User login
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
A syringe services program (SSP) is a harm reduction strategy designed to improve the quality of care provided to people who use drugs (PWUD). SSPs not only provide sterile syringes but establish a connection to medical services and resources for the safe disposal of syringes. By engaging with an SSP, patients may receive naloxone, condoms, fentanyl test strips, opioid use disorder medications, vaccinations, or testing for infectious diseases such as HIV and hepatitis C virus (HCV). Patients may also be connected to housing or social work services.
SSPs do not lead to increased drug use,1 increased improperly disposed supplies needed for drug use in the community, or increased crime.2,3 New users of SSPs are 5 times more likely to enter treatment for drug use than those who do not use SSPs.4-8 Further, SSPs have been found to reduce HIV and HCV transmission and are cost-effective in HIV prevention.9-11
Syringe Services Program
SSPs were implemented at the US Department of Veterans Affairs (VA) Alaska VA Healthcare System (AVAHCS) and VA Southern Oregon Healthcare System (VASOHCS). AVAHCS provides outpatient care across Alaska, with sites in Anchorage, Fairbanks, Homer, Juneau, Wasilla, and Soldotna. VASOHCS provides outpatient care to Southern Oregon and Northern California, with sites in White City, Grants Pass, and Klamath Falls, Oregon. Both are part of Veterans Integrated Service Network 20
Workgroups at AVAHCS and VASOHCS developed SSPs to reduce risks associated with drug use, promote positive outcomes for PWUD, and increase availability of harm reduction resources. During the July 2023 to June 2024 pharmacy residency cycle, an ambulatory care pharmacy resident from the Veterans Integrated Services Network 20 Clinical Resource Hub—a regional resource for clinical services—joined the workgroups. The workgroups established a goal that SSP resources would be made available to enrolled patients without any exclusions, prioritizing health equity.
SSP implementation needed buy-in from AVAHCS and VASOHCS leadership and key stakeholders who could participate in the workgroups. Following AVAHCS and VASOHCS leadership approval, each facility workgroup drafted standard operating procedures (SOPs). Both facilities planned to implement the program using prepackaged kits (sterile syringes, alcohol pads, cotton swabs, a sharps container, and an educational brochure on safe injection practices) supplied by the VA National Harm Reduction Program.
Each SSP offered patients direct links to additional care options at the time of kit distribution, including information regarding medications/supplies (ie, hepatitis A/B vaccines, HIV preexposure prophylaxis, substance use disorder pharmacotherapy, naloxone, and condoms), laboratory tests for infectious and sexually transmitted diseases, and referrals to substance use disorder treatment, social work, suicide prevention, mental health, and primary care.
The goal was to implement both SSPs during the July 2023 to June 2024 residency year. Other goals included tracking the quantity of supplies distributed, the number of patients reached, the impact of clinician education on the distribution of supplies, and comparing the implementation of the SSPs in the electronic health record (EHR) systems.
Alaska VA Healthcare System
An SOP was approved on December 20, 2023, and national supply kits were stocked in collaboration with the logistics department at the Anchorage AVAHCS campus. Social and behavioral health teams, primary care social workers, primary care clinicians, and nursing staff received training on the resources available through the SSP. A local adaptation of a template was created in the Computerized Patient Records System (CPRS) EHR. The template facilitates SSP kit distribution and patient screening for additional resources. Patients can engage with the SSP through any trained staff member. The staff member then completes the template and helps to distribute the SSP kit, in collaboration with the logistics department. The SSP does not operate in a dedicated physical space. The behavioral health team is most actively engaged in the SSP. The goal of SSP is to have resources available anywhere a patient requests services, including primary care and specialty clinics and to empower staff to meet patients’ needs. One patient has utilized the SSP as of June 2025.
Southern Oregon Healthcare System
Kits were ordered and stocked as pharmacy items in preparation for dispensing while awaiting medical center policy approval. Education began with the primary care mental health integration team. After initial education, an interdisciplinary presentation was given to VASOHCS clinicians to increase knowledge of the SSP. To enable documentation of SSP engagement, a local template was developed in the Cerner EHR to be shared among care team members at the facility. Similar to AVAHCS, the SSP does not have a physical space. All trained facility staff may engage in the SSP and distribute SSP kits. The workgroup that implemented this program remains available to support staff. Five patients have accessed the SSP since November 2024 and 7 SSP kits have been distributed as of June 2025.
Discussion
The SSP workgroups sought to expand the program through additional education. A number of factors should be considered when implementing an SSP. Across facilities, program implementation can be time-consuming and the timeline for administrative processes may be long. The workgroups met weekly or monthly depending on the status of the program and the administrative processes. Materials developed included SOP and MCP documents, a 1-page educational handout on SSP offerings, and a PowerPoint presentation for initial clinician education. Involving a pharmacy resident supported professional development and accelerated implementation timelines.
The facilities differed in implementation. AVAHCS collaborated with the logistics department to distribute kits, while VASOHCS worked with the Pharmacy service. A benefit of collaborating with logistics is that patients can receive a kit at the point of contact with the health care system, receiving it directly from the clinic the patient is visiting while eliminating the need to make an additional stop at the pharmacy. Conversely, partnering with the Pharmacy service allowed supply kits to be distributed by mail, enabling patients direct access to kits without having to present in-person. This is particularly valuable considering the large geographical area and remote care services available at VASOHCS.
Implementation varied significantly because AVAHCS operated on CPRS while VASOHCS used Cerner, a newer EHR. AVAHCS adapted a national template produced for CPRS sites, while VASOHCS had to prepare a local template (auto-text) for SSP documentation. Future plans at AVAHCS may include adding fentanyl test strips as an orderable item in the EHR given that AVAHCS has a local instance of CPRS; however, VASOHCS cannot order fentanyl test strips through the Pharmacy service due to legal restrictions. While Oregon permits fentanyl test strip use, the Cerner instance used by VA is a national program, and therefore the addition of fentanyl test strips as an orderable item in the EHR would carry national implications, including for VA health care systems in states where fentanyl test strip legality is variable. Despite the challenges, efforts to include fentanyl test strips in both SSPs are ongoing.
No significant EHR changes were needed to make the national supply kits available in the Cerner EHR through the VASOHCS Pharmacy service. To have national supply kits available through the AVAHCS Pharmacy service, the EHR would need to be manipulated by adding a local drug file in CPRS. Differences between the EHRs often facilitated the need for adaptation from existing models of SSPs within VA, which were all based in CPRS.
Conclusions
The implementation of SSPs at AVAHCS and VASOHCS enable clinicians to provide quality harm reduction services to PWUD. Despite variations in EHR systems, AVAHCS and VASOHCS implemented SSP within 1 year. Tracking of program engagement via the number of patients interacting with the program and the number of SSP kits distributed will continue. SSP implementation in states where it is permitted may help provide optimal patient care for PWUD.
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
- Hagan H, McGough JP, Thiede H, Hopkins S, Duchin J, Alexander ER. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. J Subst Abuse Treat. 2000;19(3):247-252. doi:10.1016/s0740-5472(00)00104-5
- Marx MA, Crape B, Brookmeyer RS, et al. Trends in crime and the introduction of a needle exchange program. Am J Public Health. 2000;90(12):1933-1936. doi:10.2105/ajph.90.12.1933
- Galea S, Ahern J, Fuller C, Freudenberg N, Vlahov D. Needle exchange programs and experience of violence in an inner city neighborhood. J Acquir Immune Defic Syndr. 2001;28(3):282-288. doi:10.1097/00042560-200111010-00014
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337-1341. doi:10.15585/ mmwr.mm6448a3
- Tookes HE, Kral AH, Wenger LD, et al. A comparison of syringe disposal practices among injection drug users in a city with versus a city without needle and syringe programs. Drug Alcohol Depend. 2012;123(1-3):255-259. doi:10.1016/j.drugalcdep.2011.12.001
- Klein SJ, Candelas AR, Cooper JG, et al. Increasing safe syringe collection sites in New York State. Public Health Rep. 2008;123(4):433-440. doi:10.1177/003335490812300404
- de Montigny L, Vernez Moudon A, Leigh B, Kim SY. Assessing a drop box programme: a spatial analysis of discarded needles. Int J Drug Policy. 2010;21(3):208-214. doi:10.1016/j.drugpo.2009.07.003
- Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89(2-3):214-222. doi:10.1016/j.drugalcdep.2006.12.035
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. doi:10.1002/14651858.CD012021.pub2
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe programmes in people who inject drugs — an overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi:10.1186/s12889-017-4210-2
- Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. doi:10.1371/journal.pmed.1002312
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
Implementation of Harm Reduction Syringe Services Programs at 2 Veterans Affairs Medical Centers
VA Cancer Clinical Trials as a Strategy for Increasing Accrual of Racial and Ethnic Underrepresented Groups
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.

Improving Colorectal Cancer Screening via Mailed Fecal Immunochemical Testing in a Veterans Affairs Health System
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
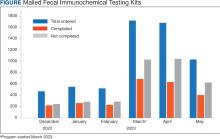
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Daily Double! Assessing the Effectiveness of Game-Based Learning on the Pharmacy Knowledge of US Coast Guard Health Services Technicians
Daily Double! Assessing the Effectiveness of Game-Based Learning on the Pharmacy Knowledge of US Coast Guard Health Services Technicians
The US Coast Guard (USCG) operates within the US Department of Homeland Security and represents a force of > 50,000 servicemembers.1 The missions of the service include maritime law enforcement (drug interdiction), search and rescue, and defense readiness.2
The USCG operates 42 clinics and numerous smaller sick bays of varying sizes and medical capabilities throughout the country to provide acute and routine medical services. Health services technicians (HSs) are the most common staffing component and provide much of the support services in each USCG health care setting. The HS rating, colloquially referred to as corpsmen, is achieved through a 22-week course known as “A” school that trains servicemembers in outpatient and acute care, including emergency medical technician training.3 There are about 750 USCG HSs.
Within USCG clinics, HSs conduct ambulatory intakes for outpatient appointments, administer immunizations and blood draws, requisition medical equipment and supplies, serve as a pharmacy technician, complete physical examinations, and manage referrals, among other duties. Their familiarity with different aspects of clinic operations and medical practice must be broad. To that end, corpsmen develop and reinforce their medical knowledge through various trainings, including additional courses to specialize in certain medical skills, such as pharmacy technician “C” school or dental assistant “C” school.
The USCG employs < 15 field pharmacists, most of whom serve in an ambulatory care environment.4 Responsibilities of USCG pharmacists include the routine reinforcement of pharmacy knowledge with HSs. For the corpsmen who are not pharmacy technicians or who have not attended pharmacy technician “C” school, the extent of their pharmacy instruction primarily came from the “A” school curriculum, of which only 1 class is specific to pharmacy. Providing routine pharmacy-related training to the HSs further cultivates their pharmacy knowledge and confidence so that they can practice more holistically. These trainings do not need to follow any specific format.
In this study, 3 pharmacists at 3 separate USCG clinics conducted a training inspired by the Jeopardy! game show with the corpsmen at their respective clinics. This study examined the effectiveness of game-based learning on the pharmacy knowledge retention of HSs at 3 USCG clinics. A secondary objective was to evaluate the baseline pharmacy knowledge of corpsmen based on specific corpsmen demographics.
Methods
As part of a USCG quality improvement study in 2024, 28 HSs at the 3 USCG clinics were provided a preintervention assessment, completed game-based educational program (intervention), and then were assessed again following the intervention.
The HSs were presented with a 25-question assessment that included 10 knowledge questions (3 on over-the-counter medications, 2 on use of medications in pregnancy, 2 on precautions and contraindications, 2 on indications, and 1 on immunizations) and 15 brand-generic matching questions. These questions were developed and reviewed by the 3 participating pharmacists to ensure that their scope was commensurate with the overall pharmacy knowledge that could be reasonably expected of corpsmen spanning various points of their HS career.
One to 7 days after the preintervention assessment, the pharmacists hosted the game-based learning modeled after Jeopardy!. The Jeopardy! categories mirrored the assessment knowledge question categories, and brand-generic nomenclature was freely discussed throughout. About 2 weeks later, the same HSs who completed the preintervention assessment and participated in the game were presented with the same assessment.
In addition to capturing the difference in scores between the 2 assessments, additional demographic data were gathered, including service time as an HS and whether they received formalized pharmacy technician training and if so, how long they have served in that capacity. Demographic data were collected to identify potential correlations between demographic characteristics and results.
Results
Twenty-eight HSs at the 3 clinics completed the game-based training and both assessments. The mean score increased from 15.1 preintervention to 17.4 postintervention (Table). Preintervention scores ranged from 1 to 24 and postintervention scores ranged from 6 to 25.
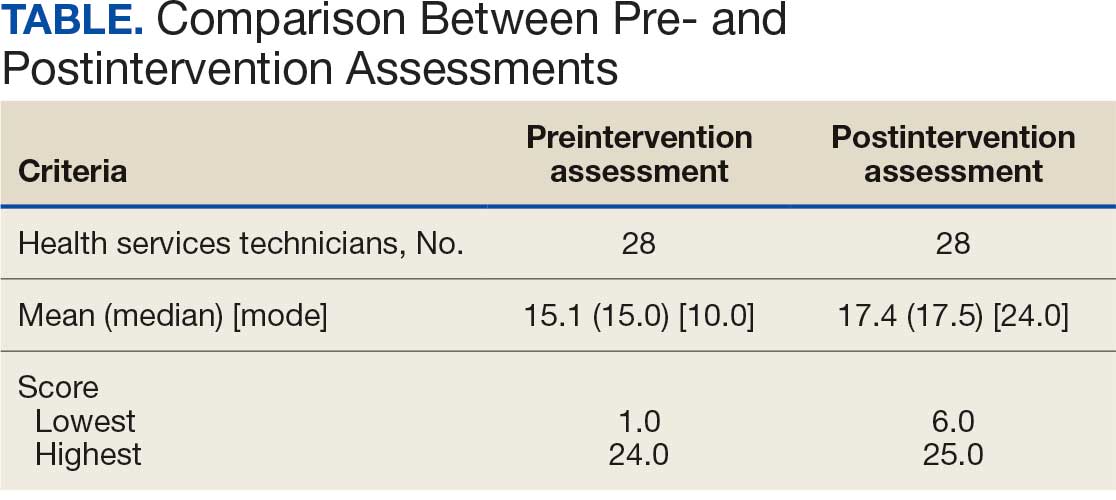
There were 19 HSs (68%) whose score increased from preintervention to postintervention and 5 (18%) had decreased scores. The largest score decrease was 4 (from 18 to 14), and the largest score increase was 11 (from 13 to 24). The mean improvement was 3.9 among the 19 HSs with increased scores
Twenty-one HSs reported no formal pharmacy technician training, 3 completed pharmacy technician “C” school, and 4 received informal on-the-job training. The mean score for the “C” school trained HSs was 23.0 preintervention and 23.7 postintervention. The mean score for HSs trained on the job was 16.0 preintervention and 18.5 postintervention. The mean score for HSs with no training was 13.9 preintervention and 16.3 postintervention.
As HSs advance in their careers, they typically assume roles with increasing technical knowledge, responsibility, and oversight, thus aligning with advancement from E-4 (third class petty officer) to E-6 (first class petty officer) and beyond. In this study, there was 1 E-3, 12 E-4s (mean time as an HS, 1.3 years), 8 E-5s (mean time as an HS, 4.8 years), and 7 E-6s (mean time as an HS, 8.6 years). The E-3 had a preintervention score of 1.0 and a postintervention score of 6.0. The E-4s had a mean change in score from pre- to postintervention of 2.4. The E-5s had a mean change in score from pre- to postintervention of 1.6. The E-6s had a mean change in score from pre- to postintervention of 2.3.
Discussion
This study is novel in its examination of the impact of game-based learning on the retention of the pharmacy knowledge of USCG corpsmen. A PubMed literature search of the phrase “((Corpsman) OR (Corpsmen)) AND (Coast Guard)” yields 135 results, though none were relevant to the USCG population described in this study. A PubMed literature search of the phrase “(Jeopardy!) AND (pharmacy)” yields 28 results, only 1 of which discusses using the game-based approach as an instructional tool.5 A PubMed literature search of the phrase “(game) AND (Coast Guard)” yields 55 results, none of which were specifically relevant to game-based learning in the USCG. This study appears to be among the first to discuss results and trends in game-based learning with USCG corpsmen.
The preponderance of literature for game-based learning strategies exists in children; more research in adults is needed.6,7 With studies showing that game-based learning may impact motivation to learn and learning gains, it is unsurprising that there is some research in professional health care education. Games modeled after everything from simulated clinical scenarios to Family Feud and Chutes and Ladders-style games have been compared with traditional learning strategies. However, the results of whether game-based learning strategies improve knowledge, clinical decision-making, and motivation to learn vary, suggesting the need for more research in this field.8
The results of this study suggest that Jeopardy! is likely an effective instructional method for USCG corpsmen on pharmacy topics. While there were some HSs whose postintervention scores decreased, 19 (68%) had increased scores. Because the second assessment was administered about 2 weeks after the game-based learning, the results suggest some level of knowledge retention. Between these results and the informally perceived level of engagement, game-based learning could be a more stimulating alternative training method to a standard slide-based presentation.
Stratifying the data by demographics revealed additional trends, although they should be interpreted with caution due to the small sample size. The baseline results strongly illustrate the value of formalized training. It is generally expected that HSs who have completed the “C” school pharmacy technician training program should have more pharmacy knowledge than those with on-the-job or less training. The results indicate that “C” school trained and on-the-job trained HSs scored higher on the preintervention assessment (mean, 23.0 and 16.0, respectively), than those with no such experiences (mean, 13.9). Such results underscore the value of formalized training—whether as a pharmacy technician or in any other “C” school—in enhancing the medical knowledge of HSs that may allow them to hold roles of increased responsibility and medical scope.
In addition to stratification by pharmacy technician training, stratification by years of HS experience (roughly correlated to rank) yields a similar result. It would be expected that as HSs advance in their careers, they gain more exposure to various medical topics, including pharmacy. That is not always the case, however, as it is possible an HS never rotated through a pharmacy technician position or has not been recently exposed to pharmacy knowledge. Nevertheless, the results suggest that increased HS experience was likely associated with an increased baseline pharmacy knowledge, with mean preintervention scores increasing from 11.9 to 18.1 to 19.3 for E-4, E-5, and E-6, respectively.
While there are many explanations for these results, the authors hypothesize that when HSs are E-4s, they might not yet have exposure to all aspects of the clinic and are perhaps not as well-versed in pharmacy practice. An E-5—now a few years into their career—would have completed pharmacy technician “C” school or on-the-job training (if applicable), which could account for the significant jump in pharmacy knowledge scores. An E-6 can still engage in direct patient care activities but take on leadership and supervisory roles within the clinic, perhaps explaining the smaller increase in score.
In terms of increasing responsibility, many USCG corpsmen complete another schooling opportunity—Independent Duty Health Services Technician (IDHS)—so they can serve in independent duty roles, many of which are on USCG cutters. While cutters are deployed, that IDHS could be the sole medical personnel on the cutter and function in a midlevel practitioner extender role. Formalized training in pharmacy—the benefits of which are suggested through these results—or another field of medical practice would strengthen the skillset and confidence of IDHSs.
Though not formally assessed, the 3 pharmacists noted that the game-based learning was met with overwhelmingly positive feedback in terms of excitement, energy, and overall engagement.
Limitations
This cohort of individuals represents a small proportion of the total number of USCG corpsmen, and it is not fully representative of all practice settings. HSs can be assigned to USCG cutters as IDHSs, which would not be captured in this cohort. Even within a single clinic, the knowledge of HSs varies, as not all HS duties consist solely of clinical skills. Additionally, while the overall game framework was consistent among the 3 sites, there may have been unquantifiable differences in overall teaching style by the 3 pharmacists that may have resulted in different levels of content retention. Given the lack of similar studies in this population, this study can best be described as a quantitative descriptor of results rather than a statistical comparison of what instructional method works best.
Conclusions
The USCG greatly benefits from having trained and experienced HSs fulfilling mission support roles in the organization. In addition to traditional slide-based trainings, game-based learning can be considered to create engaging learning environments to support the knowledge retention of pharmacy and other medical topics for USCG corpsmen.
- US Coast Guard. Organizational overview. About the US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About
- US Coast Guard. Missions. About US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About/Missions/
- US Coast Guard. Health services technician. Accessed October 14, 2025. https://www.gocoastguard.com/careers/enlisted/hs
- Zhou F, Woodward Z. Impact of pharmacist interventions at an outpatient US Coast Guard clinic. Fed Pract. 2023;40(6):174-177. doi:10.12788/fp.0383
- Cusick J. A Jeopardy-style review game using team clickers. MedEdPORTAL. 2016;12:10485. doi:10.15766/mep_2374-8265.10485
- Dahalan F, Alias N, Shaharom MSN. Gamification and game based learning for vocational education and training: a systematic literature review. Educ Inf Technol (Dordr). 2023:1-39. doi:10.1007/s10639-022-11548-w
- Wesselink LA. Testing the Effectiveness of Game-Based Learning for Adults by Designing an Educational Game: A Design and Research Study to Investigate the Effectiveness of Educational Games for Adults to Learn Basic Skills of Microsoft Excel. Master’s thesis. University of Twente; 2020. Accessed October 22, 2025. http://essay.utwentw.nl/88229
- Del Cura-González I, Ariza-Cardiel G, Polentinos-Castro E, et al. Effectiveness of a game-based educational strategy e-EDUCAGUIA for implementing antimicrobial clinical practice guidelines in family medicine residents in Spain: a randomized clinical trial by cluster. BMC Med Educ. 2022;22:893. doi:10.1186/s12909-022-03843-4
The US Coast Guard (USCG) operates within the US Department of Homeland Security and represents a force of > 50,000 servicemembers.1 The missions of the service include maritime law enforcement (drug interdiction), search and rescue, and defense readiness.2
The USCG operates 42 clinics and numerous smaller sick bays of varying sizes and medical capabilities throughout the country to provide acute and routine medical services. Health services technicians (HSs) are the most common staffing component and provide much of the support services in each USCG health care setting. The HS rating, colloquially referred to as corpsmen, is achieved through a 22-week course known as “A” school that trains servicemembers in outpatient and acute care, including emergency medical technician training.3 There are about 750 USCG HSs.
Within USCG clinics, HSs conduct ambulatory intakes for outpatient appointments, administer immunizations and blood draws, requisition medical equipment and supplies, serve as a pharmacy technician, complete physical examinations, and manage referrals, among other duties. Their familiarity with different aspects of clinic operations and medical practice must be broad. To that end, corpsmen develop and reinforce their medical knowledge through various trainings, including additional courses to specialize in certain medical skills, such as pharmacy technician “C” school or dental assistant “C” school.
The USCG employs < 15 field pharmacists, most of whom serve in an ambulatory care environment.4 Responsibilities of USCG pharmacists include the routine reinforcement of pharmacy knowledge with HSs. For the corpsmen who are not pharmacy technicians or who have not attended pharmacy technician “C” school, the extent of their pharmacy instruction primarily came from the “A” school curriculum, of which only 1 class is specific to pharmacy. Providing routine pharmacy-related training to the HSs further cultivates their pharmacy knowledge and confidence so that they can practice more holistically. These trainings do not need to follow any specific format.
In this study, 3 pharmacists at 3 separate USCG clinics conducted a training inspired by the Jeopardy! game show with the corpsmen at their respective clinics. This study examined the effectiveness of game-based learning on the pharmacy knowledge retention of HSs at 3 USCG clinics. A secondary objective was to evaluate the baseline pharmacy knowledge of corpsmen based on specific corpsmen demographics.
Methods
As part of a USCG quality improvement study in 2024, 28 HSs at the 3 USCG clinics were provided a preintervention assessment, completed game-based educational program (intervention), and then were assessed again following the intervention.
The HSs were presented with a 25-question assessment that included 10 knowledge questions (3 on over-the-counter medications, 2 on use of medications in pregnancy, 2 on precautions and contraindications, 2 on indications, and 1 on immunizations) and 15 brand-generic matching questions. These questions were developed and reviewed by the 3 participating pharmacists to ensure that their scope was commensurate with the overall pharmacy knowledge that could be reasonably expected of corpsmen spanning various points of their HS career.
One to 7 days after the preintervention assessment, the pharmacists hosted the game-based learning modeled after Jeopardy!. The Jeopardy! categories mirrored the assessment knowledge question categories, and brand-generic nomenclature was freely discussed throughout. About 2 weeks later, the same HSs who completed the preintervention assessment and participated in the game were presented with the same assessment.
In addition to capturing the difference in scores between the 2 assessments, additional demographic data were gathered, including service time as an HS and whether they received formalized pharmacy technician training and if so, how long they have served in that capacity. Demographic data were collected to identify potential correlations between demographic characteristics and results.
Results
Twenty-eight HSs at the 3 clinics completed the game-based training and both assessments. The mean score increased from 15.1 preintervention to 17.4 postintervention (Table). Preintervention scores ranged from 1 to 24 and postintervention scores ranged from 6 to 25.

There were 19 HSs (68%) whose score increased from preintervention to postintervention and 5 (18%) had decreased scores. The largest score decrease was 4 (from 18 to 14), and the largest score increase was 11 (from 13 to 24). The mean improvement was 3.9 among the 19 HSs with increased scores
Twenty-one HSs reported no formal pharmacy technician training, 3 completed pharmacy technician “C” school, and 4 received informal on-the-job training. The mean score for the “C” school trained HSs was 23.0 preintervention and 23.7 postintervention. The mean score for HSs trained on the job was 16.0 preintervention and 18.5 postintervention. The mean score for HSs with no training was 13.9 preintervention and 16.3 postintervention.
As HSs advance in their careers, they typically assume roles with increasing technical knowledge, responsibility, and oversight, thus aligning with advancement from E-4 (third class petty officer) to E-6 (first class petty officer) and beyond. In this study, there was 1 E-3, 12 E-4s (mean time as an HS, 1.3 years), 8 E-5s (mean time as an HS, 4.8 years), and 7 E-6s (mean time as an HS, 8.6 years). The E-3 had a preintervention score of 1.0 and a postintervention score of 6.0. The E-4s had a mean change in score from pre- to postintervention of 2.4. The E-5s had a mean change in score from pre- to postintervention of 1.6. The E-6s had a mean change in score from pre- to postintervention of 2.3.
Discussion
This study is novel in its examination of the impact of game-based learning on the retention of the pharmacy knowledge of USCG corpsmen. A PubMed literature search of the phrase “((Corpsman) OR (Corpsmen)) AND (Coast Guard)” yields 135 results, though none were relevant to the USCG population described in this study. A PubMed literature search of the phrase “(Jeopardy!) AND (pharmacy)” yields 28 results, only 1 of which discusses using the game-based approach as an instructional tool.5 A PubMed literature search of the phrase “(game) AND (Coast Guard)” yields 55 results, none of which were specifically relevant to game-based learning in the USCG. This study appears to be among the first to discuss results and trends in game-based learning with USCG corpsmen.
The preponderance of literature for game-based learning strategies exists in children; more research in adults is needed.6,7 With studies showing that game-based learning may impact motivation to learn and learning gains, it is unsurprising that there is some research in professional health care education. Games modeled after everything from simulated clinical scenarios to Family Feud and Chutes and Ladders-style games have been compared with traditional learning strategies. However, the results of whether game-based learning strategies improve knowledge, clinical decision-making, and motivation to learn vary, suggesting the need for more research in this field.8
The results of this study suggest that Jeopardy! is likely an effective instructional method for USCG corpsmen on pharmacy topics. While there were some HSs whose postintervention scores decreased, 19 (68%) had increased scores. Because the second assessment was administered about 2 weeks after the game-based learning, the results suggest some level of knowledge retention. Between these results and the informally perceived level of engagement, game-based learning could be a more stimulating alternative training method to a standard slide-based presentation.
Stratifying the data by demographics revealed additional trends, although they should be interpreted with caution due to the small sample size. The baseline results strongly illustrate the value of formalized training. It is generally expected that HSs who have completed the “C” school pharmacy technician training program should have more pharmacy knowledge than those with on-the-job or less training. The results indicate that “C” school trained and on-the-job trained HSs scored higher on the preintervention assessment (mean, 23.0 and 16.0, respectively), than those with no such experiences (mean, 13.9). Such results underscore the value of formalized training—whether as a pharmacy technician or in any other “C” school—in enhancing the medical knowledge of HSs that may allow them to hold roles of increased responsibility and medical scope.
In addition to stratification by pharmacy technician training, stratification by years of HS experience (roughly correlated to rank) yields a similar result. It would be expected that as HSs advance in their careers, they gain more exposure to various medical topics, including pharmacy. That is not always the case, however, as it is possible an HS never rotated through a pharmacy technician position or has not been recently exposed to pharmacy knowledge. Nevertheless, the results suggest that increased HS experience was likely associated with an increased baseline pharmacy knowledge, with mean preintervention scores increasing from 11.9 to 18.1 to 19.3 for E-4, E-5, and E-6, respectively.
While there are many explanations for these results, the authors hypothesize that when HSs are E-4s, they might not yet have exposure to all aspects of the clinic and are perhaps not as well-versed in pharmacy practice. An E-5—now a few years into their career—would have completed pharmacy technician “C” school or on-the-job training (if applicable), which could account for the significant jump in pharmacy knowledge scores. An E-6 can still engage in direct patient care activities but take on leadership and supervisory roles within the clinic, perhaps explaining the smaller increase in score.
In terms of increasing responsibility, many USCG corpsmen complete another schooling opportunity—Independent Duty Health Services Technician (IDHS)—so they can serve in independent duty roles, many of which are on USCG cutters. While cutters are deployed, that IDHS could be the sole medical personnel on the cutter and function in a midlevel practitioner extender role. Formalized training in pharmacy—the benefits of which are suggested through these results—or another field of medical practice would strengthen the skillset and confidence of IDHSs.
Though not formally assessed, the 3 pharmacists noted that the game-based learning was met with overwhelmingly positive feedback in terms of excitement, energy, and overall engagement.
Limitations
This cohort of individuals represents a small proportion of the total number of USCG corpsmen, and it is not fully representative of all practice settings. HSs can be assigned to USCG cutters as IDHSs, which would not be captured in this cohort. Even within a single clinic, the knowledge of HSs varies, as not all HS duties consist solely of clinical skills. Additionally, while the overall game framework was consistent among the 3 sites, there may have been unquantifiable differences in overall teaching style by the 3 pharmacists that may have resulted in different levels of content retention. Given the lack of similar studies in this population, this study can best be described as a quantitative descriptor of results rather than a statistical comparison of what instructional method works best.
Conclusions
The USCG greatly benefits from having trained and experienced HSs fulfilling mission support roles in the organization. In addition to traditional slide-based trainings, game-based learning can be considered to create engaging learning environments to support the knowledge retention of pharmacy and other medical topics for USCG corpsmen.
The US Coast Guard (USCG) operates within the US Department of Homeland Security and represents a force of > 50,000 servicemembers.1 The missions of the service include maritime law enforcement (drug interdiction), search and rescue, and defense readiness.2
The USCG operates 42 clinics and numerous smaller sick bays of varying sizes and medical capabilities throughout the country to provide acute and routine medical services. Health services technicians (HSs) are the most common staffing component and provide much of the support services in each USCG health care setting. The HS rating, colloquially referred to as corpsmen, is achieved through a 22-week course known as “A” school that trains servicemembers in outpatient and acute care, including emergency medical technician training.3 There are about 750 USCG HSs.
Within USCG clinics, HSs conduct ambulatory intakes for outpatient appointments, administer immunizations and blood draws, requisition medical equipment and supplies, serve as a pharmacy technician, complete physical examinations, and manage referrals, among other duties. Their familiarity with different aspects of clinic operations and medical practice must be broad. To that end, corpsmen develop and reinforce their medical knowledge through various trainings, including additional courses to specialize in certain medical skills, such as pharmacy technician “C” school or dental assistant “C” school.
The USCG employs < 15 field pharmacists, most of whom serve in an ambulatory care environment.4 Responsibilities of USCG pharmacists include the routine reinforcement of pharmacy knowledge with HSs. For the corpsmen who are not pharmacy technicians or who have not attended pharmacy technician “C” school, the extent of their pharmacy instruction primarily came from the “A” school curriculum, of which only 1 class is specific to pharmacy. Providing routine pharmacy-related training to the HSs further cultivates their pharmacy knowledge and confidence so that they can practice more holistically. These trainings do not need to follow any specific format.
In this study, 3 pharmacists at 3 separate USCG clinics conducted a training inspired by the Jeopardy! game show with the corpsmen at their respective clinics. This study examined the effectiveness of game-based learning on the pharmacy knowledge retention of HSs at 3 USCG clinics. A secondary objective was to evaluate the baseline pharmacy knowledge of corpsmen based on specific corpsmen demographics.
Methods
As part of a USCG quality improvement study in 2024, 28 HSs at the 3 USCG clinics were provided a preintervention assessment, completed game-based educational program (intervention), and then were assessed again following the intervention.
The HSs were presented with a 25-question assessment that included 10 knowledge questions (3 on over-the-counter medications, 2 on use of medications in pregnancy, 2 on precautions and contraindications, 2 on indications, and 1 on immunizations) and 15 brand-generic matching questions. These questions were developed and reviewed by the 3 participating pharmacists to ensure that their scope was commensurate with the overall pharmacy knowledge that could be reasonably expected of corpsmen spanning various points of their HS career.
One to 7 days after the preintervention assessment, the pharmacists hosted the game-based learning modeled after Jeopardy!. The Jeopardy! categories mirrored the assessment knowledge question categories, and brand-generic nomenclature was freely discussed throughout. About 2 weeks later, the same HSs who completed the preintervention assessment and participated in the game were presented with the same assessment.
In addition to capturing the difference in scores between the 2 assessments, additional demographic data were gathered, including service time as an HS and whether they received formalized pharmacy technician training and if so, how long they have served in that capacity. Demographic data were collected to identify potential correlations between demographic characteristics and results.
Results
Twenty-eight HSs at the 3 clinics completed the game-based training and both assessments. The mean score increased from 15.1 preintervention to 17.4 postintervention (Table). Preintervention scores ranged from 1 to 24 and postintervention scores ranged from 6 to 25.

There were 19 HSs (68%) whose score increased from preintervention to postintervention and 5 (18%) had decreased scores. The largest score decrease was 4 (from 18 to 14), and the largest score increase was 11 (from 13 to 24). The mean improvement was 3.9 among the 19 HSs with increased scores
Twenty-one HSs reported no formal pharmacy technician training, 3 completed pharmacy technician “C” school, and 4 received informal on-the-job training. The mean score for the “C” school trained HSs was 23.0 preintervention and 23.7 postintervention. The mean score for HSs trained on the job was 16.0 preintervention and 18.5 postintervention. The mean score for HSs with no training was 13.9 preintervention and 16.3 postintervention.
As HSs advance in their careers, they typically assume roles with increasing technical knowledge, responsibility, and oversight, thus aligning with advancement from E-4 (third class petty officer) to E-6 (first class petty officer) and beyond. In this study, there was 1 E-3, 12 E-4s (mean time as an HS, 1.3 years), 8 E-5s (mean time as an HS, 4.8 years), and 7 E-6s (mean time as an HS, 8.6 years). The E-3 had a preintervention score of 1.0 and a postintervention score of 6.0. The E-4s had a mean change in score from pre- to postintervention of 2.4. The E-5s had a mean change in score from pre- to postintervention of 1.6. The E-6s had a mean change in score from pre- to postintervention of 2.3.
Discussion
This study is novel in its examination of the impact of game-based learning on the retention of the pharmacy knowledge of USCG corpsmen. A PubMed literature search of the phrase “((Corpsman) OR (Corpsmen)) AND (Coast Guard)” yields 135 results, though none were relevant to the USCG population described in this study. A PubMed literature search of the phrase “(Jeopardy!) AND (pharmacy)” yields 28 results, only 1 of which discusses using the game-based approach as an instructional tool.5 A PubMed literature search of the phrase “(game) AND (Coast Guard)” yields 55 results, none of which were specifically relevant to game-based learning in the USCG. This study appears to be among the first to discuss results and trends in game-based learning with USCG corpsmen.
The preponderance of literature for game-based learning strategies exists in children; more research in adults is needed.6,7 With studies showing that game-based learning may impact motivation to learn and learning gains, it is unsurprising that there is some research in professional health care education. Games modeled after everything from simulated clinical scenarios to Family Feud and Chutes and Ladders-style games have been compared with traditional learning strategies. However, the results of whether game-based learning strategies improve knowledge, clinical decision-making, and motivation to learn vary, suggesting the need for more research in this field.8
The results of this study suggest that Jeopardy! is likely an effective instructional method for USCG corpsmen on pharmacy topics. While there were some HSs whose postintervention scores decreased, 19 (68%) had increased scores. Because the second assessment was administered about 2 weeks after the game-based learning, the results suggest some level of knowledge retention. Between these results and the informally perceived level of engagement, game-based learning could be a more stimulating alternative training method to a standard slide-based presentation.
Stratifying the data by demographics revealed additional trends, although they should be interpreted with caution due to the small sample size. The baseline results strongly illustrate the value of formalized training. It is generally expected that HSs who have completed the “C” school pharmacy technician training program should have more pharmacy knowledge than those with on-the-job or less training. The results indicate that “C” school trained and on-the-job trained HSs scored higher on the preintervention assessment (mean, 23.0 and 16.0, respectively), than those with no such experiences (mean, 13.9). Such results underscore the value of formalized training—whether as a pharmacy technician or in any other “C” school—in enhancing the medical knowledge of HSs that may allow them to hold roles of increased responsibility and medical scope.
In addition to stratification by pharmacy technician training, stratification by years of HS experience (roughly correlated to rank) yields a similar result. It would be expected that as HSs advance in their careers, they gain more exposure to various medical topics, including pharmacy. That is not always the case, however, as it is possible an HS never rotated through a pharmacy technician position or has not been recently exposed to pharmacy knowledge. Nevertheless, the results suggest that increased HS experience was likely associated with an increased baseline pharmacy knowledge, with mean preintervention scores increasing from 11.9 to 18.1 to 19.3 for E-4, E-5, and E-6, respectively.
While there are many explanations for these results, the authors hypothesize that when HSs are E-4s, they might not yet have exposure to all aspects of the clinic and are perhaps not as well-versed in pharmacy practice. An E-5—now a few years into their career—would have completed pharmacy technician “C” school or on-the-job training (if applicable), which could account for the significant jump in pharmacy knowledge scores. An E-6 can still engage in direct patient care activities but take on leadership and supervisory roles within the clinic, perhaps explaining the smaller increase in score.
In terms of increasing responsibility, many USCG corpsmen complete another schooling opportunity—Independent Duty Health Services Technician (IDHS)—so they can serve in independent duty roles, many of which are on USCG cutters. While cutters are deployed, that IDHS could be the sole medical personnel on the cutter and function in a midlevel practitioner extender role. Formalized training in pharmacy—the benefits of which are suggested through these results—or another field of medical practice would strengthen the skillset and confidence of IDHSs.
Though not formally assessed, the 3 pharmacists noted that the game-based learning was met with overwhelmingly positive feedback in terms of excitement, energy, and overall engagement.
Limitations
This cohort of individuals represents a small proportion of the total number of USCG corpsmen, and it is not fully representative of all practice settings. HSs can be assigned to USCG cutters as IDHSs, which would not be captured in this cohort. Even within a single clinic, the knowledge of HSs varies, as not all HS duties consist solely of clinical skills. Additionally, while the overall game framework was consistent among the 3 sites, there may have been unquantifiable differences in overall teaching style by the 3 pharmacists that may have resulted in different levels of content retention. Given the lack of similar studies in this population, this study can best be described as a quantitative descriptor of results rather than a statistical comparison of what instructional method works best.
Conclusions
The USCG greatly benefits from having trained and experienced HSs fulfilling mission support roles in the organization. In addition to traditional slide-based trainings, game-based learning can be considered to create engaging learning environments to support the knowledge retention of pharmacy and other medical topics for USCG corpsmen.
- US Coast Guard. Organizational overview. About the US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About
- US Coast Guard. Missions. About US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About/Missions/
- US Coast Guard. Health services technician. Accessed October 14, 2025. https://www.gocoastguard.com/careers/enlisted/hs
- Zhou F, Woodward Z. Impact of pharmacist interventions at an outpatient US Coast Guard clinic. Fed Pract. 2023;40(6):174-177. doi:10.12788/fp.0383
- Cusick J. A Jeopardy-style review game using team clickers. MedEdPORTAL. 2016;12:10485. doi:10.15766/mep_2374-8265.10485
- Dahalan F, Alias N, Shaharom MSN. Gamification and game based learning for vocational education and training: a systematic literature review. Educ Inf Technol (Dordr). 2023:1-39. doi:10.1007/s10639-022-11548-w
- Wesselink LA. Testing the Effectiveness of Game-Based Learning for Adults by Designing an Educational Game: A Design and Research Study to Investigate the Effectiveness of Educational Games for Adults to Learn Basic Skills of Microsoft Excel. Master’s thesis. University of Twente; 2020. Accessed October 22, 2025. http://essay.utwentw.nl/88229
- Del Cura-González I, Ariza-Cardiel G, Polentinos-Castro E, et al. Effectiveness of a game-based educational strategy e-EDUCAGUIA for implementing antimicrobial clinical practice guidelines in family medicine residents in Spain: a randomized clinical trial by cluster. BMC Med Educ. 2022;22:893. doi:10.1186/s12909-022-03843-4
- US Coast Guard. Organizational overview. About the US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About
- US Coast Guard. Missions. About US Coast Guard. Accessed October 14, 2025. https://www.uscg.mil/About/Missions/
- US Coast Guard. Health services technician. Accessed October 14, 2025. https://www.gocoastguard.com/careers/enlisted/hs
- Zhou F, Woodward Z. Impact of pharmacist interventions at an outpatient US Coast Guard clinic. Fed Pract. 2023;40(6):174-177. doi:10.12788/fp.0383
- Cusick J. A Jeopardy-style review game using team clickers. MedEdPORTAL. 2016;12:10485. doi:10.15766/mep_2374-8265.10485
- Dahalan F, Alias N, Shaharom MSN. Gamification and game based learning for vocational education and training: a systematic literature review. Educ Inf Technol (Dordr). 2023:1-39. doi:10.1007/s10639-022-11548-w
- Wesselink LA. Testing the Effectiveness of Game-Based Learning for Adults by Designing an Educational Game: A Design and Research Study to Investigate the Effectiveness of Educational Games for Adults to Learn Basic Skills of Microsoft Excel. Master’s thesis. University of Twente; 2020. Accessed October 22, 2025. http://essay.utwentw.nl/88229
- Del Cura-González I, Ariza-Cardiel G, Polentinos-Castro E, et al. Effectiveness of a game-based educational strategy e-EDUCAGUIA for implementing antimicrobial clinical practice guidelines in family medicine residents in Spain: a randomized clinical trial by cluster. BMC Med Educ. 2022;22:893. doi:10.1186/s12909-022-03843-4
Daily Double! Assessing the Effectiveness of Game-Based Learning on the Pharmacy Knowledge of US Coast Guard Health Services Technicians
Daily Double! Assessing the Effectiveness of Game-Based Learning on the Pharmacy Knowledge of US Coast Guard Health Services Technicians
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure
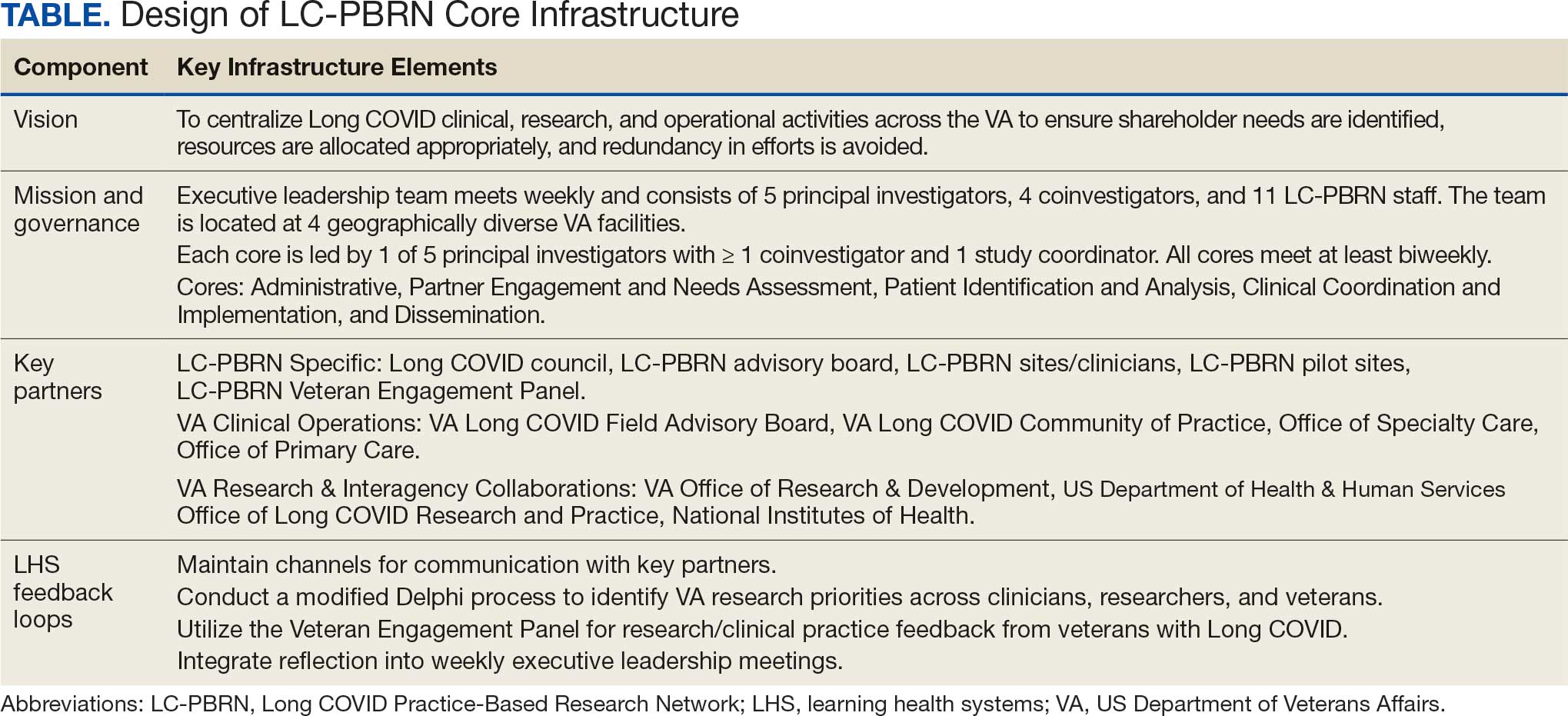
Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).
Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).
The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.
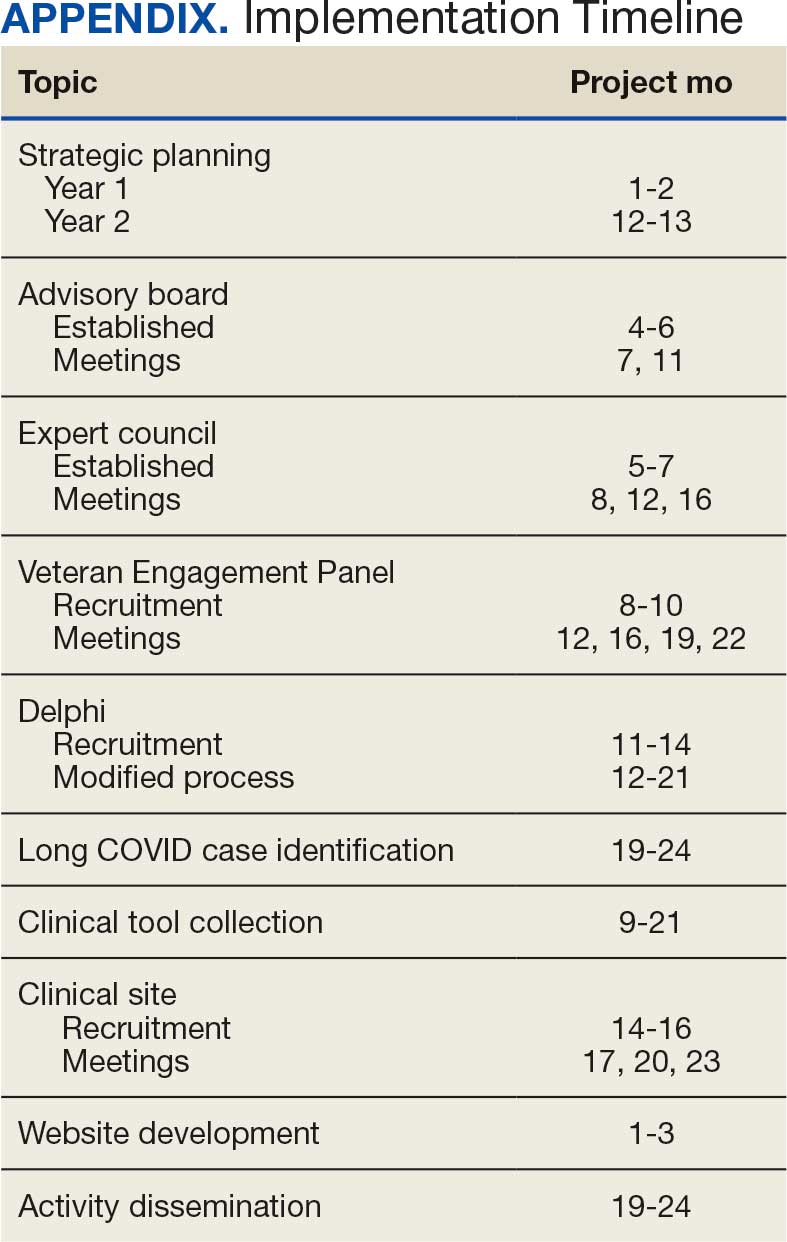
- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure

Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).

Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).

The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.

Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure

Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).

Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).

The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.

- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Negotiating the VUCA World Through Tiered Huddles
Negotiating the VUCA World Through Tiered Huddles
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2
Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.
The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.
VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2

Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.


The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.

VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2

Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.


The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.

VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
Negotiating the VUCA World Through Tiered Huddles
Negotiating the VUCA World Through Tiered Huddles
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).
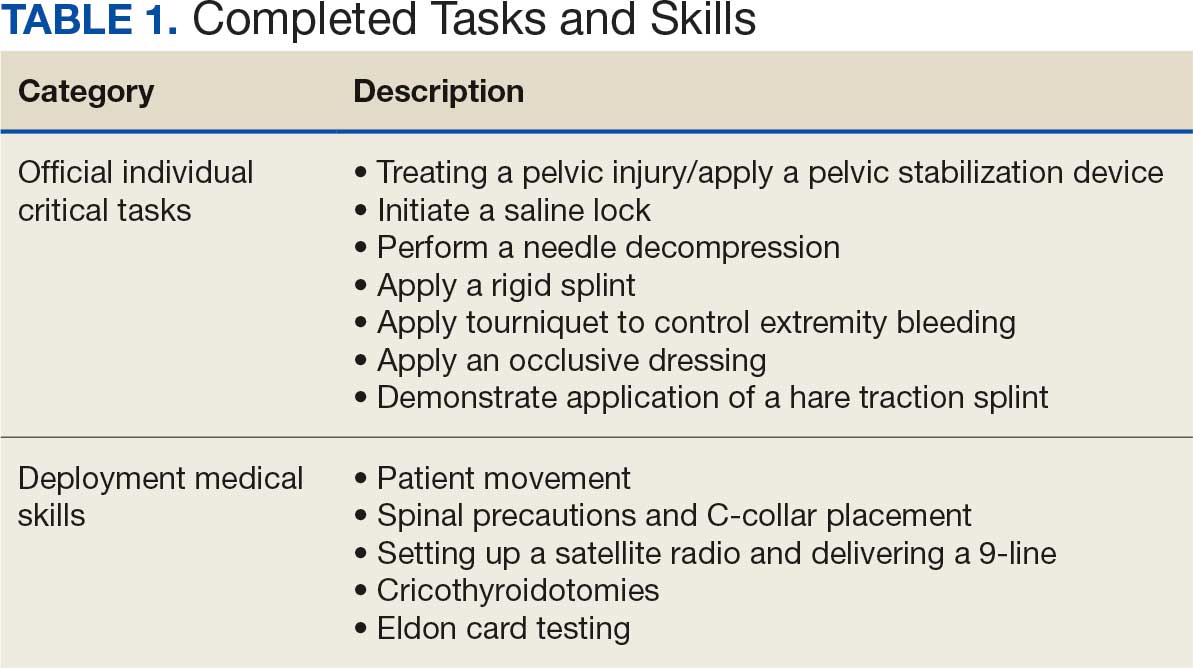
Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.
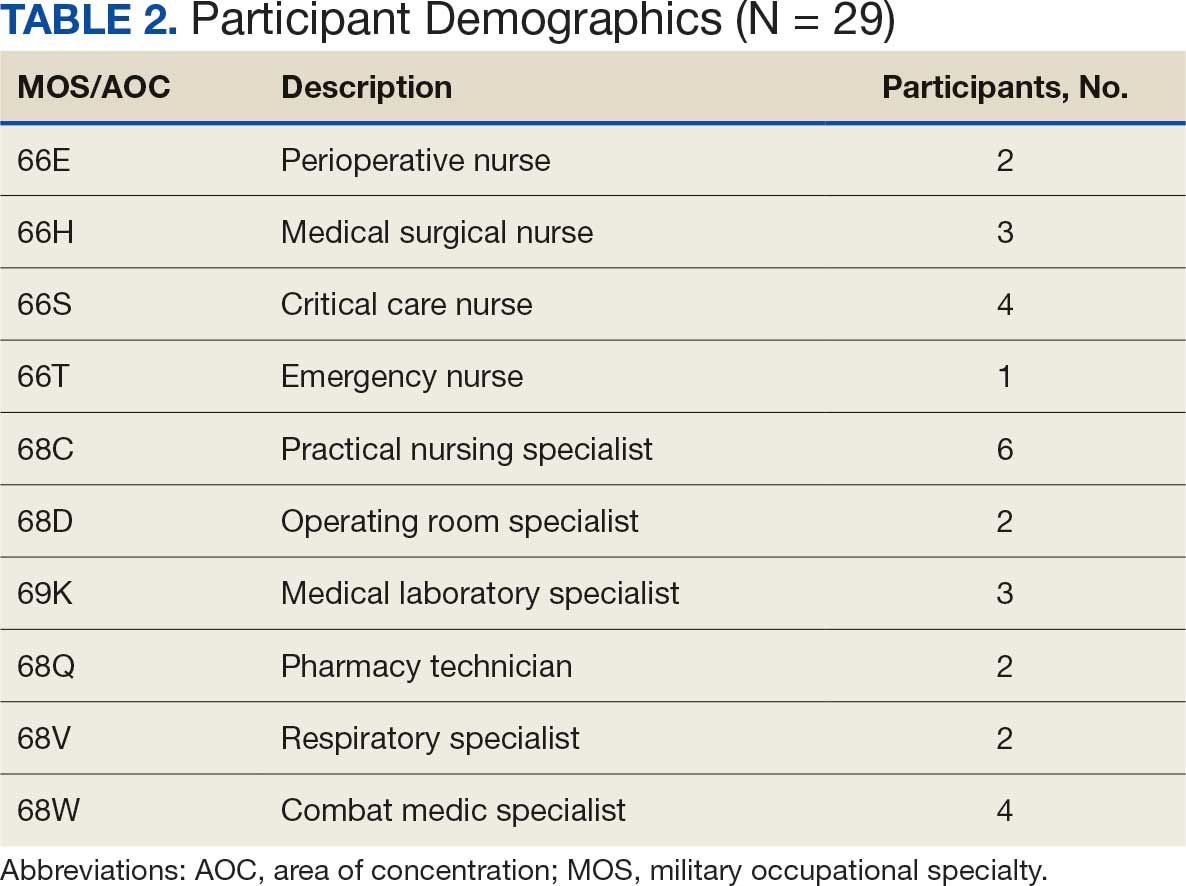
Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).
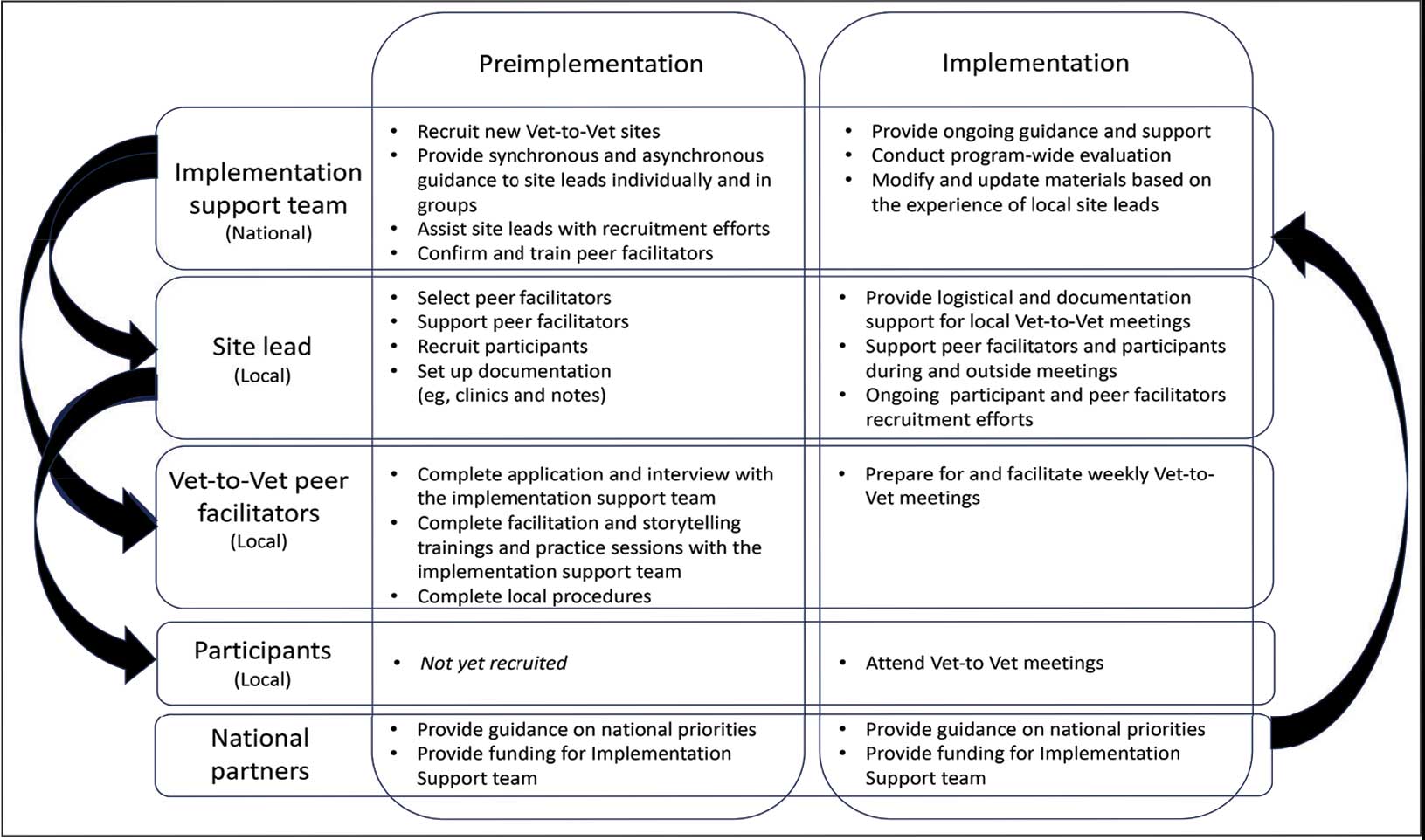
Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).
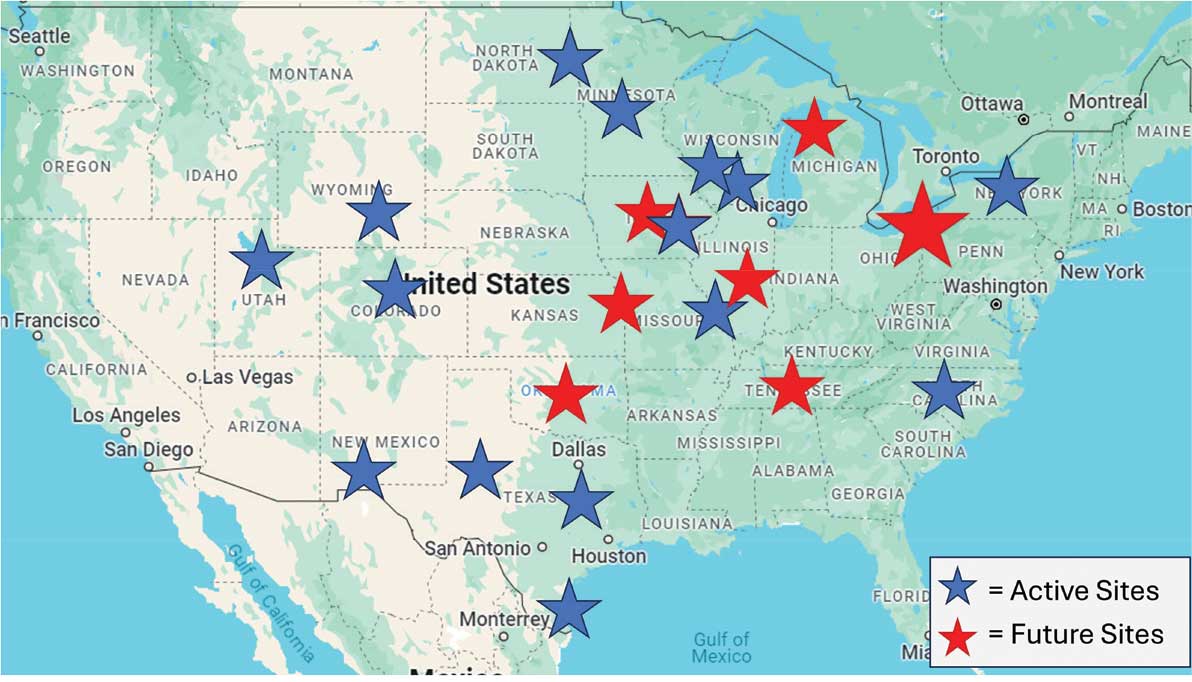
Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.
This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.
RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.
Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder