User login
Better care reduces time to successful refeeding in acute pancreatitis
Enhanced recovery approaches were safe and effective at promoting earlier restoration of gut function in acute pancreatitis patients, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Patients recruited for the trial were admitted directly from an emergency department and received either enhanced care consisting of patient-directed oral intake, early ambulation, and nonopioid analgesia or received normal care consisting of opioid analgesia, physician-directed diet, and nursing parameters, Elizabeth Dong, MD, of the Kaiser Permanente Los Angeles Medical Center and her associates said.
Among the 46 patients included in the study, 61% had an etiology of gallstones, 15% had an etiology of alcohol, 13% had hyperglyceridemia, and 11% had a different etiology. Median age was 53.1 years, Dr. Dong and her associates noted.
Time to successful oral refeeding, the primary study endpoint, was significantly reduced in the enhanced treatment group, with a median time of 13.8 hours, compared with the normal treatment group, in which median time to oral refeeding was 124.8 hours. In addition, patients in the enhanced care group had a mean pancreatitis activity score of 43.5 after 48-72 hours, while patients in the control group had a mean score of 72.1.
Length of stay and frequency of 30-day readmission did not differ significantly between study groups.
The study was not funded by industry grants, and no disclosures were reported.
Enhanced recovery approaches were safe and effective at promoting earlier restoration of gut function in acute pancreatitis patients, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Patients recruited for the trial were admitted directly from an emergency department and received either enhanced care consisting of patient-directed oral intake, early ambulation, and nonopioid analgesia or received normal care consisting of opioid analgesia, physician-directed diet, and nursing parameters, Elizabeth Dong, MD, of the Kaiser Permanente Los Angeles Medical Center and her associates said.
Among the 46 patients included in the study, 61% had an etiology of gallstones, 15% had an etiology of alcohol, 13% had hyperglyceridemia, and 11% had a different etiology. Median age was 53.1 years, Dr. Dong and her associates noted.
Time to successful oral refeeding, the primary study endpoint, was significantly reduced in the enhanced treatment group, with a median time of 13.8 hours, compared with the normal treatment group, in which median time to oral refeeding was 124.8 hours. In addition, patients in the enhanced care group had a mean pancreatitis activity score of 43.5 after 48-72 hours, while patients in the control group had a mean score of 72.1.
Length of stay and frequency of 30-day readmission did not differ significantly between study groups.
The study was not funded by industry grants, and no disclosures were reported.
Enhanced recovery approaches were safe and effective at promoting earlier restoration of gut function in acute pancreatitis patients, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Patients recruited for the trial were admitted directly from an emergency department and received either enhanced care consisting of patient-directed oral intake, early ambulation, and nonopioid analgesia or received normal care consisting of opioid analgesia, physician-directed diet, and nursing parameters, Elizabeth Dong, MD, of the Kaiser Permanente Los Angeles Medical Center and her associates said.
Among the 46 patients included in the study, 61% had an etiology of gallstones, 15% had an etiology of alcohol, 13% had hyperglyceridemia, and 11% had a different etiology. Median age was 53.1 years, Dr. Dong and her associates noted.
Time to successful oral refeeding, the primary study endpoint, was significantly reduced in the enhanced treatment group, with a median time of 13.8 hours, compared with the normal treatment group, in which median time to oral refeeding was 124.8 hours. In addition, patients in the enhanced care group had a mean pancreatitis activity score of 43.5 after 48-72 hours, while patients in the control group had a mean score of 72.1.
Length of stay and frequency of 30-day readmission did not differ significantly between study groups.
The study was not funded by industry grants, and no disclosures were reported.
FROM WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Median time to successful oral refeeding was more than 4 days faster in patients who received enhanced care.
Data source: A pilot single-blind, randomized, controlled trial of 46 patients admitted from an emergency department between July 2016 and April 2017.
Disclosures: The study was not funded by industry grants, and no disclosures were reported.
Serrated polyps alone not associated with future high-risk adenomas
The presence of serrated polyps on index colonoscopies without low-risk adenomas was not associated with metachronous high-risk adenomas on surveillance exams, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Data for the study were collected from 4,616 adults who had two colonoscopies on record with the New Hampshire Colonoscopy Registry. Patients with high-risk adenomas at the index colonoscopy were excluded from the study. The median time between index and surveillance exams was 4.9 years, and median age was 61 years, according to Joseph Anderson, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and his associates.
Overall, the risk for metachronous high-risk adenomas in the study group was 6.3% and the risk of large serrated polyps greater than or equal to 1 cm was 1.2%. After patient age, sex, smoking, body mass index, and time between the two exams were adjusted for, low-risk adenomas at the time of the index colonoscopy were associated with an increased metachronous risk of high-risk adenomas, Dr. Anderson and his colleagues noted.
Large serrated polyps and the presence of sessile serrated polyps or traditional serrated adenomas at index exam increased the risk of metachronous serrated polyps at the surveillance colonoscopy 10-fold and 14-fold, respectively, but did not increase the risk of high-risk adenomas. The presence of both low-risk adenomas and significant serrated polyps was not associated with an increased risk of high-risk adenomas over the presence of low-risk adenomas alone.
The study was not funded by industry grants, and no disclosures were reported.
The presence of serrated polyps on index colonoscopies without low-risk adenomas was not associated with metachronous high-risk adenomas on surveillance exams, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Data for the study were collected from 4,616 adults who had two colonoscopies on record with the New Hampshire Colonoscopy Registry. Patients with high-risk adenomas at the index colonoscopy were excluded from the study. The median time between index and surveillance exams was 4.9 years, and median age was 61 years, according to Joseph Anderson, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and his associates.
Overall, the risk for metachronous high-risk adenomas in the study group was 6.3% and the risk of large serrated polyps greater than or equal to 1 cm was 1.2%. After patient age, sex, smoking, body mass index, and time between the two exams were adjusted for, low-risk adenomas at the time of the index colonoscopy were associated with an increased metachronous risk of high-risk adenomas, Dr. Anderson and his colleagues noted.
Large serrated polyps and the presence of sessile serrated polyps or traditional serrated adenomas at index exam increased the risk of metachronous serrated polyps at the surveillance colonoscopy 10-fold and 14-fold, respectively, but did not increase the risk of high-risk adenomas. The presence of both low-risk adenomas and significant serrated polyps was not associated with an increased risk of high-risk adenomas over the presence of low-risk adenomas alone.
The study was not funded by industry grants, and no disclosures were reported.
The presence of serrated polyps on index colonoscopies without low-risk adenomas was not associated with metachronous high-risk adenomas on surveillance exams, according to a study presented at the World Congress of Gastroenterology at ACG 2017.
Data for the study were collected from 4,616 adults who had two colonoscopies on record with the New Hampshire Colonoscopy Registry. Patients with high-risk adenomas at the index colonoscopy were excluded from the study. The median time between index and surveillance exams was 4.9 years, and median age was 61 years, according to Joseph Anderson, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and his associates.
Overall, the risk for metachronous high-risk adenomas in the study group was 6.3% and the risk of large serrated polyps greater than or equal to 1 cm was 1.2%. After patient age, sex, smoking, body mass index, and time between the two exams were adjusted for, low-risk adenomas at the time of the index colonoscopy were associated with an increased metachronous risk of high-risk adenomas, Dr. Anderson and his colleagues noted.
Large serrated polyps and the presence of sessile serrated polyps or traditional serrated adenomas at index exam increased the risk of metachronous serrated polyps at the surveillance colonoscopy 10-fold and 14-fold, respectively, but did not increase the risk of high-risk adenomas. The presence of both low-risk adenomas and significant serrated polyps was not associated with an increased risk of high-risk adenomas over the presence of low-risk adenomas alone.
The study was not funded by industry grants, and no disclosures were reported.
FROM THE 13TH WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: After patient age, sex, smoking, body mass index, and time between index and surveillance exams were adjusted for, the presence of index serrated polyps without additional low-risk adenomas did not increase risk of high-risk adenomas at surveillance exams.
Data source: Data collected from 4,616 patients in the New Hampshire Colonoscopy Registry.
Disclosures: The study was not funded by industry grants, and no disclosures were reported.
MACRA Monday: Advance care plan
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #47: Care Plan
This measure is aimed at capturing the percentage of patients aged 65 years and older who have a documented advance care plan in their medical records.
What you need to do: Discuss with the patient the creation of an advance care plan or the naming of a surrogate decision maker and then document that in the medical record. If the patient does not wish to make a plan or is unable to name a decision maker, document that along with the fact that the issue of advance care planning was discussed.
Eligible cases include patients aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205,99212, 99213, 99214, 99215, 99218, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99234, 99235, 99236, 99291, 99304, 99305, 99306, 99307, 99308, 99309, 99310, 99324, 99325, 99326, 99327, 99328, 99334, 99335, 99336, 99337, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a Quality Data Code that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1123F indicates that advance care planning was discussed and documented, and an advance care plan or surrogate decision maker was documented in the medical record. On the other hand, CPT II 1124F should be used if advance care planning was discussed and documented in the medical record, but the patient did not wish or was not able to name a surrogate decision maker or provide an advance care plan.
CMS has a full list of measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B–allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #47: Care Plan
This measure is aimed at capturing the percentage of patients aged 65 years and older who have a documented advance care plan in their medical records.
What you need to do: Discuss with the patient the creation of an advance care plan or the naming of a surrogate decision maker and then document that in the medical record. If the patient does not wish to make a plan or is unable to name a decision maker, document that along with the fact that the issue of advance care planning was discussed.
Eligible cases include patients aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205,99212, 99213, 99214, 99215, 99218, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99234, 99235, 99236, 99291, 99304, 99305, 99306, 99307, 99308, 99309, 99310, 99324, 99325, 99326, 99327, 99328, 99334, 99335, 99336, 99337, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a Quality Data Code that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1123F indicates that advance care planning was discussed and documented, and an advance care plan or surrogate decision maker was documented in the medical record. On the other hand, CPT II 1124F should be used if advance care planning was discussed and documented in the medical record, but the patient did not wish or was not able to name a surrogate decision maker or provide an advance care plan.
CMS has a full list of measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B–allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #47: Care Plan
This measure is aimed at capturing the percentage of patients aged 65 years and older who have a documented advance care plan in their medical records.
What you need to do: Discuss with the patient the creation of an advance care plan or the naming of a surrogate decision maker and then document that in the medical record. If the patient does not wish to make a plan or is unable to name a decision maker, document that along with the fact that the issue of advance care planning was discussed.
Eligible cases include patients aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205,99212, 99213, 99214, 99215, 99218, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99234, 99235, 99236, 99291, 99304, 99305, 99306, 99307, 99308, 99309, 99310, 99324, 99325, 99326, 99327, 99328, 99334, 99335, 99336, 99337, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a Quality Data Code that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1123F indicates that advance care planning was discussed and documented, and an advance care plan or surrogate decision maker was documented in the medical record. On the other hand, CPT II 1124F should be used if advance care planning was discussed and documented in the medical record, but the patient did not wish or was not able to name a surrogate decision maker or provide an advance care plan.
CMS has a full list of measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B–allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
CBT for insomnia and hot flashes lifts mood in midlife
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT NAMS 2017
Key clinical point:
Major finding: Patient-reported and clinician-assessed depression scores dropped after the intervention (P = .001 for both).
Data source: Randomized controlled trial of 40 midlife women with insomnia and hot flashes.
Disclosures: The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
Good for something, or an American tragedy
The headline in the Oct. 13, 2017, Portland (Maine) Press Herald hinted that I was about to read a sad story: “New Hampshire doctor, 85, may lose practice because she doesn’t use computer.” Anna Konopka, MD, who has a 300-patient practice in New London, doesn’t use a computer in her office, and as a consequence can’t participate in her state’s mandated prescription drug monitoring program. She has appealed to the governor, but if her appeal is denied she will be forced to close her office.
The closure will present a hardship for the residents of this small New Hampshire town, who will have to replace their obviously committed physician who has served them for more than 30 years. And I am sure that Dr. Konopka would have preferred to end her professional career on her own terms. It isn’t going to be easy to give up that positive feedback from her patients that every primary care physician enjoys even on her worst day.
I wouldn’t be surprised to learn that Dr. Konopka has listened to other physicians in her community complain about the cost and time-gobbling inefficiencies of their EHRs. She may have been put off by her own experiences as a patient whose physician spends too much time looking at his computer screen and fails to engage with her. Or she may have simply done the math and come up with the obvious answer that a computer system would be a bad investment for her small practice.
I suspect that there are days that you wish you had followed this wise older physician’s lead and never plugged into that “good-for-nothing piece of junk” sitting on the desk in your exam room. The sadness in this story is that the computer and the Internet are (or at least could be) good for some things, including the statewide prescription drug monitoring program that Dr. Konopka can’t participate in. Immunization data banks, prescribing programs that minimize physician error, and systems for storing and plotting your patient’s lab work and metrics are just a few of the things that a good computer system is good for. And, of course, there is the real-time access to the vast store of medical and research knowledge that has made textbooks obsolete.
I’m not sure where we can go from here without throwing out the baby with the bathwater and starting from scratch. We have computer scientists and physicians who I am sure could create a patient- and physician-friendly system that could cover the whole country. The trick will be keeping the politicians out of the room.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
The headline in the Oct. 13, 2017, Portland (Maine) Press Herald hinted that I was about to read a sad story: “New Hampshire doctor, 85, may lose practice because she doesn’t use computer.” Anna Konopka, MD, who has a 300-patient practice in New London, doesn’t use a computer in her office, and as a consequence can’t participate in her state’s mandated prescription drug monitoring program. She has appealed to the governor, but if her appeal is denied she will be forced to close her office.
The closure will present a hardship for the residents of this small New Hampshire town, who will have to replace their obviously committed physician who has served them for more than 30 years. And I am sure that Dr. Konopka would have preferred to end her professional career on her own terms. It isn’t going to be easy to give up that positive feedback from her patients that every primary care physician enjoys even on her worst day.
I wouldn’t be surprised to learn that Dr. Konopka has listened to other physicians in her community complain about the cost and time-gobbling inefficiencies of their EHRs. She may have been put off by her own experiences as a patient whose physician spends too much time looking at his computer screen and fails to engage with her. Or she may have simply done the math and come up with the obvious answer that a computer system would be a bad investment for her small practice.
I suspect that there are days that you wish you had followed this wise older physician’s lead and never plugged into that “good-for-nothing piece of junk” sitting on the desk in your exam room. The sadness in this story is that the computer and the Internet are (or at least could be) good for some things, including the statewide prescription drug monitoring program that Dr. Konopka can’t participate in. Immunization data banks, prescribing programs that minimize physician error, and systems for storing and plotting your patient’s lab work and metrics are just a few of the things that a good computer system is good for. And, of course, there is the real-time access to the vast store of medical and research knowledge that has made textbooks obsolete.
I’m not sure where we can go from here without throwing out the baby with the bathwater and starting from scratch. We have computer scientists and physicians who I am sure could create a patient- and physician-friendly system that could cover the whole country. The trick will be keeping the politicians out of the room.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
The headline in the Oct. 13, 2017, Portland (Maine) Press Herald hinted that I was about to read a sad story: “New Hampshire doctor, 85, may lose practice because she doesn’t use computer.” Anna Konopka, MD, who has a 300-patient practice in New London, doesn’t use a computer in her office, and as a consequence can’t participate in her state’s mandated prescription drug monitoring program. She has appealed to the governor, but if her appeal is denied she will be forced to close her office.
The closure will present a hardship for the residents of this small New Hampshire town, who will have to replace their obviously committed physician who has served them for more than 30 years. And I am sure that Dr. Konopka would have preferred to end her professional career on her own terms. It isn’t going to be easy to give up that positive feedback from her patients that every primary care physician enjoys even on her worst day.
I wouldn’t be surprised to learn that Dr. Konopka has listened to other physicians in her community complain about the cost and time-gobbling inefficiencies of their EHRs. She may have been put off by her own experiences as a patient whose physician spends too much time looking at his computer screen and fails to engage with her. Or she may have simply done the math and come up with the obvious answer that a computer system would be a bad investment for her small practice.
I suspect that there are days that you wish you had followed this wise older physician’s lead and never plugged into that “good-for-nothing piece of junk” sitting on the desk in your exam room. The sadness in this story is that the computer and the Internet are (or at least could be) good for some things, including the statewide prescription drug monitoring program that Dr. Konopka can’t participate in. Immunization data banks, prescribing programs that minimize physician error, and systems for storing and plotting your patient’s lab work and metrics are just a few of the things that a good computer system is good for. And, of course, there is the real-time access to the vast store of medical and research knowledge that has made textbooks obsolete.
I’m not sure where we can go from here without throwing out the baby with the bathwater and starting from scratch. We have computer scientists and physicians who I am sure could create a patient- and physician-friendly system that could cover the whole country. The trick will be keeping the politicians out of the room.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
Restoring Function in Veterans With Complex Chronic Pain
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
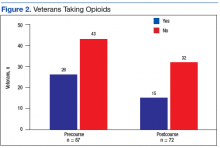
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
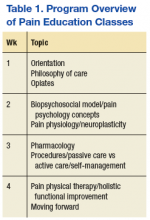
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
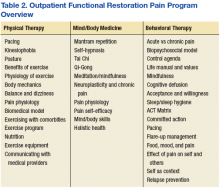
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
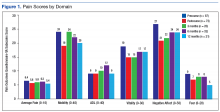
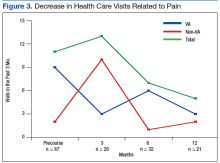
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
Compound induces selective apoptosis in AML
Researchers say they have discovered a compound that can overcome resistance to apoptosis in acute myeloid leukemia (AML).
The compound, BTSA1, works by activating the BCL-2 family protein BAX.
BTSA1 prompted apoptosis in leukemia cells while sparing healthy cells. It also suppressed AML in mice without producing side effects.
Evripidis Gavathiotis, PhD, of Albert Einstein College of Medicine in Bronx, New York, and his colleagues described these results in Cancer Cell.
The team knew that apoptosis occurs when BAX is activated by pro-apoptotic proteins. However, cancer cells can avoid apoptosis by producing anti-apoptotic proteins that suppress BAX and the proteins that activate it.
“Our novel compound revives suppressed BAX molecules in cancer cells by binding with high affinity to BAX’s activation site,” Dr Gavathiotis said. “BAX can then swing into action, killing cancer cells while leaving healthy cells unscathed.”
Dr Gavathiotis was the lead author of a paper published in Nature in 2008 that first described the structure and shape of BAX’s activation site. He has since looked for small molecules that can activate BAX strongly enough to overcome cancer cells’ resistance to apoptosis.
His team initially screened more than 1 million compounds to reveal those with BAX-binding potential. The most promising 500 compounds were then evaluated in the lab.
“A compound dubbed BTSA1 (short for BAX Trigger Site Activator 1) proved to be the most potent BAX activator, causing rapid and extensive apoptosis when added to several different human AML cell lines,” said Denis Reyna, a doctoral student in Dr Gavathiotis’s lab.
The researchers also tested BTSA1 in blood samples from patients with high-risk AML. BTSA1 induced apoptosis in the patients’ AML cells but did not affect healthy hematopoietic stem cells.
Finally, the researchers generated mouse models of AML. BTSA1 was given to half the mice, while the other half served as controls.
On average, the BTSA1-treated mice survived significantly longer than the control mice—55 days and 40 days, respectively (P=0.0009). In fact, 43% of BTSA1-treated mice were still alive after 60 days and showing no signs of AML.
In addition, the mice treated with BTSA1 showed no evidence of toxicity.
“BTSA1 activates BAX and causes apoptosis in AML cells while sparing healthy cells and tissues, probably because the cancer cells are primed for apoptosis,” Dr Gavathiotis said.
He and his colleagues found that AML cells contained significantly higher BAX levels than normal blood cells from healthy subjects.
“With more BAX available in AML cells, even low BTSA1 doses will trigger enough BAX activation to cause apoptotic death, while sparing healthy cells that contain low levels of BAX or none at all,” Dr Gavathiotis said.
He and his team plan to determine if BTSA1 will elicit similar results in other cancer types. ![]()
Researchers say they have discovered a compound that can overcome resistance to apoptosis in acute myeloid leukemia (AML).
The compound, BTSA1, works by activating the BCL-2 family protein BAX.
BTSA1 prompted apoptosis in leukemia cells while sparing healthy cells. It also suppressed AML in mice without producing side effects.
Evripidis Gavathiotis, PhD, of Albert Einstein College of Medicine in Bronx, New York, and his colleagues described these results in Cancer Cell.
The team knew that apoptosis occurs when BAX is activated by pro-apoptotic proteins. However, cancer cells can avoid apoptosis by producing anti-apoptotic proteins that suppress BAX and the proteins that activate it.
“Our novel compound revives suppressed BAX molecules in cancer cells by binding with high affinity to BAX’s activation site,” Dr Gavathiotis said. “BAX can then swing into action, killing cancer cells while leaving healthy cells unscathed.”
Dr Gavathiotis was the lead author of a paper published in Nature in 2008 that first described the structure and shape of BAX’s activation site. He has since looked for small molecules that can activate BAX strongly enough to overcome cancer cells’ resistance to apoptosis.
His team initially screened more than 1 million compounds to reveal those with BAX-binding potential. The most promising 500 compounds were then evaluated in the lab.
“A compound dubbed BTSA1 (short for BAX Trigger Site Activator 1) proved to be the most potent BAX activator, causing rapid and extensive apoptosis when added to several different human AML cell lines,” said Denis Reyna, a doctoral student in Dr Gavathiotis’s lab.
The researchers also tested BTSA1 in blood samples from patients with high-risk AML. BTSA1 induced apoptosis in the patients’ AML cells but did not affect healthy hematopoietic stem cells.
Finally, the researchers generated mouse models of AML. BTSA1 was given to half the mice, while the other half served as controls.
On average, the BTSA1-treated mice survived significantly longer than the control mice—55 days and 40 days, respectively (P=0.0009). In fact, 43% of BTSA1-treated mice were still alive after 60 days and showing no signs of AML.
In addition, the mice treated with BTSA1 showed no evidence of toxicity.
“BTSA1 activates BAX and causes apoptosis in AML cells while sparing healthy cells and tissues, probably because the cancer cells are primed for apoptosis,” Dr Gavathiotis said.
He and his colleagues found that AML cells contained significantly higher BAX levels than normal blood cells from healthy subjects.
“With more BAX available in AML cells, even low BTSA1 doses will trigger enough BAX activation to cause apoptotic death, while sparing healthy cells that contain low levels of BAX or none at all,” Dr Gavathiotis said.
He and his team plan to determine if BTSA1 will elicit similar results in other cancer types. ![]()
Researchers say they have discovered a compound that can overcome resistance to apoptosis in acute myeloid leukemia (AML).
The compound, BTSA1, works by activating the BCL-2 family protein BAX.
BTSA1 prompted apoptosis in leukemia cells while sparing healthy cells. It also suppressed AML in mice without producing side effects.
Evripidis Gavathiotis, PhD, of Albert Einstein College of Medicine in Bronx, New York, and his colleagues described these results in Cancer Cell.
The team knew that apoptosis occurs when BAX is activated by pro-apoptotic proteins. However, cancer cells can avoid apoptosis by producing anti-apoptotic proteins that suppress BAX and the proteins that activate it.
“Our novel compound revives suppressed BAX molecules in cancer cells by binding with high affinity to BAX’s activation site,” Dr Gavathiotis said. “BAX can then swing into action, killing cancer cells while leaving healthy cells unscathed.”
Dr Gavathiotis was the lead author of a paper published in Nature in 2008 that first described the structure and shape of BAX’s activation site. He has since looked for small molecules that can activate BAX strongly enough to overcome cancer cells’ resistance to apoptosis.
His team initially screened more than 1 million compounds to reveal those with BAX-binding potential. The most promising 500 compounds were then evaluated in the lab.
“A compound dubbed BTSA1 (short for BAX Trigger Site Activator 1) proved to be the most potent BAX activator, causing rapid and extensive apoptosis when added to several different human AML cell lines,” said Denis Reyna, a doctoral student in Dr Gavathiotis’s lab.
The researchers also tested BTSA1 in blood samples from patients with high-risk AML. BTSA1 induced apoptosis in the patients’ AML cells but did not affect healthy hematopoietic stem cells.
Finally, the researchers generated mouse models of AML. BTSA1 was given to half the mice, while the other half served as controls.
On average, the BTSA1-treated mice survived significantly longer than the control mice—55 days and 40 days, respectively (P=0.0009). In fact, 43% of BTSA1-treated mice were still alive after 60 days and showing no signs of AML.
In addition, the mice treated with BTSA1 showed no evidence of toxicity.
“BTSA1 activates BAX and causes apoptosis in AML cells while sparing healthy cells and tissues, probably because the cancer cells are primed for apoptosis,” Dr Gavathiotis said.
He and his colleagues found that AML cells contained significantly higher BAX levels than normal blood cells from healthy subjects.
“With more BAX available in AML cells, even low BTSA1 doses will trigger enough BAX activation to cause apoptotic death, while sparing healthy cells that contain low levels of BAX or none at all,” Dr Gavathiotis said.
He and his team plan to determine if BTSA1 will elicit similar results in other cancer types. ![]()
Depression and Heart Failure? Put Down the SSRI
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
Involving experts in S. aureus bacteremia treatment reduces mortality
SAN DIEGO – Thirty-day mortality associated with Staphylococcus aureus bacteremia is reduced if there is guidance from either an antimicrobial stewardship team (AST) or an infectious disease consultant (IDC), according to a multivariate experience at Yale New Haven Hospital presented at an annual scientific meeting on infectious diseases.
“This has been a hot area, because there have been a lot of recent studies suggesting that expert infectious disease advice improves care, but not every study has associated expert advice with a mortality benefit,” said Jacqueline Sherbuk, MD, a fellow in the division of infectious diseases and international health at the University of Virginia, Charlottesville. She was a resident at Yale University when this study was conducted.
In this study, the impact of an IDC on outcome in patients with S. aureus bacteremia was evaluated relative to no expert advice. By itself, an IDC was associated with improved adherence to standards of care for S. aureus bacteremia management, but the reduction in mortality was not statistically significant for those who received IDC guidance relative to those who did not.
“Given that patient care may be guided by consultations from the AST independent of IDC, we looked at the overall impact of expert opinion versus no expert involvement, and this achieved significance on multivariate analysis,” Dr. Sherbuk reported.
For adherence to guidelines, IDC guidance was better than no expert advice on multiple measures, including proportion obtaining an echocardiogram (89% vs. 67%; P less than .001), appropriate definitive antibiotics (98% vs. 80%; P less than .001), and appropriate treatment duration (92% vs. 35%; P less than .001). However, the advantage for 30-day mortality rates was only a trend (11% vs. 21%, P = .07). It was only when patients who received IDC guidance or a consultation from the AST were combined that the difference climbed to significance (11% vs. 23%; P = .04).
“On multivariate analysis, the OR [odds ratio] was substantial, predicting a 60% reduction [OR 0.40; P = .03) in 30-day mortality for expert advice vs. no expert advice,” Dr. Sherbuk reported.
In this retrospective observational study, 261 unique cases of S. aureus bacteremia cases in adult patients established with positive blood cultures were evaluated. The cases were collected over a 1-year period at Yale New Haven Hospital. After exclusion of those who died within 3 days of the initial positive culture or who were transferred to other facilities, 236 were included in this analysis.
IDC guidance, which is not required for S. aureus bacteremia at Yale New Haven Hospital, was provided for 74.5% of the patients. Another 4% of patients received guidance from the AST, which is an independent service often provided prior to IDC guidance, according to Dr. Sherbuk.
Relapse (3% vs. 5%) and reinfection (6% vs. 4%) rates were low in both those who did and did not receive expert advice, respectively. These rates were not significantly different. On multivariate analysis, the two factors associated with increased 30-day mortality were patient age greater than 60 years and sepsis based on sequential organ failure assessment.
Several previous studies have associated IDC advice with improved outcomes in S. aureus bacteremia, according to Dr. Sherbuk, but this study suggests that the AST “can be a meaningful adjunct” to IDC guidance to improve outcomes. She noted that several other sets of data presented at this year’s ID Week also associated AST with improved infection management.
SAN DIEGO – Thirty-day mortality associated with Staphylococcus aureus bacteremia is reduced if there is guidance from either an antimicrobial stewardship team (AST) or an infectious disease consultant (IDC), according to a multivariate experience at Yale New Haven Hospital presented at an annual scientific meeting on infectious diseases.
“This has been a hot area, because there have been a lot of recent studies suggesting that expert infectious disease advice improves care, but not every study has associated expert advice with a mortality benefit,” said Jacqueline Sherbuk, MD, a fellow in the division of infectious diseases and international health at the University of Virginia, Charlottesville. She was a resident at Yale University when this study was conducted.
In this study, the impact of an IDC on outcome in patients with S. aureus bacteremia was evaluated relative to no expert advice. By itself, an IDC was associated with improved adherence to standards of care for S. aureus bacteremia management, but the reduction in mortality was not statistically significant for those who received IDC guidance relative to those who did not.
“Given that patient care may be guided by consultations from the AST independent of IDC, we looked at the overall impact of expert opinion versus no expert involvement, and this achieved significance on multivariate analysis,” Dr. Sherbuk reported.
For adherence to guidelines, IDC guidance was better than no expert advice on multiple measures, including proportion obtaining an echocardiogram (89% vs. 67%; P less than .001), appropriate definitive antibiotics (98% vs. 80%; P less than .001), and appropriate treatment duration (92% vs. 35%; P less than .001). However, the advantage for 30-day mortality rates was only a trend (11% vs. 21%, P = .07). It was only when patients who received IDC guidance or a consultation from the AST were combined that the difference climbed to significance (11% vs. 23%; P = .04).
“On multivariate analysis, the OR [odds ratio] was substantial, predicting a 60% reduction [OR 0.40; P = .03) in 30-day mortality for expert advice vs. no expert advice,” Dr. Sherbuk reported.
In this retrospective observational study, 261 unique cases of S. aureus bacteremia cases in adult patients established with positive blood cultures were evaluated. The cases were collected over a 1-year period at Yale New Haven Hospital. After exclusion of those who died within 3 days of the initial positive culture or who were transferred to other facilities, 236 were included in this analysis.
IDC guidance, which is not required for S. aureus bacteremia at Yale New Haven Hospital, was provided for 74.5% of the patients. Another 4% of patients received guidance from the AST, which is an independent service often provided prior to IDC guidance, according to Dr. Sherbuk.
Relapse (3% vs. 5%) and reinfection (6% vs. 4%) rates were low in both those who did and did not receive expert advice, respectively. These rates were not significantly different. On multivariate analysis, the two factors associated with increased 30-day mortality were patient age greater than 60 years and sepsis based on sequential organ failure assessment.
Several previous studies have associated IDC advice with improved outcomes in S. aureus bacteremia, according to Dr. Sherbuk, but this study suggests that the AST “can be a meaningful adjunct” to IDC guidance to improve outcomes. She noted that several other sets of data presented at this year’s ID Week also associated AST with improved infection management.
SAN DIEGO – Thirty-day mortality associated with Staphylococcus aureus bacteremia is reduced if there is guidance from either an antimicrobial stewardship team (AST) or an infectious disease consultant (IDC), according to a multivariate experience at Yale New Haven Hospital presented at an annual scientific meeting on infectious diseases.
“This has been a hot area, because there have been a lot of recent studies suggesting that expert infectious disease advice improves care, but not every study has associated expert advice with a mortality benefit,” said Jacqueline Sherbuk, MD, a fellow in the division of infectious diseases and international health at the University of Virginia, Charlottesville. She was a resident at Yale University when this study was conducted.
In this study, the impact of an IDC on outcome in patients with S. aureus bacteremia was evaluated relative to no expert advice. By itself, an IDC was associated with improved adherence to standards of care for S. aureus bacteremia management, but the reduction in mortality was not statistically significant for those who received IDC guidance relative to those who did not.
“Given that patient care may be guided by consultations from the AST independent of IDC, we looked at the overall impact of expert opinion versus no expert involvement, and this achieved significance on multivariate analysis,” Dr. Sherbuk reported.
For adherence to guidelines, IDC guidance was better than no expert advice on multiple measures, including proportion obtaining an echocardiogram (89% vs. 67%; P less than .001), appropriate definitive antibiotics (98% vs. 80%; P less than .001), and appropriate treatment duration (92% vs. 35%; P less than .001). However, the advantage for 30-day mortality rates was only a trend (11% vs. 21%, P = .07). It was only when patients who received IDC guidance or a consultation from the AST were combined that the difference climbed to significance (11% vs. 23%; P = .04).
“On multivariate analysis, the OR [odds ratio] was substantial, predicting a 60% reduction [OR 0.40; P = .03) in 30-day mortality for expert advice vs. no expert advice,” Dr. Sherbuk reported.
In this retrospective observational study, 261 unique cases of S. aureus bacteremia cases in adult patients established with positive blood cultures were evaluated. The cases were collected over a 1-year period at Yale New Haven Hospital. After exclusion of those who died within 3 days of the initial positive culture or who were transferred to other facilities, 236 were included in this analysis.
IDC guidance, which is not required for S. aureus bacteremia at Yale New Haven Hospital, was provided for 74.5% of the patients. Another 4% of patients received guidance from the AST, which is an independent service often provided prior to IDC guidance, according to Dr. Sherbuk.
Relapse (3% vs. 5%) and reinfection (6% vs. 4%) rates were low in both those who did and did not receive expert advice, respectively. These rates were not significantly different. On multivariate analysis, the two factors associated with increased 30-day mortality were patient age greater than 60 years and sepsis based on sequential organ failure assessment.
Several previous studies have associated IDC advice with improved outcomes in S. aureus bacteremia, according to Dr. Sherbuk, but this study suggests that the AST “can be a meaningful adjunct” to IDC guidance to improve outcomes. She noted that several other sets of data presented at this year’s ID Week also associated AST with improved infection management.
AT ID WEEK 2017
Key clinical point:
Major finding: For those receiving expert involvement in S. aureus bacteremia management, the odds ratio for 30-day mortality was reduced 60% (OR 0.40; P = .03) on multivariate analysis.
Data source: Retrospective, single-center study exploring the management of 236 S. aureus bacteremia cases in adult patients.
Disclosures: Dr. Sherbuk reported no financial relationships relevant to this study.
Cases of Legionnaires’ continue to rise in the United States
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
REPORTING FROM ID WEEK 2017