User login
VA Gets it Right on Suicide
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
Climate change: Commentary in four dermatology journals calls for emergency action
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
A farewell to arms? Drug approvals based on single-arm trials can be flawed
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
AT ESMO CONGRESS 2022
Biden’s Cancer Moonshot turns its focus to early-detection blood tests
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
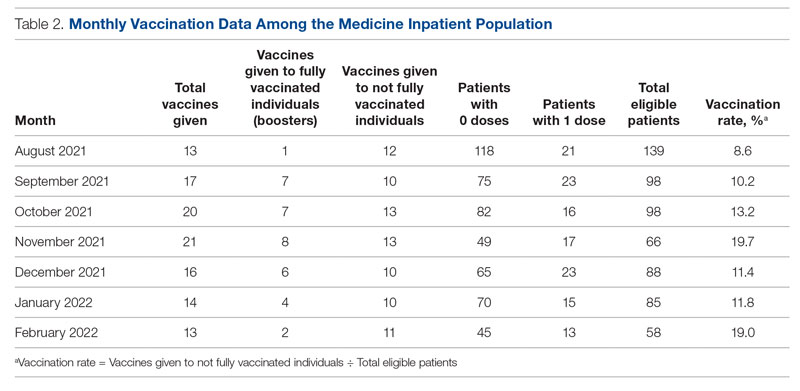
Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
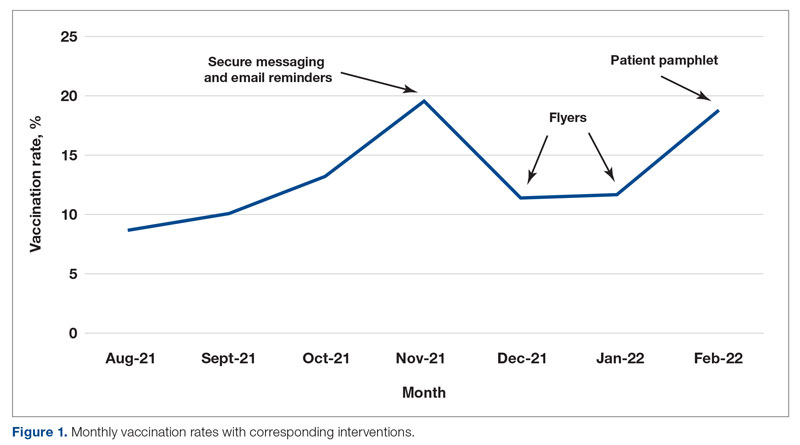
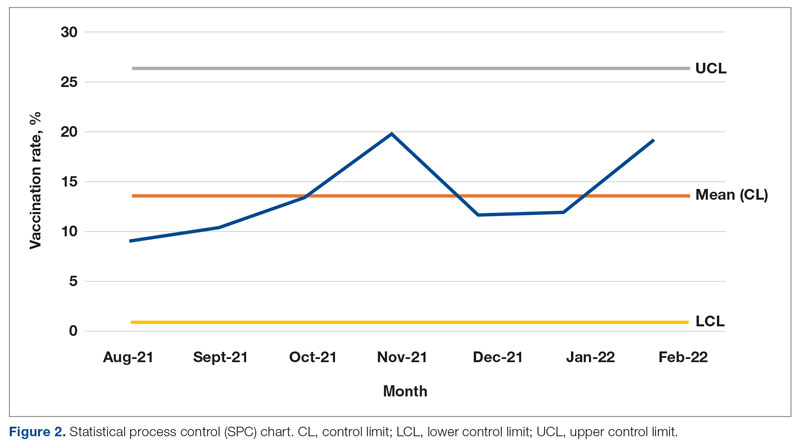
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
Diabetes Population Health Innovations in the Age of COVID-19: Insights From the T1D Exchange Quality Improvement Collaborative
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.
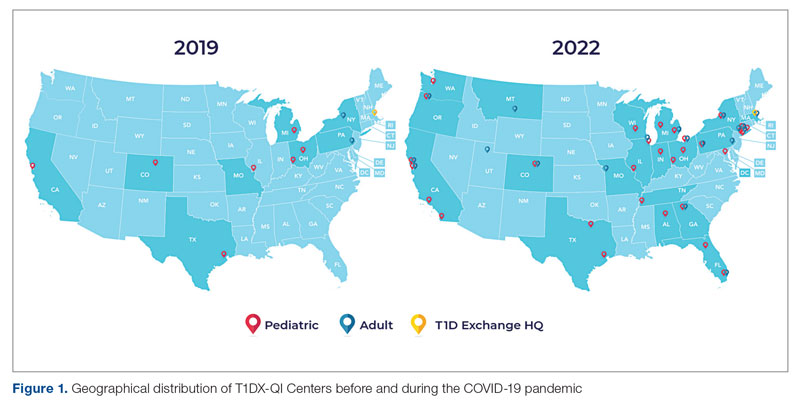
Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.
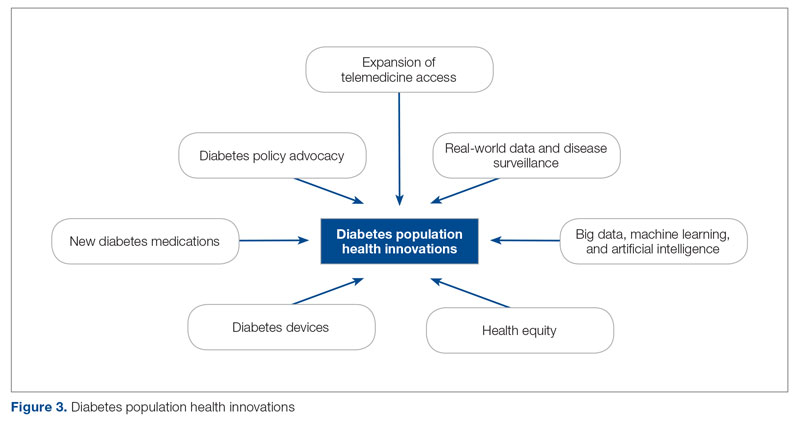
Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.
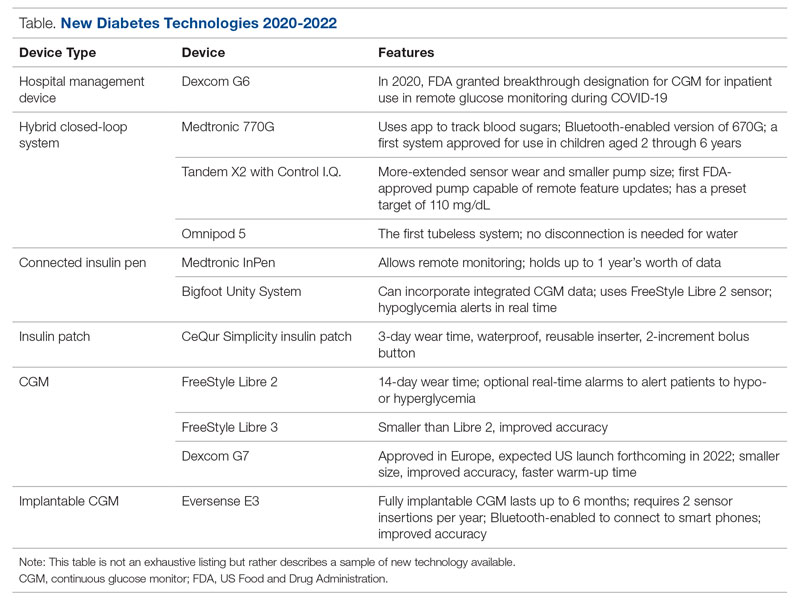
The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: amungmode@t1dexchange.org
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: amungmode@t1dexchange.org
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: amungmode@t1dexchange.org
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
Deprescribing in Older Adults in Community and Nursing Home Settings
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
Abbreviated Delirium Screening Instruments: Plausible Tool to Improve Delirium Detection in Hospitalized Older Patients
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x










