User login
Clinical Trial Summary: Ergonomics of robotic surgery
The Ergonomics in Robotic Surgery clinical trial is being conducted to study the role of ergonomics in adjusting robotic surgery consoles to individual body types of operators. The study will look at comfort and physical support of gynecologic surgeons after performing a hysterectomy using a da Vinci robotic surgery console. One group of surgeons will adjust their own console and another group will have the console adjusted by an ergonomist.
For a further description of the study, go to www.clinicaltrials.gov.
tborden@frontlinemedcom.com
On Twitter @ThereseBorden
The Ergonomics in Robotic Surgery clinical trial is being conducted to study the role of ergonomics in adjusting robotic surgery consoles to individual body types of operators. The study will look at comfort and physical support of gynecologic surgeons after performing a hysterectomy using a da Vinci robotic surgery console. One group of surgeons will adjust their own console and another group will have the console adjusted by an ergonomist.
For a further description of the study, go to www.clinicaltrials.gov.
tborden@frontlinemedcom.com
On Twitter @ThereseBorden
The Ergonomics in Robotic Surgery clinical trial is being conducted to study the role of ergonomics in adjusting robotic surgery consoles to individual body types of operators. The study will look at comfort and physical support of gynecologic surgeons after performing a hysterectomy using a da Vinci robotic surgery console. One group of surgeons will adjust their own console and another group will have the console adjusted by an ergonomist.
For a further description of the study, go to www.clinicaltrials.gov.
tborden@frontlinemedcom.com
On Twitter @ThereseBorden
FROM CLINICALTRIALS.GOV
SHM pushes to protect patients from ‘surprise’ out-of-network expenses
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.
VA study finds high MRSA infection risk among those colonized with the bacterium
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Key clinical point: About half of postdischarge MRSA infections were in patients who acquired the organism before discharge.
Major finding: The proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
Study details: An analysis of more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015.
Disclosures: The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
Patient and physician outreach boost CRC screening rates
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
Malpractice: Communication and compensation program helps to minimize lawsuits
Communication and resolution programs at four Massachusetts medical centers helped resolve adverse medical events without increasing lawsuits or leading to excessive payouts to patients, according to Michelle M. Mello, PhD, and her colleagues.
They evaluated a communication and resolution program (CRP) model known as CARe (Communication, Apology, and Resolution) implemented at Beth Israel Deaconess Medical Center in Boston and Baystate Medical Center in Springfield, Mass., and at two of each center’s community hospitals. As part of the CARe model, hospital staff and insurers communicate with patients when adverse events occur, investigate and explain what happened, and, when appropriate, apologize and offer compensation.
Of 989 total events studied, 929 (90%) entered the program because an adverse event that allegedly exceeded the severity threshold was reported and 60 (6%) entered CARe because a prelitigation notice or claim was received, said Dr. Mello, professor of law and health research and policy at Stanford (Calif.) University.
Few events that entered the CARe process met the criteria for compensation. The standard of care was violated in 26% of cases where a determination could be reached. No determination could be reached in 59 cases, 9 cases were pending at the close of data collection, and 5 were referred directly to the insurer. Of the 241 cases involving standard-of-care violations, 55% were potentially eligible for compensation because they involved significant harm. After further review, monetary compensation was offered in 43 cases and paid in 40 cases by August 2016, with $75,000 as the median payment (Health Aff. 2017 Oct 2;36[10]:1795-1803).
As of August 2016, 5% of the 929 adverse events led to claims or lawsuits. Insurers deemed 14 of the 47 events that ultimately resulted in legal action ineligible for compensation because of a lack of negligence or lack of harm. They deemed 22 of the cases compensable, offered compensation in all of them, and had settled 20 by August 2016.
During the CARe process, patient safety improvements were frequently identified and improvements made, the investigators said. Of 132 cases in which review progressed far enough for patient safety questions to have been answered, 41% of the incidents gave rise to a safety improvement action. Actions included sharing investigation findings with clinical staff members, clinical staff educational efforts, policy changes, safety alerts sent to staff members, input into the quality improvement system for further analysis, new process flow diagrams, and human factor engineering analysis, among others.
Investigators also surveyed clinicians on their satisfaction with the CARe program. Of 162 clinicians (124 physicians), nearly 40% were either not very or not at all familiar with the program. More than two-thirds (69%) of those who felt well informed about the program gave strongly positive ratings and 10% gave a negative rating to the program. The most commonly suggested improvement to CARe was to improve communication with clinicians.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Communication and resolution programs at four Massachusetts medical centers helped resolve adverse medical events without increasing lawsuits or leading to excessive payouts to patients, according to Michelle M. Mello, PhD, and her colleagues.
They evaluated a communication and resolution program (CRP) model known as CARe (Communication, Apology, and Resolution) implemented at Beth Israel Deaconess Medical Center in Boston and Baystate Medical Center in Springfield, Mass., and at two of each center’s community hospitals. As part of the CARe model, hospital staff and insurers communicate with patients when adverse events occur, investigate and explain what happened, and, when appropriate, apologize and offer compensation.
Of 989 total events studied, 929 (90%) entered the program because an adverse event that allegedly exceeded the severity threshold was reported and 60 (6%) entered CARe because a prelitigation notice or claim was received, said Dr. Mello, professor of law and health research and policy at Stanford (Calif.) University.
Few events that entered the CARe process met the criteria for compensation. The standard of care was violated in 26% of cases where a determination could be reached. No determination could be reached in 59 cases, 9 cases were pending at the close of data collection, and 5 were referred directly to the insurer. Of the 241 cases involving standard-of-care violations, 55% were potentially eligible for compensation because they involved significant harm. After further review, monetary compensation was offered in 43 cases and paid in 40 cases by August 2016, with $75,000 as the median payment (Health Aff. 2017 Oct 2;36[10]:1795-1803).
As of August 2016, 5% of the 929 adverse events led to claims or lawsuits. Insurers deemed 14 of the 47 events that ultimately resulted in legal action ineligible for compensation because of a lack of negligence or lack of harm. They deemed 22 of the cases compensable, offered compensation in all of them, and had settled 20 by August 2016.
During the CARe process, patient safety improvements were frequently identified and improvements made, the investigators said. Of 132 cases in which review progressed far enough for patient safety questions to have been answered, 41% of the incidents gave rise to a safety improvement action. Actions included sharing investigation findings with clinical staff members, clinical staff educational efforts, policy changes, safety alerts sent to staff members, input into the quality improvement system for further analysis, new process flow diagrams, and human factor engineering analysis, among others.
Investigators also surveyed clinicians on their satisfaction with the CARe program. Of 162 clinicians (124 physicians), nearly 40% were either not very or not at all familiar with the program. More than two-thirds (69%) of those who felt well informed about the program gave strongly positive ratings and 10% gave a negative rating to the program. The most commonly suggested improvement to CARe was to improve communication with clinicians.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Communication and resolution programs at four Massachusetts medical centers helped resolve adverse medical events without increasing lawsuits or leading to excessive payouts to patients, according to Michelle M. Mello, PhD, and her colleagues.
They evaluated a communication and resolution program (CRP) model known as CARe (Communication, Apology, and Resolution) implemented at Beth Israel Deaconess Medical Center in Boston and Baystate Medical Center in Springfield, Mass., and at two of each center’s community hospitals. As part of the CARe model, hospital staff and insurers communicate with patients when adverse events occur, investigate and explain what happened, and, when appropriate, apologize and offer compensation.
Of 989 total events studied, 929 (90%) entered the program because an adverse event that allegedly exceeded the severity threshold was reported and 60 (6%) entered CARe because a prelitigation notice or claim was received, said Dr. Mello, professor of law and health research and policy at Stanford (Calif.) University.
Few events that entered the CARe process met the criteria for compensation. The standard of care was violated in 26% of cases where a determination could be reached. No determination could be reached in 59 cases, 9 cases were pending at the close of data collection, and 5 were referred directly to the insurer. Of the 241 cases involving standard-of-care violations, 55% were potentially eligible for compensation because they involved significant harm. After further review, monetary compensation was offered in 43 cases and paid in 40 cases by August 2016, with $75,000 as the median payment (Health Aff. 2017 Oct 2;36[10]:1795-1803).
As of August 2016, 5% of the 929 adverse events led to claims or lawsuits. Insurers deemed 14 of the 47 events that ultimately resulted in legal action ineligible for compensation because of a lack of negligence or lack of harm. They deemed 22 of the cases compensable, offered compensation in all of them, and had settled 20 by August 2016.
During the CARe process, patient safety improvements were frequently identified and improvements made, the investigators said. Of 132 cases in which review progressed far enough for patient safety questions to have been answered, 41% of the incidents gave rise to a safety improvement action. Actions included sharing investigation findings with clinical staff members, clinical staff educational efforts, policy changes, safety alerts sent to staff members, input into the quality improvement system for further analysis, new process flow diagrams, and human factor engineering analysis, among others.
Investigators also surveyed clinicians on their satisfaction with the CARe program. Of 162 clinicians (124 physicians), nearly 40% were either not very or not at all familiar with the program. More than two-thirds (69%) of those who felt well informed about the program gave strongly positive ratings and 10% gave a negative rating to the program. The most commonly suggested improvement to CARe was to improve communication with clinicians.
agallegos@frontlinemedcom.com
On Twitter @legal_med
FROM HEALTH AFFAIRS
Key clinical point:
Major finding: Out of 989 events, monetary compensation was paid in 40 cases, with a $75,000 median payment.
Data source: Review of 989 adverse events at four Massachusetts hospitals.
Disclosures: The project was funded by Baystate Health Insurance Company, Blue Cross Blue Shield of Massachusetts, CRICO RMF, Coverys, Harvard Pilgrim Health Care, Massachusetts Medical Society, and Tufts Health Plan. The authors listed no relevant conflicts of interest.
Irregular Erythematous Patch on the Face of an Infant
The Diagnosis: Phakomatosis Pigmentovascularis With Sturge-Weber Syndrome
The erythematous patches were identified as capillary malformations (port-wine stains) and the slate gray pigmentary changes as dermal melanocytosis (Mongolian spots)(Figure). In fact, the diagnosis of phakomatosis pigmentovascularis (PPV) type II requires dermal melanocytosis and capillary malformation with and without nevus anemicus.1 In one case series, 46% (7/15) of patients with PPV had nevus anemicus2 but our patient did not.

Phakomatosis pigmentovascularis was divided into 4 types in 1985,3 then later 5 types.4 Subcategories of the 5 types include type A, which denotes a lack of extracutaneous involvement, and type B, which is used when internal manifestations have been exhibited. Since 1947, approximately 222 cases of PPV have been described in the literature.2
A case of PPV associated with Sturge-Weber syndrome (SWS) was reported in 1997.5 Since then, PPV occasionally has been linked with SWS,5-9 though there have been other syndromic associations including Klippel-Trenaunay-Weber syndrome and melanosis oculi.2 The incidence and prevalence of overlap of PPV and SWS is unknown but is likely to be rare. In our case, magnetic resonance imaging of the patient's brain did not reveal the characteristic tram-track appearance of SWS; however, the diagnosis of SWS type II only requires facial angioma with or without glaucoma.9,10 Most cases of PPV originate from Japan, Argentina, and Mexico.2 Interestingly, our patient's parents were both of Mexican ancestry. Phakomatosis pigmentovascularis type IIb is the most common, followed by type IIa.2 Most cases have been described as sporadic, though our patient's mother also exhibited a port-wine stain on the right neck, suggesting a possible genetic association.
The etiology of PPV has been postulated as twin spotting or didymosis (Greek for twin), most commonly seen in plants and animals. A previous review defined twin spotting as 2 mutant tissues situated adjacent to one another and unique from the normal tissue surrounding both of them.2 When the cell loses its heterozygosity, this phenomenon appears. An alternative etiology supplants that a drug or virus toxic to the nervous system causes aberrant angioblasts and melanoblasts.11,12 The etiology of SWS also is unknown, though vasomotor instability has been postulated as a cause.6,13
It is important to exclude associated internal organ involvement with both of these syndromes because approximately 50% of PPV cases have extracutaneous organ involvement.2,14 In fact, PPV is known to involve the brain, skeletal system, and eye, potentially manifesting as deafness, hydrocephalus, extremity overgrowth, scoliosis, cataracts, and more.2 Patients with SWS often exhibit brain and eye symptoms including seizures.1 To screen for extracutaneous involvement, multiple imaging studies should be performed. In our patient, an echocardiogram revealed a patent foramen ovale and normal cardiac anatomy for his age. Brain imaging revealed a hypoplastic left sigmoid and transverse sinus without venous thrombosis and unremarkable appearance of the brain. An ultrasound of the liver, spleen, kidneys, and pancreas revealed no evidence of solid, cystic, or vascular lesions, though the gallbladder exhibited hyperechoic areas.
To manage the skin lesions, some authors recommend Q-switched lasers for pigmented lesions and pulsed dye lasers for capillary malformations.15 Paller and Mancini1 cited evidence that pulsed dye laser treatment before the age of 1 year may offer a psychological advantage, while other views have been offered.16 Some physicians believe that no urgent treatment of capillary malformations is needed unless internal organs are involved.2,15
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. New York, NY: Elsevier/Saunders; 2011.
- Fernández-Guarino M, Boixeda P, de Las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Hasegawa Y, Yasuhara M. Phakomatosis pigmentovascularis type VIa. Arch Dermatol. 1985;121:651-655.
- Torrelo A, Zambrano A, Happle R. Cutis marmorata telangiectatica congenita and extensive Mongolian spots: type V phacomatosis pigmentovascularis. Br J Dermatol. 2003;148:342-345.
- Teekhasaenee C, Ritch R. Glaucoma in phakomatosis pigmentovascularis. Ophthalmology. 1997;104:150-157.
- Patil B, Sinha G, Nayak B, et al. Bilateral Sturge-Weber and phakomatosis pigmentovascularis with glaucoma, an overlap syndrome [published online May 6, 2015]. Case Rep Ophthalmol Med. 2015;2015:106932.
- Hagiwara K, Uezato H, Nonaka S. Phacomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome and pyogenic granuloma. J Dermatol. 1998;25:721-729.
- Al Robaee A, Banka N, Alfadley A. Phakomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome. Pediatr Dermatol. 2004;21:642-645.
- Yang Y, Guo X, Xu J, et al. Phakomatosis pigmentovascularis associated with Sturge-Weber syndrome, ota nevus, and congenital glaucoma. Medicine (Baltimore). 2015;94:E1025.
- Roach ES. Neurocutaneous syndromes. Pediatr Clin North Am. 1992;39:591-620.
- Happle R. Mosaicism in human skin, understanding the patterns and mechanisms. Arch Dermatol. 1993;129:1460-1470.
- Happle R. Loss of heterozygosity in human skin. J Am Acad Dermatol. 1999;85:355-358.
- Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
- Kim YC, Park HJ, Cinn YW. Phakomatosis pigmentovascularis type IIa with generalized vitiligo. Br J Dermatol. 2002;147:1028-1029.
- Brittain P, Walsh EJ, Smidt AC. Blotchy baby: a case of phakomatosis pigmentovascularis [published online February 1, 2013]. J Pediatr. 2013;162:1293.
- Van der Horst CM, Koster PH, de Borgie CA, et al. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338:1028-1033.
The Diagnosis: Phakomatosis Pigmentovascularis With Sturge-Weber Syndrome
The erythematous patches were identified as capillary malformations (port-wine stains) and the slate gray pigmentary changes as dermal melanocytosis (Mongolian spots)(Figure). In fact, the diagnosis of phakomatosis pigmentovascularis (PPV) type II requires dermal melanocytosis and capillary malformation with and without nevus anemicus.1 In one case series, 46% (7/15) of patients with PPV had nevus anemicus2 but our patient did not.

Phakomatosis pigmentovascularis was divided into 4 types in 1985,3 then later 5 types.4 Subcategories of the 5 types include type A, which denotes a lack of extracutaneous involvement, and type B, which is used when internal manifestations have been exhibited. Since 1947, approximately 222 cases of PPV have been described in the literature.2
A case of PPV associated with Sturge-Weber syndrome (SWS) was reported in 1997.5 Since then, PPV occasionally has been linked with SWS,5-9 though there have been other syndromic associations including Klippel-Trenaunay-Weber syndrome and melanosis oculi.2 The incidence and prevalence of overlap of PPV and SWS is unknown but is likely to be rare. In our case, magnetic resonance imaging of the patient's brain did not reveal the characteristic tram-track appearance of SWS; however, the diagnosis of SWS type II only requires facial angioma with or without glaucoma.9,10 Most cases of PPV originate from Japan, Argentina, and Mexico.2 Interestingly, our patient's parents were both of Mexican ancestry. Phakomatosis pigmentovascularis type IIb is the most common, followed by type IIa.2 Most cases have been described as sporadic, though our patient's mother also exhibited a port-wine stain on the right neck, suggesting a possible genetic association.
The etiology of PPV has been postulated as twin spotting or didymosis (Greek for twin), most commonly seen in plants and animals. A previous review defined twin spotting as 2 mutant tissues situated adjacent to one another and unique from the normal tissue surrounding both of them.2 When the cell loses its heterozygosity, this phenomenon appears. An alternative etiology supplants that a drug or virus toxic to the nervous system causes aberrant angioblasts and melanoblasts.11,12 The etiology of SWS also is unknown, though vasomotor instability has been postulated as a cause.6,13
It is important to exclude associated internal organ involvement with both of these syndromes because approximately 50% of PPV cases have extracutaneous organ involvement.2,14 In fact, PPV is known to involve the brain, skeletal system, and eye, potentially manifesting as deafness, hydrocephalus, extremity overgrowth, scoliosis, cataracts, and more.2 Patients with SWS often exhibit brain and eye symptoms including seizures.1 To screen for extracutaneous involvement, multiple imaging studies should be performed. In our patient, an echocardiogram revealed a patent foramen ovale and normal cardiac anatomy for his age. Brain imaging revealed a hypoplastic left sigmoid and transverse sinus without venous thrombosis and unremarkable appearance of the brain. An ultrasound of the liver, spleen, kidneys, and pancreas revealed no evidence of solid, cystic, or vascular lesions, though the gallbladder exhibited hyperechoic areas.
To manage the skin lesions, some authors recommend Q-switched lasers for pigmented lesions and pulsed dye lasers for capillary malformations.15 Paller and Mancini1 cited evidence that pulsed dye laser treatment before the age of 1 year may offer a psychological advantage, while other views have been offered.16 Some physicians believe that no urgent treatment of capillary malformations is needed unless internal organs are involved.2,15
The Diagnosis: Phakomatosis Pigmentovascularis With Sturge-Weber Syndrome
The erythematous patches were identified as capillary malformations (port-wine stains) and the slate gray pigmentary changes as dermal melanocytosis (Mongolian spots)(Figure). In fact, the diagnosis of phakomatosis pigmentovascularis (PPV) type II requires dermal melanocytosis and capillary malformation with and without nevus anemicus.1 In one case series, 46% (7/15) of patients with PPV had nevus anemicus2 but our patient did not.

Phakomatosis pigmentovascularis was divided into 4 types in 1985,3 then later 5 types.4 Subcategories of the 5 types include type A, which denotes a lack of extracutaneous involvement, and type B, which is used when internal manifestations have been exhibited. Since 1947, approximately 222 cases of PPV have been described in the literature.2
A case of PPV associated with Sturge-Weber syndrome (SWS) was reported in 1997.5 Since then, PPV occasionally has been linked with SWS,5-9 though there have been other syndromic associations including Klippel-Trenaunay-Weber syndrome and melanosis oculi.2 The incidence and prevalence of overlap of PPV and SWS is unknown but is likely to be rare. In our case, magnetic resonance imaging of the patient's brain did not reveal the characteristic tram-track appearance of SWS; however, the diagnosis of SWS type II only requires facial angioma with or without glaucoma.9,10 Most cases of PPV originate from Japan, Argentina, and Mexico.2 Interestingly, our patient's parents were both of Mexican ancestry. Phakomatosis pigmentovascularis type IIb is the most common, followed by type IIa.2 Most cases have been described as sporadic, though our patient's mother also exhibited a port-wine stain on the right neck, suggesting a possible genetic association.
The etiology of PPV has been postulated as twin spotting or didymosis (Greek for twin), most commonly seen in plants and animals. A previous review defined twin spotting as 2 mutant tissues situated adjacent to one another and unique from the normal tissue surrounding both of them.2 When the cell loses its heterozygosity, this phenomenon appears. An alternative etiology supplants that a drug or virus toxic to the nervous system causes aberrant angioblasts and melanoblasts.11,12 The etiology of SWS also is unknown, though vasomotor instability has been postulated as a cause.6,13
It is important to exclude associated internal organ involvement with both of these syndromes because approximately 50% of PPV cases have extracutaneous organ involvement.2,14 In fact, PPV is known to involve the brain, skeletal system, and eye, potentially manifesting as deafness, hydrocephalus, extremity overgrowth, scoliosis, cataracts, and more.2 Patients with SWS often exhibit brain and eye symptoms including seizures.1 To screen for extracutaneous involvement, multiple imaging studies should be performed. In our patient, an echocardiogram revealed a patent foramen ovale and normal cardiac anatomy for his age. Brain imaging revealed a hypoplastic left sigmoid and transverse sinus without venous thrombosis and unremarkable appearance of the brain. An ultrasound of the liver, spleen, kidneys, and pancreas revealed no evidence of solid, cystic, or vascular lesions, though the gallbladder exhibited hyperechoic areas.
To manage the skin lesions, some authors recommend Q-switched lasers for pigmented lesions and pulsed dye lasers for capillary malformations.15 Paller and Mancini1 cited evidence that pulsed dye laser treatment before the age of 1 year may offer a psychological advantage, while other views have been offered.16 Some physicians believe that no urgent treatment of capillary malformations is needed unless internal organs are involved.2,15
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. New York, NY: Elsevier/Saunders; 2011.
- Fernández-Guarino M, Boixeda P, de Las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Hasegawa Y, Yasuhara M. Phakomatosis pigmentovascularis type VIa. Arch Dermatol. 1985;121:651-655.
- Torrelo A, Zambrano A, Happle R. Cutis marmorata telangiectatica congenita and extensive Mongolian spots: type V phacomatosis pigmentovascularis. Br J Dermatol. 2003;148:342-345.
- Teekhasaenee C, Ritch R. Glaucoma in phakomatosis pigmentovascularis. Ophthalmology. 1997;104:150-157.
- Patil B, Sinha G, Nayak B, et al. Bilateral Sturge-Weber and phakomatosis pigmentovascularis with glaucoma, an overlap syndrome [published online May 6, 2015]. Case Rep Ophthalmol Med. 2015;2015:106932.
- Hagiwara K, Uezato H, Nonaka S. Phacomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome and pyogenic granuloma. J Dermatol. 1998;25:721-729.
- Al Robaee A, Banka N, Alfadley A. Phakomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome. Pediatr Dermatol. 2004;21:642-645.
- Yang Y, Guo X, Xu J, et al. Phakomatosis pigmentovascularis associated with Sturge-Weber syndrome, ota nevus, and congenital glaucoma. Medicine (Baltimore). 2015;94:E1025.
- Roach ES. Neurocutaneous syndromes. Pediatr Clin North Am. 1992;39:591-620.
- Happle R. Mosaicism in human skin, understanding the patterns and mechanisms. Arch Dermatol. 1993;129:1460-1470.
- Happle R. Loss of heterozygosity in human skin. J Am Acad Dermatol. 1999;85:355-358.
- Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
- Kim YC, Park HJ, Cinn YW. Phakomatosis pigmentovascularis type IIa with generalized vitiligo. Br J Dermatol. 2002;147:1028-1029.
- Brittain P, Walsh EJ, Smidt AC. Blotchy baby: a case of phakomatosis pigmentovascularis [published online February 1, 2013]. J Pediatr. 2013;162:1293.
- Van der Horst CM, Koster PH, de Borgie CA, et al. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338:1028-1033.
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. New York, NY: Elsevier/Saunders; 2011.
- Fernández-Guarino M, Boixeda P, de Las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Hasegawa Y, Yasuhara M. Phakomatosis pigmentovascularis type VIa. Arch Dermatol. 1985;121:651-655.
- Torrelo A, Zambrano A, Happle R. Cutis marmorata telangiectatica congenita and extensive Mongolian spots: type V phacomatosis pigmentovascularis. Br J Dermatol. 2003;148:342-345.
- Teekhasaenee C, Ritch R. Glaucoma in phakomatosis pigmentovascularis. Ophthalmology. 1997;104:150-157.
- Patil B, Sinha G, Nayak B, et al. Bilateral Sturge-Weber and phakomatosis pigmentovascularis with glaucoma, an overlap syndrome [published online May 6, 2015]. Case Rep Ophthalmol Med. 2015;2015:106932.
- Hagiwara K, Uezato H, Nonaka S. Phacomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome and pyogenic granuloma. J Dermatol. 1998;25:721-729.
- Al Robaee A, Banka N, Alfadley A. Phakomatosis pigmentovascularis type IIb associated with Sturge-Weber syndrome. Pediatr Dermatol. 2004;21:642-645.
- Yang Y, Guo X, Xu J, et al. Phakomatosis pigmentovascularis associated with Sturge-Weber syndrome, ota nevus, and congenital glaucoma. Medicine (Baltimore). 2015;94:E1025.
- Roach ES. Neurocutaneous syndromes. Pediatr Clin North Am. 1992;39:591-620.
- Happle R. Mosaicism in human skin, understanding the patterns and mechanisms. Arch Dermatol. 1993;129:1460-1470.
- Happle R. Loss of heterozygosity in human skin. J Am Acad Dermatol. 1999;85:355-358.
- Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
- Kim YC, Park HJ, Cinn YW. Phakomatosis pigmentovascularis type IIa with generalized vitiligo. Br J Dermatol. 2002;147:1028-1029.
- Brittain P, Walsh EJ, Smidt AC. Blotchy baby: a case of phakomatosis pigmentovascularis [published online February 1, 2013]. J Pediatr. 2013;162:1293.
- Van der Horst CM, Koster PH, de Borgie CA, et al. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338:1028-1033.
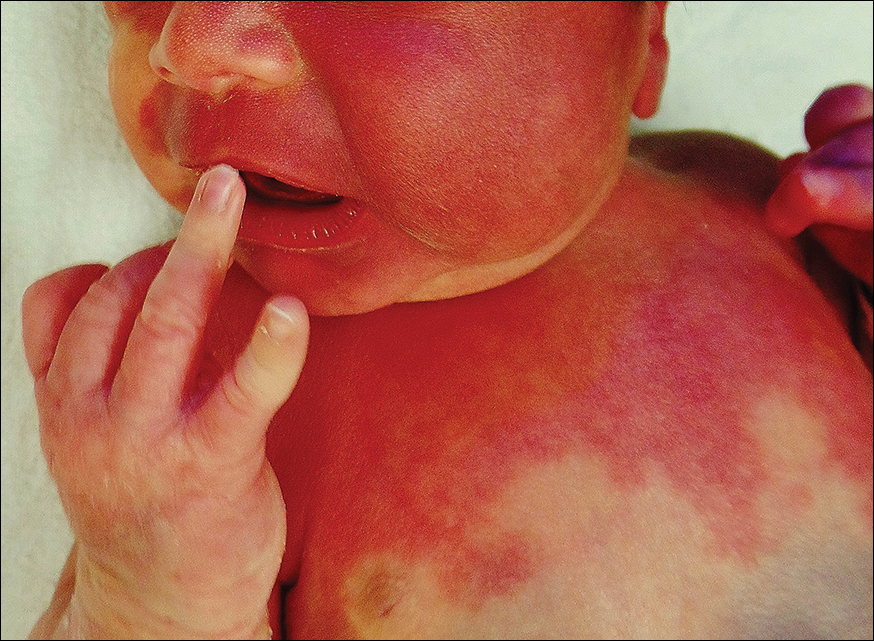
A newborn presented with an irregular and well-demarcated erythematous patch on the face, trunk, buttocks, and toes on the left foot. Another red patch was present on the right side of the face, while a slate gray patch covered the flanks and back. The limbs appeared symmetric and he exhibited no gross deformities. On close physical examination, he was noted to have a cloudy left eye. An ophthalmology consultation revealed a choroidal hemangioma and congenital glaucoma in the left eye.
A Case of Streptococcus pyogenes Sepsis of Possible Oral Origin
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region.1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn, can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes (S pyogenes).
Case Presentation
A 57-year-old female with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the emergency department (ED) by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills. On examination the patient was noted to have a fever of 104° F, heart rate of 160 bpm, respirations of 22 breaths per minute, blood pressure (BP) 109/58, and a white blood cell count (WBC) of 8.7 X 103. There was a noted skin abrasion on her right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on 2 vasopressors for control of low BP and assistance with low urine output. After a 6 L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology was consulted for possible acute respiratory distress syndrome secondary to sepsis. General surgery was consulted for possible necrotizing fasciitis of the chest wall, and cardiology was consulted for low cardiac output.
On day 4 of her hospitalization, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures with lack of organism growth was noted. While in the MICU, she was followed by the Infectious Disease service as her WBC remained elevated and peaking at 32.6 X 103, while blood cultures were negative for bacterial growth. The dental service was consulted on day 5 to evaluate for other possible sources of infection.
The patient’s oral condition was noted as having advanced chronic periodontal disease that required full mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50,000 until day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. On extraction the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the U.S. health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care.2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000.3
Conclusion
This case demonstrates the successful collaboration of dentistry for the overall medical management of the patient. An integrated approach highlights the need for and the value of integrating dental programs within large tertiary hospital systems. Such integration will likely improve earlier recognition and better management of oral infections resulting in systemic illness and improve patient outcomes, reduced length of hospital stay, and reduction of overall costs.
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993; 51(8):868-873.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed September 5, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region.1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn, can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes (S pyogenes).
Case Presentation
A 57-year-old female with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the emergency department (ED) by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills. On examination the patient was noted to have a fever of 104° F, heart rate of 160 bpm, respirations of 22 breaths per minute, blood pressure (BP) 109/58, and a white blood cell count (WBC) of 8.7 X 103. There was a noted skin abrasion on her right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on 2 vasopressors for control of low BP and assistance with low urine output. After a 6 L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology was consulted for possible acute respiratory distress syndrome secondary to sepsis. General surgery was consulted for possible necrotizing fasciitis of the chest wall, and cardiology was consulted for low cardiac output.
On day 4 of her hospitalization, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures with lack of organism growth was noted. While in the MICU, she was followed by the Infectious Disease service as her WBC remained elevated and peaking at 32.6 X 103, while blood cultures were negative for bacterial growth. The dental service was consulted on day 5 to evaluate for other possible sources of infection.
The patient’s oral condition was noted as having advanced chronic periodontal disease that required full mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50,000 until day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. On extraction the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the U.S. health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care.2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000.3
Conclusion
This case demonstrates the successful collaboration of dentistry for the overall medical management of the patient. An integrated approach highlights the need for and the value of integrating dental programs within large tertiary hospital systems. Such integration will likely improve earlier recognition and better management of oral infections resulting in systemic illness and improve patient outcomes, reduced length of hospital stay, and reduction of overall costs.
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region.1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn, can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes (S pyogenes).
Case Presentation
A 57-year-old female with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the emergency department (ED) by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills. On examination the patient was noted to have a fever of 104° F, heart rate of 160 bpm, respirations of 22 breaths per minute, blood pressure (BP) 109/58, and a white blood cell count (WBC) of 8.7 X 103. There was a noted skin abrasion on her right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on 2 vasopressors for control of low BP and assistance with low urine output. After a 6 L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology was consulted for possible acute respiratory distress syndrome secondary to sepsis. General surgery was consulted for possible necrotizing fasciitis of the chest wall, and cardiology was consulted for low cardiac output.
On day 4 of her hospitalization, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures with lack of organism growth was noted. While in the MICU, she was followed by the Infectious Disease service as her WBC remained elevated and peaking at 32.6 X 103, while blood cultures were negative for bacterial growth. The dental service was consulted on day 5 to evaluate for other possible sources of infection.
The patient’s oral condition was noted as having advanced chronic periodontal disease that required full mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50,000 until day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. On extraction the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the U.S. health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care.2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000.3
Conclusion
This case demonstrates the successful collaboration of dentistry for the overall medical management of the patient. An integrated approach highlights the need for and the value of integrating dental programs within large tertiary hospital systems. Such integration will likely improve earlier recognition and better management of oral infections resulting in systemic illness and improve patient outcomes, reduced length of hospital stay, and reduction of overall costs.
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993; 51(8):868-873.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed September 5, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993; 51(8):868-873.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed September 5, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
Natural selection opportunities tied to cancer rates
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
Countries with the lowest opportunities for natural selection have higher cancer rates than countries with the highest opportunities for natural selection, according to a study published in Evolutionary Applications.
Researchers said this is because modern medicine is enabling people to survive cancers, and their genetic backgrounds are passing from one generation to the next.
The team said the rate of some cancers has doubled and even quadrupled over the past 100 to 150 years, and human evolution has moved away from “survival of the fittest.”
“Modern medicine has enabled the human species to live much longer than would otherwise be expected in the natural world,” said study author Maciej Henneberg, PhD, DSc, of the University of Adelaide in South Australia.
“Besides the obvious benefits that modern medicine gives, it also brings with it an unexpected side-effect—allowing genetic material to be passed from one generation to the next that predisposes people to have poor health, such as type 1 diabetes or cancer.”
“Because of the quality of our healthcare in western society, we have almost removed natural selection as the ‘janitor of the gene pool.’ Unfortunately, the accumulation of genetic mutations over time and across multiple generations is like a delayed death sentence.”
Country comparison
The researchers studied global cancer data from the World Health Organization as well as other health and socioeconomic data from the United Nations and the World Bank of 173 countries. The team compared the top 10 countries with the highest opportunities for natural selection to the 10 countries with the lowest opportunities for natural selection.
“We looked at countries that offered the greatest opportunity to survive cancer compared with those that didn’t,” said study author Wenpeng You, a PhD student at the University of Adelaide. “This does not only take into account factors such as socioeconomic status, urbanization, and quality of medical services but also low mortality and fertility rates, which are the 2 distinguishing features in the ‘better’ world.”
“Countries with low mortality rates may allow more people with cancer genetic background to reproduce and pass cancer genes/mutations to the next generation. Meanwhile, low fertility rates in these countries may not be able to have diverse biological variations to provide the opportunity for selecting a naturally fit population—for example, people without or with less cancer genetic background. Low mortality rate and low fertility rate in the ‘better’ world may have formed a self-reinforcing cycle which has accumulated cancer genetic background at a greater rate than previously thought.”
Based on the researchers’ analysis, the 20 countries are:
| Lowest opportunities for natural selection | Highest opportunities for natural selection |
| Iceland | Burkina Faso |
| Singapore | Chad |
| Japan | Central African Republic |
| Switzerland | Afghanistan |
| Sweden | Somalia |
| Luxembourg | Sierra Leone |
| Germany | Democratic Republic of the Congo |
| Italy | Guinea-Bissau |
| Cyprus | Burundi |
| Andorra | Cameroon |
Cancer incidence
The researchers found the rates of most cancers were higher in the 10 countries with the lowest opportunities for natural selection. The incidence of all cancers was 2.326 times higher in the low-opportunity countries than the high-opportunity ones.
The increased incidences of hematologic malignancies were as follows:
- Non-Hodgkin lymphoma—2.019 times higher in the low-opportunity countries
- Hodgkin lymphoma—3.314 times higher in the low-opportunity countries
- Leukemia—3.574 times higher in the low-opportunity countries
- Multiple myeloma—4.257 times higher in the low-opportunity countries .
Dr Henneberg said that, having removed natural selection as the “janitor of the gene pool,” our modern society is faced with a controversial issue.
“It may be that the only way humankind can be rid of cancer once and for all is through genetic engineering—to repair our genes and take cancer out of the equation,” he said. ![]()
Study supports prophylaxis in kids with ALL
Results of an observational study support targeted antibacterial prophylaxis in children undergoing induction therapy for acute lymphoblastic leukemia (ALL).
Prophylaxis effectively prevented febrile neutropenia and systemic infection in the children studied.
Prophylaxis with the drug levofloxacin reduced the use of treatment antibiotics and the incidence of Clostridium difficile infection.
“This research provides the first major evidence supporting targeted use of antibacterial prophylaxis for at-risk pediatric ALL patients, particularly use of the broad-spectrum antibiotic levofloxacin,” said study author Joshua Wolf, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Prophylactic antibiotic therapy with levofloxacin is routine for at-risk adult ALL patients, but it has remained controversial in children. Until this study, evidence supporting the safety and efficacy of prophylactic antibiotic therapy in children with ALL has been sparse.”
Dr Wolf and his colleagues described their study in Clinical Infectious Diseases.
The study included 344 patients newly diagnosed with ALL who were enrolled in the St. Jude Total XVI clinical trial (NCT00549848). Patients were enrolled from 2007 to 2016.
Until July 2014, the patients received prophylactic antibiotic therapy at the discretion of their physicians. Patients typically received cefepime, ciprofloxacin, or vancomycin plus cefepime or ciprofloxacin. And prophylaxis was typically started at the onset of neutropenia after chemotherapy.
Beginning in August 2014, hospital treatment guidelines changed to recommend prophylactic levofloxacin during induction for ALL patients who develop neutropenia expected to last at least 7 days.
Dr Wolf and his colleagues used the change to compare infection rates and other questions in the following patient groups.
| Patient characteristics | No prophylaxis (n=173) | Levofloxacin prophylaxis (n=69) | Other prophylaxis (n=102) |
| Median age in years (range) | 5.8 (3-11.9) | 6.8 (3.9-11.1) | 7 (3.6-11.9) |
| B-ALL | 83% | 78% | 79% |
| Low-risk ALL | 51% | 54% | 50% |
| Standard-risk ALL | 47% | 41% | 42% |
| High-risk ALL | 2% | 6% | 8% |
| Median duration of neutropenia in days (range) | 17 (11-24) | 18 (12-23) | 20 (17-25) |
| Median duration of profound neutropenia in days (range) | 6 (2-13) | 7 (4-12) | 11 (5-16) |
Results
Researchers reported that patients with neutropenia who received any prophylactic therapy were far less likely than those who did not to develop fever, documented or likely infections, or bloodstream infections.
In a multivariate analysis, the adjusted odds ratios in patients who received prophylaxis, compared to those who did not, were as follows.
- Febrile neutropenia—0.23, P<0.001
- Febrile neutropenia with clinically documented infection—0.30, P=0.002
- Febrile neutropenia with microbiologically documented infection—0.25, P<0.001
- Clinically documented infection—0.54, P=0.02
- Microbiologically documented infection—0.40, P<0.001
- Bloodstream infection—0.30, P=0.008
- C difficile infection—0.38, P=0.04
- Likely bacterial infection—0.26, P<0.001
- Any enterocolitis—0.44, P=0.03.
Analysis also revealed that patients who received levofloxacin had a greater reduction in C difficile infection than patients who received other prophylaxis. The adjusted odds ratio was 0.04 (P<0.001).
However, there was no significant difference between the prophylaxis groups when it came to other infections.
Patients who received levofloxacin prophylaxis had significantly less exposure to other antibiotics than patients who received other prophylaxis or no prophylaxis.
This included exposure to cefepime/ceftazidime (P<0.001 for both comparisons), vancomycin (P<0.001 for both), meropenem (P<0.001 for both), and aminoglycosides (P=0.002 for no prophylaxis, P=0.04 for other prophylaxis).
The reduction in exposure to other antibiotics may partly explain why C difficile infections declined in levofloxacin-treated patients, Dr Wolf said.
He also noted that antibiotic resistance did not significantly increase in this study, despite the greater use of levofloxacin to prevent infections.
“We are cautiously optimistic that any impact of levofloxacin on antibacterial resistance will be balanced by the reduction in use of other antibiotics,” Dr Wolf said, “but long-term monitoring of antibiotic resistance patterns in young ALL patients will be needed to prove this.” ![]()
Results of an observational study support targeted antibacterial prophylaxis in children undergoing induction therapy for acute lymphoblastic leukemia (ALL).
Prophylaxis effectively prevented febrile neutropenia and systemic infection in the children studied.
Prophylaxis with the drug levofloxacin reduced the use of treatment antibiotics and the incidence of Clostridium difficile infection.
“This research provides the first major evidence supporting targeted use of antibacterial prophylaxis for at-risk pediatric ALL patients, particularly use of the broad-spectrum antibiotic levofloxacin,” said study author Joshua Wolf, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Prophylactic antibiotic therapy with levofloxacin is routine for at-risk adult ALL patients, but it has remained controversial in children. Until this study, evidence supporting the safety and efficacy of prophylactic antibiotic therapy in children with ALL has been sparse.”
Dr Wolf and his colleagues described their study in Clinical Infectious Diseases.
The study included 344 patients newly diagnosed with ALL who were enrolled in the St. Jude Total XVI clinical trial (NCT00549848). Patients were enrolled from 2007 to 2016.
Until July 2014, the patients received prophylactic antibiotic therapy at the discretion of their physicians. Patients typically received cefepime, ciprofloxacin, or vancomycin plus cefepime or ciprofloxacin. And prophylaxis was typically started at the onset of neutropenia after chemotherapy.
Beginning in August 2014, hospital treatment guidelines changed to recommend prophylactic levofloxacin during induction for ALL patients who develop neutropenia expected to last at least 7 days.
Dr Wolf and his colleagues used the change to compare infection rates and other questions in the following patient groups.
| Patient characteristics | No prophylaxis (n=173) | Levofloxacin prophylaxis (n=69) | Other prophylaxis (n=102) |
| Median age in years (range) | 5.8 (3-11.9) | 6.8 (3.9-11.1) | 7 (3.6-11.9) |
| B-ALL | 83% | 78% | 79% |
| Low-risk ALL | 51% | 54% | 50% |
| Standard-risk ALL | 47% | 41% | 42% |
| High-risk ALL | 2% | 6% | 8% |
| Median duration of neutropenia in days (range) | 17 (11-24) | 18 (12-23) | 20 (17-25) |
| Median duration of profound neutropenia in days (range) | 6 (2-13) | 7 (4-12) | 11 (5-16) |
Results
Researchers reported that patients with neutropenia who received any prophylactic therapy were far less likely than those who did not to develop fever, documented or likely infections, or bloodstream infections.
In a multivariate analysis, the adjusted odds ratios in patients who received prophylaxis, compared to those who did not, were as follows.
- Febrile neutropenia—0.23, P<0.001
- Febrile neutropenia with clinically documented infection—0.30, P=0.002
- Febrile neutropenia with microbiologically documented infection—0.25, P<0.001
- Clinically documented infection—0.54, P=0.02
- Microbiologically documented infection—0.40, P<0.001
- Bloodstream infection—0.30, P=0.008
- C difficile infection—0.38, P=0.04
- Likely bacterial infection—0.26, P<0.001
- Any enterocolitis—0.44, P=0.03.
Analysis also revealed that patients who received levofloxacin had a greater reduction in C difficile infection than patients who received other prophylaxis. The adjusted odds ratio was 0.04 (P<0.001).
However, there was no significant difference between the prophylaxis groups when it came to other infections.
Patients who received levofloxacin prophylaxis had significantly less exposure to other antibiotics than patients who received other prophylaxis or no prophylaxis.
This included exposure to cefepime/ceftazidime (P<0.001 for both comparisons), vancomycin (P<0.001 for both), meropenem (P<0.001 for both), and aminoglycosides (P=0.002 for no prophylaxis, P=0.04 for other prophylaxis).
The reduction in exposure to other antibiotics may partly explain why C difficile infections declined in levofloxacin-treated patients, Dr Wolf said.
He also noted that antibiotic resistance did not significantly increase in this study, despite the greater use of levofloxacin to prevent infections.
“We are cautiously optimistic that any impact of levofloxacin on antibacterial resistance will be balanced by the reduction in use of other antibiotics,” Dr Wolf said, “but long-term monitoring of antibiotic resistance patterns in young ALL patients will be needed to prove this.” ![]()
Results of an observational study support targeted antibacterial prophylaxis in children undergoing induction therapy for acute lymphoblastic leukemia (ALL).
Prophylaxis effectively prevented febrile neutropenia and systemic infection in the children studied.
Prophylaxis with the drug levofloxacin reduced the use of treatment antibiotics and the incidence of Clostridium difficile infection.
“This research provides the first major evidence supporting targeted use of antibacterial prophylaxis for at-risk pediatric ALL patients, particularly use of the broad-spectrum antibiotic levofloxacin,” said study author Joshua Wolf, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Prophylactic antibiotic therapy with levofloxacin is routine for at-risk adult ALL patients, but it has remained controversial in children. Until this study, evidence supporting the safety and efficacy of prophylactic antibiotic therapy in children with ALL has been sparse.”
Dr Wolf and his colleagues described their study in Clinical Infectious Diseases.
The study included 344 patients newly diagnosed with ALL who were enrolled in the St. Jude Total XVI clinical trial (NCT00549848). Patients were enrolled from 2007 to 2016.
Until July 2014, the patients received prophylactic antibiotic therapy at the discretion of their physicians. Patients typically received cefepime, ciprofloxacin, or vancomycin plus cefepime or ciprofloxacin. And prophylaxis was typically started at the onset of neutropenia after chemotherapy.
Beginning in August 2014, hospital treatment guidelines changed to recommend prophylactic levofloxacin during induction for ALL patients who develop neutropenia expected to last at least 7 days.
Dr Wolf and his colleagues used the change to compare infection rates and other questions in the following patient groups.
| Patient characteristics | No prophylaxis (n=173) | Levofloxacin prophylaxis (n=69) | Other prophylaxis (n=102) |
| Median age in years (range) | 5.8 (3-11.9) | 6.8 (3.9-11.1) | 7 (3.6-11.9) |
| B-ALL | 83% | 78% | 79% |
| Low-risk ALL | 51% | 54% | 50% |
| Standard-risk ALL | 47% | 41% | 42% |
| High-risk ALL | 2% | 6% | 8% |
| Median duration of neutropenia in days (range) | 17 (11-24) | 18 (12-23) | 20 (17-25) |
| Median duration of profound neutropenia in days (range) | 6 (2-13) | 7 (4-12) | 11 (5-16) |
Results
Researchers reported that patients with neutropenia who received any prophylactic therapy were far less likely than those who did not to develop fever, documented or likely infections, or bloodstream infections.
In a multivariate analysis, the adjusted odds ratios in patients who received prophylaxis, compared to those who did not, were as follows.
- Febrile neutropenia—0.23, P<0.001
- Febrile neutropenia with clinically documented infection—0.30, P=0.002
- Febrile neutropenia with microbiologically documented infection—0.25, P<0.001
- Clinically documented infection—0.54, P=0.02
- Microbiologically documented infection—0.40, P<0.001
- Bloodstream infection—0.30, P=0.008
- C difficile infection—0.38, P=0.04
- Likely bacterial infection—0.26, P<0.001
- Any enterocolitis—0.44, P=0.03.
Analysis also revealed that patients who received levofloxacin had a greater reduction in C difficile infection than patients who received other prophylaxis. The adjusted odds ratio was 0.04 (P<0.001).
However, there was no significant difference between the prophylaxis groups when it came to other infections.
Patients who received levofloxacin prophylaxis had significantly less exposure to other antibiotics than patients who received other prophylaxis or no prophylaxis.
This included exposure to cefepime/ceftazidime (P<0.001 for both comparisons), vancomycin (P<0.001 for both), meropenem (P<0.001 for both), and aminoglycosides (P=0.002 for no prophylaxis, P=0.04 for other prophylaxis).
The reduction in exposure to other antibiotics may partly explain why C difficile infections declined in levofloxacin-treated patients, Dr Wolf said.
He also noted that antibiotic resistance did not significantly increase in this study, despite the greater use of levofloxacin to prevent infections.
“We are cautiously optimistic that any impact of levofloxacin on antibacterial resistance will be balanced by the reduction in use of other antibiotics,” Dr Wolf said, “but long-term monitoring of antibiotic resistance patterns in young ALL patients will be needed to prove this.” ![]()
NCCN completes resource on radiation therapy
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018. ![]()
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018. ![]()
The National Comprehensive Cancer Network® (NCCN) has announced the release of the newly completed NCCN Radiation Therapy Compendium™.
This resource includes information designed to support clinical decision-making regarding the use of radiation therapy in cancer patients.
The content is based on the NCCN Clinical Practice Guidelines in Oncology and includes information from the 41 guidelines that reference radiation therapy.
“By compiling every recommendation for radiation therapy in one place, we’ve made it significantly easier for specialists . . . to stay up-to-date on the very latest recommendations, regardless of how many different cancer types they treat,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“This targeted content provides radiation oncologists with the specific, cutting-edge information they need, without forcing them to sift through any extraneous information. It’s part of our ongoing effort to always provide the most pertinent data on emerging treatment practices in the clearest, most efficient way possible.”
The NCCN Radiation Therapy Compendium includes a full complement of radiation therapy recommendations found in the current NCCN guidelines, including specific treatment modalities such as 2D/3D conformal external beam radiation therapy, intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative body radiotherapy, image-guided radiation therapy, low dose-rate/high dose-rate brachytherapy, radioisotope, and particle therapy.
NCCN first announced the launch of the Radiation Therapy Compendium in March at the NCCN Annual Conference: Improving the Quality, Effectiveness, and Efficiency of Cancer Care.
At the time, the NCCN released a preliminary version of the compendium featuring 24 cancer types. The newly completed version now contains all 41 disease sites that are currently being treated using radiation therapy.
The compendium will be updated on a continual basis in conjunction with the library of clinical guidelines.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. The compendium is available free-of-charge through March 2018. ![]()