User login
Chronic Pain: How to Approach These 3 Common Conditions
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
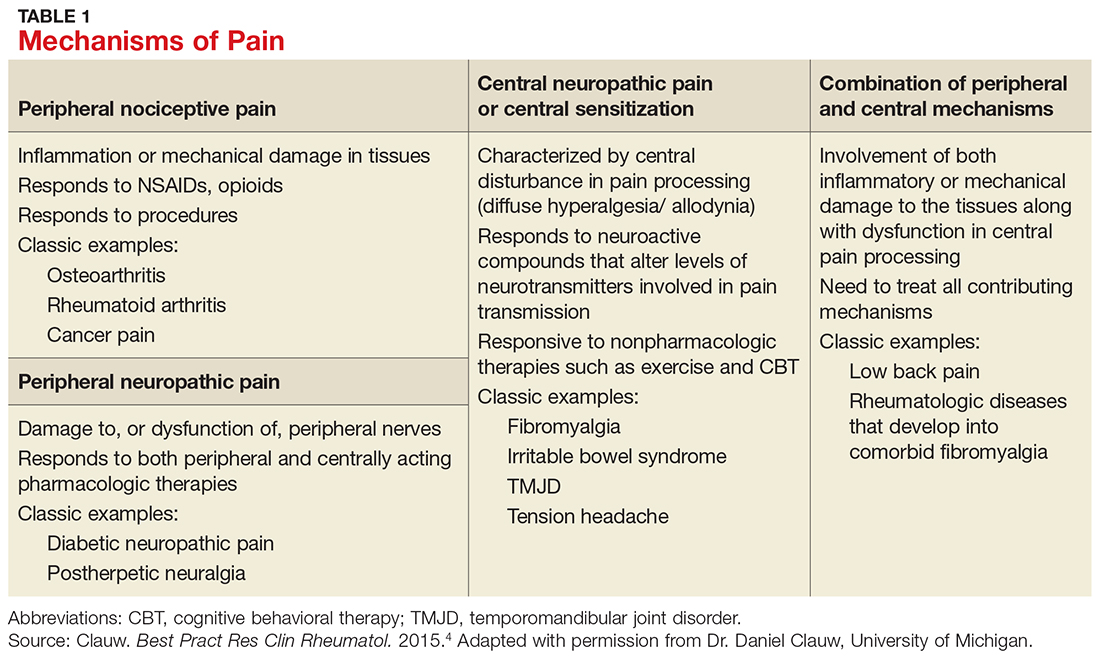
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
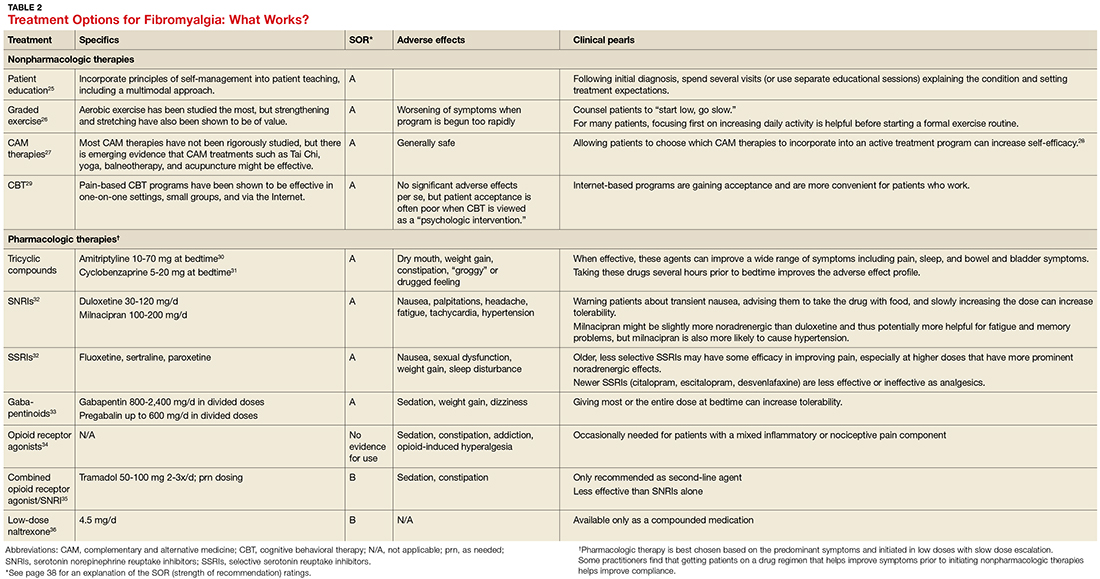
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
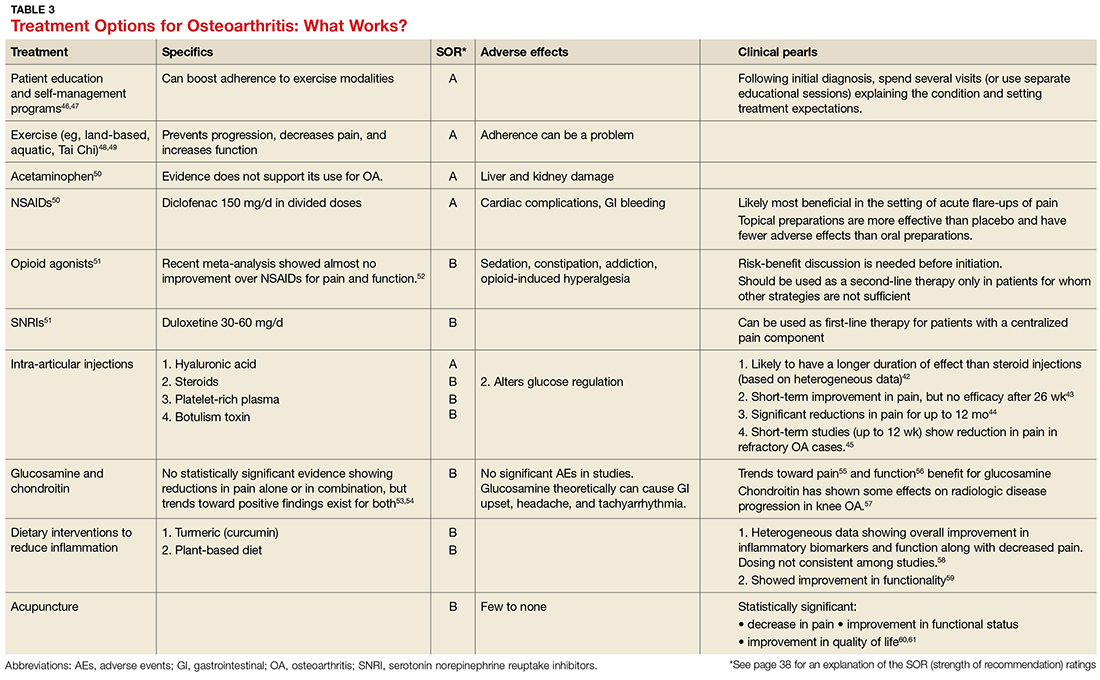
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
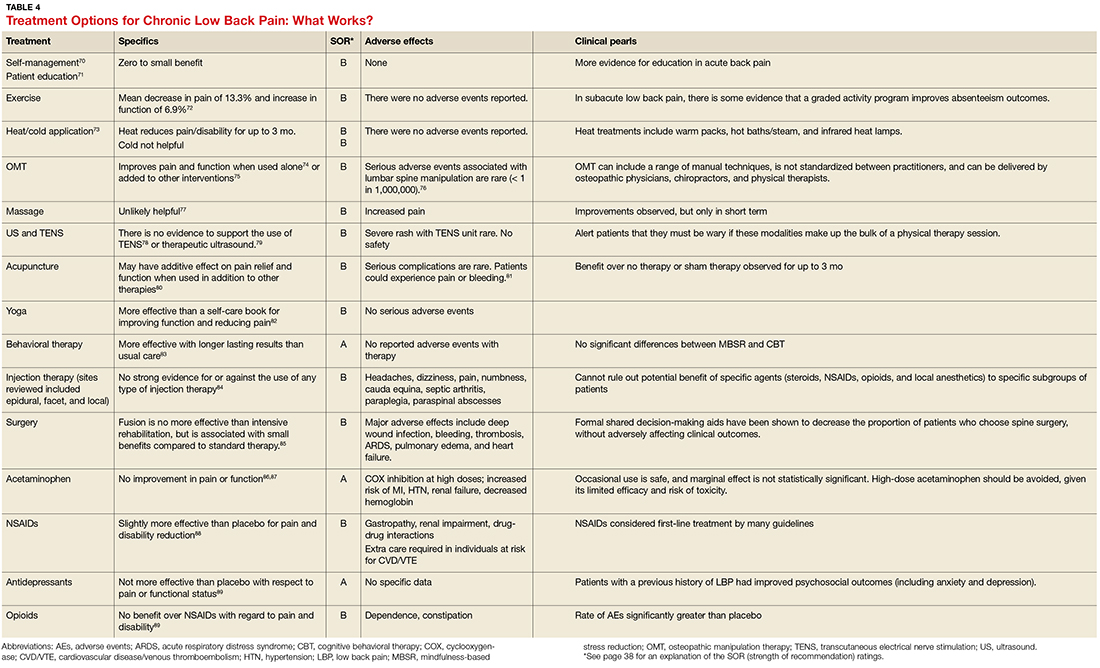
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
Reconstruction becoming more common after mastectomy
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
PFS better with first-line pazopanib vs. sorafenib in mRCC
MADRID – The drug sequence matters when it comes to the treatment of advanced or metastatic renal cell carcinoma (mRCC) investigators have found.
The median progression-free survival (PFS) for patients treated with first-line pazopanib (Votrient) followed by sorafenib (Nexavar) as a second-line therapy at the time of first progression was 12.9 months, compared with 8.6 months for patients started on sorafenib and then crossed over to pazopanib at progression, reported Margitta Retz, MD, of the Technical University of Munich.
The trial design specified that to show noninferiority for the sorafenib-pazopanib combination, the upper limit of the one-sided 95% confidence interval of the hazard ratio would have to be less than 1.225, but the upper limit was instead 1.68.
However, two key secondary endpoints – progression-free survival during first line-therapy and disease-control rate – clearly favored the pazopanib-first sequence, although there were no significant differences in overall survival (OS), Dr. Retz said at the European Society for Medical Oncology Congress.
SWITCH-2 was a phase 3, randomized, open-label crossover trial designed to test the combination and sequencing of sorafenib and pazopanib, each of which is approved for the treatment of mRCC.
In the SWITCH-1 trial, investigators looked at sorafenib followed by sunitinib (Sutent) or vice-versa and found no significant differences in either total PFS or OS. Based on these results, they conducted a similar trial using sorafenib and pazopanib.
A total of 377 patients in Germany, Austria, and the Netherlands with mRCC who were not good candidates for cytokines and had no prior systemic therapies were enrolled and randomly assigned to sorafenib 400 mg twice daily or pazopanib 800 mg once daily until progression or intolerable toxicity. In each arm, patients were crossed over to the other drug at the time of first progression.
After 42 months of follow-up, the hazard ratio for the primary endpoint of total PFS was 1.36 trending in favor of pazopanib.
For the secondary endpoint of first-line PFS, pazopanib-first was clearly superior, with a median PFS of 9.3 months, compared with 5.6 months for sorafenib-first (HR, 1.56, P = .0017). There was no difference in second-line PFS, at 2.2 vs. 2.9 months, respectively.
Overall survival trended in favor of up-front pazopanib, with a median of 28 months vs. a median of 22.7 months for up-front sorafenib, but this difference, as noted before, was not significant.
An analysis of tumor response and disease-control rates showed that in the first line, the pazopanib-sorafenib sequence was associated with a disease-control rate (composite of complete and partial responses and stable disease) of 77.7%, compared with 67.7% for sorafenib-pazopanib (P = .0304).
In the second line, however, the disease control rate favored the sorafenib-followed-by-pazopanib arm, at 56.6% vs. 43.6% (P = .0112).
A subgroup analysis showed that, in terms of total PFS, the pazopanib-sorafenib sequence offered greater benefit to patients older than 65, those with favorable Memorial Sloan Kettering Cancer Center (MSKCC/Motzer) scores, patients with good Karnofsky Performance Status, and patients whose tumors had a non–clear cell histology.
In each study arm, adverse events were more commonly seen during first-line therapy. With sorafenib, the most frequent adverse events were hand-foot skin reaction, alopecia, and rash. For pazopanib, the most common adverse events were fatigue, hypertension, nausea, abdominal pain, and elevation of liver enzymes.
Although the investigators reported no differences in overall survival, “I question that: I think half a year of survival is a meaningful difference. Although it’s statistically insignificant, it might be important for the patients,” he said.
He suggested that ESMO guidelines regarding treatment of patients with mRCC should be revised to reflect data from this and other trials suggesting that sorafenib should be dropped as a treatment option in either first- or second-line therapy.
The trial was supported by grants from Bayer and Novartis. Dr. Retz disclosed honoraria from those companies and others, and advisory board participation for other drug makers. Dr. Staehler disclosed but did not specify relationships with Bayer, Novartis, and others.
MADRID – The drug sequence matters when it comes to the treatment of advanced or metastatic renal cell carcinoma (mRCC) investigators have found.
The median progression-free survival (PFS) for patients treated with first-line pazopanib (Votrient) followed by sorafenib (Nexavar) as a second-line therapy at the time of first progression was 12.9 months, compared with 8.6 months for patients started on sorafenib and then crossed over to pazopanib at progression, reported Margitta Retz, MD, of the Technical University of Munich.
The trial design specified that to show noninferiority for the sorafenib-pazopanib combination, the upper limit of the one-sided 95% confidence interval of the hazard ratio would have to be less than 1.225, but the upper limit was instead 1.68.
However, two key secondary endpoints – progression-free survival during first line-therapy and disease-control rate – clearly favored the pazopanib-first sequence, although there were no significant differences in overall survival (OS), Dr. Retz said at the European Society for Medical Oncology Congress.
SWITCH-2 was a phase 3, randomized, open-label crossover trial designed to test the combination and sequencing of sorafenib and pazopanib, each of which is approved for the treatment of mRCC.
In the SWITCH-1 trial, investigators looked at sorafenib followed by sunitinib (Sutent) or vice-versa and found no significant differences in either total PFS or OS. Based on these results, they conducted a similar trial using sorafenib and pazopanib.
A total of 377 patients in Germany, Austria, and the Netherlands with mRCC who were not good candidates for cytokines and had no prior systemic therapies were enrolled and randomly assigned to sorafenib 400 mg twice daily or pazopanib 800 mg once daily until progression or intolerable toxicity. In each arm, patients were crossed over to the other drug at the time of first progression.
After 42 months of follow-up, the hazard ratio for the primary endpoint of total PFS was 1.36 trending in favor of pazopanib.
For the secondary endpoint of first-line PFS, pazopanib-first was clearly superior, with a median PFS of 9.3 months, compared with 5.6 months for sorafenib-first (HR, 1.56, P = .0017). There was no difference in second-line PFS, at 2.2 vs. 2.9 months, respectively.
Overall survival trended in favor of up-front pazopanib, with a median of 28 months vs. a median of 22.7 months for up-front sorafenib, but this difference, as noted before, was not significant.
An analysis of tumor response and disease-control rates showed that in the first line, the pazopanib-sorafenib sequence was associated with a disease-control rate (composite of complete and partial responses and stable disease) of 77.7%, compared with 67.7% for sorafenib-pazopanib (P = .0304).
In the second line, however, the disease control rate favored the sorafenib-followed-by-pazopanib arm, at 56.6% vs. 43.6% (P = .0112).
A subgroup analysis showed that, in terms of total PFS, the pazopanib-sorafenib sequence offered greater benefit to patients older than 65, those with favorable Memorial Sloan Kettering Cancer Center (MSKCC/Motzer) scores, patients with good Karnofsky Performance Status, and patients whose tumors had a non–clear cell histology.
In each study arm, adverse events were more commonly seen during first-line therapy. With sorafenib, the most frequent adverse events were hand-foot skin reaction, alopecia, and rash. For pazopanib, the most common adverse events were fatigue, hypertension, nausea, abdominal pain, and elevation of liver enzymes.
Although the investigators reported no differences in overall survival, “I question that: I think half a year of survival is a meaningful difference. Although it’s statistically insignificant, it might be important for the patients,” he said.
He suggested that ESMO guidelines regarding treatment of patients with mRCC should be revised to reflect data from this and other trials suggesting that sorafenib should be dropped as a treatment option in either first- or second-line therapy.
The trial was supported by grants from Bayer and Novartis. Dr. Retz disclosed honoraria from those companies and others, and advisory board participation for other drug makers. Dr. Staehler disclosed but did not specify relationships with Bayer, Novartis, and others.
MADRID – The drug sequence matters when it comes to the treatment of advanced or metastatic renal cell carcinoma (mRCC) investigators have found.
The median progression-free survival (PFS) for patients treated with first-line pazopanib (Votrient) followed by sorafenib (Nexavar) as a second-line therapy at the time of first progression was 12.9 months, compared with 8.6 months for patients started on sorafenib and then crossed over to pazopanib at progression, reported Margitta Retz, MD, of the Technical University of Munich.
The trial design specified that to show noninferiority for the sorafenib-pazopanib combination, the upper limit of the one-sided 95% confidence interval of the hazard ratio would have to be less than 1.225, but the upper limit was instead 1.68.
However, two key secondary endpoints – progression-free survival during first line-therapy and disease-control rate – clearly favored the pazopanib-first sequence, although there were no significant differences in overall survival (OS), Dr. Retz said at the European Society for Medical Oncology Congress.
SWITCH-2 was a phase 3, randomized, open-label crossover trial designed to test the combination and sequencing of sorafenib and pazopanib, each of which is approved for the treatment of mRCC.
In the SWITCH-1 trial, investigators looked at sorafenib followed by sunitinib (Sutent) or vice-versa and found no significant differences in either total PFS or OS. Based on these results, they conducted a similar trial using sorafenib and pazopanib.
A total of 377 patients in Germany, Austria, and the Netherlands with mRCC who were not good candidates for cytokines and had no prior systemic therapies were enrolled and randomly assigned to sorafenib 400 mg twice daily or pazopanib 800 mg once daily until progression or intolerable toxicity. In each arm, patients were crossed over to the other drug at the time of first progression.
After 42 months of follow-up, the hazard ratio for the primary endpoint of total PFS was 1.36 trending in favor of pazopanib.
For the secondary endpoint of first-line PFS, pazopanib-first was clearly superior, with a median PFS of 9.3 months, compared with 5.6 months for sorafenib-first (HR, 1.56, P = .0017). There was no difference in second-line PFS, at 2.2 vs. 2.9 months, respectively.
Overall survival trended in favor of up-front pazopanib, with a median of 28 months vs. a median of 22.7 months for up-front sorafenib, but this difference, as noted before, was not significant.
An analysis of tumor response and disease-control rates showed that in the first line, the pazopanib-sorafenib sequence was associated with a disease-control rate (composite of complete and partial responses and stable disease) of 77.7%, compared with 67.7% for sorafenib-pazopanib (P = .0304).
In the second line, however, the disease control rate favored the sorafenib-followed-by-pazopanib arm, at 56.6% vs. 43.6% (P = .0112).
A subgroup analysis showed that, in terms of total PFS, the pazopanib-sorafenib sequence offered greater benefit to patients older than 65, those with favorable Memorial Sloan Kettering Cancer Center (MSKCC/Motzer) scores, patients with good Karnofsky Performance Status, and patients whose tumors had a non–clear cell histology.
In each study arm, adverse events were more commonly seen during first-line therapy. With sorafenib, the most frequent adverse events were hand-foot skin reaction, alopecia, and rash. For pazopanib, the most common adverse events were fatigue, hypertension, nausea, abdominal pain, and elevation of liver enzymes.
Although the investigators reported no differences in overall survival, “I question that: I think half a year of survival is a meaningful difference. Although it’s statistically insignificant, it might be important for the patients,” he said.
He suggested that ESMO guidelines regarding treatment of patients with mRCC should be revised to reflect data from this and other trials suggesting that sorafenib should be dropped as a treatment option in either first- or second-line therapy.
The trial was supported by grants from Bayer and Novartis. Dr. Retz disclosed honoraria from those companies and others, and advisory board participation for other drug makers. Dr. Staehler disclosed but did not specify relationships with Bayer, Novartis, and others.
AT ESMO 2017
Key clinical point: than when the drug sequence was reversed.
Major finding: Total PFS with sorafenib-pazopanib did not meet its primary endpoint of noninferiority to pazopanib-sorafenib.
Data source: Randomized, sequential open-label, phase 3 trial in 377 patients with advanced/metastatic RCC.
Disclosures: The trial was supported by grants from Bayer and Novartis. Dr. Retz disclosed honoraria from those companies and others, and advisory board participation for other drug makers. Dr. Staehler disclosed but did not specify relationships with Bayer, Novartis, and others.
Painless Telangiectatic Lesion on the Wrist
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.
Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.


Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.


Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
A 91-year-old white man with a history of atrial fibrillation, benign prostatic hyperplasia, dysphagia, gastroesophageal reflux disease, hypertension, hypothyroidism, osteoarthritis, and laryngeal cancer presented with an 8-mm firm, painless, pink lesion with telangiectasia on the left wrist. The lesion had been present for an unknown period of time and was asymptomatic at presentation.
Eye movement, not CT or MRI, rules out posterior stroke
SAN DIEGO – When a patient with vertigo and dizziness presents to the emergency department, the first order of business is to figure out if they’re due to benign inner ear problems or a posterior fossa stroke.
Emergency department physicians usually use noncontrast CT (NCCT) to rule out stroke, while neurologists turn to MRI with diffusion-weighted imaging (MRI-DWI) to make the call.
Neither are good enough. The sensitivity of NCCT for acute posterior fossa strokes is just 30.8%. The sensitivity of MRI-DWI is better at 76.4%, but “false negatives [occur] in roughly one [of] four brainstem strokes in the first 48 hours,” according to a meta-analysis from Johns Hopkins University, Baltimore. The study, presented at the annual meeting of the American Neurological Association, involved more than 800 patients in the 14 strongest studies to look into the issue since 1990.
“Anterior circulation strokes are obvious, but posterior circulation strokes are subtle. We’ve surveyed ED physicians, and there’s a certain rate in the population who just don’t understand how bad CTs are for detecting acute stroke.” Meanwhile, “neurologists know” they can’t rely on CTs, “but they think MRIs are good enough. That turns out not to be true either,” he said.
Dr. Newman-Toker’s comments were sparked by a discussion about the meta-analysis, but he spoke from years of work trying to improve the situation. He was clear about what’s at stake: The early recognition of posterior fossa strokes, treatment with thrombolytics and surgical decompression (when warranted), and prevention of a second, larger stroke, can mean the difference between walking out of the hospital and dying or being wheelchair bound for life.
He and his colleagues have developed a way to detect posterior fossa strokes using eye movement abnormalities. They call it HINTS, which stands for “head impulse, nystagmus, and test of skew. “It turns out that the eye movements of inner ear disease look slightly different than the eye movements of brain disease; the subtle differences are enough to distinguish between the two.” When the technique is mastered, “our best estimate is that the sensitivity for posterior circulation stroke is around 99%,” Dr. Newman-Toker said. He is working to get the message out and train people; a video of the technique is online (Semin Neurol. 2015 Oct;35[5]:506-21).
As for the meta-analysis, “none of us were terribly surprised that CT wasn’t much good, and I knew that MRI wasn’t going to be perfect, but it was worse than I expected when we crunched all the numbers,” he said.
It’s no surprise that eye movement trumps imaging. “Physiology beats anatomy in the acute phase. It’s takes a little while for anatomy to change” on imaging, but eye movements change immediately “when patients become symptomatic with dizziness and vertigo,” he said.
Meanwhile, “if you don’t know how to evaluate peoples’ eye movements, I think MRIs are the next best thing,” he said.
There was no industry funding for the work. Johns Hopkins is working with a company called Natus to develop HINTS training. Dr. Newman-Toker might earn royalties, but so far “I haven’t made any money off it, and there’s no document saying I’m ever going to make any money from it,” he said.
SAN DIEGO – When a patient with vertigo and dizziness presents to the emergency department, the first order of business is to figure out if they’re due to benign inner ear problems or a posterior fossa stroke.
Emergency department physicians usually use noncontrast CT (NCCT) to rule out stroke, while neurologists turn to MRI with diffusion-weighted imaging (MRI-DWI) to make the call.
Neither are good enough. The sensitivity of NCCT for acute posterior fossa strokes is just 30.8%. The sensitivity of MRI-DWI is better at 76.4%, but “false negatives [occur] in roughly one [of] four brainstem strokes in the first 48 hours,” according to a meta-analysis from Johns Hopkins University, Baltimore. The study, presented at the annual meeting of the American Neurological Association, involved more than 800 patients in the 14 strongest studies to look into the issue since 1990.
“Anterior circulation strokes are obvious, but posterior circulation strokes are subtle. We’ve surveyed ED physicians, and there’s a certain rate in the population who just don’t understand how bad CTs are for detecting acute stroke.” Meanwhile, “neurologists know” they can’t rely on CTs, “but they think MRIs are good enough. That turns out not to be true either,” he said.
Dr. Newman-Toker’s comments were sparked by a discussion about the meta-analysis, but he spoke from years of work trying to improve the situation. He was clear about what’s at stake: The early recognition of posterior fossa strokes, treatment with thrombolytics and surgical decompression (when warranted), and prevention of a second, larger stroke, can mean the difference between walking out of the hospital and dying or being wheelchair bound for life.
He and his colleagues have developed a way to detect posterior fossa strokes using eye movement abnormalities. They call it HINTS, which stands for “head impulse, nystagmus, and test of skew. “It turns out that the eye movements of inner ear disease look slightly different than the eye movements of brain disease; the subtle differences are enough to distinguish between the two.” When the technique is mastered, “our best estimate is that the sensitivity for posterior circulation stroke is around 99%,” Dr. Newman-Toker said. He is working to get the message out and train people; a video of the technique is online (Semin Neurol. 2015 Oct;35[5]:506-21).
As for the meta-analysis, “none of us were terribly surprised that CT wasn’t much good, and I knew that MRI wasn’t going to be perfect, but it was worse than I expected when we crunched all the numbers,” he said.
It’s no surprise that eye movement trumps imaging. “Physiology beats anatomy in the acute phase. It’s takes a little while for anatomy to change” on imaging, but eye movements change immediately “when patients become symptomatic with dizziness and vertigo,” he said.
Meanwhile, “if you don’t know how to evaluate peoples’ eye movements, I think MRIs are the next best thing,” he said.
There was no industry funding for the work. Johns Hopkins is working with a company called Natus to develop HINTS training. Dr. Newman-Toker might earn royalties, but so far “I haven’t made any money off it, and there’s no document saying I’m ever going to make any money from it,” he said.
SAN DIEGO – When a patient with vertigo and dizziness presents to the emergency department, the first order of business is to figure out if they’re due to benign inner ear problems or a posterior fossa stroke.
Emergency department physicians usually use noncontrast CT (NCCT) to rule out stroke, while neurologists turn to MRI with diffusion-weighted imaging (MRI-DWI) to make the call.
Neither are good enough. The sensitivity of NCCT for acute posterior fossa strokes is just 30.8%. The sensitivity of MRI-DWI is better at 76.4%, but “false negatives [occur] in roughly one [of] four brainstem strokes in the first 48 hours,” according to a meta-analysis from Johns Hopkins University, Baltimore. The study, presented at the annual meeting of the American Neurological Association, involved more than 800 patients in the 14 strongest studies to look into the issue since 1990.
“Anterior circulation strokes are obvious, but posterior circulation strokes are subtle. We’ve surveyed ED physicians, and there’s a certain rate in the population who just don’t understand how bad CTs are for detecting acute stroke.” Meanwhile, “neurologists know” they can’t rely on CTs, “but they think MRIs are good enough. That turns out not to be true either,” he said.
Dr. Newman-Toker’s comments were sparked by a discussion about the meta-analysis, but he spoke from years of work trying to improve the situation. He was clear about what’s at stake: The early recognition of posterior fossa strokes, treatment with thrombolytics and surgical decompression (when warranted), and prevention of a second, larger stroke, can mean the difference between walking out of the hospital and dying or being wheelchair bound for life.
He and his colleagues have developed a way to detect posterior fossa strokes using eye movement abnormalities. They call it HINTS, which stands for “head impulse, nystagmus, and test of skew. “It turns out that the eye movements of inner ear disease look slightly different than the eye movements of brain disease; the subtle differences are enough to distinguish between the two.” When the technique is mastered, “our best estimate is that the sensitivity for posterior circulation stroke is around 99%,” Dr. Newman-Toker said. He is working to get the message out and train people; a video of the technique is online (Semin Neurol. 2015 Oct;35[5]:506-21).
As for the meta-analysis, “none of us were terribly surprised that CT wasn’t much good, and I knew that MRI wasn’t going to be perfect, but it was worse than I expected when we crunched all the numbers,” he said.
It’s no surprise that eye movement trumps imaging. “Physiology beats anatomy in the acute phase. It’s takes a little while for anatomy to change” on imaging, but eye movements change immediately “when patients become symptomatic with dizziness and vertigo,” he said.
Meanwhile, “if you don’t know how to evaluate peoples’ eye movements, I think MRIs are the next best thing,” he said.
There was no industry funding for the work. Johns Hopkins is working with a company called Natus to develop HINTS training. Dr. Newman-Toker might earn royalties, but so far “I haven’t made any money off it, and there’s no document saying I’m ever going to make any money from it,” he said.
AT ANA 2017
Key clinical point:
Major finding: The sensitivity of CT for acute posterior strokes was just 30.8%. The sensitivity of MRI was better at 76.4%, but it still missed one in four.
Data source: Meta-analysis of more than 800 patients
Disclosures: There was no industry funding for the work. The senior investigator might profit from commercialization of HINTS training.
CDC data show decline in some hospital-acquired infections
SAN DIEGO – There was an encouraging 22% reduction in hospital-acquired infections (HAIs) after adjustment for clinical variables when 2015 and 2011 data from national Centers for Disease Control and Prevention hospital surveys were compared.
“The data suggest that national efforts toward preventing HAIs are succeeding,” reported Shelley S. Magill, MD, PhD, a medical epidemiologist in the Division of Healthcare Quality Promotion at the CDC who summarized the data at an annual scientific meeting on infectious diseases .
The comparative data were drawn from point prevalence surveys conducted in 2011 and 2015 as part of the CDC’s Emerging Infections Program. In this type of survey, the data are collected over 1 day, providing a snapshot in time among selected hospitals. The analysis presented by Dr. Magill was restricted to the 148 hospitals that participated in both the 2011 and 2015 surveys, although the 2015 survey included a total of 199 hospitals, of which other data analyses are planned.
Due to the change in incidence, the rank order of HAIs was different in 2015 relative to 2011. While surgical site infections (SSIs) represented the most frequent HAI in 2011, they fell to the third most frequent HAI in 2015; pneumonia and gastrointestinal (GI) infections assumed the first and second spots, respectively. The GI HAI infection category includes Clostridium difficile infection.
The incidence of SSI HAI among all hospitalized patients in the survey fell by 41% between 2011 and 2015 (from 1.00% to 0.59%; P = .001). The other big contributor to the overall reduction in HAIs was the fall in the incidence of urinary tract infections, which fell 36% (from 0.55% to 0.35%; P = .04). The decrease in pneumonia (from 0.97% to 0.89%) was not significant, nor was the even more modest reduction in bloodstream HAI (from 0.45% to 0.43%). There was a modest increase in GI/Clostridium difficile infections (from 0.56% to 0.59%).
The surveys do not permit the reduction in HAI rates to be attributed to any specific prevention practices, but Dr. Magill pointed out that the overall reductions correlate with reduced use of urinary catheters and central lines; reductions of both have been advocated as a means for improved infection control. Of several factors that might contribute to a reduction in SSI HAI, Dr. Magill speculated that better adherence to guidelines and more rigorous steps at preoperative infection control strategies might be among them.
Detailed analyses of the data collected from all of the hospitals that participated in the 2015 survey are planned, including an evaluation of which antibiotics were used to treat the HAIs found in this survey. Although the findings so far encourage speculation that infection control practices, such as prudent use of urinary catheters, are having a positive effect, Dr. Magill said that the data also point out the challenges.
“Given that pneumonia continues to represent a large proportion of HAIs in hospitals, more work is needed to identify risk factors; understand the factors that are preventable, particularly in the nonventilated patients; and develop better preventive approaches,” Dr. Magill said.
Dr. Magill reported no financial relationships relevant to this study.
SAN DIEGO – There was an encouraging 22% reduction in hospital-acquired infections (HAIs) after adjustment for clinical variables when 2015 and 2011 data from national Centers for Disease Control and Prevention hospital surveys were compared.
“The data suggest that national efforts toward preventing HAIs are succeeding,” reported Shelley S. Magill, MD, PhD, a medical epidemiologist in the Division of Healthcare Quality Promotion at the CDC who summarized the data at an annual scientific meeting on infectious diseases .
The comparative data were drawn from point prevalence surveys conducted in 2011 and 2015 as part of the CDC’s Emerging Infections Program. In this type of survey, the data are collected over 1 day, providing a snapshot in time among selected hospitals. The analysis presented by Dr. Magill was restricted to the 148 hospitals that participated in both the 2011 and 2015 surveys, although the 2015 survey included a total of 199 hospitals, of which other data analyses are planned.
Due to the change in incidence, the rank order of HAIs was different in 2015 relative to 2011. While surgical site infections (SSIs) represented the most frequent HAI in 2011, they fell to the third most frequent HAI in 2015; pneumonia and gastrointestinal (GI) infections assumed the first and second spots, respectively. The GI HAI infection category includes Clostridium difficile infection.
The incidence of SSI HAI among all hospitalized patients in the survey fell by 41% between 2011 and 2015 (from 1.00% to 0.59%; P = .001). The other big contributor to the overall reduction in HAIs was the fall in the incidence of urinary tract infections, which fell 36% (from 0.55% to 0.35%; P = .04). The decrease in pneumonia (from 0.97% to 0.89%) was not significant, nor was the even more modest reduction in bloodstream HAI (from 0.45% to 0.43%). There was a modest increase in GI/Clostridium difficile infections (from 0.56% to 0.59%).
The surveys do not permit the reduction in HAI rates to be attributed to any specific prevention practices, but Dr. Magill pointed out that the overall reductions correlate with reduced use of urinary catheters and central lines; reductions of both have been advocated as a means for improved infection control. Of several factors that might contribute to a reduction in SSI HAI, Dr. Magill speculated that better adherence to guidelines and more rigorous steps at preoperative infection control strategies might be among them.
Detailed analyses of the data collected from all of the hospitals that participated in the 2015 survey are planned, including an evaluation of which antibiotics were used to treat the HAIs found in this survey. Although the findings so far encourage speculation that infection control practices, such as prudent use of urinary catheters, are having a positive effect, Dr. Magill said that the data also point out the challenges.
“Given that pneumonia continues to represent a large proportion of HAIs in hospitals, more work is needed to identify risk factors; understand the factors that are preventable, particularly in the nonventilated patients; and develop better preventive approaches,” Dr. Magill said.
Dr. Magill reported no financial relationships relevant to this study.
SAN DIEGO – There was an encouraging 22% reduction in hospital-acquired infections (HAIs) after adjustment for clinical variables when 2015 and 2011 data from national Centers for Disease Control and Prevention hospital surveys were compared.
“The data suggest that national efforts toward preventing HAIs are succeeding,” reported Shelley S. Magill, MD, PhD, a medical epidemiologist in the Division of Healthcare Quality Promotion at the CDC who summarized the data at an annual scientific meeting on infectious diseases .
The comparative data were drawn from point prevalence surveys conducted in 2011 and 2015 as part of the CDC’s Emerging Infections Program. In this type of survey, the data are collected over 1 day, providing a snapshot in time among selected hospitals. The analysis presented by Dr. Magill was restricted to the 148 hospitals that participated in both the 2011 and 2015 surveys, although the 2015 survey included a total of 199 hospitals, of which other data analyses are planned.
Due to the change in incidence, the rank order of HAIs was different in 2015 relative to 2011. While surgical site infections (SSIs) represented the most frequent HAI in 2011, they fell to the third most frequent HAI in 2015; pneumonia and gastrointestinal (GI) infections assumed the first and second spots, respectively. The GI HAI infection category includes Clostridium difficile infection.
The incidence of SSI HAI among all hospitalized patients in the survey fell by 41% between 2011 and 2015 (from 1.00% to 0.59%; P = .001). The other big contributor to the overall reduction in HAIs was the fall in the incidence of urinary tract infections, which fell 36% (from 0.55% to 0.35%; P = .04). The decrease in pneumonia (from 0.97% to 0.89%) was not significant, nor was the even more modest reduction in bloodstream HAI (from 0.45% to 0.43%). There was a modest increase in GI/Clostridium difficile infections (from 0.56% to 0.59%).
The surveys do not permit the reduction in HAI rates to be attributed to any specific prevention practices, but Dr. Magill pointed out that the overall reductions correlate with reduced use of urinary catheters and central lines; reductions of both have been advocated as a means for improved infection control. Of several factors that might contribute to a reduction in SSI HAI, Dr. Magill speculated that better adherence to guidelines and more rigorous steps at preoperative infection control strategies might be among them.
Detailed analyses of the data collected from all of the hospitals that participated in the 2015 survey are planned, including an evaluation of which antibiotics were used to treat the HAIs found in this survey. Although the findings so far encourage speculation that infection control practices, such as prudent use of urinary catheters, are having a positive effect, Dr. Magill said that the data also point out the challenges.
“Given that pneumonia continues to represent a large proportion of HAIs in hospitals, more work is needed to identify risk factors; understand the factors that are preventable, particularly in the nonventilated patients; and develop better preventive approaches,” Dr. Magill said.
Dr. Magill reported no financial relationships relevant to this study.
AT ID WEEK 2017
Key clinical point:
Major finding: In two point prevalence surveys conducted in the same hospitals, the rate of HAI was 22% lower in 2015 (P = .001), compared with 2011.
Data source: CDC national surveys of HAIs in 148 hospitals in two different years (2011 and 2015) were compared.
Disclosures: Dr. Magill reported no financial relationships relevant to this study.
ExteNET: Benefit of extended neratinib in HER2+ breast cancer sustained
MADRID – who received postoperative trastuzumab (Herceptin) and chemotherapy, long-term follow-up results from the ExteNET trial show.
In a planned intention-to-treat analysis at 5 years of follow-up, extended adjuvant therapy with the tyrosine kinase inhibitor neratinib was associated with a small but significant improvement in invasive disease-free survival (iDFS), compared with placebo, with most of the benefit occurring in women with hormone receptor–positive disease, reported Miguel Martin, MD, of the Gregorio Marañón Health Research Institute in Madrid.
Data from an earlier analysis of the trial supported the Food and Drug Administration’s decision to approve neratinib in the extended adjuvant setting in July 2017.
In the ExteNET trial, 2,840 women with early HER2-positive breast cancer who had undergone surgery and adjuvant treatment with trastuzumab and chemotherapy were stratified by nodal and hormone receptor status and by concurrent vs. sequential chemotherapy and trastuzumab, and were then randomly assigned to receive oral neratinib 240 mg/day for 1 year, or placebo. Analyses of iDFS were planned for 2 and 5 years, and an overall survival analysis was planned after 248 patient deaths had occurred. Overall survival data have not matured as yet, Dr. Martin noted.
Results of an unspecified 3-year analysis of the trial, presented at the San Antonio Breast Cancer Symposium in 2015, showed a continued benefit for the addition of neratinib, a finding that has now been extended out to 5 years.
At ESMO 2017, Dr. Martin presented data on all efficacy endpoints except overall survival in the intention-to-treat population.
By the cutoff date in March 2017, 2,117 of the original 2,840 patients (76%) gave consent for collection of additional data, including 1,028 who had been assigned to neratinib, and 1,089 assigned to placebo.
The 5-year iDFS rate was 90.2% for patients assigned to neratinib, compared with 87.7% for those assigned to placebo, an absolute difference of 2.5%. This translated into a hazard ratio favoring neratinib of 0.73 (P = .008). Neratinib was also significantly better than placebo for DFS in patients with ductal carcinoma in situ (89.7% vs. 86.8, HR, 0.71, P = .004).
However, there were no significant differences at 5 years between trial arms in either distant DFS, time to distant recurrence, or central nervous system recurrences. Dr. Martin noted that the although there were fewer CNS recurrences with neratinib (1.30% vs. 1.82%), the total number of cases was too small to detect a possible difference.
In a subgroup analysis, neratinib trended toward better performance in all categories, but was significantly better than placebo only among patients from Asia, Eastern Europe, and South America, and among patients with four or more positive lymph nodes.
An analysis of iDFS by hormone receptor status showed that for HR-positive patients, the 5-year iDFS rate was 91.2% with neratinib vs. 86.8% with placebo, translating into a hazard ratio of 0.60, P = .002). In contrast, iDFS rates were nearly identical among HR-negative patients, at 88.9% vs. 88.8%, respectively.
Following treatment discontinuation, there was no evidence of increased symptomatic cardiotoxicity or second primary malignancies vs. placebo, and no late-term consequences of neratinib-associated diarrhea, Dr. Martin said.
A separate poster on health-related quality of life, also presented at ESMO 2017, showed that patients assigned to neratinib had a drop in quality-of-life measures during the first month of treatment, possibly because of diarrhea, but then had a steady improvement toward baseline. There is an ongoing study to evaluate whether loperamide-based regimens can reduce or prevent neratinib-associated diarrhea, the investigators noted.
“In ExteNET, we’ve seen continued demonstration of clinically significant benefit, particularly in higher-risk, hormone receptor–positive disease, despite many limitations, with change in sponsor and initial plan for only 2 years of follow-up,” said Hope S. Rugo, MD, from the University of California, San Francisco, the invited discussant.
“Survival data is pending, and we’re looking forward to seeing that in 2019, but the reduction in distant events, although small, is still encouraging,” she said.
The trial is sponsored by Puma Biotechnology. Dr. Martin disclosed honoraria from Roche/Genentech, Novartis, Amgen, AstraZeneca, Pfizer, PharmaMar, and Lilly, and research grants from Roche and Novartis. Dr Rugo disclosed travel support from PUMA and Mylan, research support from Genentech/Roche, and honoraria from Biotheranostics. She also serves on the Oncology Practice Advisory Board.
MADRID – who received postoperative trastuzumab (Herceptin) and chemotherapy, long-term follow-up results from the ExteNET trial show.
In a planned intention-to-treat analysis at 5 years of follow-up, extended adjuvant therapy with the tyrosine kinase inhibitor neratinib was associated with a small but significant improvement in invasive disease-free survival (iDFS), compared with placebo, with most of the benefit occurring in women with hormone receptor–positive disease, reported Miguel Martin, MD, of the Gregorio Marañón Health Research Institute in Madrid.
Data from an earlier analysis of the trial supported the Food and Drug Administration’s decision to approve neratinib in the extended adjuvant setting in July 2017.
In the ExteNET trial, 2,840 women with early HER2-positive breast cancer who had undergone surgery and adjuvant treatment with trastuzumab and chemotherapy were stratified by nodal and hormone receptor status and by concurrent vs. sequential chemotherapy and trastuzumab, and were then randomly assigned to receive oral neratinib 240 mg/day for 1 year, or placebo. Analyses of iDFS were planned for 2 and 5 years, and an overall survival analysis was planned after 248 patient deaths had occurred. Overall survival data have not matured as yet, Dr. Martin noted.
Results of an unspecified 3-year analysis of the trial, presented at the San Antonio Breast Cancer Symposium in 2015, showed a continued benefit for the addition of neratinib, a finding that has now been extended out to 5 years.
At ESMO 2017, Dr. Martin presented data on all efficacy endpoints except overall survival in the intention-to-treat population.
By the cutoff date in March 2017, 2,117 of the original 2,840 patients (76%) gave consent for collection of additional data, including 1,028 who had been assigned to neratinib, and 1,089 assigned to placebo.
The 5-year iDFS rate was 90.2% for patients assigned to neratinib, compared with 87.7% for those assigned to placebo, an absolute difference of 2.5%. This translated into a hazard ratio favoring neratinib of 0.73 (P = .008). Neratinib was also significantly better than placebo for DFS in patients with ductal carcinoma in situ (89.7% vs. 86.8, HR, 0.71, P = .004).
However, there were no significant differences at 5 years between trial arms in either distant DFS, time to distant recurrence, or central nervous system recurrences. Dr. Martin noted that the although there were fewer CNS recurrences with neratinib (1.30% vs. 1.82%), the total number of cases was too small to detect a possible difference.
In a subgroup analysis, neratinib trended toward better performance in all categories, but was significantly better than placebo only among patients from Asia, Eastern Europe, and South America, and among patients with four or more positive lymph nodes.
An analysis of iDFS by hormone receptor status showed that for HR-positive patients, the 5-year iDFS rate was 91.2% with neratinib vs. 86.8% with placebo, translating into a hazard ratio of 0.60, P = .002). In contrast, iDFS rates were nearly identical among HR-negative patients, at 88.9% vs. 88.8%, respectively.
Following treatment discontinuation, there was no evidence of increased symptomatic cardiotoxicity or second primary malignancies vs. placebo, and no late-term consequences of neratinib-associated diarrhea, Dr. Martin said.
A separate poster on health-related quality of life, also presented at ESMO 2017, showed that patients assigned to neratinib had a drop in quality-of-life measures during the first month of treatment, possibly because of diarrhea, but then had a steady improvement toward baseline. There is an ongoing study to evaluate whether loperamide-based regimens can reduce or prevent neratinib-associated diarrhea, the investigators noted.
“In ExteNET, we’ve seen continued demonstration of clinically significant benefit, particularly in higher-risk, hormone receptor–positive disease, despite many limitations, with change in sponsor and initial plan for only 2 years of follow-up,” said Hope S. Rugo, MD, from the University of California, San Francisco, the invited discussant.
“Survival data is pending, and we’re looking forward to seeing that in 2019, but the reduction in distant events, although small, is still encouraging,” she said.
The trial is sponsored by Puma Biotechnology. Dr. Martin disclosed honoraria from Roche/Genentech, Novartis, Amgen, AstraZeneca, Pfizer, PharmaMar, and Lilly, and research grants from Roche and Novartis. Dr Rugo disclosed travel support from PUMA and Mylan, research support from Genentech/Roche, and honoraria from Biotheranostics. She also serves on the Oncology Practice Advisory Board.
MADRID – who received postoperative trastuzumab (Herceptin) and chemotherapy, long-term follow-up results from the ExteNET trial show.
In a planned intention-to-treat analysis at 5 years of follow-up, extended adjuvant therapy with the tyrosine kinase inhibitor neratinib was associated with a small but significant improvement in invasive disease-free survival (iDFS), compared with placebo, with most of the benefit occurring in women with hormone receptor–positive disease, reported Miguel Martin, MD, of the Gregorio Marañón Health Research Institute in Madrid.
Data from an earlier analysis of the trial supported the Food and Drug Administration’s decision to approve neratinib in the extended adjuvant setting in July 2017.
In the ExteNET trial, 2,840 women with early HER2-positive breast cancer who had undergone surgery and adjuvant treatment with trastuzumab and chemotherapy were stratified by nodal and hormone receptor status and by concurrent vs. sequential chemotherapy and trastuzumab, and were then randomly assigned to receive oral neratinib 240 mg/day for 1 year, or placebo. Analyses of iDFS were planned for 2 and 5 years, and an overall survival analysis was planned after 248 patient deaths had occurred. Overall survival data have not matured as yet, Dr. Martin noted.
Results of an unspecified 3-year analysis of the trial, presented at the San Antonio Breast Cancer Symposium in 2015, showed a continued benefit for the addition of neratinib, a finding that has now been extended out to 5 years.
At ESMO 2017, Dr. Martin presented data on all efficacy endpoints except overall survival in the intention-to-treat population.
By the cutoff date in March 2017, 2,117 of the original 2,840 patients (76%) gave consent for collection of additional data, including 1,028 who had been assigned to neratinib, and 1,089 assigned to placebo.
The 5-year iDFS rate was 90.2% for patients assigned to neratinib, compared with 87.7% for those assigned to placebo, an absolute difference of 2.5%. This translated into a hazard ratio favoring neratinib of 0.73 (P = .008). Neratinib was also significantly better than placebo for DFS in patients with ductal carcinoma in situ (89.7% vs. 86.8, HR, 0.71, P = .004).
However, there were no significant differences at 5 years between trial arms in either distant DFS, time to distant recurrence, or central nervous system recurrences. Dr. Martin noted that the although there were fewer CNS recurrences with neratinib (1.30% vs. 1.82%), the total number of cases was too small to detect a possible difference.
In a subgroup analysis, neratinib trended toward better performance in all categories, but was significantly better than placebo only among patients from Asia, Eastern Europe, and South America, and among patients with four or more positive lymph nodes.
An analysis of iDFS by hormone receptor status showed that for HR-positive patients, the 5-year iDFS rate was 91.2% with neratinib vs. 86.8% with placebo, translating into a hazard ratio of 0.60, P = .002). In contrast, iDFS rates were nearly identical among HR-negative patients, at 88.9% vs. 88.8%, respectively.
Following treatment discontinuation, there was no evidence of increased symptomatic cardiotoxicity or second primary malignancies vs. placebo, and no late-term consequences of neratinib-associated diarrhea, Dr. Martin said.
A separate poster on health-related quality of life, also presented at ESMO 2017, showed that patients assigned to neratinib had a drop in quality-of-life measures during the first month of treatment, possibly because of diarrhea, but then had a steady improvement toward baseline. There is an ongoing study to evaluate whether loperamide-based regimens can reduce or prevent neratinib-associated diarrhea, the investigators noted.
“In ExteNET, we’ve seen continued demonstration of clinically significant benefit, particularly in higher-risk, hormone receptor–positive disease, despite many limitations, with change in sponsor and initial plan for only 2 years of follow-up,” said Hope S. Rugo, MD, from the University of California, San Francisco, the invited discussant.
“Survival data is pending, and we’re looking forward to seeing that in 2019, but the reduction in distant events, although small, is still encouraging,” she said.
The trial is sponsored by Puma Biotechnology. Dr. Martin disclosed honoraria from Roche/Genentech, Novartis, Amgen, AstraZeneca, Pfizer, PharmaMar, and Lilly, and research grants from Roche and Novartis. Dr Rugo disclosed travel support from PUMA and Mylan, research support from Genentech/Roche, and honoraria from Biotheranostics. She also serves on the Oncology Practice Advisory Board.
AT ESMO 2017
Key clinical point: Neratinib after adjuvant trastuzumab and chemotherapy continued to show improved invasive disease-free survival at 5 years in women with early HER2+ breast cancer.
Major finding: The 5-year iDFS rate was 90.2% for patients assigned to neratinib, compared with 87.7% for those assigned to placebo.
Data source: 5-year follow-up of randomized phase 3 trial in 2,840 women with HER2+ breast cancer treated with surgery and adjuvant chemotherapy/trastuzumab.
Disclosures: The trial is sponsored by Puma Biotechnology. Dr. Martin disclosed honoraria from Roche/Genentech, Novartis, Amgen, AstraZeneca, Pfizer, PharmaMar, and Lilly, and research grants from Roche and Novartis. Dr Rugo disclosed travel support from PUMA and Mylan, research support from Genentech/Roche, and honoraria from Biotheranostics. She also serves on the Oncology Practice Advisory Board.
More IBD remissions with higher induction vedolizumab levels
ORLANDO – Higher vedolizumab levels during induction were associated with better responses to therapy at 22 weeks in patients with inflammatory bowel diseases in a prospective cohort study.
The findings suggest that therapeutic drug monitoring and early optimization could play an important role in improving outcomes in patients with Crohn’s disease or ulcerative colitis who are receiving treatment with the monoclonal antibody, Andres J. Yarur, MD, reported in a poster at the World Congress of Gastroenterology at ACG 2017.
Patients with a VTL of 24 mcg/mL or greater at week 2, and 10.6 mcg/mL or greater at week 6, were more likely to be in remission at week 22 (odds ratios, 5 and 13.5, respectively).
Of note, VTLs were numerically higher in patients receiving combination therapy, compared with those receiving vedolizumab monotherapy, but the difference was statistically significant only at week 2 (24.7 vs. 21.8 mcg/mL, respectively), he said.
Similar correlations between trough levels and response rates have been seen with other biologics, but data on such correlations has been lacking for vedolizumab. Since some patients develop primary or secondary nonresponse, Dr. Yarur and his colleagues assessed the relationship between serum VTLs during induction and disease remission after 22 weeks, he explained in an interview.
They also investigated the presence of antibodies to vedolizumab .
The primary outcome of deep remission at 22 weeks was defined as normal C-reactive protein levels and Simple Endoscopic Score for Crohn’s Disease of 2 or less in patients with Crohn’s disease, and Mayo Endoscopic score of 1 or less in patients with ulcerative colitis, plus clinical remission (Harvey-Bradshaw Index score of less than 5 in patients with Crohn’s disease and Mayo Clinical Score of less than 3 in ulcerative colitis).
Three patients developed antibodies to vedolizumab during induction, but the antibodies were undetectable by week 14 in all three, he said.
“The findings open the question of whether higher doses during induction will improve the rate of remission,” he said, noting that such early optimization is currently being evaluated in ongoing studies.
Dr. Yarur reported having no relevant disclosures.
ORLANDO – Higher vedolizumab levels during induction were associated with better responses to therapy at 22 weeks in patients with inflammatory bowel diseases in a prospective cohort study.
The findings suggest that therapeutic drug monitoring and early optimization could play an important role in improving outcomes in patients with Crohn’s disease or ulcerative colitis who are receiving treatment with the monoclonal antibody, Andres J. Yarur, MD, reported in a poster at the World Congress of Gastroenterology at ACG 2017.
Patients with a VTL of 24 mcg/mL or greater at week 2, and 10.6 mcg/mL or greater at week 6, were more likely to be in remission at week 22 (odds ratios, 5 and 13.5, respectively).
Of note, VTLs were numerically higher in patients receiving combination therapy, compared with those receiving vedolizumab monotherapy, but the difference was statistically significant only at week 2 (24.7 vs. 21.8 mcg/mL, respectively), he said.
Similar correlations between trough levels and response rates have been seen with other biologics, but data on such correlations has been lacking for vedolizumab. Since some patients develop primary or secondary nonresponse, Dr. Yarur and his colleagues assessed the relationship between serum VTLs during induction and disease remission after 22 weeks, he explained in an interview.
They also investigated the presence of antibodies to vedolizumab .
The primary outcome of deep remission at 22 weeks was defined as normal C-reactive protein levels and Simple Endoscopic Score for Crohn’s Disease of 2 or less in patients with Crohn’s disease, and Mayo Endoscopic score of 1 or less in patients with ulcerative colitis, plus clinical remission (Harvey-Bradshaw Index score of less than 5 in patients with Crohn’s disease and Mayo Clinical Score of less than 3 in ulcerative colitis).
Three patients developed antibodies to vedolizumab during induction, but the antibodies were undetectable by week 14 in all three, he said.
“The findings open the question of whether higher doses during induction will improve the rate of remission,” he said, noting that such early optimization is currently being evaluated in ongoing studies.
Dr. Yarur reported having no relevant disclosures.
ORLANDO – Higher vedolizumab levels during induction were associated with better responses to therapy at 22 weeks in patients with inflammatory bowel diseases in a prospective cohort study.
The findings suggest that therapeutic drug monitoring and early optimization could play an important role in improving outcomes in patients with Crohn’s disease or ulcerative colitis who are receiving treatment with the monoclonal antibody, Andres J. Yarur, MD, reported in a poster at the World Congress of Gastroenterology at ACG 2017.
Patients with a VTL of 24 mcg/mL or greater at week 2, and 10.6 mcg/mL or greater at week 6, were more likely to be in remission at week 22 (odds ratios, 5 and 13.5, respectively).
Of note, VTLs were numerically higher in patients receiving combination therapy, compared with those receiving vedolizumab monotherapy, but the difference was statistically significant only at week 2 (24.7 vs. 21.8 mcg/mL, respectively), he said.
Similar correlations between trough levels and response rates have been seen with other biologics, but data on such correlations has been lacking for vedolizumab. Since some patients develop primary or secondary nonresponse, Dr. Yarur and his colleagues assessed the relationship between serum VTLs during induction and disease remission after 22 weeks, he explained in an interview.
They also investigated the presence of antibodies to vedolizumab .
The primary outcome of deep remission at 22 weeks was defined as normal C-reactive protein levels and Simple Endoscopic Score for Crohn’s Disease of 2 or less in patients with Crohn’s disease, and Mayo Endoscopic score of 1 or less in patients with ulcerative colitis, plus clinical remission (Harvey-Bradshaw Index score of less than 5 in patients with Crohn’s disease and Mayo Clinical Score of less than 3 in ulcerative colitis).
Three patients developed antibodies to vedolizumab during induction, but the antibodies were undetectable by week 14 in all three, he said.
“The findings open the question of whether higher doses during induction will improve the rate of remission,” he said, noting that such early optimization is currently being evaluated in ongoing studies.
Dr. Yarur reported having no relevant disclosures.
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Vedolizumab trough levels at weeks 2 and 6 were higher among those who achieved remission at week 22, compared with those who did not (25 vs. 21.8 mcg/mL and 26.1 vs. 12.7 mcg/mL, respectively).
Data source: A prospective cohort study of 45 patients.
Disclosures: Dr. Yarur reported having no relevant disclosures.
VIDEO: What’s next in women’s health policy?
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.
AT NAMS 2017
Young adult stroke survivors have distinct risk profile
SAN DIEGO – Young adults who have suffered a stroke are at greater risk of a second stroke than other cardiovascular events, at least in the first year. That’s the conclusion drawn from a new analysis of 2013 data drawn from the Nationwide Readmissions Database.
The results suggest that younger adults who have a first-time stroke have a different risk profile than older adults and could require different management to improve long-term outcomes. The incidence of stroke has increased in recent years to the point that this population now accounts for about 10% of all strokes.
The analysis looked at all admissions for ischemic stroke in patients aged 18-45. The researchers found a cumulative risk of rehospitalization of 5.5% at 300 days, compared with 3.6% for cardiovascular disease.
The study can’t explain the association, nor can it prove causation. “Our thought was that the effects of hypertension might take longer to manifest in terms of cardiovascular outcomes as compared to hypercholesterolemia and diabetes,” said Dr. Jin, who is chief resident in the department of neurology at Icahn School of Medicine at Mount Sinai, New York. He presented the research at the annual meeting of the American Neurological Association.
The result sends a clear message for physicians caring for young adults who have experienced a first-time stroke. “They should be rigorously worked up and managed for any disorders in blood sugar and lipid disorders,” Dr. Jin said. Their care is vital because these younger adults have more time to accumulate second, third, or fourth strokes that could dramatically increase the burden of disease. “It’s just a matter of time. Optimizing their secondary prevention is crucial,” Dr. Jin said.
The study included data from 12,392 young adults in the Nationwide Readmissions Database who had suffered a first-time stroke. The researchers identified a higher readmission rate for stroke than cardiovascular events at 90 days (2,913.3 vs. 1,132.4 per 100,000 index hospitalizations). This pattern held when the analysis was restricted to patients who had no cardiovascular risk factors prior to the index hospitalization (2,534.9 vs. 676 per 100,000 index hospitalizations).
At 100 days, the cumulative risks were 3.2% for stroke and 2.5% for cardiovascular events. The risks were 4.3% and 3.2% at 200 days, and 5.5% and 3.6% at 300 days.
A multivariate analysis showed that patients with baseline diabetes were at a heightened risk of cardiovascular events (hazard ratio, 1.49; 95% confidence interval, 1.17-1.88), as were patients with hypercholesterolemia (HR, 1.43; 95% CI, 1.15-1.79) and those with atrial fibrillation or flutter (HR, 3.86; 95% CI, 2.74-5.43). Only diabetes was significantly associated with increased risk for hospitalization for recurrent stroke (HR, 1.5; 95% CI, 1.22-1.84).
Dr. Jin is eager to see if future research might establish a causative link between these risk factors and outcomes. The current work grew out of an administrative data set, but registry data or a prospective study could be more robust.
The study received no external funding. Dr. Jin reported having no financial disclosures.
SAN DIEGO – Young adults who have suffered a stroke are at greater risk of a second stroke than other cardiovascular events, at least in the first year. That’s the conclusion drawn from a new analysis of 2013 data drawn from the Nationwide Readmissions Database.
The results suggest that younger adults who have a first-time stroke have a different risk profile than older adults and could require different management to improve long-term outcomes. The incidence of stroke has increased in recent years to the point that this population now accounts for about 10% of all strokes.
The analysis looked at all admissions for ischemic stroke in patients aged 18-45. The researchers found a cumulative risk of rehospitalization of 5.5% at 300 days, compared with 3.6% for cardiovascular disease.
The study can’t explain the association, nor can it prove causation. “Our thought was that the effects of hypertension might take longer to manifest in terms of cardiovascular outcomes as compared to hypercholesterolemia and diabetes,” said Dr. Jin, who is chief resident in the department of neurology at Icahn School of Medicine at Mount Sinai, New York. He presented the research at the annual meeting of the American Neurological Association.
The result sends a clear message for physicians caring for young adults who have experienced a first-time stroke. “They should be rigorously worked up and managed for any disorders in blood sugar and lipid disorders,” Dr. Jin said. Their care is vital because these younger adults have more time to accumulate second, third, or fourth strokes that could dramatically increase the burden of disease. “It’s just a matter of time. Optimizing their secondary prevention is crucial,” Dr. Jin said.
The study included data from 12,392 young adults in the Nationwide Readmissions Database who had suffered a first-time stroke. The researchers identified a higher readmission rate for stroke than cardiovascular events at 90 days (2,913.3 vs. 1,132.4 per 100,000 index hospitalizations). This pattern held when the analysis was restricted to patients who had no cardiovascular risk factors prior to the index hospitalization (2,534.9 vs. 676 per 100,000 index hospitalizations).
At 100 days, the cumulative risks were 3.2% for stroke and 2.5% for cardiovascular events. The risks were 4.3% and 3.2% at 200 days, and 5.5% and 3.6% at 300 days.
A multivariate analysis showed that patients with baseline diabetes were at a heightened risk of cardiovascular events (hazard ratio, 1.49; 95% confidence interval, 1.17-1.88), as were patients with hypercholesterolemia (HR, 1.43; 95% CI, 1.15-1.79) and those with atrial fibrillation or flutter (HR, 3.86; 95% CI, 2.74-5.43). Only diabetes was significantly associated with increased risk for hospitalization for recurrent stroke (HR, 1.5; 95% CI, 1.22-1.84).
Dr. Jin is eager to see if future research might establish a causative link between these risk factors and outcomes. The current work grew out of an administrative data set, but registry data or a prospective study could be more robust.
The study received no external funding. Dr. Jin reported having no financial disclosures.
SAN DIEGO – Young adults who have suffered a stroke are at greater risk of a second stroke than other cardiovascular events, at least in the first year. That’s the conclusion drawn from a new analysis of 2013 data drawn from the Nationwide Readmissions Database.
The results suggest that younger adults who have a first-time stroke have a different risk profile than older adults and could require different management to improve long-term outcomes. The incidence of stroke has increased in recent years to the point that this population now accounts for about 10% of all strokes.
The analysis looked at all admissions for ischemic stroke in patients aged 18-45. The researchers found a cumulative risk of rehospitalization of 5.5% at 300 days, compared with 3.6% for cardiovascular disease.
The study can’t explain the association, nor can it prove causation. “Our thought was that the effects of hypertension might take longer to manifest in terms of cardiovascular outcomes as compared to hypercholesterolemia and diabetes,” said Dr. Jin, who is chief resident in the department of neurology at Icahn School of Medicine at Mount Sinai, New York. He presented the research at the annual meeting of the American Neurological Association.
The result sends a clear message for physicians caring for young adults who have experienced a first-time stroke. “They should be rigorously worked up and managed for any disorders in blood sugar and lipid disorders,” Dr. Jin said. Their care is vital because these younger adults have more time to accumulate second, third, or fourth strokes that could dramatically increase the burden of disease. “It’s just a matter of time. Optimizing their secondary prevention is crucial,” Dr. Jin said.
The study included data from 12,392 young adults in the Nationwide Readmissions Database who had suffered a first-time stroke. The researchers identified a higher readmission rate for stroke than cardiovascular events at 90 days (2,913.3 vs. 1,132.4 per 100,000 index hospitalizations). This pattern held when the analysis was restricted to patients who had no cardiovascular risk factors prior to the index hospitalization (2,534.9 vs. 676 per 100,000 index hospitalizations).
At 100 days, the cumulative risks were 3.2% for stroke and 2.5% for cardiovascular events. The risks were 4.3% and 3.2% at 200 days, and 5.5% and 3.6% at 300 days.
A multivariate analysis showed that patients with baseline diabetes were at a heightened risk of cardiovascular events (hazard ratio, 1.49; 95% confidence interval, 1.17-1.88), as were patients with hypercholesterolemia (HR, 1.43; 95% CI, 1.15-1.79) and those with atrial fibrillation or flutter (HR, 3.86; 95% CI, 2.74-5.43). Only diabetes was significantly associated with increased risk for hospitalization for recurrent stroke (HR, 1.5; 95% CI, 1.22-1.84).
Dr. Jin is eager to see if future research might establish a causative link between these risk factors and outcomes. The current work grew out of an administrative data set, but registry data or a prospective study could be more robust.
The study received no external funding. Dr. Jin reported having no financial disclosures.
AT ANA 2017
Key clinical point:
Major finding: Hospitalization for cardiovascular disease was associated with baseline diabetes (HR, 1.49) and hypercholesterolemia (HR, 1.43).
Data source: A retrospective analysis of data from the Nationwide Readmissions Database (n = 12,392).
Disclosures: The study received no external funding. Dr. Jin reported having no financial disclosures.