User login
The better mammogram: Experts explore sensitivity of new modalities
PHILADELPHIA – Is it time to think about “the better mammogram” as the new standard of care? Can nuclear medicine provide a cost-effective workaround for imaging of women with dense breasts? According to two leading breast imaging researchers,
“Digital breast tomosynthesis is the new kid on the block for screening,” said Emily F. Conant, MD, professor of radiology and chief of breast imaging at the University of Pennsylvania, Philadelphia. “It’s becoming the new standard of care in mammography,” she said, speaking during a plenary session at the annual meeting of the North American Menopause Society.
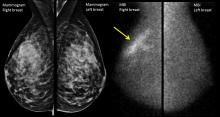
Digital breast tomosynthesis (DBT) can involve simultaneous acquisition of a conventional 2D mammogram along with a series of images to create a 3D image. Another protocol, which delivers a lower radiation dose, produces a “synthetic” 2D reconstruction of 3D mammography.*
In addition to making visible tumors that otherwise might be obscured by the overlay of dense breast tissue, DBT can help reduce the recall rate, with the 3D images providing immediate clarification at the initial appointment. Studies show that the recall rate can go down by up to 31%, Dr. Conant said.
DBT has been shown to increase detection of invasive cancers, but it does not pick up more ductal carcinoma in situ, Dr. Conant said. This fact helps address the problem of overdiagnosis of small tumors that might regress. Overall, cancer detection is reported to increase by up to 53% with DBT, Dr. Conant said.
When primarily retrospective American studies are taken together with smaller prospective European studies, “the improvement in outcomes achieved with DBT directly addresses the major concerns regarding screening for breast cancer with mammography,” she said.
However, so far the studies have not offered DBT routinely to all comers. Since 2011, DBT has been offered to every woman screened at the University of Pennsylvania, at no additional cost. This created “a sort of natural experiment – there was no bias as to who got it.” Three consecutive years’ worth of outcomes have now been analyzed, Dr. Conant said.
Patient-level data from the University of Pennsylvania experience show statistically significant reductions in recall rate from diagnostic mammography alone. Also, researchers saw a steady increase in the rate of cancers detected per 1,000 patients, from 4.6 with digital mammography alone, to 6.1 by year three of DBT (JAMA Oncol. 2016 Jun 1;2[6]:737-43). This reflected the institutional learning curve with DBT, Dr. Conant said.
She said that the data also showed “a promising trend down in false negatives,” with an early reduction in cancers that were missed by DBT. Time is needed for mature cancer registry data to bear out these early trends, she added.
Other recent data show that DBT has promise to improve detection rates in a population of great interest – younger women, where there are often too many false positives and not enough cancers found, Dr. Conant said. If the risk-benefit ratio for DBT continues to play out as the data pile up, “I would strongly suggest that we should be doing screening in the 40s,” she said.
An important caveat, noted Dr. Conant, is that whether tomosynthesis is used or not, mammography captures anatomy, not physiology, and very dense breast tissue may still obscure a tumor, even when the tomographic slices are peeled back.
Though “DBT is ‘the better mammogram,’ additional outcome data are needed,” she said, including studies that compare modalities, include subgroup analyses, and better delineate the effect of cancer biology.
Molecular breast imaging
Another imaging modality uses nuclear medicine to capture the physiologic changes that accompany cancer. Molecular breast imaging (MBI), or scintimammography, can help “unveil the reservoir of hidden cancers in dense breasts,” said Deborah J. Rhodes, MD, professor of medicine at the Mayo Clinic, Rochester, Minn.
Dr. Rhodes – along with Michael O’Connor, PhD, Connie Hruska, PhD, Katie Hunt, MD, and Amy Conners, MD, her collaborators at the Mayo Clinic – uses a specialized array of gamma cameras to detect uptake of an injected radionuclide that’s preferentially avid for tumor tissue. This technique can unmask smaller tumors not seen on mammogram because it’s not impeded by having to “see” through dense breast tissue.
The radiation dose for an MBI study is a bit more than for DBT, but less than a coronary calcium score scan. The cost is about one-tenth that of breast magnetic resonance imaging (MRI), and interpretation is relatively straightforward, said Dr. Rhodes, who also presented data at the North American Menopause Society plenary.
“The traditional measure of mammography’s performance inflates its effectiveness,” especially in dense breast tissue, said Dr. Rhodes. “What is the sensitivity of mammography in the dense breast? It depends on what you measure it against.”
When cancers detected by MRI or MBI are added, the sensitivity of mammography drops from the 86.9% reported by the Breast Cancer Surveillance Consortium to 21%-31%, according to several published studies.
In one study, Dr. Rhodes and her Mayo colleagues found that the diagnostic yield per 1,000 patients with dense breasts by mammogram alone was 1.9 cancers. When MBI was added, that figure jumped to 8.8 cancers per 1,000 patients, an incremental gain of 363%.
“Tumor size matters profoundly,” she added. “If a tumor is detected above 2 cm, long-term survival drops below 50%.”
That contrasts with the better-than-80% long-term survival rate seen for those with sub-centimeter tumors, even in node-positive disease. “Only a third of tumors are detected when they are less than 1 cm” with regular screening mammography, Dr. Rhodes said.
However, in 2016 the U.S. Preventive Services Task Force concluded that the current evidence was insufficient to assess whether adjunctive screening for breast cancer using breast ultrasonography, MRI, DBT, or other methods should be used in women with dense breasts. The USPSTF noted that there weren’t studies that addressed the effect of supplemental screening on breast cancer morbidity or mortality.
The problem is that it can take 20 years or more to demonstrate mortality reduction, meaning that “no other imaging modality can compete” with mammography when this yardstick is used, Dr. Rhodes said. “This insistence on a mortality endpoint before we change practice” is impeding progress in screening, she said.
The American College of Obstetricians and Gynecologists “does not recommend adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.” However, the organization “strongly supports additional research to identify more effective screening methods” that will improve outcomes and minimize false positives in women with dense breasts.
Though DBT is becoming more widely available, MBI is still primarily used in research centers. Both Dr. Conant and Dr. Rhodes acknowledged that since these techniques are not required to be covered by insurance, payment – and patient access – may vary. Both physicians said their home institutions have worked hard to keep costs down for their studies.
Dr. Conant is consultant or advisory board member for Hologic. Dr. Rhodes reported having no conflicts of interest.
*Correction, 11/15/2017: An earlier version of this story misstated the synthetic mammography protocol.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Is it time to think about “the better mammogram” as the new standard of care? Can nuclear medicine provide a cost-effective workaround for imaging of women with dense breasts? According to two leading breast imaging researchers,
“Digital breast tomosynthesis is the new kid on the block for screening,” said Emily F. Conant, MD, professor of radiology and chief of breast imaging at the University of Pennsylvania, Philadelphia. “It’s becoming the new standard of care in mammography,” she said, speaking during a plenary session at the annual meeting of the North American Menopause Society.
Digital breast tomosynthesis (DBT) can involve simultaneous acquisition of a conventional 2D mammogram along with a series of images to create a 3D image. Another protocol, which delivers a lower radiation dose, produces a “synthetic” 2D reconstruction of 3D mammography.*
In addition to making visible tumors that otherwise might be obscured by the overlay of dense breast tissue, DBT can help reduce the recall rate, with the 3D images providing immediate clarification at the initial appointment. Studies show that the recall rate can go down by up to 31%, Dr. Conant said.
DBT has been shown to increase detection of invasive cancers, but it does not pick up more ductal carcinoma in situ, Dr. Conant said. This fact helps address the problem of overdiagnosis of small tumors that might regress. Overall, cancer detection is reported to increase by up to 53% with DBT, Dr. Conant said.
When primarily retrospective American studies are taken together with smaller prospective European studies, “the improvement in outcomes achieved with DBT directly addresses the major concerns regarding screening for breast cancer with mammography,” she said.
However, so far the studies have not offered DBT routinely to all comers. Since 2011, DBT has been offered to every woman screened at the University of Pennsylvania, at no additional cost. This created “a sort of natural experiment – there was no bias as to who got it.” Three consecutive years’ worth of outcomes have now been analyzed, Dr. Conant said.
Patient-level data from the University of Pennsylvania experience show statistically significant reductions in recall rate from diagnostic mammography alone. Also, researchers saw a steady increase in the rate of cancers detected per 1,000 patients, from 4.6 with digital mammography alone, to 6.1 by year three of DBT (JAMA Oncol. 2016 Jun 1;2[6]:737-43). This reflected the institutional learning curve with DBT, Dr. Conant said.
She said that the data also showed “a promising trend down in false negatives,” with an early reduction in cancers that were missed by DBT. Time is needed for mature cancer registry data to bear out these early trends, she added.
Other recent data show that DBT has promise to improve detection rates in a population of great interest – younger women, where there are often too many false positives and not enough cancers found, Dr. Conant said. If the risk-benefit ratio for DBT continues to play out as the data pile up, “I would strongly suggest that we should be doing screening in the 40s,” she said.
An important caveat, noted Dr. Conant, is that whether tomosynthesis is used or not, mammography captures anatomy, not physiology, and very dense breast tissue may still obscure a tumor, even when the tomographic slices are peeled back.
Though “DBT is ‘the better mammogram,’ additional outcome data are needed,” she said, including studies that compare modalities, include subgroup analyses, and better delineate the effect of cancer biology.
Molecular breast imaging
Another imaging modality uses nuclear medicine to capture the physiologic changes that accompany cancer. Molecular breast imaging (MBI), or scintimammography, can help “unveil the reservoir of hidden cancers in dense breasts,” said Deborah J. Rhodes, MD, professor of medicine at the Mayo Clinic, Rochester, Minn.
Dr. Rhodes – along with Michael O’Connor, PhD, Connie Hruska, PhD, Katie Hunt, MD, and Amy Conners, MD, her collaborators at the Mayo Clinic – uses a specialized array of gamma cameras to detect uptake of an injected radionuclide that’s preferentially avid for tumor tissue. This technique can unmask smaller tumors not seen on mammogram because it’s not impeded by having to “see” through dense breast tissue.
The radiation dose for an MBI study is a bit more than for DBT, but less than a coronary calcium score scan. The cost is about one-tenth that of breast magnetic resonance imaging (MRI), and interpretation is relatively straightforward, said Dr. Rhodes, who also presented data at the North American Menopause Society plenary.
“The traditional measure of mammography’s performance inflates its effectiveness,” especially in dense breast tissue, said Dr. Rhodes. “What is the sensitivity of mammography in the dense breast? It depends on what you measure it against.”
When cancers detected by MRI or MBI are added, the sensitivity of mammography drops from the 86.9% reported by the Breast Cancer Surveillance Consortium to 21%-31%, according to several published studies.
In one study, Dr. Rhodes and her Mayo colleagues found that the diagnostic yield per 1,000 patients with dense breasts by mammogram alone was 1.9 cancers. When MBI was added, that figure jumped to 8.8 cancers per 1,000 patients, an incremental gain of 363%.
“Tumor size matters profoundly,” she added. “If a tumor is detected above 2 cm, long-term survival drops below 50%.”
That contrasts with the better-than-80% long-term survival rate seen for those with sub-centimeter tumors, even in node-positive disease. “Only a third of tumors are detected when they are less than 1 cm” with regular screening mammography, Dr. Rhodes said.
However, in 2016 the U.S. Preventive Services Task Force concluded that the current evidence was insufficient to assess whether adjunctive screening for breast cancer using breast ultrasonography, MRI, DBT, or other methods should be used in women with dense breasts. The USPSTF noted that there weren’t studies that addressed the effect of supplemental screening on breast cancer morbidity or mortality.
The problem is that it can take 20 years or more to demonstrate mortality reduction, meaning that “no other imaging modality can compete” with mammography when this yardstick is used, Dr. Rhodes said. “This insistence on a mortality endpoint before we change practice” is impeding progress in screening, she said.
The American College of Obstetricians and Gynecologists “does not recommend adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.” However, the organization “strongly supports additional research to identify more effective screening methods” that will improve outcomes and minimize false positives in women with dense breasts.
Though DBT is becoming more widely available, MBI is still primarily used in research centers. Both Dr. Conant and Dr. Rhodes acknowledged that since these techniques are not required to be covered by insurance, payment – and patient access – may vary. Both physicians said their home institutions have worked hard to keep costs down for their studies.
Dr. Conant is consultant or advisory board member for Hologic. Dr. Rhodes reported having no conflicts of interest.
*Correction, 11/15/2017: An earlier version of this story misstated the synthetic mammography protocol.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Is it time to think about “the better mammogram” as the new standard of care? Can nuclear medicine provide a cost-effective workaround for imaging of women with dense breasts? According to two leading breast imaging researchers,
“Digital breast tomosynthesis is the new kid on the block for screening,” said Emily F. Conant, MD, professor of radiology and chief of breast imaging at the University of Pennsylvania, Philadelphia. “It’s becoming the new standard of care in mammography,” she said, speaking during a plenary session at the annual meeting of the North American Menopause Society.
Digital breast tomosynthesis (DBT) can involve simultaneous acquisition of a conventional 2D mammogram along with a series of images to create a 3D image. Another protocol, which delivers a lower radiation dose, produces a “synthetic” 2D reconstruction of 3D mammography.*
In addition to making visible tumors that otherwise might be obscured by the overlay of dense breast tissue, DBT can help reduce the recall rate, with the 3D images providing immediate clarification at the initial appointment. Studies show that the recall rate can go down by up to 31%, Dr. Conant said.
DBT has been shown to increase detection of invasive cancers, but it does not pick up more ductal carcinoma in situ, Dr. Conant said. This fact helps address the problem of overdiagnosis of small tumors that might regress. Overall, cancer detection is reported to increase by up to 53% with DBT, Dr. Conant said.
When primarily retrospective American studies are taken together with smaller prospective European studies, “the improvement in outcomes achieved with DBT directly addresses the major concerns regarding screening for breast cancer with mammography,” she said.
However, so far the studies have not offered DBT routinely to all comers. Since 2011, DBT has been offered to every woman screened at the University of Pennsylvania, at no additional cost. This created “a sort of natural experiment – there was no bias as to who got it.” Three consecutive years’ worth of outcomes have now been analyzed, Dr. Conant said.
Patient-level data from the University of Pennsylvania experience show statistically significant reductions in recall rate from diagnostic mammography alone. Also, researchers saw a steady increase in the rate of cancers detected per 1,000 patients, from 4.6 with digital mammography alone, to 6.1 by year three of DBT (JAMA Oncol. 2016 Jun 1;2[6]:737-43). This reflected the institutional learning curve with DBT, Dr. Conant said.
She said that the data also showed “a promising trend down in false negatives,” with an early reduction in cancers that were missed by DBT. Time is needed for mature cancer registry data to bear out these early trends, she added.
Other recent data show that DBT has promise to improve detection rates in a population of great interest – younger women, where there are often too many false positives and not enough cancers found, Dr. Conant said. If the risk-benefit ratio for DBT continues to play out as the data pile up, “I would strongly suggest that we should be doing screening in the 40s,” she said.
An important caveat, noted Dr. Conant, is that whether tomosynthesis is used or not, mammography captures anatomy, not physiology, and very dense breast tissue may still obscure a tumor, even when the tomographic slices are peeled back.
Though “DBT is ‘the better mammogram,’ additional outcome data are needed,” she said, including studies that compare modalities, include subgroup analyses, and better delineate the effect of cancer biology.
Molecular breast imaging
Another imaging modality uses nuclear medicine to capture the physiologic changes that accompany cancer. Molecular breast imaging (MBI), or scintimammography, can help “unveil the reservoir of hidden cancers in dense breasts,” said Deborah J. Rhodes, MD, professor of medicine at the Mayo Clinic, Rochester, Minn.
Dr. Rhodes – along with Michael O’Connor, PhD, Connie Hruska, PhD, Katie Hunt, MD, and Amy Conners, MD, her collaborators at the Mayo Clinic – uses a specialized array of gamma cameras to detect uptake of an injected radionuclide that’s preferentially avid for tumor tissue. This technique can unmask smaller tumors not seen on mammogram because it’s not impeded by having to “see” through dense breast tissue.
The radiation dose for an MBI study is a bit more than for DBT, but less than a coronary calcium score scan. The cost is about one-tenth that of breast magnetic resonance imaging (MRI), and interpretation is relatively straightforward, said Dr. Rhodes, who also presented data at the North American Menopause Society plenary.
“The traditional measure of mammography’s performance inflates its effectiveness,” especially in dense breast tissue, said Dr. Rhodes. “What is the sensitivity of mammography in the dense breast? It depends on what you measure it against.”
When cancers detected by MRI or MBI are added, the sensitivity of mammography drops from the 86.9% reported by the Breast Cancer Surveillance Consortium to 21%-31%, according to several published studies.
In one study, Dr. Rhodes and her Mayo colleagues found that the diagnostic yield per 1,000 patients with dense breasts by mammogram alone was 1.9 cancers. When MBI was added, that figure jumped to 8.8 cancers per 1,000 patients, an incremental gain of 363%.
“Tumor size matters profoundly,” she added. “If a tumor is detected above 2 cm, long-term survival drops below 50%.”
That contrasts with the better-than-80% long-term survival rate seen for those with sub-centimeter tumors, even in node-positive disease. “Only a third of tumors are detected when they are less than 1 cm” with regular screening mammography, Dr. Rhodes said.
However, in 2016 the U.S. Preventive Services Task Force concluded that the current evidence was insufficient to assess whether adjunctive screening for breast cancer using breast ultrasonography, MRI, DBT, or other methods should be used in women with dense breasts. The USPSTF noted that there weren’t studies that addressed the effect of supplemental screening on breast cancer morbidity or mortality.
The problem is that it can take 20 years or more to demonstrate mortality reduction, meaning that “no other imaging modality can compete” with mammography when this yardstick is used, Dr. Rhodes said. “This insistence on a mortality endpoint before we change practice” is impeding progress in screening, she said.
The American College of Obstetricians and Gynecologists “does not recommend adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.” However, the organization “strongly supports additional research to identify more effective screening methods” that will improve outcomes and minimize false positives in women with dense breasts.
Though DBT is becoming more widely available, MBI is still primarily used in research centers. Both Dr. Conant and Dr. Rhodes acknowledged that since these techniques are not required to be covered by insurance, payment – and patient access – may vary. Both physicians said their home institutions have worked hard to keep costs down for their studies.
Dr. Conant is consultant or advisory board member for Hologic. Dr. Rhodes reported having no conflicts of interest.
*Correction, 11/15/2017: An earlier version of this story misstated the synthetic mammography protocol.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
Beyond the Kegel: the who, why, and how of pelvic floor PT
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
Morphology index guides adnexal mass workup in postmenopausal women
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
VIDEO: Expert roundtable on hormone therapy
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – The updated hormone therapy position statement from the North American Menopause Society tells clinicians to move away from “lowest dose for the shortest time” and toward prescribing the appropriate dose, formulation, and route of administration to meet treatment goals.
At the group’s annual meeting, some of the authors of the position statement outlined the latest evidence for the safety of hormone therapy and special clinical considerations based on age or time of menopause and unique health risks like breast cancer. Additionally, the authors said there should not be an arbitrary “stop date” for hormone therapy.
The authors also discussed the risks of using compounded bioidentical hormones to treat menopausal symptoms.
This roundtable discussion includes JoAnn V. Pinkerton, MD, NAMS executive director and professor of obstetrics and gynecology at the University of Virginia, Charlottesville; Andrew Kaunitz, MD, associate chairman of the department of obstetrics and gynecology at the University of Florida, Jacksonville; and Cynthia A. Stuenkel, MD, clinical professor of medicine at the University of California, San Diego.
Dr. Pinkerton reported institutional research support from TherapeuticsMD. Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has also received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics. Dr. Stuenkel reported having no financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
EXPERT ANALYSIS FROM NAMS 2017
VIDEO: New sexual desire drugs coming for women
PHILADELPHIA – Despite the slow start that flibanserin had since being approved to treat hypoactive sexual desire disorder (HSDD) in premenopausal women in 2015, more drugs are in the pipeline to help women address low desire.
One drug – bremelanotide – has completed phase 3 trials and could be considered by the Food and Drug Administration as early as 2018, Sheryl A. Kingsberg, PhD, said during an interview at the annual meeting of the North American Menopause Society.
Bremelanotide is a first-in-class melanocortin receptor 4 agonist being developed for premenopausal women to use on an as-needed basis and is delivered using a single-dose, auto injector.
Another drug, prasterone, is also being studied to treat HSDD. The intravaginal DHEA treatment is already approved to treat dyspareunia due to vulvovaginal atrophy in menopause. The manufacturer is beginning phase 3 trials for HSDD in postmenopausal women, said Dr. Kingsberg, who is chief of the division of behavioral medicine at MacDonald Women’s Hospital/University Hospitals Cleveland Medical Center and the president of NAMS.
Additional drugs are in earlier stages of development for HSDD. While flibanserin hasn’t been a blockbuster drug, its approval by the FDA paved the way for additional drug development in this area, Dr. Kingsberg said.
Dr. Kingsberg reported consultant/advisory board work for Amag Pharmaceuticals, Duchesnay, Emotional Brain, EndoCeutics, Materna Medical, Palatin Technologies, Pfizer, Shionogi, TherapeuticsMD, Valeant Pharmaceuticals, and Viveve. She is on the speakers bureau for Valeant Pharmaceuticals and owns stock in Viveve.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Despite the slow start that flibanserin had since being approved to treat hypoactive sexual desire disorder (HSDD) in premenopausal women in 2015, more drugs are in the pipeline to help women address low desire.
One drug – bremelanotide – has completed phase 3 trials and could be considered by the Food and Drug Administration as early as 2018, Sheryl A. Kingsberg, PhD, said during an interview at the annual meeting of the North American Menopause Society.
Bremelanotide is a first-in-class melanocortin receptor 4 agonist being developed for premenopausal women to use on an as-needed basis and is delivered using a single-dose, auto injector.
Another drug, prasterone, is also being studied to treat HSDD. The intravaginal DHEA treatment is already approved to treat dyspareunia due to vulvovaginal atrophy in menopause. The manufacturer is beginning phase 3 trials for HSDD in postmenopausal women, said Dr. Kingsberg, who is chief of the division of behavioral medicine at MacDonald Women’s Hospital/University Hospitals Cleveland Medical Center and the president of NAMS.
Additional drugs are in earlier stages of development for HSDD. While flibanserin hasn’t been a blockbuster drug, its approval by the FDA paved the way for additional drug development in this area, Dr. Kingsberg said.
Dr. Kingsberg reported consultant/advisory board work for Amag Pharmaceuticals, Duchesnay, Emotional Brain, EndoCeutics, Materna Medical, Palatin Technologies, Pfizer, Shionogi, TherapeuticsMD, Valeant Pharmaceuticals, and Viveve. She is on the speakers bureau for Valeant Pharmaceuticals and owns stock in Viveve.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Despite the slow start that flibanserin had since being approved to treat hypoactive sexual desire disorder (HSDD) in premenopausal women in 2015, more drugs are in the pipeline to help women address low desire.
One drug – bremelanotide – has completed phase 3 trials and could be considered by the Food and Drug Administration as early as 2018, Sheryl A. Kingsberg, PhD, said during an interview at the annual meeting of the North American Menopause Society.
Bremelanotide is a first-in-class melanocortin receptor 4 agonist being developed for premenopausal women to use on an as-needed basis and is delivered using a single-dose, auto injector.
Another drug, prasterone, is also being studied to treat HSDD. The intravaginal DHEA treatment is already approved to treat dyspareunia due to vulvovaginal atrophy in menopause. The manufacturer is beginning phase 3 trials for HSDD in postmenopausal women, said Dr. Kingsberg, who is chief of the division of behavioral medicine at MacDonald Women’s Hospital/University Hospitals Cleveland Medical Center and the president of NAMS.
Additional drugs are in earlier stages of development for HSDD. While flibanserin hasn’t been a blockbuster drug, its approval by the FDA paved the way for additional drug development in this area, Dr. Kingsberg said.
Dr. Kingsberg reported consultant/advisory board work for Amag Pharmaceuticals, Duchesnay, Emotional Brain, EndoCeutics, Materna Medical, Palatin Technologies, Pfizer, Shionogi, TherapeuticsMD, Valeant Pharmaceuticals, and Viveve. She is on the speakers bureau for Valeant Pharmaceuticals and owns stock in Viveve.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
EXPERT ANALYSIS FROM NAMS 2017
VIDEO: Dr. Andrew Kaunitz’s top lessons from NAMS 2017
PHILADELPHIA – Andrew Kaunitz, MD, the chair of the 2017 scientific program committee for the annual meeting of the North American Menopause Society, shared his top take-home messages from the meeting.
New anabolic medications that increase bone mineral density and dramatically reduce fracture risk are in the pipeline, Dr. Kaunitz, a professor in the department of obstetrics and gynecology at the University of Florida, Jacksonville, said in a video interview.
Another finding from the meeting is that type 2 diabetes, despite being associated with an increased body mass index, actually elevates a woman’s risk for fracture. “That was something new for me, and I think it was something new for a lot of the practitioners attending the NAMS meeting,” Dr. Kaunitz said.
The meeting also offered tips for managing polycystic ovarian syndrome in women who are in midlife, including the importance of screening for diabetes and assessing for lipid disorders. Additionally, attendees learned about the management of migraines in menopausal women and older reproductive-age women.
A well-attended session on breast imaging explored how breast tomosynthesis can reduce false positives and recalls, as well as how new technology can reduce the radiation exposure associated with tomosynthesis. The session also featured evidence that screening mammography has lower-than-reported sensitivity, but offered a hopeful note on the promise of improved sensitivity through molecular breast imaging.
Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Andrew Kaunitz, MD, the chair of the 2017 scientific program committee for the annual meeting of the North American Menopause Society, shared his top take-home messages from the meeting.
New anabolic medications that increase bone mineral density and dramatically reduce fracture risk are in the pipeline, Dr. Kaunitz, a professor in the department of obstetrics and gynecology at the University of Florida, Jacksonville, said in a video interview.
Another finding from the meeting is that type 2 diabetes, despite being associated with an increased body mass index, actually elevates a woman’s risk for fracture. “That was something new for me, and I think it was something new for a lot of the practitioners attending the NAMS meeting,” Dr. Kaunitz said.
The meeting also offered tips for managing polycystic ovarian syndrome in women who are in midlife, including the importance of screening for diabetes and assessing for lipid disorders. Additionally, attendees learned about the management of migraines in menopausal women and older reproductive-age women.
A well-attended session on breast imaging explored how breast tomosynthesis can reduce false positives and recalls, as well as how new technology can reduce the radiation exposure associated with tomosynthesis. The session also featured evidence that screening mammography has lower-than-reported sensitivity, but offered a hopeful note on the promise of improved sensitivity through molecular breast imaging.
Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Andrew Kaunitz, MD, the chair of the 2017 scientific program committee for the annual meeting of the North American Menopause Society, shared his top take-home messages from the meeting.
New anabolic medications that increase bone mineral density and dramatically reduce fracture risk are in the pipeline, Dr. Kaunitz, a professor in the department of obstetrics and gynecology at the University of Florida, Jacksonville, said in a video interview.
Another finding from the meeting is that type 2 diabetes, despite being associated with an increased body mass index, actually elevates a woman’s risk for fracture. “That was something new for me, and I think it was something new for a lot of the practitioners attending the NAMS meeting,” Dr. Kaunitz said.
The meeting also offered tips for managing polycystic ovarian syndrome in women who are in midlife, including the importance of screening for diabetes and assessing for lipid disorders. Additionally, attendees learned about the management of migraines in menopausal women and older reproductive-age women.
A well-attended session on breast imaging explored how breast tomosynthesis can reduce false positives and recalls, as well as how new technology can reduce the radiation exposure associated with tomosynthesis. The session also featured evidence that screening mammography has lower-than-reported sensitivity, but offered a hopeful note on the promise of improved sensitivity through molecular breast imaging.
Dr. Kaunitz reported consultant/advisory board work for Allergan, Amag Pharmaceuticals, Bayer, Mithra Pharmaceuticals, Pfizer, and Shionogi. He has received grant/research support from Bayer, Radius Health, TherapeuticsMD, and Millendo Therapeutics.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
EXPERT ANALYSIS FROM NAMS 2017
VIDEO: Does genitourinary syndrome of menopause capture all the symptoms?
PHILADELPHIA – Genitourinary syndrome of menopause (GSM) replaced vulvovaginal atrophy in 2014 as a way to describe the changes to the genital and urinary tracts after menopause, but preliminary research shows it may be missing some symptoms.
In 2015, Amanda Clark, MD, a urogynecologist at the Kaiser Center for Health Research in Portland, Ore., and her colleagues surveyed women aged 55 years and older about their vulvar, vaginal, urinary, and sexual symptoms within 2 weeks of a well-woman visit to their primary care physician or gynecologist in the Kaiser system. In total, 1,533 provided valid data.
The researchers then used factor analysis to see if the symptoms matched up with GSM. If GSM is a true syndrome and only a single syndrome, then all of the factors would fit together in a one-factor model, Dr. Clark explained at the annual meeting of the North American Menopause Society. Instead, the researchers found that a three-factor model – with vulvovaginal symptoms of irritation and pain in one group, urinary symptoms in another group, and vaginal discharge and odor in a third group – fit best with the symptoms reported in their survey.
“This work is very preliminary and needs to be replicated in many other samples and looked at carefully,” Dr. Clark said in an interview. “But what we think is that genitourinary syndrome of menopause is a starting point.”
The study was funded by a Pfizer Independent Grant for Learning & Change and the North American Menopause Society. Dr. Clark reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Genitourinary syndrome of menopause (GSM) replaced vulvovaginal atrophy in 2014 as a way to describe the changes to the genital and urinary tracts after menopause, but preliminary research shows it may be missing some symptoms.
In 2015, Amanda Clark, MD, a urogynecologist at the Kaiser Center for Health Research in Portland, Ore., and her colleagues surveyed women aged 55 years and older about their vulvar, vaginal, urinary, and sexual symptoms within 2 weeks of a well-woman visit to their primary care physician or gynecologist in the Kaiser system. In total, 1,533 provided valid data.
The researchers then used factor analysis to see if the symptoms matched up with GSM. If GSM is a true syndrome and only a single syndrome, then all of the factors would fit together in a one-factor model, Dr. Clark explained at the annual meeting of the North American Menopause Society. Instead, the researchers found that a three-factor model – with vulvovaginal symptoms of irritation and pain in one group, urinary symptoms in another group, and vaginal discharge and odor in a third group – fit best with the symptoms reported in their survey.
“This work is very preliminary and needs to be replicated in many other samples and looked at carefully,” Dr. Clark said in an interview. “But what we think is that genitourinary syndrome of menopause is a starting point.”
The study was funded by a Pfizer Independent Grant for Learning & Change and the North American Menopause Society. Dr. Clark reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – Genitourinary syndrome of menopause (GSM) replaced vulvovaginal atrophy in 2014 as a way to describe the changes to the genital and urinary tracts after menopause, but preliminary research shows it may be missing some symptoms.
In 2015, Amanda Clark, MD, a urogynecologist at the Kaiser Center for Health Research in Portland, Ore., and her colleagues surveyed women aged 55 years and older about their vulvar, vaginal, urinary, and sexual symptoms within 2 weeks of a well-woman visit to their primary care physician or gynecologist in the Kaiser system. In total, 1,533 provided valid data.
The researchers then used factor analysis to see if the symptoms matched up with GSM. If GSM is a true syndrome and only a single syndrome, then all of the factors would fit together in a one-factor model, Dr. Clark explained at the annual meeting of the North American Menopause Society. Instead, the researchers found that a three-factor model – with vulvovaginal symptoms of irritation and pain in one group, urinary symptoms in another group, and vaginal discharge and odor in a third group – fit best with the symptoms reported in their survey.
“This work is very preliminary and needs to be replicated in many other samples and looked at carefully,” Dr. Clark said in an interview. “But what we think is that genitourinary syndrome of menopause is a starting point.”
The study was funded by a Pfizer Independent Grant for Learning & Change and the North American Menopause Society. Dr. Clark reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
AT NAMS 2017
VIDEO: What’s next in women’s health policy?
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.

mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.

mschneider@frontlinemedcom.com
On Twitter @maryellenny
PHILADELPHIA – The Affordable Care Act (ACA) largely has delivered on its promises to expand access to care for women, but those benefits are in jeopardy because of actions by the Trump administration, one health policy expert said.
President Trump’s announcement that he plans to end the ACA’s cost-sharing reduction payments, which help subsidize the cost of insurance for low-income Americans, combined with new federal regulations expanding religious exemptions to the health law’s contraception mandate, would make it harder for women to obtain health care, said Michael Policar, MD, MPH, a clinical professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
In an interview at the annual meeting of the North American Menopause Society, Dr. Policar said it’s unclear whether these executive actions actually will go into effect because they are being challenged in court. But Dr. Policar said his concern is that this is just the “leading edge of more proposals and more changes” to come from the administration, which could target family planning funding.
Dr. Policar reported that he is a litigation consultant for Bayer.

mschneider@frontlinemedcom.com
On Twitter @maryellenny
AT NAMS 2017
CBT for insomnia and hot flashes lifts mood in midlife
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – Cognitive-behavioral therapy tailored to perimenopausal and postmenopausal women who were experiencing both insomnia and vasomotor symptoms effectively improved both sleep and mood, according to a small controlled study.
When 40 women were randomized to receive either cognitive-behavioral therapy for menopausal insomnia (CBTMI) or education about menopause and sleep, those who received CBTMI had significantly reduced scores on both objective and subjective scales of depression, and their sleep also improved.
The CBTMI intervention was effective even for women with high scores on the depression scales at baseline, Sara Nowakowski, PhD, said at a top abstracts session at the annual meeting of the North American Menopause Society.
Over the 8 weeks of the study intervention, women in the CBTMI arm received four 50-minute individual sessions with either a social worker or a psychologist in a gynecology clinic outpatient setting. Counseling during the sessions focused both on hot flashes and insomnia, using evidence-based CBT techniques to address both. These included sleep restriction, changing behaviors to strengthen the association of the bed with sleep, cognitive therapy to address maladaptive beliefs about both sleep and host flashes, general sleep hygiene, hot flash coping mechanisms, and relaxation training.
Those in the menopause education control arm had a 1-hour educational session about menopausal symptoms and sleep hygiene, received written material, and were told they could make any changes desired.
Participants, whose mean age was 55 years, were included if they reported at least one nocturnal hot flash per night and met criteria for the sleep disorder of insomnia. Although patients who met criteria for major depression were not excluded, women with surgical menopause or cancer treatment–related menopause were excluded, as were those with substance use disorder, significant other psychiatric comorbidities, and those with obstructive sleep apnea or periodic limb movements/restless leg syndrome.
Dr. Nowakowski, a clinical psychologist in the department of obstetrics and gynecology, University of Texas, Galveston, and her colleagues administered the Insomnia Severity Index (ISI), the Center for Epidemiologic Studies Depression Scale (CES-D), and the Hamilton Depression Rating Scale (HDRS) both before and after the 8-week intervention.
The investigators used a mixed-models statistical analysis, finding a significant improvement over time during the study period in both patient-reported (P = .001) and clinician-assessed (P = .001) ratings of depression for the CBTMI group.
When the effect of the treatment arm was analyzed, CBTMI also offered significantly greater improvement in patient-reported (P = .009) and clinician-assessed (P = .022) depression ratings.
Patients were divided into high and low depression severity, with a score over 8 on the CES-D and 16 on the HDRS putting the participant into the high-severity category. Both groups had significant improvement on the ISI from baseline. “Treatment response for insomnia severity did not differ based on baseline depression severity,” Dr. Nowakowski said.
The efficacy of the relatively brief intervention has clinical relevance to those caring for the 39%-60% of women in midlife who have symptoms of insomnia and the 8%-40% of midlife women who report elevated depression symptoms. “Comprehensive interventions that simultaneously improve sleep and mood in midlife women are greatly needed,” she said.
The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT NAMS 2017
Key clinical point:
Major finding: Patient-reported and clinician-assessed depression scores dropped after the intervention (P = .001 for both).
Data source: Randomized controlled trial of 40 midlife women with insomnia and hot flashes.
Disclosures: The National Institutes of Health and the Hogg Foundation for Mental Health funded the study. Dr. Nowakowski reported no conflicts of interest.
Oral bioidentical combo improves quality of life, vasomotor symptoms
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT NAMS 2017
Key clinical point:
Major finding: Patients taking TX-100HR had significant improvements in a menopause-related quality of life scale at all study time points.
Data source: REPLENISH, a phase 3, randomized, double blind, placebo-controlled study of 1,833 postmenopausal women.
Disclosures: Dr. Simon reported financial relationships with multiple pharmaceutical companies, including TherapeuticsMD, sponsor of the REPLENISH trial.