gyn.s without urogynecology training may not prioritize those questions amid all the other information they need to cover.
She says she tells her residents to ask patients pointedly, “Are you having any urine leakage? Patients may not think it’s a problem that can be addressed. We do patients a disservice when we don’t ask the important questions that might potentially impact patients’ lives.”
The authors and Dr. Giugale and Dr. Dunivan report no relevant financial relationships.
Bothersome urinary symptoms and incontinence at 12 months post partum are common and treatable, so screening for those symptoms as well as associated depression and anxiety is essential, write authors of a new study.
Sonia Bhandari Randhawa, MD, with the Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center in Dallas, led the study published in Urogynecology, which identified factors associated with persistent stress urinary incontinence (SUI), marked by leakage from sudden movements such as coughing or jumping; urgency UI (UUI), leakage after a sudden and intense need to urinate, even if the bladder isn’t full; and other overall bothersome urinary symptoms 1 year after delivery.
Associations by Subtype
Dr. Randhawa analyzed data provided by 419 patients (77% Hispanic White and 22% non-Hispanic Black). After multivariable analysis, SUI (n = 136, 32.5%) was significantly associated with greater body mass index (BMI) at the time of delivery and greater depression screening scores. Factors not associated included fetal birth weight, mode of delivery, degree of laceration, and breastfeeding status.
UUI (n = 69, 16.5%) was significantly associated with more births and higher anxiety screening scores. Women with overall urinary symptom bother also had significantly more births and higher anxiety screening scores.
“These findings support the [American College of Obstetricians and Gynecologists] recommendations for routine mental health and urinary incontinence screening in the postpartum period,” said Gena Dunivan, MD, director of the Division of Urogynecology and Pelvic Reconstructive Surgery at University of Alabama–Birmingham, who was not part of the study. “Routine screening for these issues will hopefully reduce the stigma, allowing more patients to receive the help they deserve.”
1 in 3 Postpartum Patients Affected by Urinary Incontinence
About one third of postpartum patients are affected by urinary incontinence, which is linked with poorer quality of life and mental health outcomes, the authors note.
Estimates of incontinence frequency post partum vary depending on the population studied, differences in subgroups, and definition of urinary incontinence. A strength of the study was its sizable population, made up almost entirely of Hispanic White and non-Hispanic Black women receiving care at a large safety-net hospital.
“This study has important clinical implications for postpartum patients,” the authors write. “Given an array of proven treatment options for both UUI and SUI, maternal health surveillance needs to include routine inquiry about UI to overcome patients’ reluctance for seeking care. Next, as elevated BMI was identified as a risk factor for persistent postpartum SUI, maintaining a healthy weight should be routinely encouraged during antenatal and postpartum clinic visits.”
Lauren Giugale, MD, director of UPMC’s Magee-Womens Hospital Postpartum Pelvic Floor Healing Clinic in Pittsburgh, Pennsylvania, says an important aspect of the study is that it measured urinary symptoms 1 year after delivery and shows that these symptoms persist. “A lot of studies look more short term,” she noted.
She also pointed to the study’s population of Black and Hispanic women, populations which “have been pretty hard to capture in urogynecology research. It’s important for us to understand these urinary symptoms are affecting those women as well as White women.”
Association With Anxiety
The association between postpartum depression scores and SUI is important, she says, but Dr. Randhawa’s team also “uniquely looked at anxiety scores in postpartum women. They showed an association between anxiety scores and UUI, so there’s certainly a potential impact of postpartum urinary symptoms on maternal mental health and maternal well-being.” The relationship between anxiety and depression and postpartum urinary symptoms is not well understood and warrants further research, she says.
In her role, Dr. Giugale says, she always asks about urinary symptoms, particularly in postpartum women. But she notes that some ob.gyn.s without urogynecology training may not prioritize those questions amid all the other information they need to cover.
She says she tells her residents to ask patients pointedly, “Are you having any urine leakage? Patients may not think it’s a problem that can be addressed. We do patients a disservice when we don’t ask the important questions that might potentially impact patients’ lives.”
The authors and Dr. Giugale and Dr. Dunivan report no relevant financial relationships.
Bothersome urinary symptoms and incontinence at 12 months post partum are common and treatable, so screening for those symptoms as well as associated depression and anxiety is essential, write authors of a new study.
Sonia Bhandari Randhawa, MD, with the Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center in Dallas, led the study published in Urogynecology, which identified factors associated with persistent stress urinary incontinence (SUI), marked by leakage from sudden movements such as coughing or jumping; urgency UI (UUI), leakage after a sudden and intense need to urinate, even if the bladder isn’t full; and other overall bothersome urinary symptoms 1 year after delivery.
Associations by Subtype
Dr. Randhawa analyzed data provided by 419 patients (77% Hispanic White and 22% non-Hispanic Black). After multivariable analysis, SUI (n = 136, 32.5%) was significantly associated with greater body mass index (BMI) at the time of delivery and greater depression screening scores. Factors not associated included fetal birth weight, mode of delivery, degree of laceration, and breastfeeding status.
UUI (n = 69, 16.5%) was significantly associated with more births and higher anxiety screening scores. Women with overall urinary symptom bother also had significantly more births and higher anxiety screening scores.
“These findings support the [American College of Obstetricians and Gynecologists] recommendations for routine mental health and urinary incontinence screening in the postpartum period,” said Gena Dunivan, MD, director of the Division of Urogynecology and Pelvic Reconstructive Surgery at University of Alabama–Birmingham, who was not part of the study. “Routine screening for these issues will hopefully reduce the stigma, allowing more patients to receive the help they deserve.”
1 in 3 Postpartum Patients Affected by Urinary Incontinence
About one third of postpartum patients are affected by urinary incontinence, which is linked with poorer quality of life and mental health outcomes, the authors note.
Estimates of incontinence frequency post partum vary depending on the population studied, differences in subgroups, and definition of urinary incontinence. A strength of the study was its sizable population, made up almost entirely of Hispanic White and non-Hispanic Black women receiving care at a large safety-net hospital.
“This study has important clinical implications for postpartum patients,” the authors write. “Given an array of proven treatment options for both UUI and SUI, maternal health surveillance needs to include routine inquiry about UI to overcome patients’ reluctance for seeking care. Next, as elevated BMI was identified as a risk factor for persistent postpartum SUI, maintaining a healthy weight should be routinely encouraged during antenatal and postpartum clinic visits.”
Lauren Giugale, MD, director of UPMC’s Magee-Womens Hospital Postpartum Pelvic Floor Healing Clinic in Pittsburgh, Pennsylvania, says an important aspect of the study is that it measured urinary symptoms 1 year after delivery and shows that these symptoms persist. “A lot of studies look more short term,” she noted.
She also pointed to the study’s population of Black and Hispanic women, populations which “have been pretty hard to capture in urogynecology research. It’s important for us to understand these urinary symptoms are affecting those women as well as White women.”
Association With Anxiety
The association between postpartum depression scores and SUI is important, she says, but Dr. Randhawa’s team also “uniquely looked at anxiety scores in postpartum women. They showed an association between anxiety scores and UUI, so there’s certainly a potential impact of postpartum urinary symptoms on maternal mental health and maternal well-being.” The relationship between anxiety and depression and postpartum urinary symptoms is not well understood and warrants further research, she says.
In her role, Dr. Giugale says, she always asks about urinary symptoms, particularly in postpartum women. But she notes that some ob.gyn.s without urogynecology training may not prioritize those questions amid all the other information they need to cover.
She says she tells her residents to ask patients pointedly, “Are you having any urine leakage? Patients may not think it’s a problem that can be addressed. We do patients a disservice when we don’t ask the important questions that might potentially impact patients’ lives.”
The authors and Dr. Giugale and Dr. Dunivan report no relevant financial relationships.
UROGYNECOLOGY
Does An Elevated Lp(a) Call for Low-dose Aspirin?
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
GLP-1 Thyroid Warning Could Increase Overdiagnosis
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
FROM ADA 2024