User login
NSAIDs Offer No Relief for Pain From IUD Placement
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Do Cannabis Users Need More Anesthesia During Surgery?
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
The Prohibitive Price Tag
Earlier in 2024 the American Headache Society issued a position statement that CGRP (calcitonin gene-related peptide) agents are a first-line option for migraine prevention.
No Shinola, Sherlock.
Any of us working frontline neurology have figured that out, including me. And I was, honestly, pretty skeptical of them when they hit the pharmacy shelves. But these days, to quote The Monkees (and Neil Diamond), “I’m a Believer.”
Unfortunately, things don’t quite work out that way. Just because a drug is clearly successful doesn’t make it practical to use first line. Most insurances won’t even let family doctors prescribe them, so they have to send patients to a neurologist (which I’m not complaining about).
Then me and my neuro-brethren have to jump through hoops because of their cost. One month of any of these drugs costs the same as a few years (or more) of generic Topamax, Nortriptyline, Nadolol, etc. Granted, I shouldn’t complain about that, either. If everyone with migraines was getting them it would drive up insurance premiums across the board — including mine.
So, after patients have tried and failed at least two to four other options (depending on their plan) I can usually get a CGRP covered. This involves filling out some forms online and submitting them ... then waiting.
Even if the drug is approved, and successful, that’s still not the end of the story. Depending on the plan I have to get them reauthorized anywhere from every 3 to 12 months. There’s also the chance that in December I’ll get a letter saying the drug won’t be covered starting January, and to try one of the recommended alternatives, like generic Topamax, Nortriptyline, Nadolol, etc. Wash, rinse, repeat.
Having celebrities like Lady Gaga pushing them doesn’t help. The commercials never mention that getting the medication isn’t as easy as “ask your doctor.” Nor does it point out that Lady Gaga won’t have an issue with a CGRP agent’s price tag of $800-$1000 per month, while most of her fans need that money for rent and groceries.
The guidelines, in essence, are useful, but only apply to a perfect world where drug cost doesn’t matter. We aren’t in one. I’m not knocking the pharmaceutical companies — research and development take A LOT of money, and every drug that comes to market has to pay not only for itself, but for several others that failed. Innovation isn’t cheap.
That doesn’t make it any easier to explain to patients, who see ads, or news blurbs on Facebook, or whatever. I just wish the advertisements would have more transparency about how the pricing works.
After all, regardless of how good an automobile may be, don’t car ads show an MSRP?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Earlier in 2024 the American Headache Society issued a position statement that CGRP (calcitonin gene-related peptide) agents are a first-line option for migraine prevention.
No Shinola, Sherlock.
Any of us working frontline neurology have figured that out, including me. And I was, honestly, pretty skeptical of them when they hit the pharmacy shelves. But these days, to quote The Monkees (and Neil Diamond), “I’m a Believer.”
Unfortunately, things don’t quite work out that way. Just because a drug is clearly successful doesn’t make it practical to use first line. Most insurances won’t even let family doctors prescribe them, so they have to send patients to a neurologist (which I’m not complaining about).
Then me and my neuro-brethren have to jump through hoops because of their cost. One month of any of these drugs costs the same as a few years (or more) of generic Topamax, Nortriptyline, Nadolol, etc. Granted, I shouldn’t complain about that, either. If everyone with migraines was getting them it would drive up insurance premiums across the board — including mine.
So, after patients have tried and failed at least two to four other options (depending on their plan) I can usually get a CGRP covered. This involves filling out some forms online and submitting them ... then waiting.
Even if the drug is approved, and successful, that’s still not the end of the story. Depending on the plan I have to get them reauthorized anywhere from every 3 to 12 months. There’s also the chance that in December I’ll get a letter saying the drug won’t be covered starting January, and to try one of the recommended alternatives, like generic Topamax, Nortriptyline, Nadolol, etc. Wash, rinse, repeat.
Having celebrities like Lady Gaga pushing them doesn’t help. The commercials never mention that getting the medication isn’t as easy as “ask your doctor.” Nor does it point out that Lady Gaga won’t have an issue with a CGRP agent’s price tag of $800-$1000 per month, while most of her fans need that money for rent and groceries.
The guidelines, in essence, are useful, but only apply to a perfect world where drug cost doesn’t matter. We aren’t in one. I’m not knocking the pharmaceutical companies — research and development take A LOT of money, and every drug that comes to market has to pay not only for itself, but for several others that failed. Innovation isn’t cheap.
That doesn’t make it any easier to explain to patients, who see ads, or news blurbs on Facebook, or whatever. I just wish the advertisements would have more transparency about how the pricing works.
After all, regardless of how good an automobile may be, don’t car ads show an MSRP?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Earlier in 2024 the American Headache Society issued a position statement that CGRP (calcitonin gene-related peptide) agents are a first-line option for migraine prevention.
No Shinola, Sherlock.
Any of us working frontline neurology have figured that out, including me. And I was, honestly, pretty skeptical of them when they hit the pharmacy shelves. But these days, to quote The Monkees (and Neil Diamond), “I’m a Believer.”
Unfortunately, things don’t quite work out that way. Just because a drug is clearly successful doesn’t make it practical to use first line. Most insurances won’t even let family doctors prescribe them, so they have to send patients to a neurologist (which I’m not complaining about).
Then me and my neuro-brethren have to jump through hoops because of their cost. One month of any of these drugs costs the same as a few years (or more) of generic Topamax, Nortriptyline, Nadolol, etc. Granted, I shouldn’t complain about that, either. If everyone with migraines was getting them it would drive up insurance premiums across the board — including mine.
So, after patients have tried and failed at least two to four other options (depending on their plan) I can usually get a CGRP covered. This involves filling out some forms online and submitting them ... then waiting.
Even if the drug is approved, and successful, that’s still not the end of the story. Depending on the plan I have to get them reauthorized anywhere from every 3 to 12 months. There’s also the chance that in December I’ll get a letter saying the drug won’t be covered starting January, and to try one of the recommended alternatives, like generic Topamax, Nortriptyline, Nadolol, etc. Wash, rinse, repeat.
Having celebrities like Lady Gaga pushing them doesn’t help. The commercials never mention that getting the medication isn’t as easy as “ask your doctor.” Nor does it point out that Lady Gaga won’t have an issue with a CGRP agent’s price tag of $800-$1000 per month, while most of her fans need that money for rent and groceries.
The guidelines, in essence, are useful, but only apply to a perfect world where drug cost doesn’t matter. We aren’t in one. I’m not knocking the pharmaceutical companies — research and development take A LOT of money, and every drug that comes to market has to pay not only for itself, but for several others that failed. Innovation isn’t cheap.
That doesn’t make it any easier to explain to patients, who see ads, or news blurbs on Facebook, or whatever. I just wish the advertisements would have more transparency about how the pricing works.
After all, regardless of how good an automobile may be, don’t car ads show an MSRP?
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Gabapentin: The Hope, the Harm, the Myth, the Reality
Since gabapentin was approved by the US Food and Drug Administration (FDA) for treatment of partial-onset seizures and postherpetic neuralgia, it has been used in many different ways, many off-label indications, and with several recent safety warnings.
Early Problems
After FDA approval in 1993 (for partial seizures), gabapentin was promoted by its maker (Park-Davis) for off-label indications, especially for pain. There was no FDA approval for that indication and the studies the company had done were deemed to have been manipulated in a subsequent lawsuit.1 Gabapentin became the nonopioid go-to medication for treatment of pain despite underwhelming evidence.
Studies on Neuropathy
In the largest trial of gabapentin for diabetic peripheral neuropathy, Rauck and colleagues found no significant difference in pain relief between gabapentin and placebo.2 A Cochrane review of gabapentin for neuropathic pain concluded that about 30%-40% of patients taking gabapentin for diabetic neuropathy achieved meaningful pain relief with gabapentin use, with a number needed to treat (NNT) of 6.6.3 The review also concluded that for postherpetic neuralgia (an FDA-approved indication) 78% of patients had moderate to substantial benefit with gabapentin (NNT 4.8 for moderate benefit).
Side Effects of Gabapentin
From the Cochrane review, the most common side effects were: dizziness (19%), somnolence (14%), peripheral edema (7%), and gait disturbance (14%). The number needed to harm for gabapentin was 7.5 The two side effects listed here that are often overlooked that I want to highlight are peripheral edema and gait disturbance. I have seen these both fairly frequently over the years. A side effect not found in the Cochrane review was weight gain. Weight gain with gabapentin was reported in a meta-analysis of drugs that can cause weight gain.4
New Warnings
In December 2019, the FDA released a warning on the potential for serious respiratory problems with gabapentin and pregabalin in patients with certain risk factors: opioid use or use of other drugs that depress the central nervous system, COPD, and other severe lung diseases.5 Rahman and colleagues found that compared with nonuse, gabapentinoid use was associated with increased risk for severe COPD exacerbation (hazard ratio, 1.39; 95% confidence interval, 1.29-1.50).6
Off-Label Uses
Primary care professionals frequently use gabapentin for two off-label indications that are incorporated into practice guidelines. Ryan et al. studied gabapentin in patients with refractory, unexplained chronic cough.7 In a randomized, placebo-controlled trial, gabapentin improved cough-specific quality of life compared with placebo (P = .004; NNT 3.58). Use of gabapentin for treatment of unexplained, refractory cough has been included in several chronic cough practice guidelines.8,9
Gabapentin has been studied for the treatment of restless legs syndrome and has been recommended as an option to treat moderate to severe restless legs syndrome in the American Academy of Sleep Medicine Guidelines.10
Pearl of the Month:
Gabapentin is used widely for many different pain syndromes. The best evidence is for postherpetic neuralgia and diabetic neuropathy. Be aware of the side effects and risks of use in patients with pulmonary disease and who are taking CNS-depressant medications.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Landefeld CS, Steinman MA. The Neurontin legacy: marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-6.
2. Rauck R et al. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract. 2013;13(6):485-96.
3. Wiffen PJ et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938.
4. Domecq JP et al. Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015 Feb;100(2):363-70.
5. 12-19-2019 FDA Drug Safety Communication. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR).
6. Rahman AA et al. Gabapentinoids and risk for severe exacerbation in chronic obstructive pulmonary disease: A population-based cohort study. Ann Intern Med. 2024 Feb;177(2):144-54.
7. Ryan NM et al. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380(9853):1583-9.
8. Gibson P et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016 Jan;149(1):27-44.
9. De Vincentis A et al. Chronic cough in adults: recommendations from an Italian intersociety consensus. Aging Clin Exp Res 2022;34:1529.
10. Aurora RN et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults — an update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039.
Since gabapentin was approved by the US Food and Drug Administration (FDA) for treatment of partial-onset seizures and postherpetic neuralgia, it has been used in many different ways, many off-label indications, and with several recent safety warnings.
Early Problems
After FDA approval in 1993 (for partial seizures), gabapentin was promoted by its maker (Park-Davis) for off-label indications, especially for pain. There was no FDA approval for that indication and the studies the company had done were deemed to have been manipulated in a subsequent lawsuit.1 Gabapentin became the nonopioid go-to medication for treatment of pain despite underwhelming evidence.
Studies on Neuropathy
In the largest trial of gabapentin for diabetic peripheral neuropathy, Rauck and colleagues found no significant difference in pain relief between gabapentin and placebo.2 A Cochrane review of gabapentin for neuropathic pain concluded that about 30%-40% of patients taking gabapentin for diabetic neuropathy achieved meaningful pain relief with gabapentin use, with a number needed to treat (NNT) of 6.6.3 The review also concluded that for postherpetic neuralgia (an FDA-approved indication) 78% of patients had moderate to substantial benefit with gabapentin (NNT 4.8 for moderate benefit).
Side Effects of Gabapentin
From the Cochrane review, the most common side effects were: dizziness (19%), somnolence (14%), peripheral edema (7%), and gait disturbance (14%). The number needed to harm for gabapentin was 7.5 The two side effects listed here that are often overlooked that I want to highlight are peripheral edema and gait disturbance. I have seen these both fairly frequently over the years. A side effect not found in the Cochrane review was weight gain. Weight gain with gabapentin was reported in a meta-analysis of drugs that can cause weight gain.4
New Warnings
In December 2019, the FDA released a warning on the potential for serious respiratory problems with gabapentin and pregabalin in patients with certain risk factors: opioid use or use of other drugs that depress the central nervous system, COPD, and other severe lung diseases.5 Rahman and colleagues found that compared with nonuse, gabapentinoid use was associated with increased risk for severe COPD exacerbation (hazard ratio, 1.39; 95% confidence interval, 1.29-1.50).6
Off-Label Uses
Primary care professionals frequently use gabapentin for two off-label indications that are incorporated into practice guidelines. Ryan et al. studied gabapentin in patients with refractory, unexplained chronic cough.7 In a randomized, placebo-controlled trial, gabapentin improved cough-specific quality of life compared with placebo (P = .004; NNT 3.58). Use of gabapentin for treatment of unexplained, refractory cough has been included in several chronic cough practice guidelines.8,9
Gabapentin has been studied for the treatment of restless legs syndrome and has been recommended as an option to treat moderate to severe restless legs syndrome in the American Academy of Sleep Medicine Guidelines.10
Pearl of the Month:
Gabapentin is used widely for many different pain syndromes. The best evidence is for postherpetic neuralgia and diabetic neuropathy. Be aware of the side effects and risks of use in patients with pulmonary disease and who are taking CNS-depressant medications.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Landefeld CS, Steinman MA. The Neurontin legacy: marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-6.
2. Rauck R et al. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract. 2013;13(6):485-96.
3. Wiffen PJ et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938.
4. Domecq JP et al. Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015 Feb;100(2):363-70.
5. 12-19-2019 FDA Drug Safety Communication. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR).
6. Rahman AA et al. Gabapentinoids and risk for severe exacerbation in chronic obstructive pulmonary disease: A population-based cohort study. Ann Intern Med. 2024 Feb;177(2):144-54.
7. Ryan NM et al. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380(9853):1583-9.
8. Gibson P et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016 Jan;149(1):27-44.
9. De Vincentis A et al. Chronic cough in adults: recommendations from an Italian intersociety consensus. Aging Clin Exp Res 2022;34:1529.
10. Aurora RN et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults — an update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039.
Since gabapentin was approved by the US Food and Drug Administration (FDA) for treatment of partial-onset seizures and postherpetic neuralgia, it has been used in many different ways, many off-label indications, and with several recent safety warnings.
Early Problems
After FDA approval in 1993 (for partial seizures), gabapentin was promoted by its maker (Park-Davis) for off-label indications, especially for pain. There was no FDA approval for that indication and the studies the company had done were deemed to have been manipulated in a subsequent lawsuit.1 Gabapentin became the nonopioid go-to medication for treatment of pain despite underwhelming evidence.
Studies on Neuropathy
In the largest trial of gabapentin for diabetic peripheral neuropathy, Rauck and colleagues found no significant difference in pain relief between gabapentin and placebo.2 A Cochrane review of gabapentin for neuropathic pain concluded that about 30%-40% of patients taking gabapentin for diabetic neuropathy achieved meaningful pain relief with gabapentin use, with a number needed to treat (NNT) of 6.6.3 The review also concluded that for postherpetic neuralgia (an FDA-approved indication) 78% of patients had moderate to substantial benefit with gabapentin (NNT 4.8 for moderate benefit).
Side Effects of Gabapentin
From the Cochrane review, the most common side effects were: dizziness (19%), somnolence (14%), peripheral edema (7%), and gait disturbance (14%). The number needed to harm for gabapentin was 7.5 The two side effects listed here that are often overlooked that I want to highlight are peripheral edema and gait disturbance. I have seen these both fairly frequently over the years. A side effect not found in the Cochrane review was weight gain. Weight gain with gabapentin was reported in a meta-analysis of drugs that can cause weight gain.4
New Warnings
In December 2019, the FDA released a warning on the potential for serious respiratory problems with gabapentin and pregabalin in patients with certain risk factors: opioid use or use of other drugs that depress the central nervous system, COPD, and other severe lung diseases.5 Rahman and colleagues found that compared with nonuse, gabapentinoid use was associated with increased risk for severe COPD exacerbation (hazard ratio, 1.39; 95% confidence interval, 1.29-1.50).6
Off-Label Uses
Primary care professionals frequently use gabapentin for two off-label indications that are incorporated into practice guidelines. Ryan et al. studied gabapentin in patients with refractory, unexplained chronic cough.7 In a randomized, placebo-controlled trial, gabapentin improved cough-specific quality of life compared with placebo (P = .004; NNT 3.58). Use of gabapentin for treatment of unexplained, refractory cough has been included in several chronic cough practice guidelines.8,9
Gabapentin has been studied for the treatment of restless legs syndrome and has been recommended as an option to treat moderate to severe restless legs syndrome in the American Academy of Sleep Medicine Guidelines.10
Pearl of the Month:
Gabapentin is used widely for many different pain syndromes. The best evidence is for postherpetic neuralgia and diabetic neuropathy. Be aware of the side effects and risks of use in patients with pulmonary disease and who are taking CNS-depressant medications.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Landefeld CS, Steinman MA. The Neurontin legacy: marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-6.
2. Rauck R et al. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract. 2013;13(6):485-96.
3. Wiffen PJ et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938.
4. Domecq JP et al. Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015 Feb;100(2):363-70.
5. 12-19-2019 FDA Drug Safety Communication. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR).
6. Rahman AA et al. Gabapentinoids and risk for severe exacerbation in chronic obstructive pulmonary disease: A population-based cohort study. Ann Intern Med. 2024 Feb;177(2):144-54.
7. Ryan NM et al. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380(9853):1583-9.
8. Gibson P et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016 Jan;149(1):27-44.
9. De Vincentis A et al. Chronic cough in adults: recommendations from an Italian intersociety consensus. Aging Clin Exp Res 2022;34:1529.
10. Aurora RN et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults — an update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012;35:1039.
Veterans Found Relief From Chronic Pain Through Telehealth Mindfulness
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
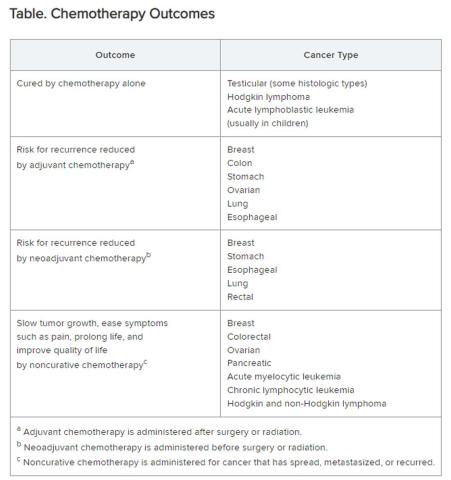
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Mobile App Shows Promise in Managing Fibromyalgia Symptoms
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Which Medications Can Cause Edema?
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Recurrent Pancreatitis Triples Risk for Chronic Disease
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.