User login
Colorectal Cancer Awareness Month is Here!
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
New Risk Score Might Improve HCC Surveillance Among Cirrhosis Patients
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
FROM GASTROENTEROLOGY
Fecal Hemoglobin Levels From Negative FITs Signal CRC Risk
, according to a large international dose-response meta-analysis.
Although the association with neoplasia decreased as f-Hb levels rose, the findings support the development of risk-stratified screening strategies based on these concentrations, according to researchers led by Danica M.N. van den Berg, MSc, a PhD candidate and econometrics researcher in the Department of Public Health at Erasmus MC, University Medical Center in Rotterdam, the Netherlands.
Higher f-Hb concentrations in prior negative screening tests are strongly associated with an increased risk of detecting colorectal neoplasia in subsequent screenings, van den Berg said in an interview. “Gastroenterologists and other clinicians should consider the value of f-Hb concentrations in refining screening protocols and personalizing patient care to detect colorectal neoplasia earlier and more accurately.”
Published in Gastroenterology, the study was prompted by prior research showing individuals with f-Hb concentrations just below the positivity cutoff had an elevated CRC risk vs those with low or no f-Hb. “However, global variations in FIT positivity cutoffs and f-Hb category definitions complicated cross-study comparisons,” van den Berg said. Given the lack of an established dose-response relationship, the study aimed to clarify how f-Hb levels in previous screenings correlate with colorectal neoplasia detection. “Understanding this relationship is crucial for developing risk-stratified colorectal cancer screening strategies based on prior FIT results, which could improve the harm-benefit balance of screening,” she said.
According to van den Berg, f-Hb concentrations could help determine optimal CRC screening intervals by identifying higher-risk individuals who could benefit from more frequent testing, while those with lower concentrations could be screened less frequently.
Study Details
The systematic review and meta-analysis are the first to focus on the dose-response relationship between f-Hb levels in prior FIT screenings and colorectal neoplasia detection, van den Berg said. It included 13 ethnically diverse studies published during 2011-2023 with 4,493,223 individuals from Spain, France, the Netherlands, Taiwan, Denmark, Scotland, Ireland, Korea, Italy, and Norway. Most studies were cohort-based, and one was a randomized controlled trial.
All studies demonstrated a positive association between f-Hb in previous screenings and colorectal neoplasia detection. Almost all reported the f-Hb concentration measured in the prior screening round, while one study combined the f-Hb concentration of two previous screening rounds by using the cumulative f-Hb value. There was, however, wide variability in the stool positivity cut-offs in the included studies, ranging from 10 μg f-Hb/g to 80 μg f-Hb/g.
With an overall effect size of 0.69 (95% CI, 0.59-0.79), pooled analysis revealed that in the next screening round, individuals with f-Hb concentrations in stool of 5, 10, 20, and 40 μg/g had a threefold, fivefold, eightfold, and 13-fold higher risk for colorectal neoplasia, respectively, vs individuals showing 0 μg/g. Although there was significant study heterogeneity (I2 = 97.5%, P < .001), sensitivity analyses confirmed the consistency of findings. Interestingly, subgroup analyses indicated that f-Hb concentrations from a previous negative test were especially predictive of advanced neoplasia in subsequent screenings.
“This is a strategy worth pursuing and evaluating in the United States,” said gastroenterologist Theodore R. Levin, MD, a research scientist at Kaiser Permanente Division of Research in Northern California, commenting on the study but not involved in it. “However, there is no currently available FIT brand in the US that reports f-Hb concentration. All FITs in the US report as a qualitative positive-negative result.”
The Dutch investigation aligns with prior studies demonstrating a positive association between f-Hb concentrations in previous screenings and the detection of colorectal neoplasia. “Our working hypothesis was that risk increases in a decreasing manner as f-Hb concentrations rise, and the findings supported this hypothesis,” van den Berg said.
Other research has projected f-Hb level risk stratification to be effective and perhaps cost-effective in reducing delayed diagnosis of CRC.
Feasibility of Implementation
In large national screening programs in Europe, Asia, and Australia, as well as those of Kaiser Permanente and the Veterans Health Administration in the United States, information on f-Hb concentrations is already available.
“Therefore, incorporating an Hb-based approach should be relatively easy and affordable,” van den Berg said, and may help to optimize resource use while maintaining high detection rates. “However, the more critical question is whether such an approach would be acceptable to the target population.” To that end, randomized controlled trials in Italy and the Netherlands are offering tailored invitation intervals based on prior f-Hb concentrations and may provide insight into the real-world application of risk-stratified screening.
Among the many variables to be considered in the context of population-wide screening are cost-effectiveness, acceptability, and practicality, as well as invitation intervals, positivity cut-off levels, and start and stop ages for screening. “A key focus will be understanding the acceptability of risk-stratified colorectal cancer screening based on f-Hb among the target population and addressing any information needs they may have, as these are critical factors for successful implementation,” said van den Berg. Her group is currently studying the most effective and cost-effective risk-based strategy for CRC screening based on f-Hb levels.
The authors cautioned that since individuals with undetectable f-Hb levels make up the majority of those with negative FIT results, care must be taken that reducing screening frequency for this low-risk group does not lead to unfavorable outcomes at the population level.
This study was funded by the Dutch Organization for Scientific Research, which had no role in study design, data collection, analysis, interpretation, or writing.
The authors declared no competing interests. Levin disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
, according to a large international dose-response meta-analysis.
Although the association with neoplasia decreased as f-Hb levels rose, the findings support the development of risk-stratified screening strategies based on these concentrations, according to researchers led by Danica M.N. van den Berg, MSc, a PhD candidate and econometrics researcher in the Department of Public Health at Erasmus MC, University Medical Center in Rotterdam, the Netherlands.
Higher f-Hb concentrations in prior negative screening tests are strongly associated with an increased risk of detecting colorectal neoplasia in subsequent screenings, van den Berg said in an interview. “Gastroenterologists and other clinicians should consider the value of f-Hb concentrations in refining screening protocols and personalizing patient care to detect colorectal neoplasia earlier and more accurately.”
Published in Gastroenterology, the study was prompted by prior research showing individuals with f-Hb concentrations just below the positivity cutoff had an elevated CRC risk vs those with low or no f-Hb. “However, global variations in FIT positivity cutoffs and f-Hb category definitions complicated cross-study comparisons,” van den Berg said. Given the lack of an established dose-response relationship, the study aimed to clarify how f-Hb levels in previous screenings correlate with colorectal neoplasia detection. “Understanding this relationship is crucial for developing risk-stratified colorectal cancer screening strategies based on prior FIT results, which could improve the harm-benefit balance of screening,” she said.
According to van den Berg, f-Hb concentrations could help determine optimal CRC screening intervals by identifying higher-risk individuals who could benefit from more frequent testing, while those with lower concentrations could be screened less frequently.
Study Details
The systematic review and meta-analysis are the first to focus on the dose-response relationship between f-Hb levels in prior FIT screenings and colorectal neoplasia detection, van den Berg said. It included 13 ethnically diverse studies published during 2011-2023 with 4,493,223 individuals from Spain, France, the Netherlands, Taiwan, Denmark, Scotland, Ireland, Korea, Italy, and Norway. Most studies were cohort-based, and one was a randomized controlled trial.
All studies demonstrated a positive association between f-Hb in previous screenings and colorectal neoplasia detection. Almost all reported the f-Hb concentration measured in the prior screening round, while one study combined the f-Hb concentration of two previous screening rounds by using the cumulative f-Hb value. There was, however, wide variability in the stool positivity cut-offs in the included studies, ranging from 10 μg f-Hb/g to 80 μg f-Hb/g.
With an overall effect size of 0.69 (95% CI, 0.59-0.79), pooled analysis revealed that in the next screening round, individuals with f-Hb concentrations in stool of 5, 10, 20, and 40 μg/g had a threefold, fivefold, eightfold, and 13-fold higher risk for colorectal neoplasia, respectively, vs individuals showing 0 μg/g. Although there was significant study heterogeneity (I2 = 97.5%, P < .001), sensitivity analyses confirmed the consistency of findings. Interestingly, subgroup analyses indicated that f-Hb concentrations from a previous negative test were especially predictive of advanced neoplasia in subsequent screenings.
“This is a strategy worth pursuing and evaluating in the United States,” said gastroenterologist Theodore R. Levin, MD, a research scientist at Kaiser Permanente Division of Research in Northern California, commenting on the study but not involved in it. “However, there is no currently available FIT brand in the US that reports f-Hb concentration. All FITs in the US report as a qualitative positive-negative result.”
The Dutch investigation aligns with prior studies demonstrating a positive association between f-Hb concentrations in previous screenings and the detection of colorectal neoplasia. “Our working hypothesis was that risk increases in a decreasing manner as f-Hb concentrations rise, and the findings supported this hypothesis,” van den Berg said.
Other research has projected f-Hb level risk stratification to be effective and perhaps cost-effective in reducing delayed diagnosis of CRC.
Feasibility of Implementation
In large national screening programs in Europe, Asia, and Australia, as well as those of Kaiser Permanente and the Veterans Health Administration in the United States, information on f-Hb concentrations is already available.
“Therefore, incorporating an Hb-based approach should be relatively easy and affordable,” van den Berg said, and may help to optimize resource use while maintaining high detection rates. “However, the more critical question is whether such an approach would be acceptable to the target population.” To that end, randomized controlled trials in Italy and the Netherlands are offering tailored invitation intervals based on prior f-Hb concentrations and may provide insight into the real-world application of risk-stratified screening.
Among the many variables to be considered in the context of population-wide screening are cost-effectiveness, acceptability, and practicality, as well as invitation intervals, positivity cut-off levels, and start and stop ages for screening. “A key focus will be understanding the acceptability of risk-stratified colorectal cancer screening based on f-Hb among the target population and addressing any information needs they may have, as these are critical factors for successful implementation,” said van den Berg. Her group is currently studying the most effective and cost-effective risk-based strategy for CRC screening based on f-Hb levels.
The authors cautioned that since individuals with undetectable f-Hb levels make up the majority of those with negative FIT results, care must be taken that reducing screening frequency for this low-risk group does not lead to unfavorable outcomes at the population level.
This study was funded by the Dutch Organization for Scientific Research, which had no role in study design, data collection, analysis, interpretation, or writing.
The authors declared no competing interests. Levin disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
, according to a large international dose-response meta-analysis.
Although the association with neoplasia decreased as f-Hb levels rose, the findings support the development of risk-stratified screening strategies based on these concentrations, according to researchers led by Danica M.N. van den Berg, MSc, a PhD candidate and econometrics researcher in the Department of Public Health at Erasmus MC, University Medical Center in Rotterdam, the Netherlands.
Higher f-Hb concentrations in prior negative screening tests are strongly associated with an increased risk of detecting colorectal neoplasia in subsequent screenings, van den Berg said in an interview. “Gastroenterologists and other clinicians should consider the value of f-Hb concentrations in refining screening protocols and personalizing patient care to detect colorectal neoplasia earlier and more accurately.”
Published in Gastroenterology, the study was prompted by prior research showing individuals with f-Hb concentrations just below the positivity cutoff had an elevated CRC risk vs those with low or no f-Hb. “However, global variations in FIT positivity cutoffs and f-Hb category definitions complicated cross-study comparisons,” van den Berg said. Given the lack of an established dose-response relationship, the study aimed to clarify how f-Hb levels in previous screenings correlate with colorectal neoplasia detection. “Understanding this relationship is crucial for developing risk-stratified colorectal cancer screening strategies based on prior FIT results, which could improve the harm-benefit balance of screening,” she said.
According to van den Berg, f-Hb concentrations could help determine optimal CRC screening intervals by identifying higher-risk individuals who could benefit from more frequent testing, while those with lower concentrations could be screened less frequently.
Study Details
The systematic review and meta-analysis are the first to focus on the dose-response relationship between f-Hb levels in prior FIT screenings and colorectal neoplasia detection, van den Berg said. It included 13 ethnically diverse studies published during 2011-2023 with 4,493,223 individuals from Spain, France, the Netherlands, Taiwan, Denmark, Scotland, Ireland, Korea, Italy, and Norway. Most studies were cohort-based, and one was a randomized controlled trial.
All studies demonstrated a positive association between f-Hb in previous screenings and colorectal neoplasia detection. Almost all reported the f-Hb concentration measured in the prior screening round, while one study combined the f-Hb concentration of two previous screening rounds by using the cumulative f-Hb value. There was, however, wide variability in the stool positivity cut-offs in the included studies, ranging from 10 μg f-Hb/g to 80 μg f-Hb/g.
With an overall effect size of 0.69 (95% CI, 0.59-0.79), pooled analysis revealed that in the next screening round, individuals with f-Hb concentrations in stool of 5, 10, 20, and 40 μg/g had a threefold, fivefold, eightfold, and 13-fold higher risk for colorectal neoplasia, respectively, vs individuals showing 0 μg/g. Although there was significant study heterogeneity (I2 = 97.5%, P < .001), sensitivity analyses confirmed the consistency of findings. Interestingly, subgroup analyses indicated that f-Hb concentrations from a previous negative test were especially predictive of advanced neoplasia in subsequent screenings.
“This is a strategy worth pursuing and evaluating in the United States,” said gastroenterologist Theodore R. Levin, MD, a research scientist at Kaiser Permanente Division of Research in Northern California, commenting on the study but not involved in it. “However, there is no currently available FIT brand in the US that reports f-Hb concentration. All FITs in the US report as a qualitative positive-negative result.”
The Dutch investigation aligns with prior studies demonstrating a positive association between f-Hb concentrations in previous screenings and the detection of colorectal neoplasia. “Our working hypothesis was that risk increases in a decreasing manner as f-Hb concentrations rise, and the findings supported this hypothesis,” van den Berg said.
Other research has projected f-Hb level risk stratification to be effective and perhaps cost-effective in reducing delayed diagnosis of CRC.
Feasibility of Implementation
In large national screening programs in Europe, Asia, and Australia, as well as those of Kaiser Permanente and the Veterans Health Administration in the United States, information on f-Hb concentrations is already available.
“Therefore, incorporating an Hb-based approach should be relatively easy and affordable,” van den Berg said, and may help to optimize resource use while maintaining high detection rates. “However, the more critical question is whether such an approach would be acceptable to the target population.” To that end, randomized controlled trials in Italy and the Netherlands are offering tailored invitation intervals based on prior f-Hb concentrations and may provide insight into the real-world application of risk-stratified screening.
Among the many variables to be considered in the context of population-wide screening are cost-effectiveness, acceptability, and practicality, as well as invitation intervals, positivity cut-off levels, and start and stop ages for screening. “A key focus will be understanding the acceptability of risk-stratified colorectal cancer screening based on f-Hb among the target population and addressing any information needs they may have, as these are critical factors for successful implementation,” said van den Berg. Her group is currently studying the most effective and cost-effective risk-based strategy for CRC screening based on f-Hb levels.
The authors cautioned that since individuals with undetectable f-Hb levels make up the majority of those with negative FIT results, care must be taken that reducing screening frequency for this low-risk group does not lead to unfavorable outcomes at the population level.
This study was funded by the Dutch Organization for Scientific Research, which had no role in study design, data collection, analysis, interpretation, or writing.
The authors declared no competing interests. Levin disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
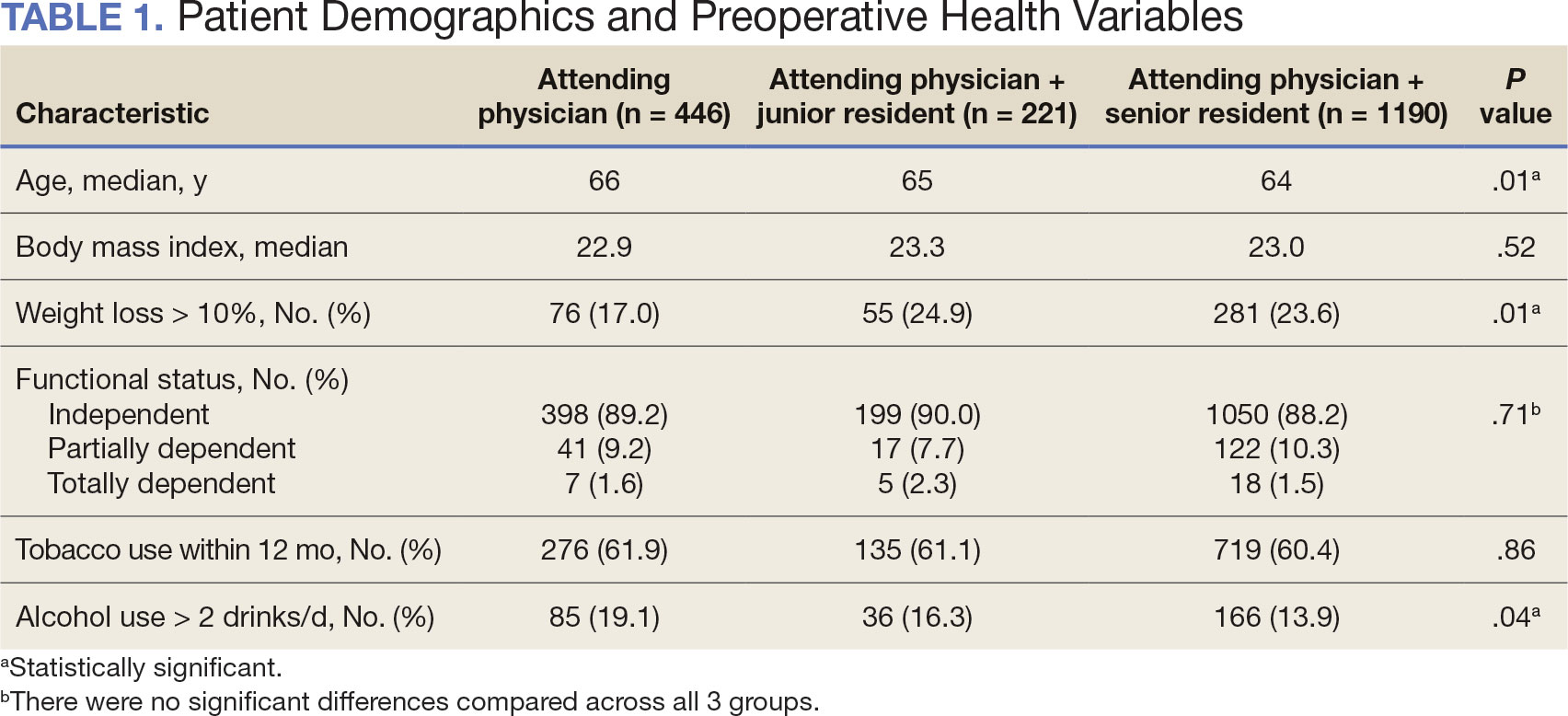
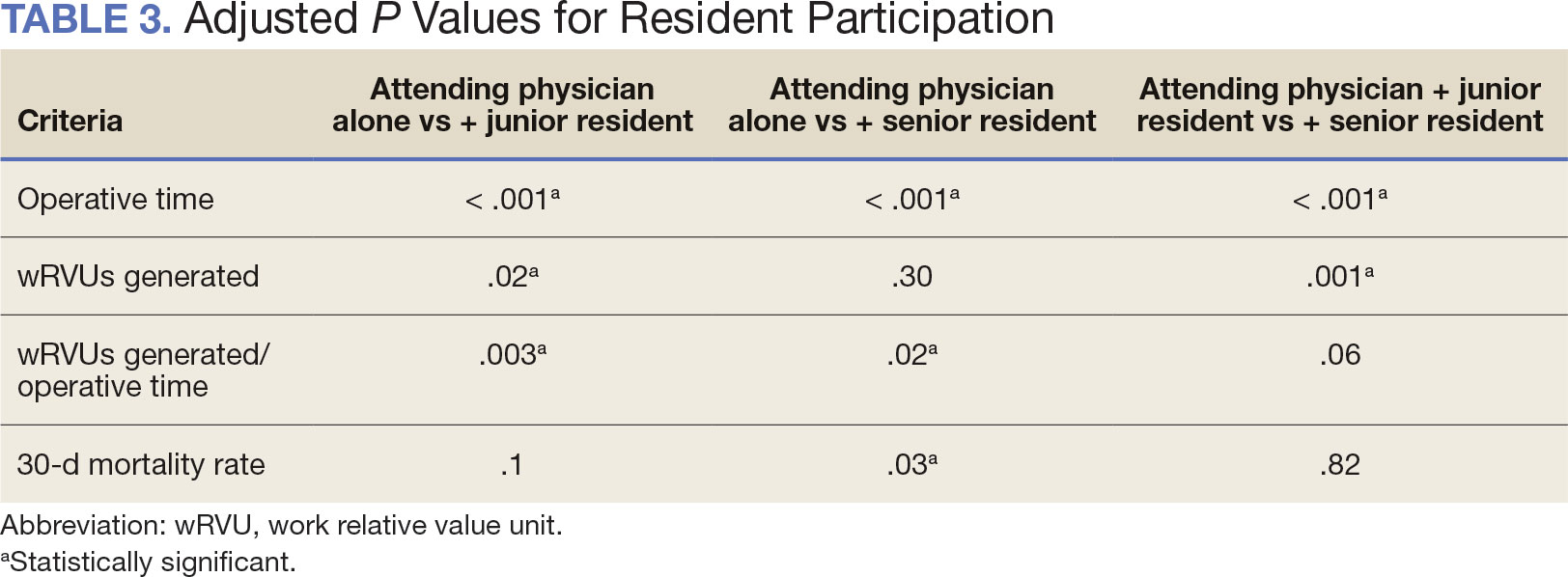
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).
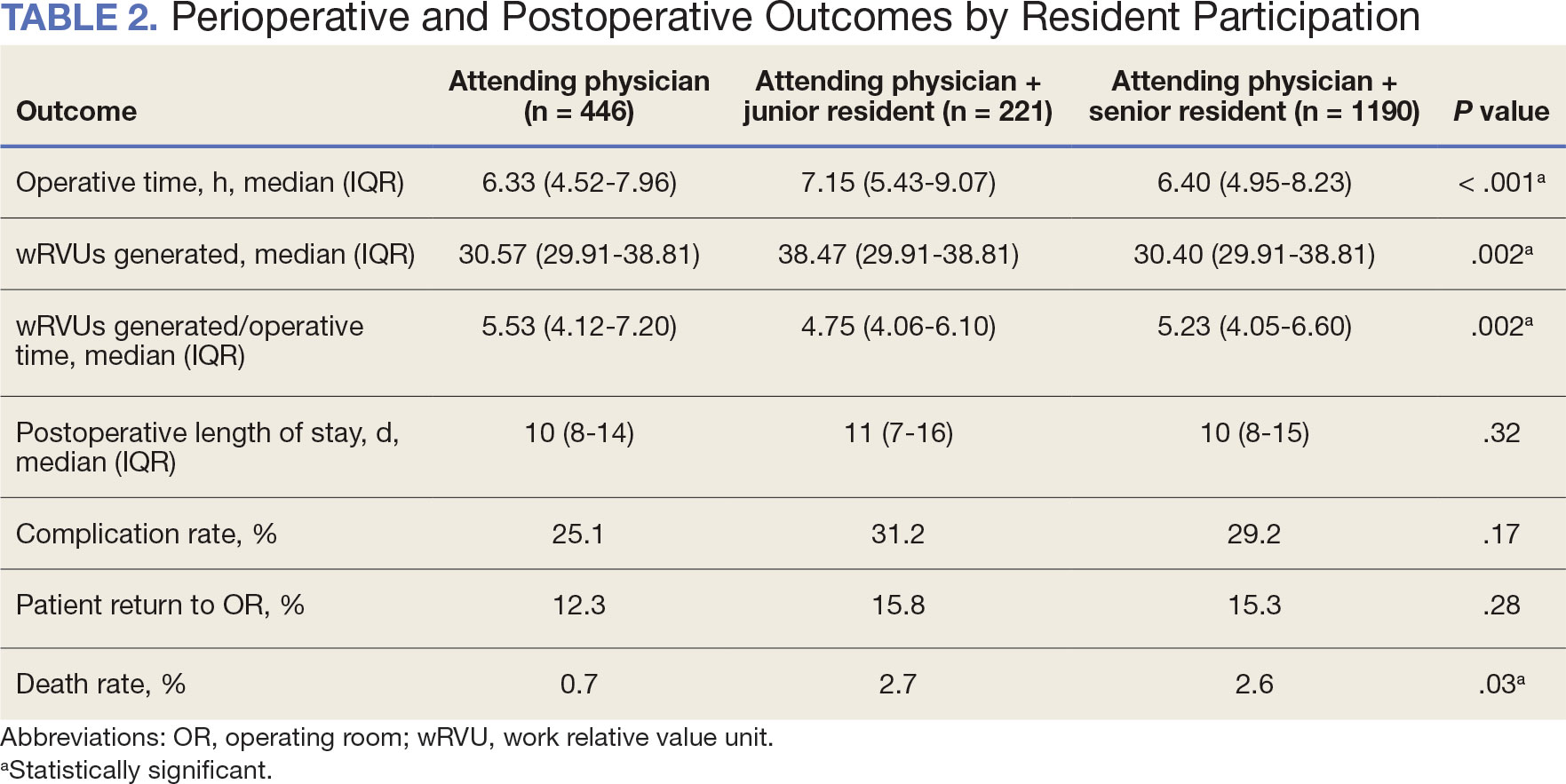
When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.
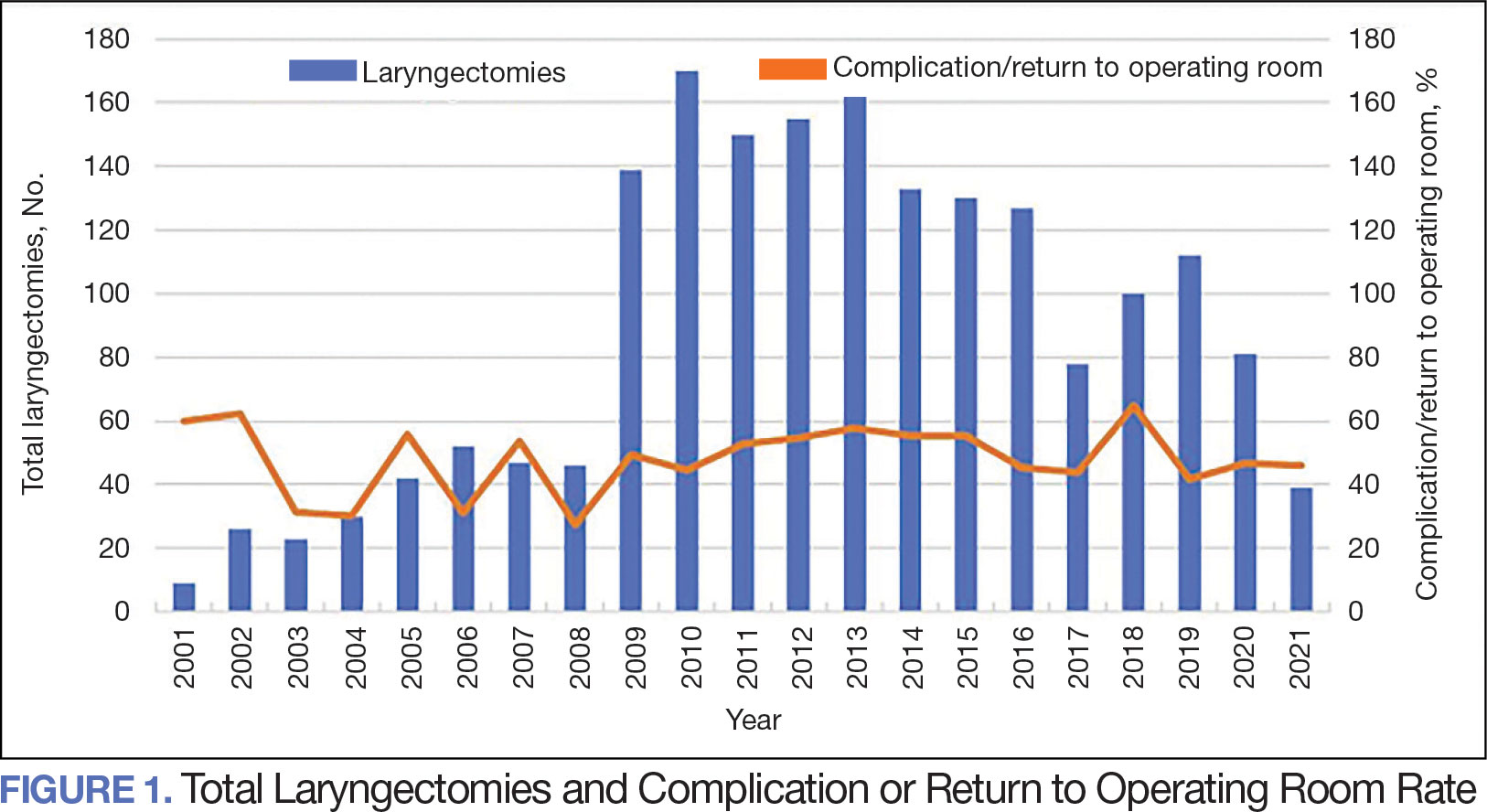
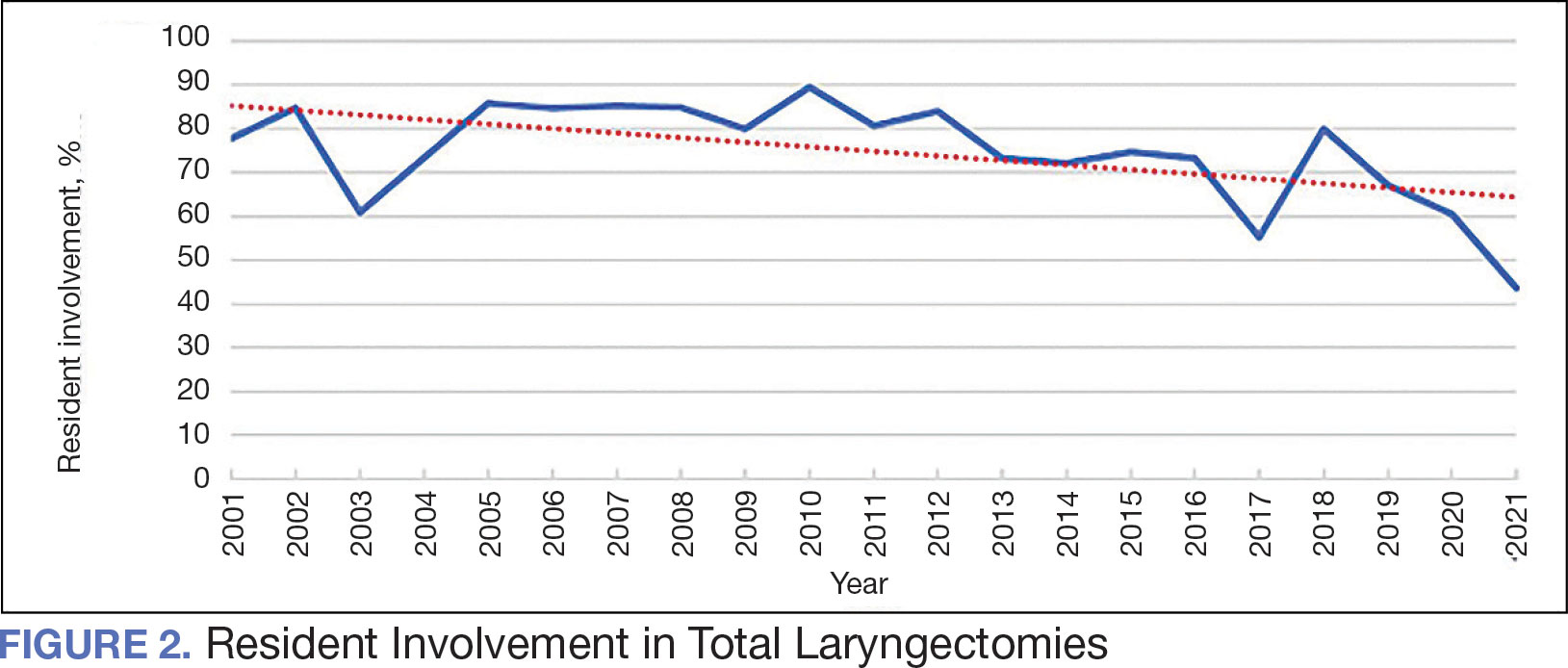
The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).
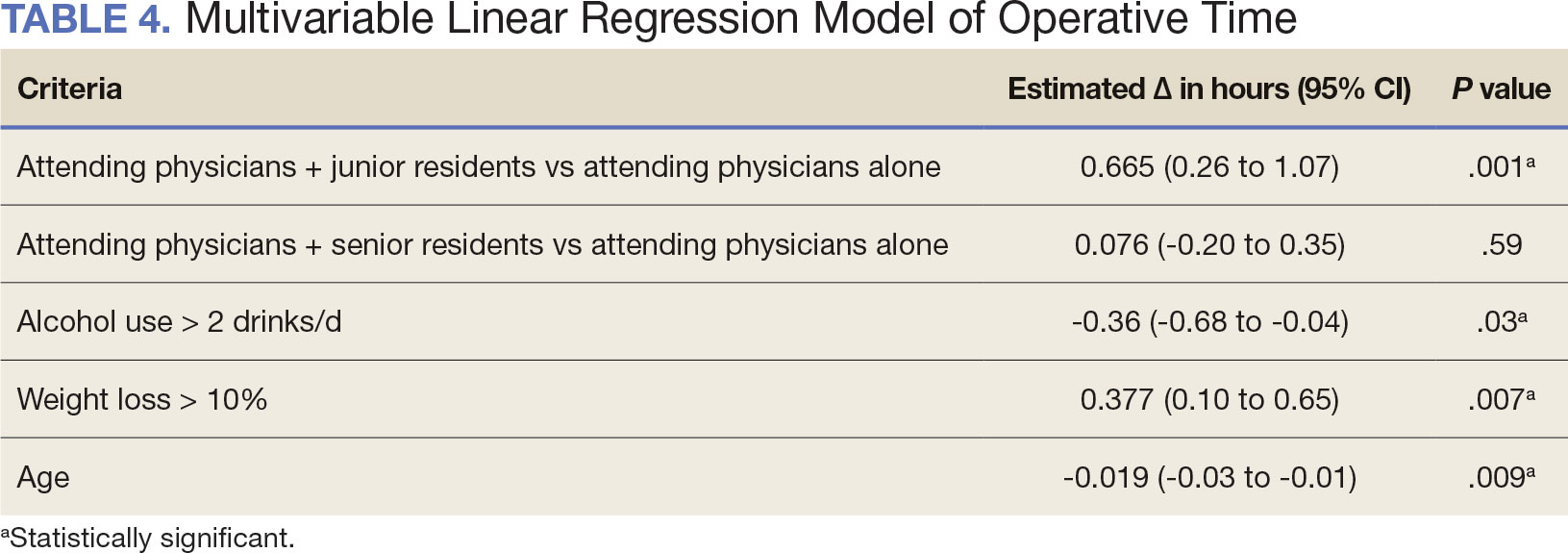
The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).
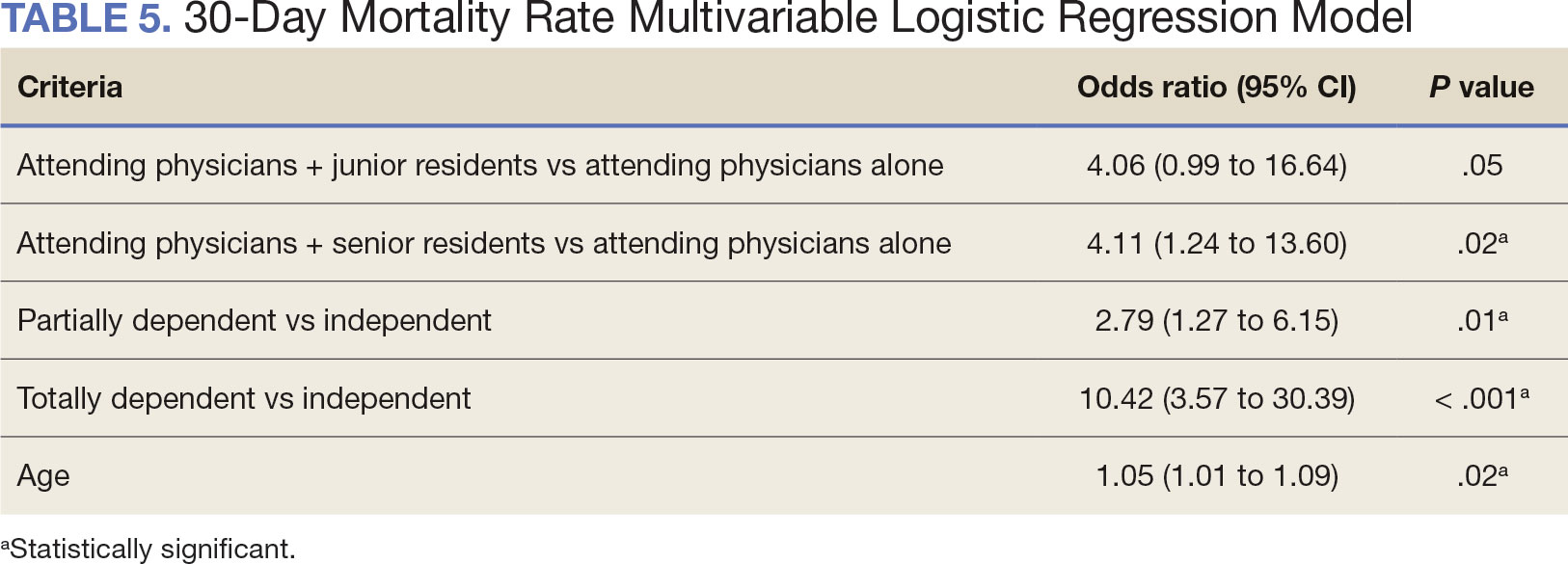
A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.
Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
Random Biopsy Improves IBD Dysplasia Detection, With Caveats
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY