User login
Chronic Pain: How to Approach These 3 Common Conditions
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
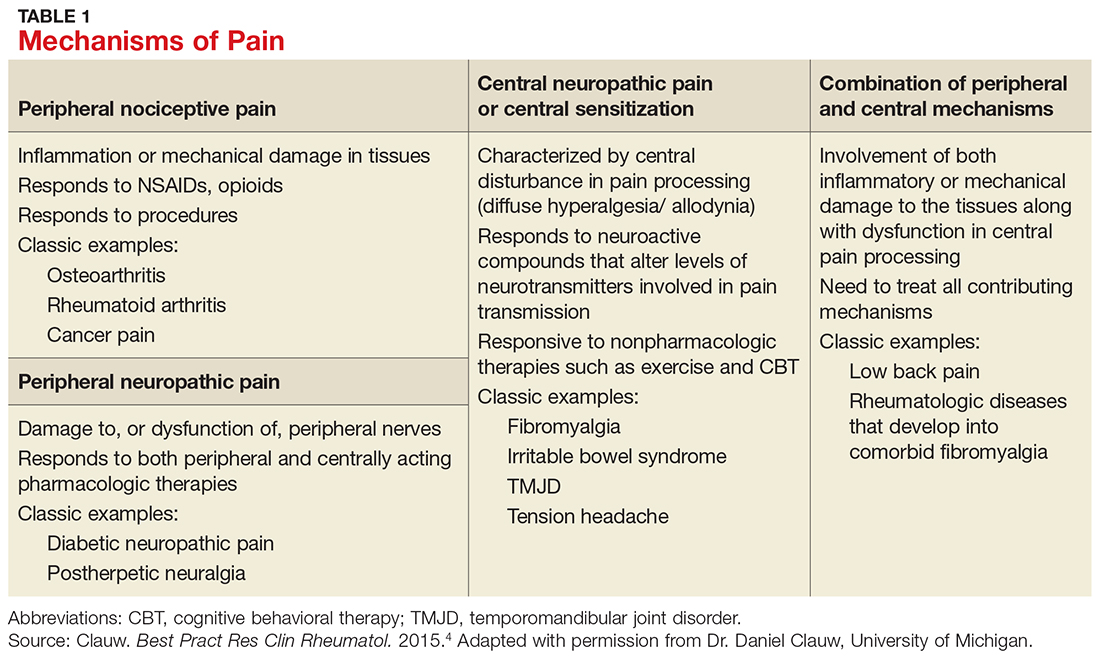
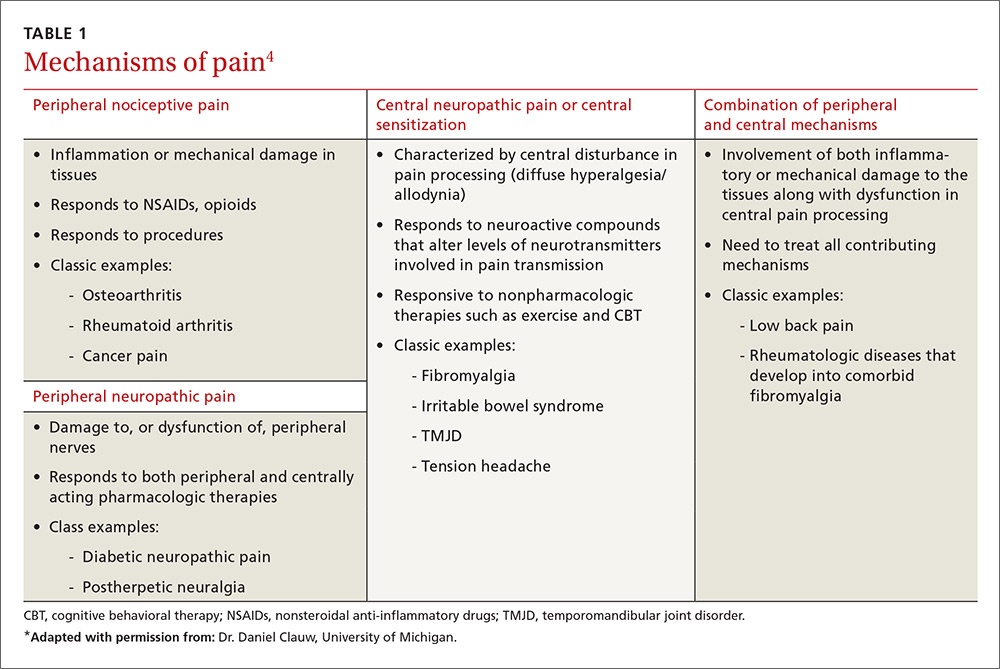
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
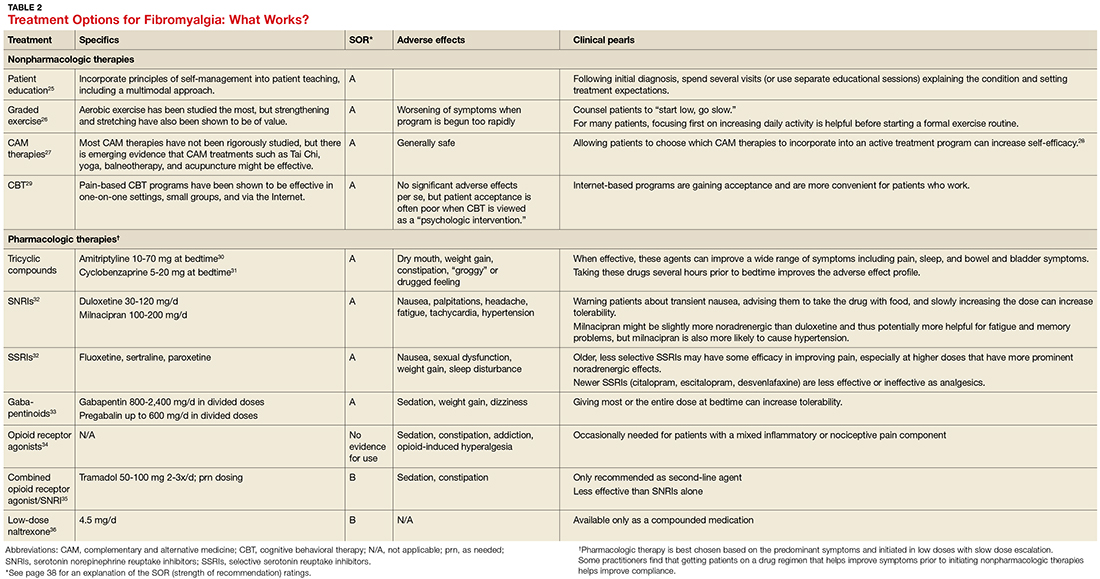
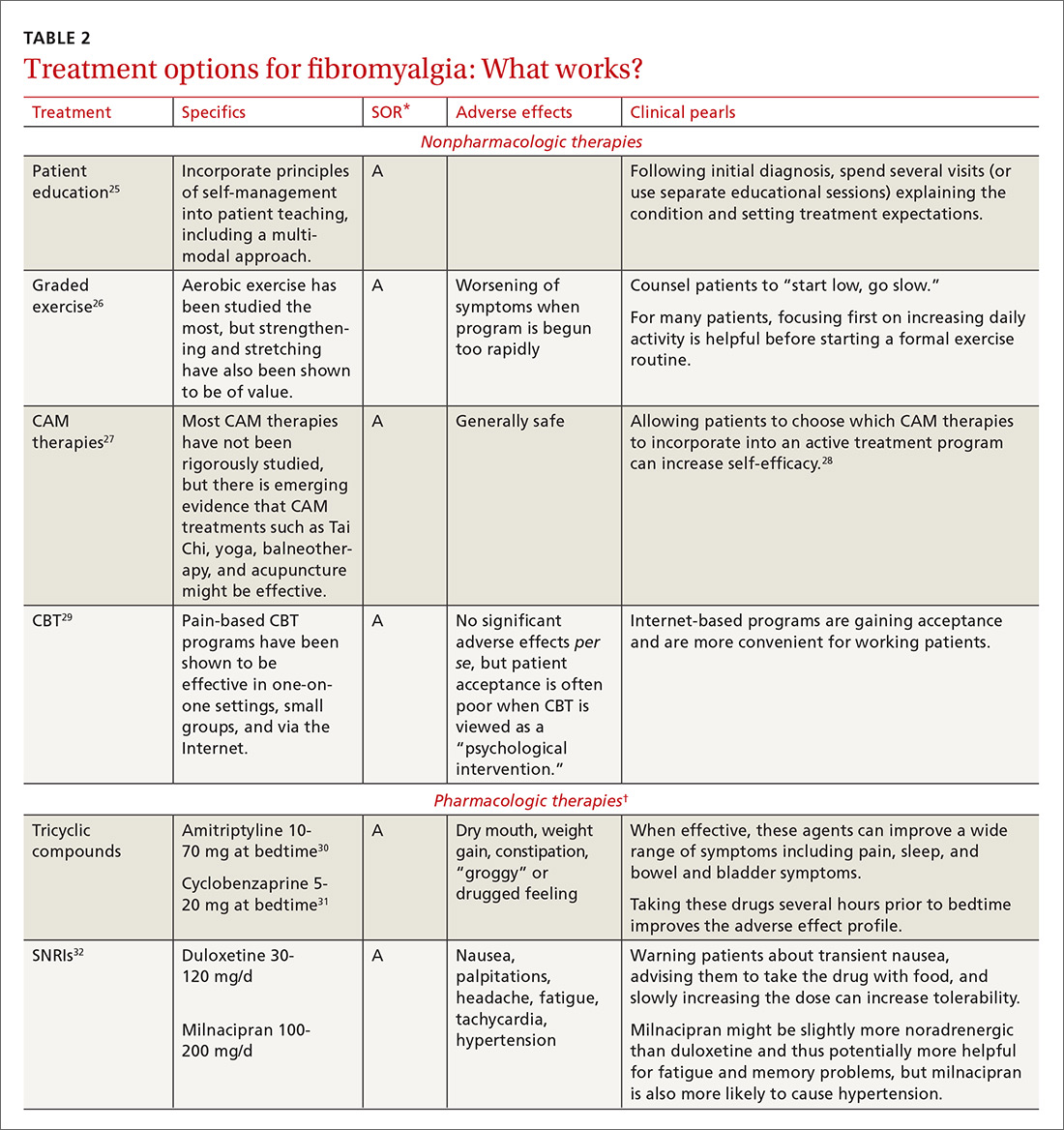
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
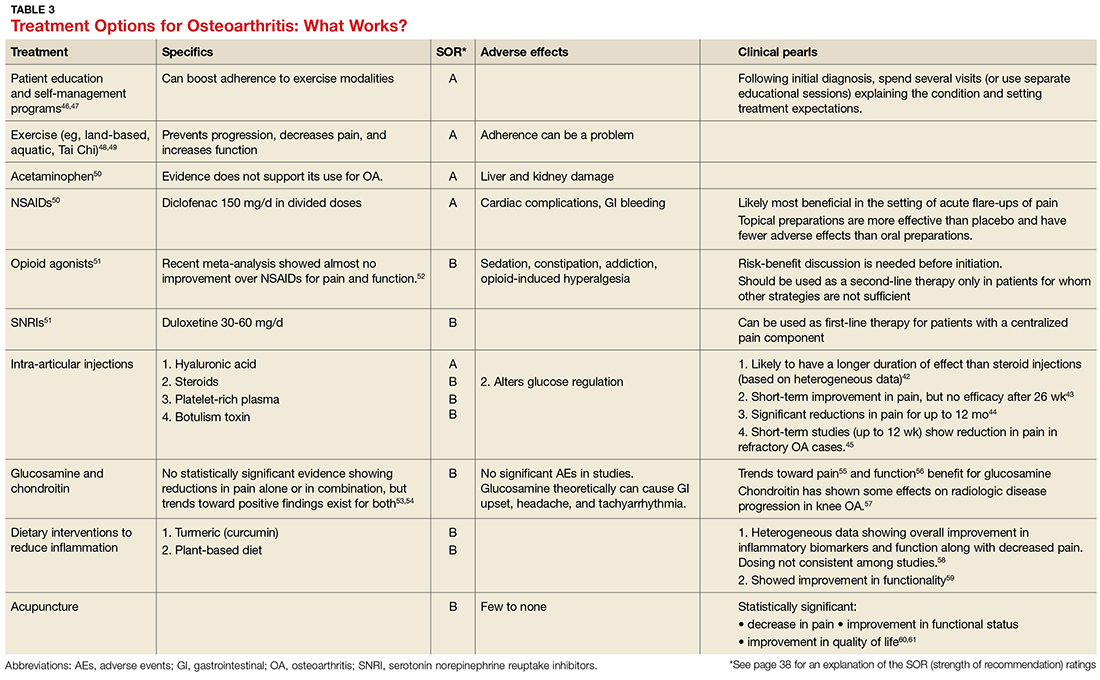
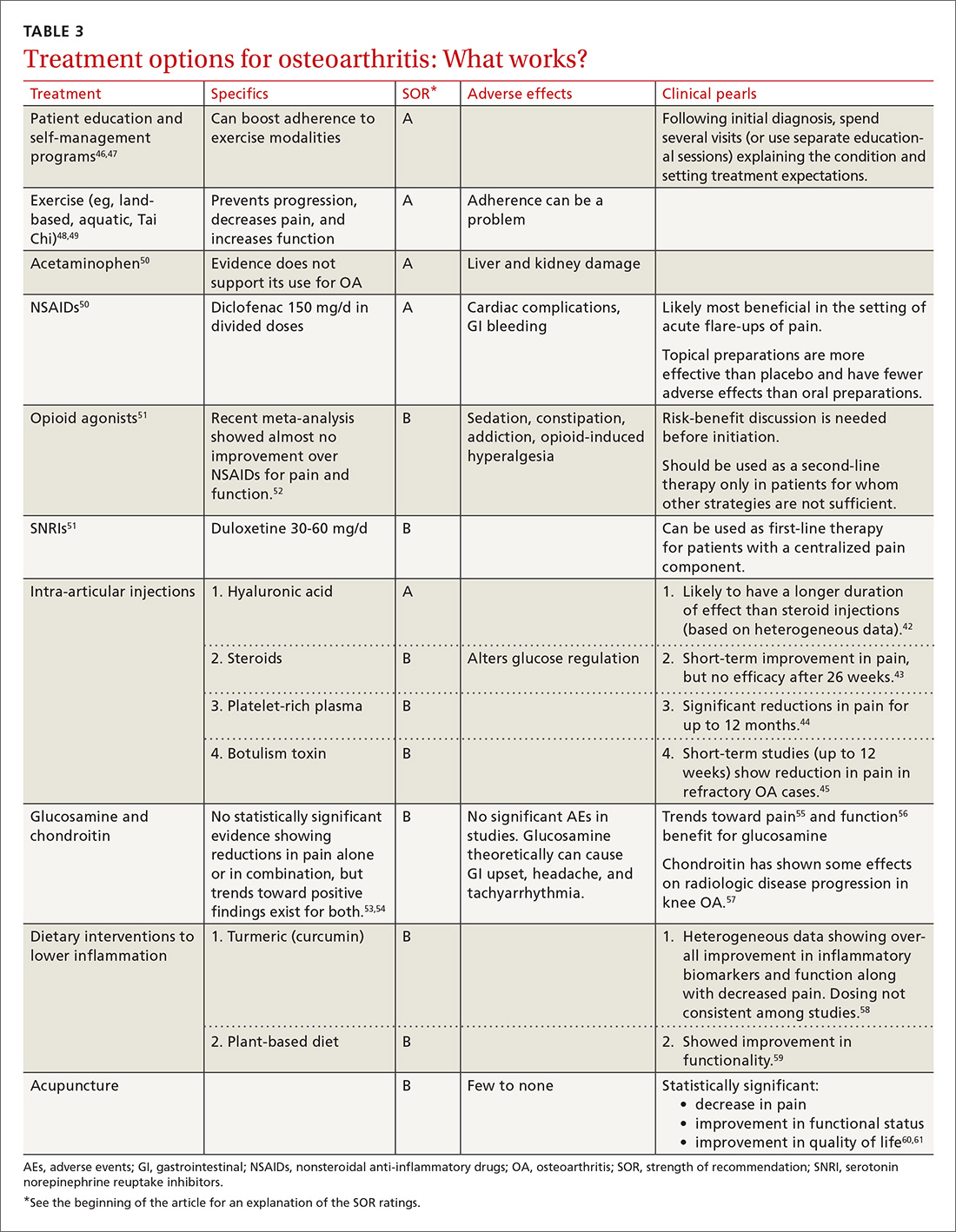
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
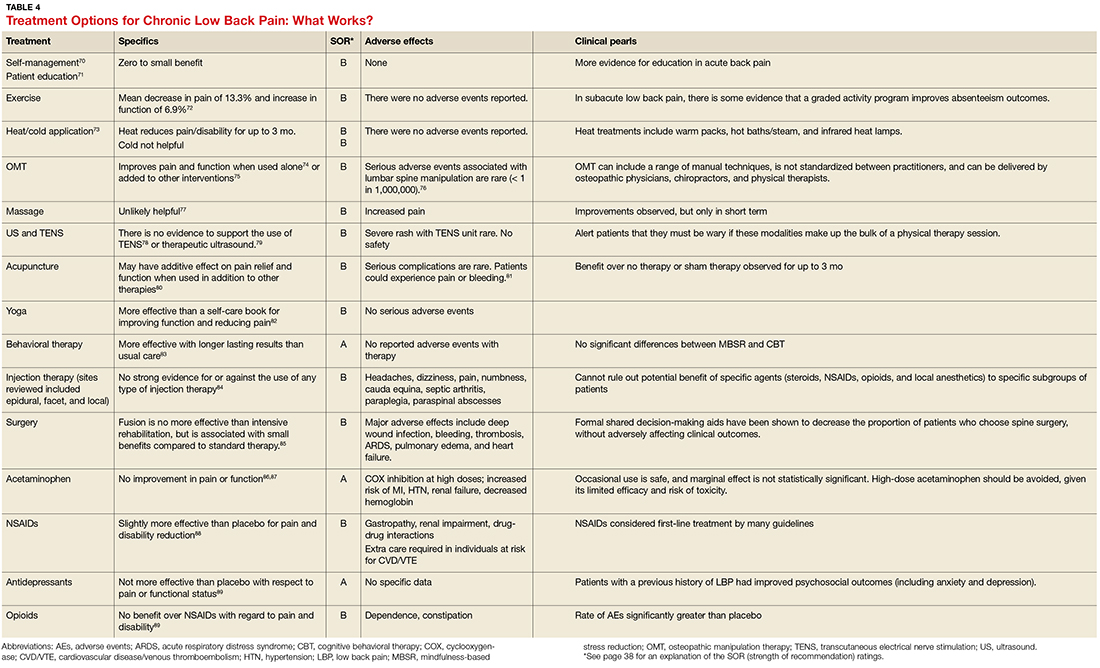
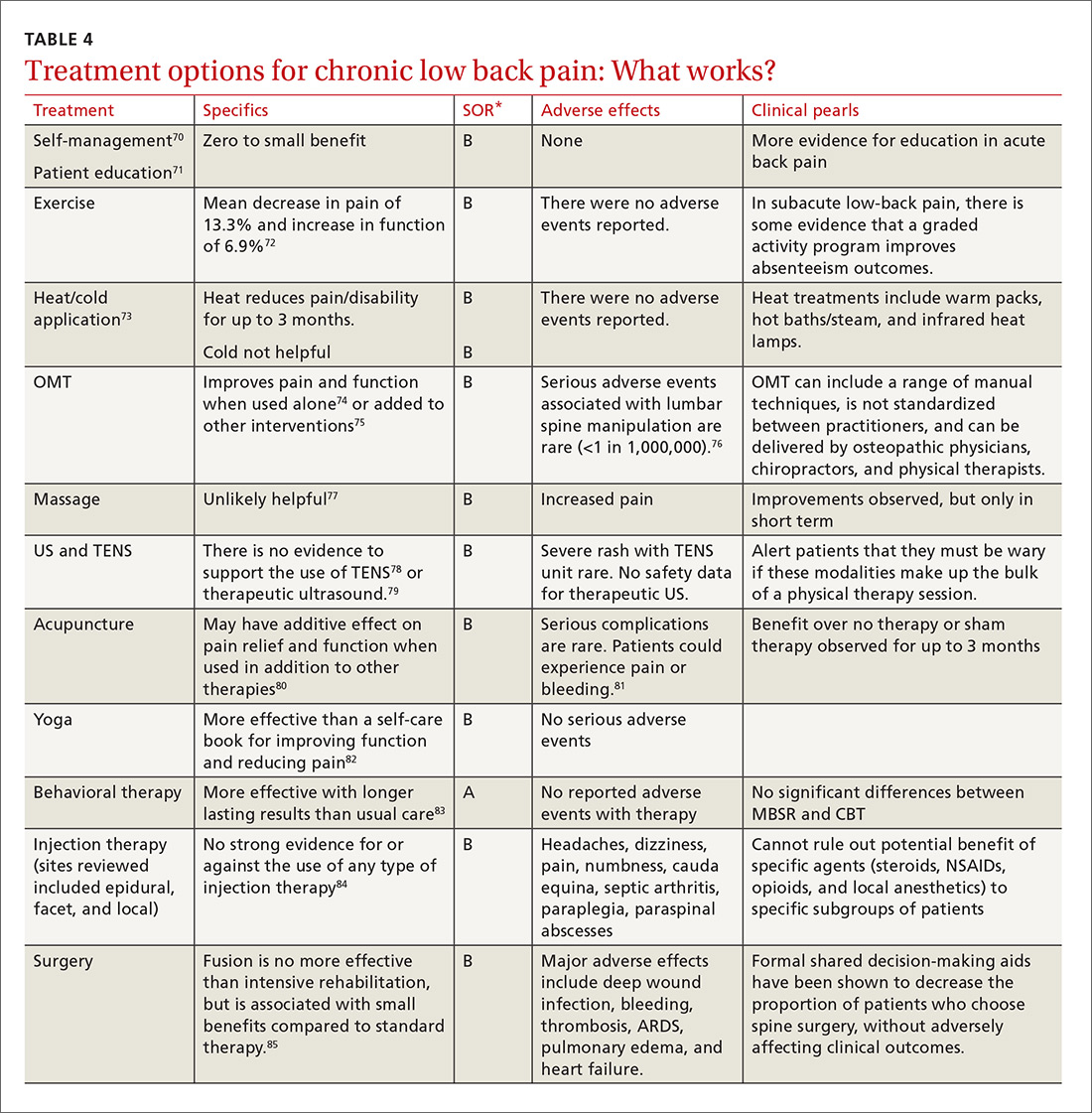
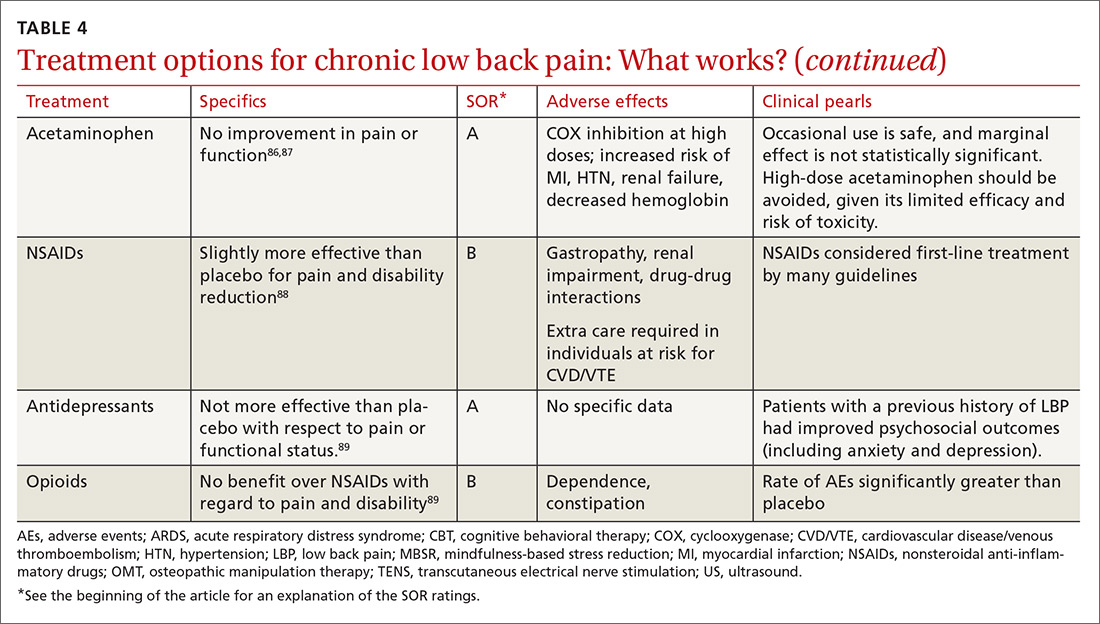
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
Case 1 Lola, 28, has a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. A rheumatologist diagnosed fibromyalgia, but Lola just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
Case 2 Matt is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
Case 3 Keith is a 56-year-old construction worker who has been experiencing back pain for many years. The pain has become more debilitating over time; it is now constant, and Keith can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency departments, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as > 3 mo in duration) is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family practice providers (FPPs) on the frontlines of two epidemics: that of chronic pain and that of the abuse and misuse of opioid pain medications.
To improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the CDC, have published guidelines on the use of opioids for nonmalignant chronic pain.2 With these guidelines in mind—and in light of the latest evidence—we propose the following paradigm for the treatment of chronic pain. A critical aspect is determining the underlying pathophysiology of a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of three common diagnoses: fibromyalgia, osteoarthritis, and low back pain.
LOOK TO THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system (CNS) can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the CNS contributing to the experience of pain.4 This alteration is thought to result from peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers, which then continue to respond to low, or absent, sensory stimuli.
Central sensitization is when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by systemic symptoms such as fatigue and slowed cognitive processing, often with little to no actual stimulation of the peripheral nociceptors.3
Table 1 lists the possible mechanisms of pain, which can be broken down into four categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (when the CNS is the primary entity involved in maintaining the pain), or any combination of the three.4
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportionate to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that patients with a higher degree of centralization of pain (measured by widespread pain index and modified fibromyalgia screening scales) were less likely to report improvement in the affected body part and in overall body pain following surgery.6,7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
TAILOR TREATMENT TO THE UNDERLYING MECHANISMS OF PAIN
As with any chronic condition, a thorough workup (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify the primary mechanism, as well as secondary factors that may contribute to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders, a history of trauma, poor sleep, and tobacco use.9-12 A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments, such as those dealing in emotional awareness and understanding of the CNS’s role in chronic pain, need to be considered.13
Three common conditions. Below we present evidence-based treatment approaches for conditions typically associated with each of the major mechanisms of chronic pain: fibromyalgia (central sensitization), OA (peripheral nociceptive), and low back pain (mixed pain state).
Fibromyalgia: a case of central sensitization
Fibromyalgia is a hallmark diagnosis for patients in whom central sensitization is the dominant cause of pain. They usually present with widespread, diffuse pain and somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
Genetic factors are thought to contribute to a predisposition for amplification. To date, five sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
Imbalances of neurotransmitters (high glutamate; low norepinephrine, serotonin, and gamma-aminobutyric acid [GABA]) play a role in central amplification.20-22 These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in Table 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies, which have high degrees of efficacy with few adverse effects.26,29
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28
OA: an example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7,000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization, and, in some patients, a centralized pain component.40 The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms, such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See Table 3 for a summary of evidence-based treatment options.42-61
Low back pain: a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific, with patients experiencing varying degrees of nociceptive (myofascial LBP), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia, augmented central pain processing, involvement of the emotional brain, and delayed recovery influenced by poor coping strategies.64-67
When developing a treatment plan for a patient with chronic LBP, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain, as well as having higher levels of depression/anxiety and a lower level of physical function.
Because LBP is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications, supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See Table 4 for a summary of evidence-based treatment options.70-89
Case 1 Lola is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FPP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
Case 2 Matt is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent NSAIDs has allowed Matt to maintain his function and his job.
Case 3 Because Keith was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean him off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
The authors thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Michigan), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
24. Wolfe F. Fibromyalgianess. Arthritis Rheum. 2009;61:715-716.
25. Hauser W, Bernardy K, Arnold B, et al. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216-224.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;(8 Suppl 2):S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(4):435-443.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
47. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
67. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14:2639-2657.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
Chronic pain: How to approach these 3 common conditions
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@med.umich.edu.
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@med.umich.edu.
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@med.umich.edu.
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy for most patients suffering from chronic pain. A
› Recommend movement and exercise therapies for all patients with chronic pain. A
› Prescribe anti-inflammatory medications for patients with peripheral nociceptive pain and centrally-acting agents, such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, and alpha 2 delta ligands, for patients with centralized pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Targeting gut flora to treat and prevent disease
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@umich.edu.
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@umich.edu.
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; jillsch@umich.edu.
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].