User login
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
THE DIAGNOSIS: Seborrheic Keratosis
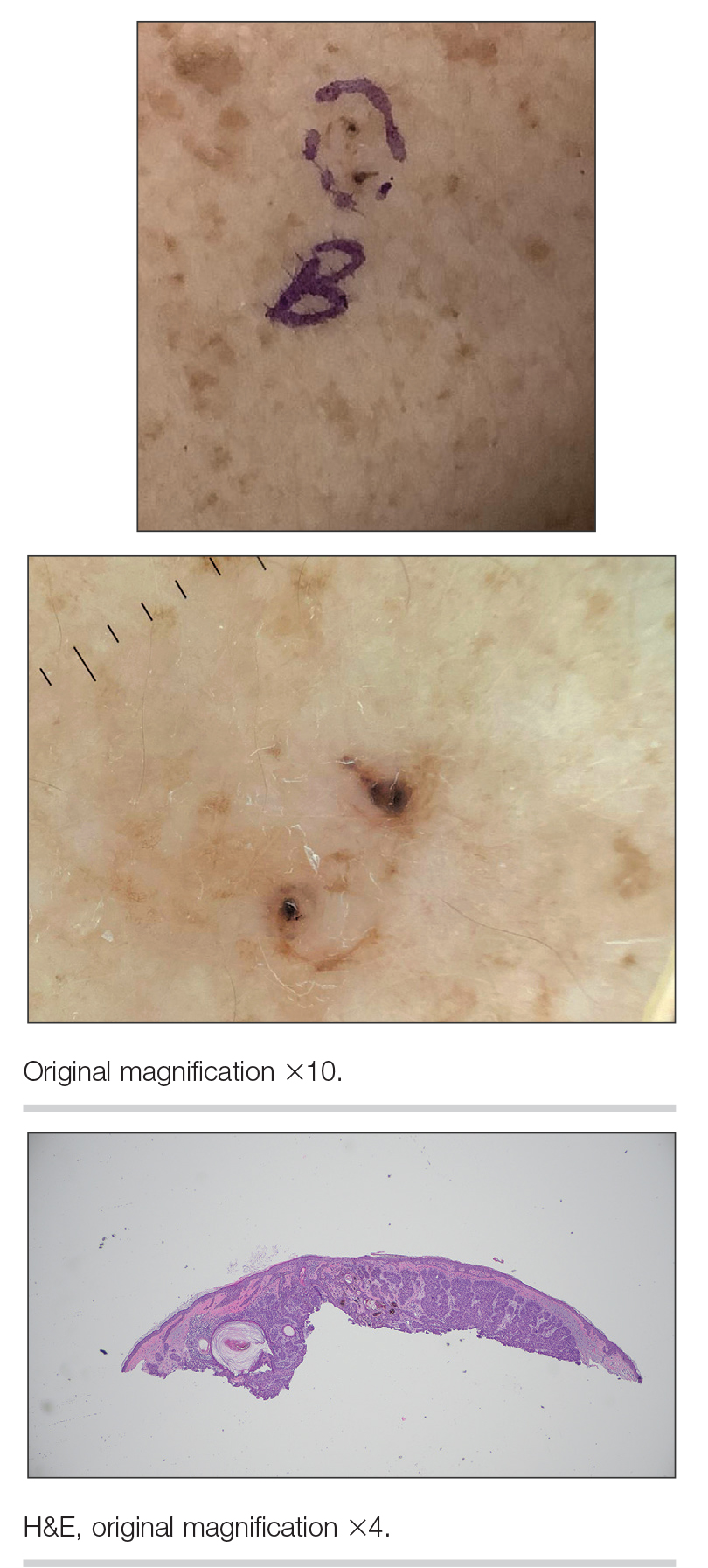
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.
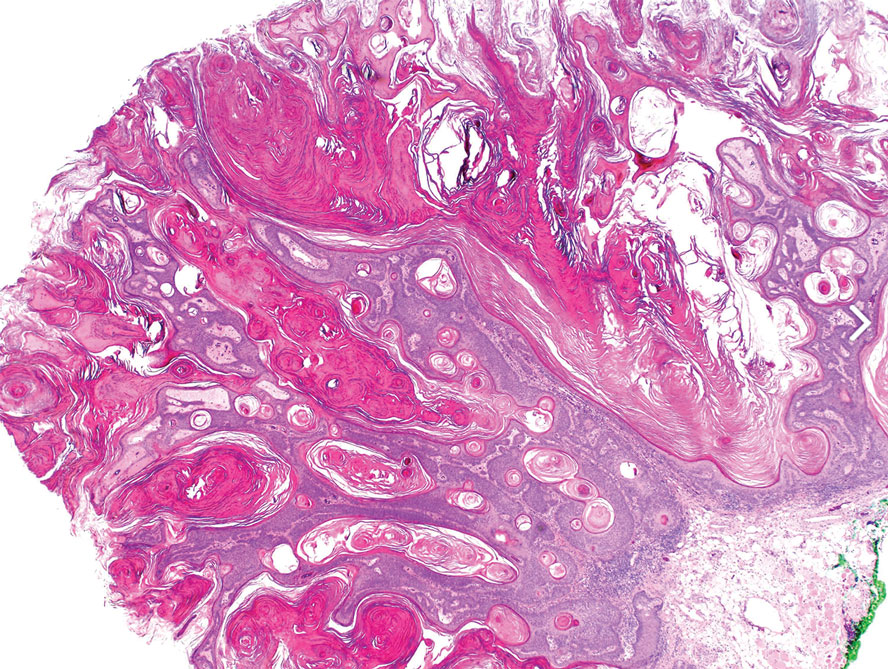
Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
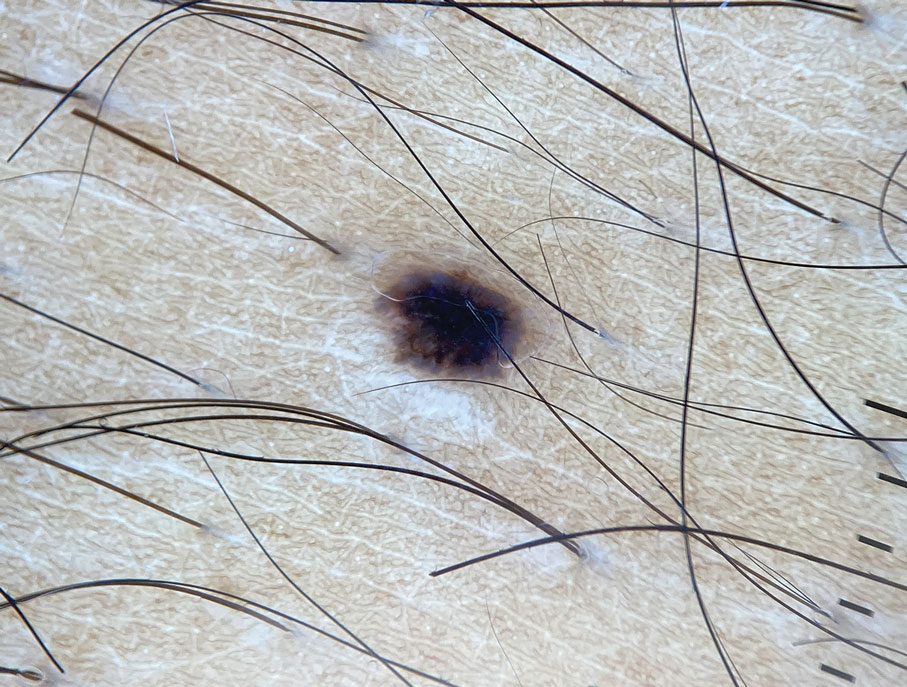
A 33-year-old man with moderately to deeply pigmented skin presented to the dermatology department with a dark-brown macule in the periumbilical area of more than 1 year’s duration. The patient was otherwise healthy and reported no personal or family history of atypical nevi, nonmelanoma skin cancer, or melanoma. Dermoscopy of the lesion showed a dark brown macule less than 2 mm in diameter with a starburst like pattern and a blue-hued border. A shave biopsy of the lesion was performed.
Solitary Plaque on the Nose
Solitary Plaque on the Nose
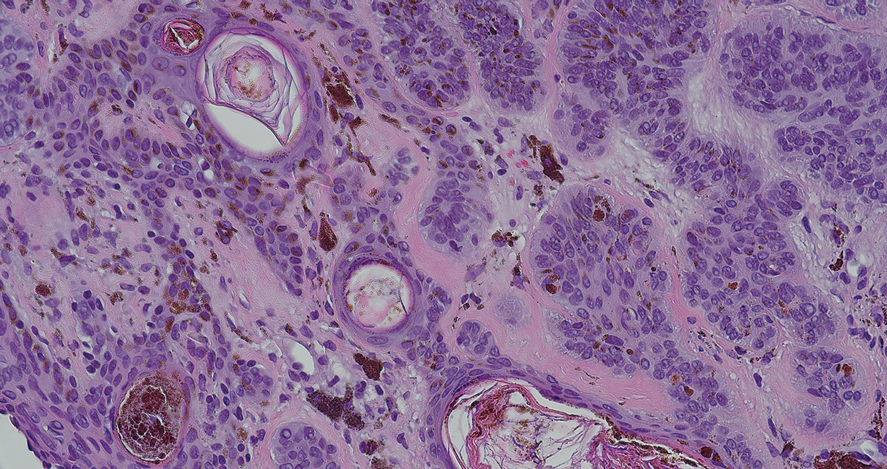
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.
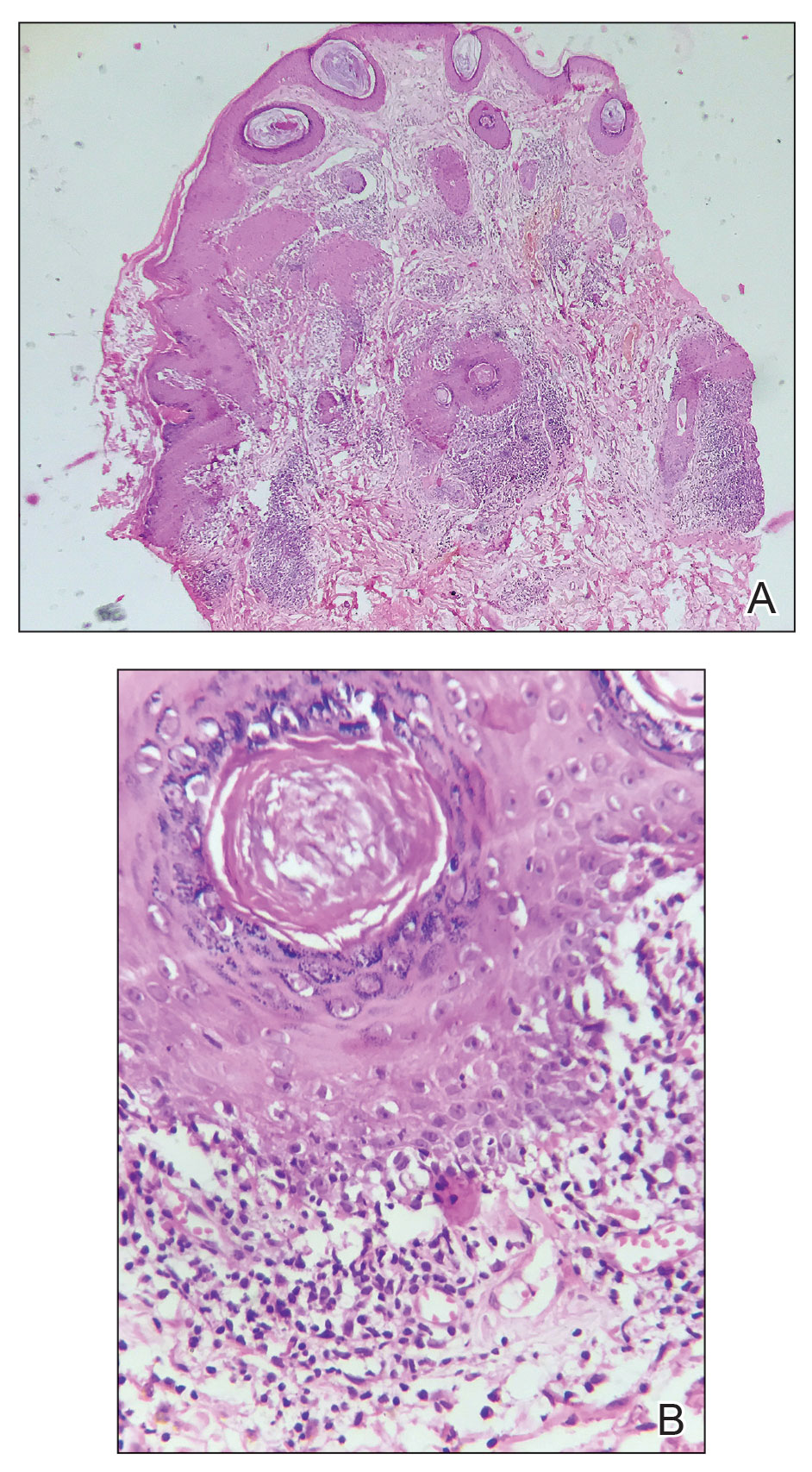
Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
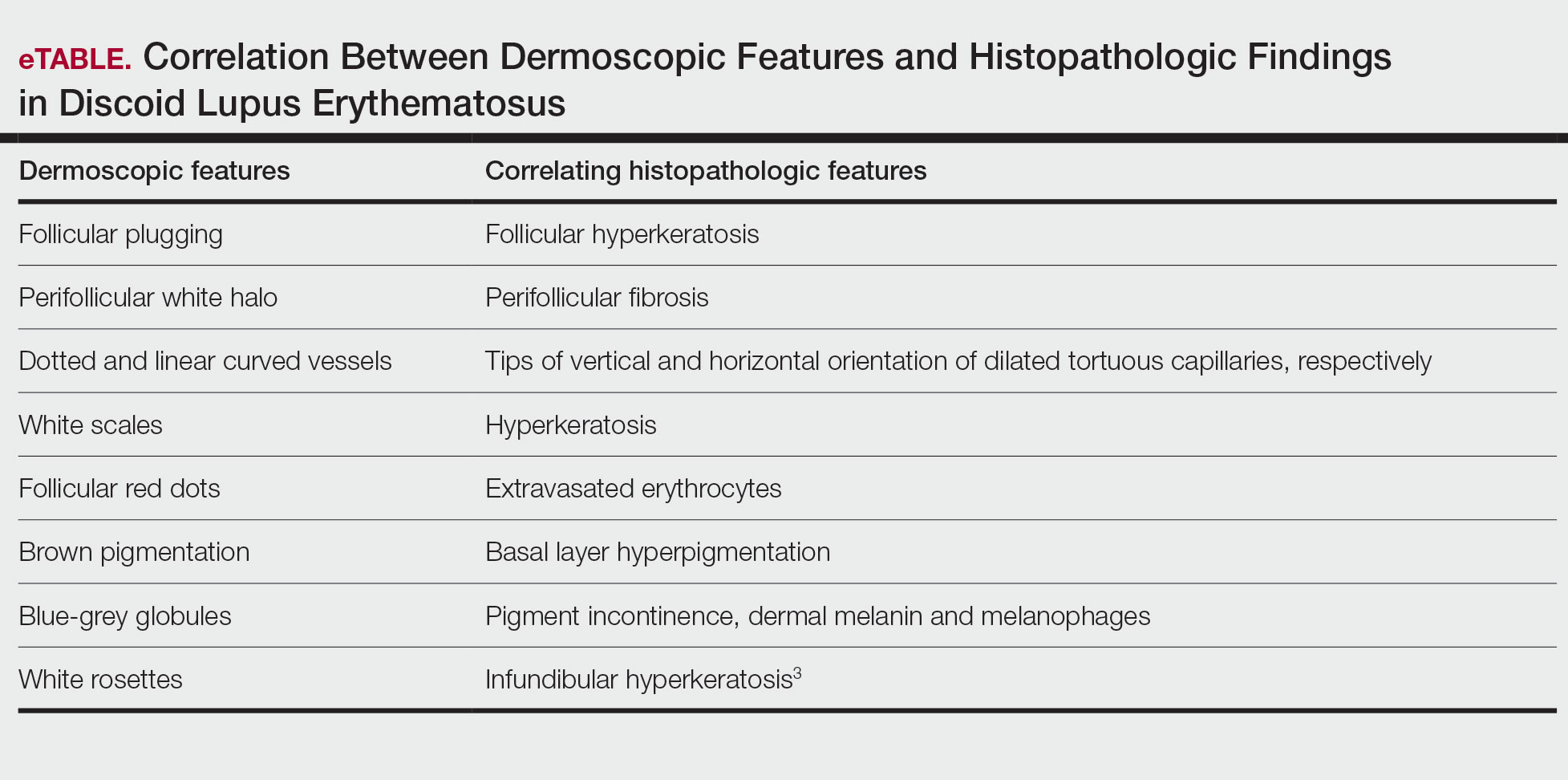
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.
On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.

Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.

On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.

Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.

On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
Solitary Plaque on the Nose
Solitary Plaque on the Nose
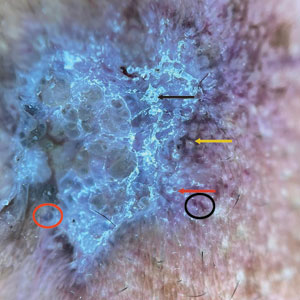
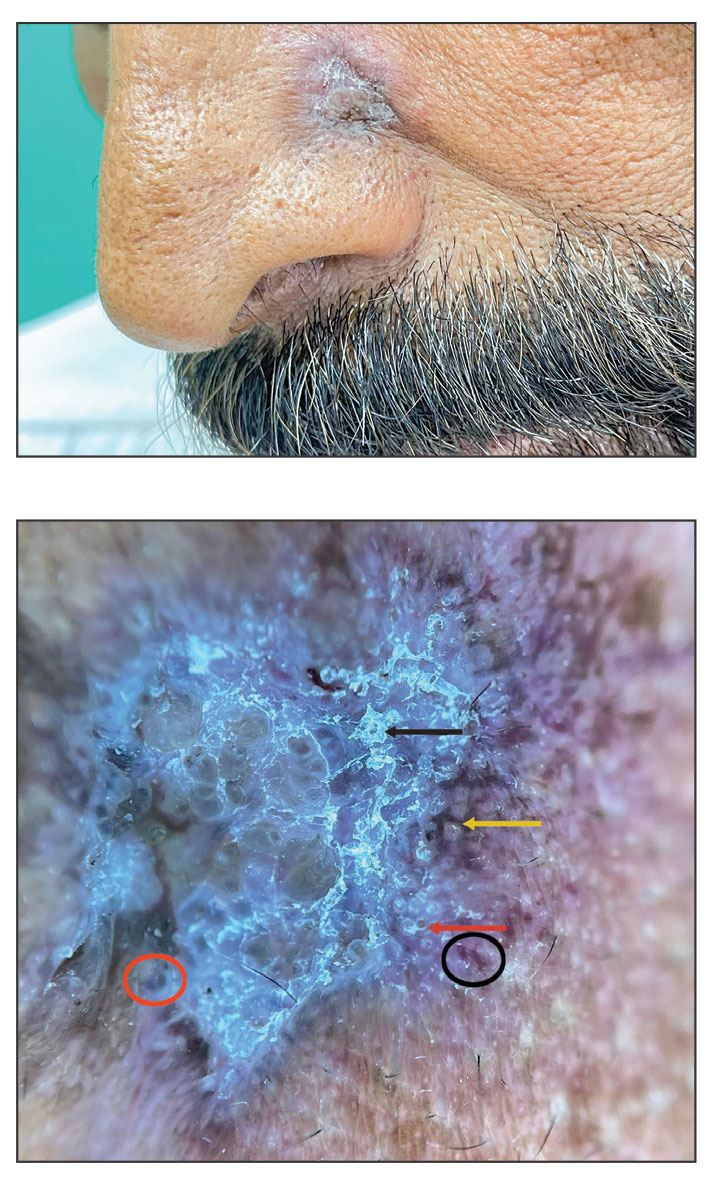
A 50-year-old Southeast Asian-Indian man presented to the dermatology clinic with a slightly elevated reddish-purple lesion on the left side of the nose accompanied by intense itching, occasional discharge, and crusting of 5 months’ duration. The patient reported applying multiple unknown topical agents initially prescribed to him by a physician; however, he subsequently continued applying these medications without regular follow-up visits. He had a history of smoking 2 packs per day for 25 years. His family history was unremarkable. Physical examination revealed a well-defined, 1.5×1.5-cm, nontender, scaly, erythematous to violaceous plaque with slightly raised margins, peripheral hyperpigmentation, and slight central atrophy on the left side of the nose. Dermoscopy revealed prominent follicles with a perifollicular halo (red arrow), white scales (black arrow), linear curved and dotted vessels (black circle), blue-grey globules (red circle), brown reticular lines (yellow arrow), and background erythema. General and systemic examination and routine laboratory workup were normal. A biopsy of the lesion was performed.
Pigmented Lesion on the Left Shoulder in an Older Woman
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.
Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
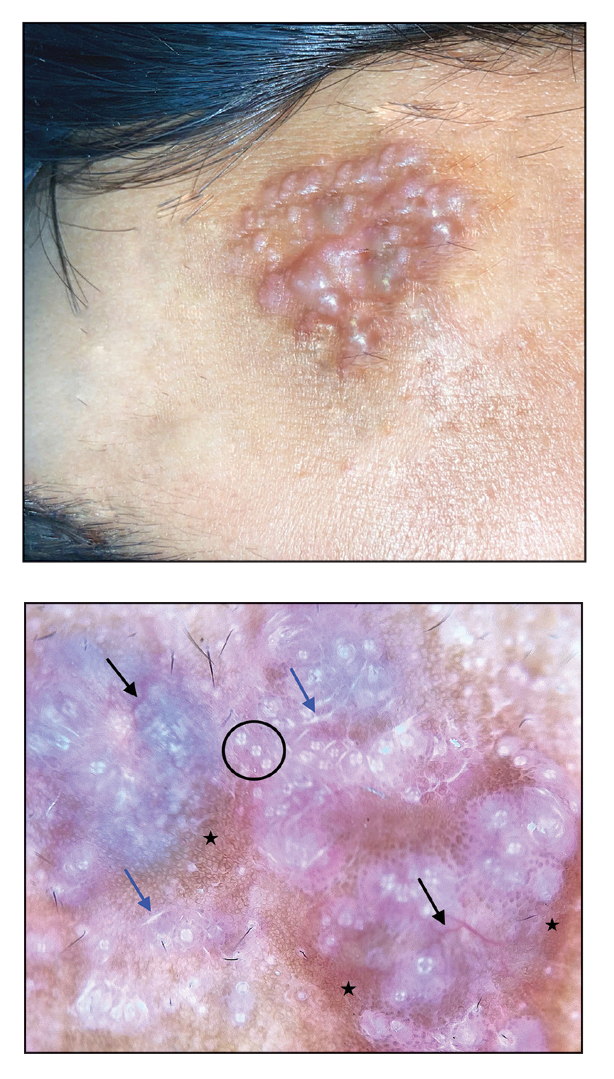
A 92-year-old woman presented to dermatology as a new patient for a full-body skin examination. She had a history of sarcoidosis and a liposarcoma that had been excised more than 20 years prior. She had no history of skin cancer; however, her granddaughter recently was diagnosed with melanoma. Physical examination revealed a 5-mm, irregular, dark brown papule on the left shoulder (top) that was evaluated by dermoscopy (middle). A tangential biopsy was performed for histopathologic analysis (bottom).
Noduloplaque on the Forehead
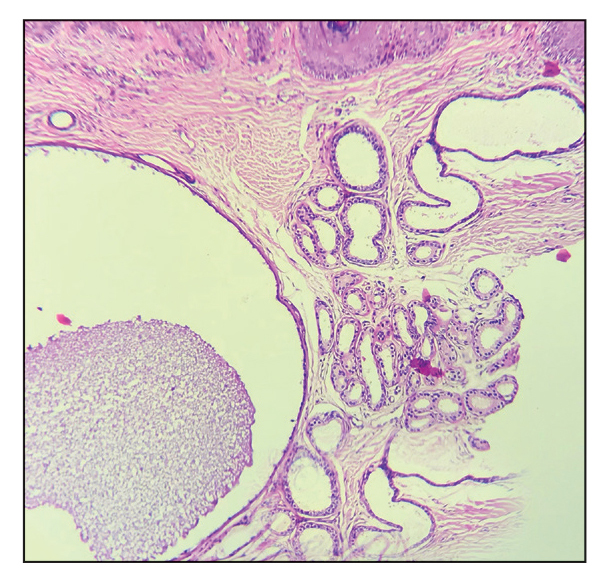
The Diagnosis: Giant Apocrine Hidrocystoma
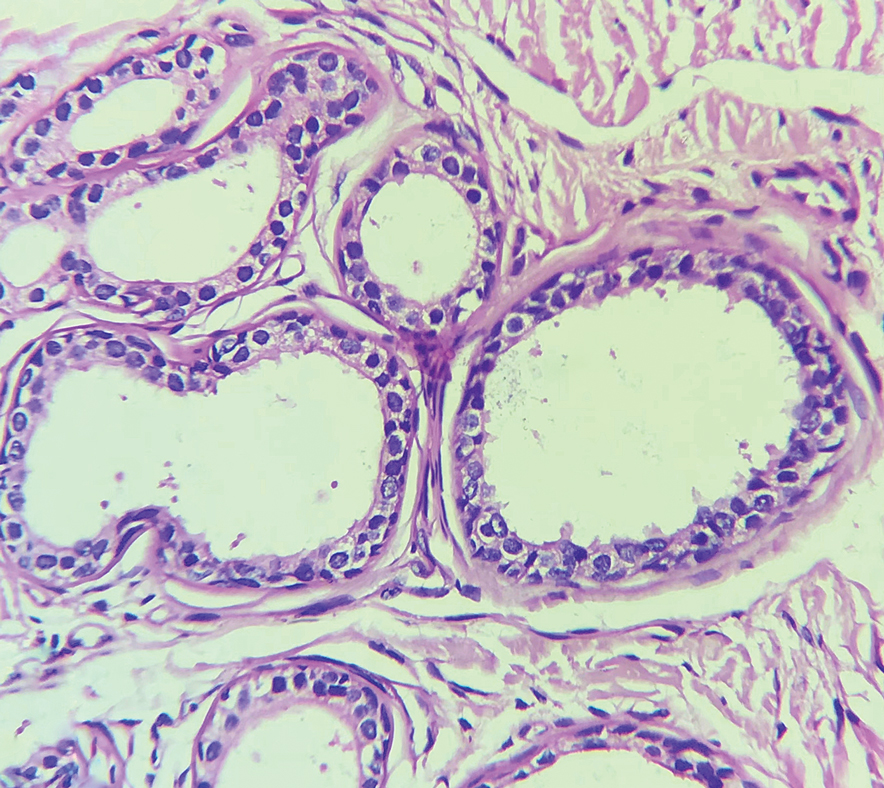
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.
Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.
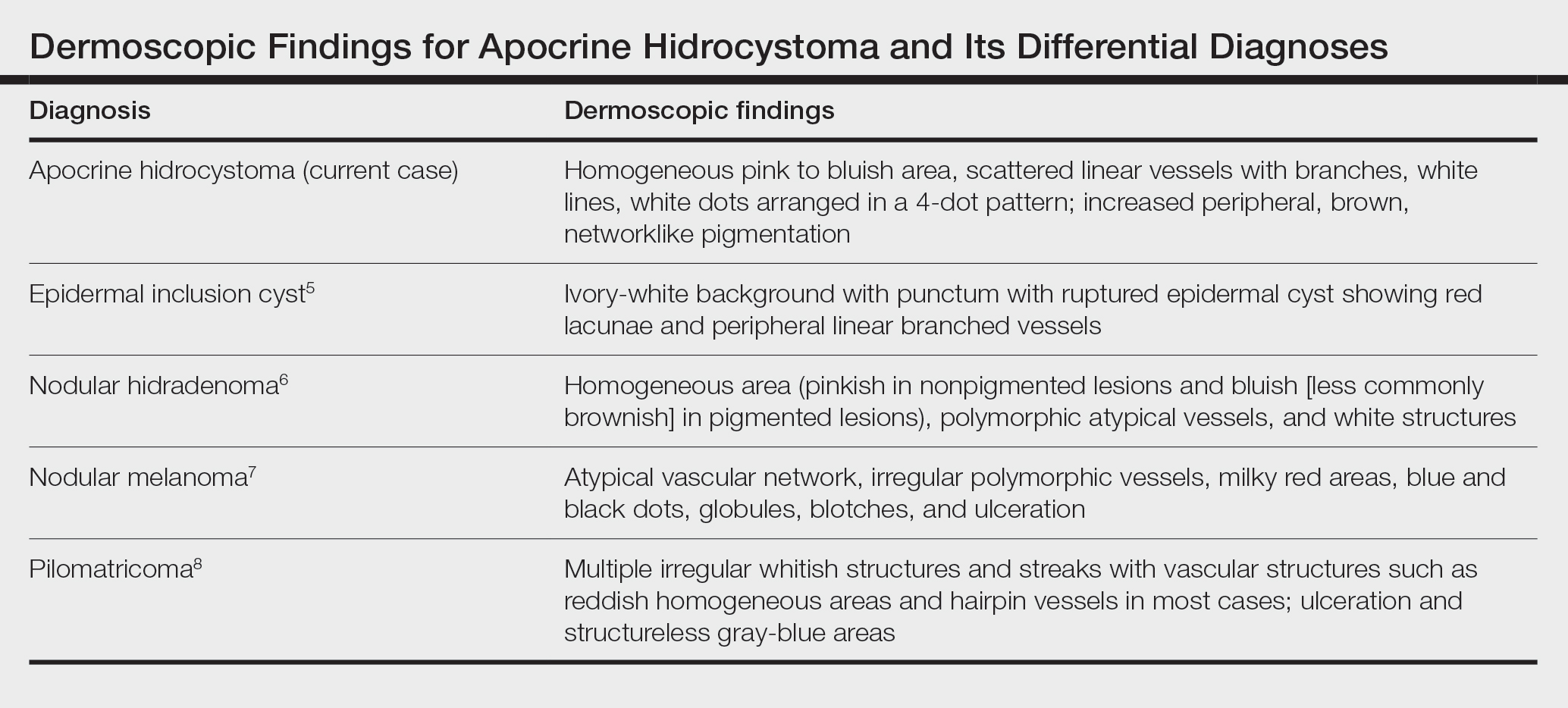
The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
A 21-year-old man presented with a raised lesion on the forehead that had started as a single papule 16 years prior and gradually increased in number and size. There were no associated symptoms and no history of seasonal variation in the size of the lesions. Physical examination revealed multiple erythematous to slightly bluish translucent papules that coalesced to form a 3×3-cm noduloplaque with cystic consistency on the right side of the forehead (top). Dermoscopic examination (middle) (polarized noncontact mode) revealed a homogeneous pink to bluish background, scattered linear vessels with branches (black arrows), multiple chrysalislike shiny white lines (blue arrows), and dots arranged in a 4-dot pattern (black circle) resembling a four-leaf clover. Increased peripheral, brown, networklike pigmentation (black stars) also was noted on dermoscopy. Histopathologic examination of the noduloplaque was performed (bottom).