User login
How Much Does Long COVID Cost Society? New Data Shed Light
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
Long COVID, a major public health crisis, is also becoming a significant economic crisis. A new study in Nature reports that the global annual economic impact of long COVID has hit $1 trillion — or about 1% of the global economy.
Long COVID is estimated to affect 6%-7% of adults. Those afflicted are often unable to work for extended periods, and some simply stop working altogether.
Besides damaging individual lives, long COVID is having wide-ranging impacts on health systems and economies worldwide, as those who suffer from it have large absences from work, leading to lower productivity. Even those who return to work after weeks, months, or even up to a year out of work may come back with worse productivity and some functional impairment — as a few of the condition’s common symptoms include fatigue and brain fog.
Experts say more is needed not only in terms of scientific research into new treatments for long COVID but also from a public policy perspective.
Long COVID’s impact on the labor force is already having ripple effects throughout the economy of the United States and other countries. Earlier this year, the US Government Accountability Office stated long COVID potentially affects up to 23 million Americans, with as many as a million people out of work. The healthcare industry is particularly hard hit.
The latest survey from the National Center for Health Statistics estimated 17.3%-18.6% of adults have experienced long COVID. This isn’t the same as those who have it now, only a broad indicator of people who’ve ever experienced symptoms.
Public health experts, economists, researchers, and physicians say they are only beginning to focus on ways to reduce long COVID’s impact.
They suggest a range of potential solutions to address the public health crisis and the economic impacts — including implementing a more thorough surveillance system to track long COVID cases, building better ventilation systems in hospitals and buildings to reduce the spread of the virus, increasing vaccination efforts as new viral strains continuously emerge, and more funding for long COVID research to better quantify and qualify the disease’s impact.
Shaky Statistics, Inconsistent Surveillance
David Smith, MD, an infectious disease specialist at the University of California, San Diego, said more needs to be done to survey, quantify, and qualify the impacts of long COVID on the economy before practical solutions can be identified.
“Our surveillance system sucks,” Smith said. “I can see how many people test positive for COVID, but how many of those people have long COVID?”
Long COVID also doesn’t have a true definition or standard diagnosis, which complicates surveillance efforts. It includes a spectrum of symptoms such as shortness of breath, chronic fatigue, and brain fog that linger for 2-3 months after an acute infection. But there’s no “concrete case definition,” Smith said. “And not everybody’s long COVID is exactly the same as everybody else’s.”
As a result, epidemiologists can’t effectively characterize the disease, and health economists can’t measure its exact economic impact.
Few countries have established comprehensive surveillance systems to estimate the burden of long COVID at the population level.
The United States currently tracks new cases by measuring wastewater levels, which isn’t as comprehensive as the tracking that was done during the pandemic. But positive wastewater samples can’t tell us who is infected in an area, nor can it distinguish whether a visitor/tourist or resident is mostly contributing to the wastewater analysis — an important distinction in public health studies.
Wastewater surveillance is an excellent complement to traditional disease surveillance with advantages and disadvantages, but it shouldn’t be the sole way to measure disease.
What Research Best Informs the Debate?
A study by Economist Impact — a think tank that partners with corporations, foundations, NGOs, and governments to help drive policy — estimated between a 0.5% and 2.3% gross domestic product (GDP) loss across eight separate countries in 2024. The study included the United Kingdom and United States.
Meanwhile, Australian researchers recently detailed how long COVID-related reductions in labor supply affected its productivity and GDP from 2022 to 2024. The study found that long COVID could be costing the Australian economy about 0.5% of its GDP, which researchers deemed a conservative estimate.
Public health researchers in New Zealand used the estimate of GDP loss in Australia to measure their own potential losses and advocated for strengthening occupational support across all sectors to protect health.
But these studies can’t quite compare with what would have to be done for the United States economy.
“New Zealand is small ... and has an excellent public health system with good delivery of vaccines and treatments…so how do we compare that to us?” Smith said. “They do better in all of their public health metrics than we do.”
Measuring the Economic Impact
Gopi Shah Goda, PhD, a health economist and senior fellow in economic studies at the Brookings Institution, co-authored a 2023 study that found COVID-19 reduced the US labor force by about 500,000 people.
Plus, workers who missed a full week due to COVID-19 absences became 7% less likely to return to the labor force a year later compared with workers who didn’t miss work for health reasons. That amounts to 0.2% of the labor force, a significant number.
“Even a small percent of the labor force is a big number…it’s like an extra year of populating aging,” Goda said.
“Some people who get long COVID might have dropped out of the labor force anyway,” Goda added.
The study concluded that average individual earnings lost from long COVID were $9000, and the total lost labor supply amounted to $62 billion annually — about half the estimated productivity losses from cancer or diabetes.
But research into long COVID research continues to be underfunded compared with other health conditions, experts noted.
Cancer and diabetes both receive billions of research dollars annually from the National Institutes of Health. Long COVID research gets only a few million, according to Goda.
Informing Public Health Policy
When it comes to caring for patients with long COVID, the big issue facing every nation’s public policy leaders is how best to allocate limited health resources.
“Public health never has enough money ... Do they buy more vaccines? Do they do educational programs? Who do they target the most?” Smith said.
Though Smith thinks the best preventative measure is increased vaccination, vaccination rates remain low in the United States.
“Unfortunately, as last fall demonstrated, there’s a lot of vaccine indifference and skepticism,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University School of Medicine, Nashville, Tennessee.
Over the past year, only 14% of eligible children and 22% of adults received the 2023-2024 COVID vaccine boosters.
Schaffner said public health experts wrestle with ways to assure the public vaccines are safe and effective.
“They’re trying to provide a level of comfort that [getting vaccinated] is the socially appropriate thing to do,” which remains a significant challenge, Schaffner said.
Some people don’t have access to vaccines and comprehensive medical services because they lack insurance, Medicaid, and Medicare. And the United States still doesn’t distribute vaccines as well as other countries, Schaffner added.
“In other countries, every doctor’s office gets vaccines for free ... here, we have a large commercial enterprise that basically runs it…there are still populations who aren’t reached,” he said.
Long COVID clinics that have opened around the country have offered help to some patients with long COVID. A year and a half ago, Yale University, New Haven, Connecticut, established its Long COVID Care Center. Stanford University, Stanford, California, opened its Long COVID Clinic back in 2021. Vanderbilt University now has its own, as well — the Adult Post-COVID Clinic.
But these clinics have faced declining federal resources, forcing some to close and others to face questions about whether they will be able to continue to operate without more aggressive federal direction and policy planning.
“With some central direction, we could provide better supportive care for the many patients with long COVID out there,” Schaffner said.
For countries with universal healthcare systems, services such as occupational health, extended sick leave, extended time for disability, and workers’ compensation benefits are readily available.
But in the United States, it’s often left to the physicians and their patients to figure out a plan.
“I think we could make physicians more aware of options for their patients…for example, regularly check eligibility for workers compensation,” Schaffner said.
A version of this article first appeared on Medscape.com.
FROM NATURE
Beware the Manchineel: A Case of Irritant Contact Dermatitis
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
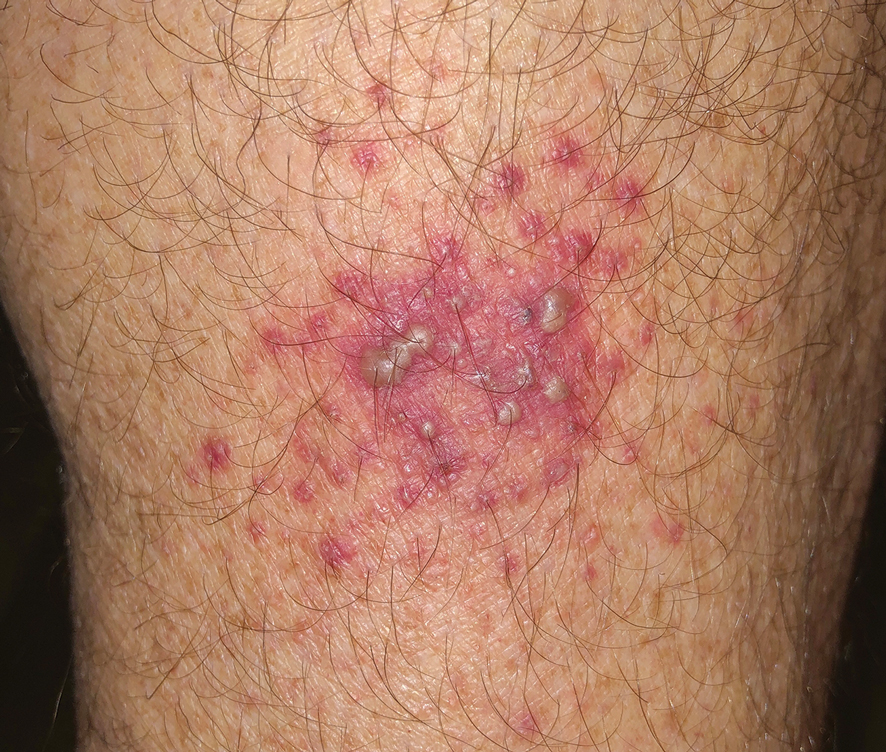
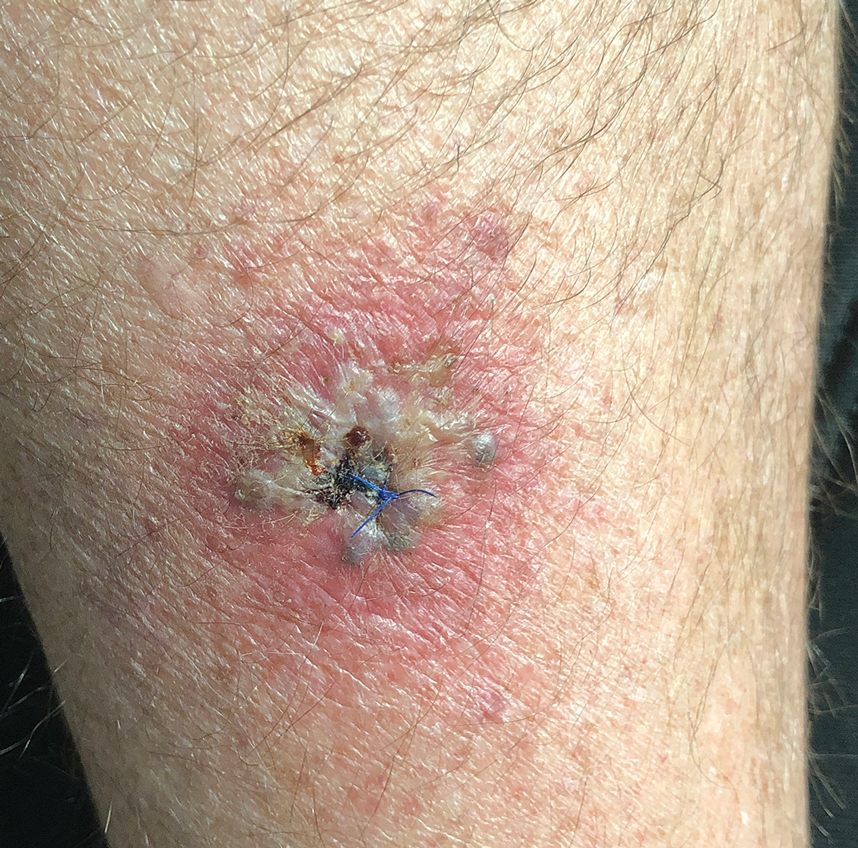
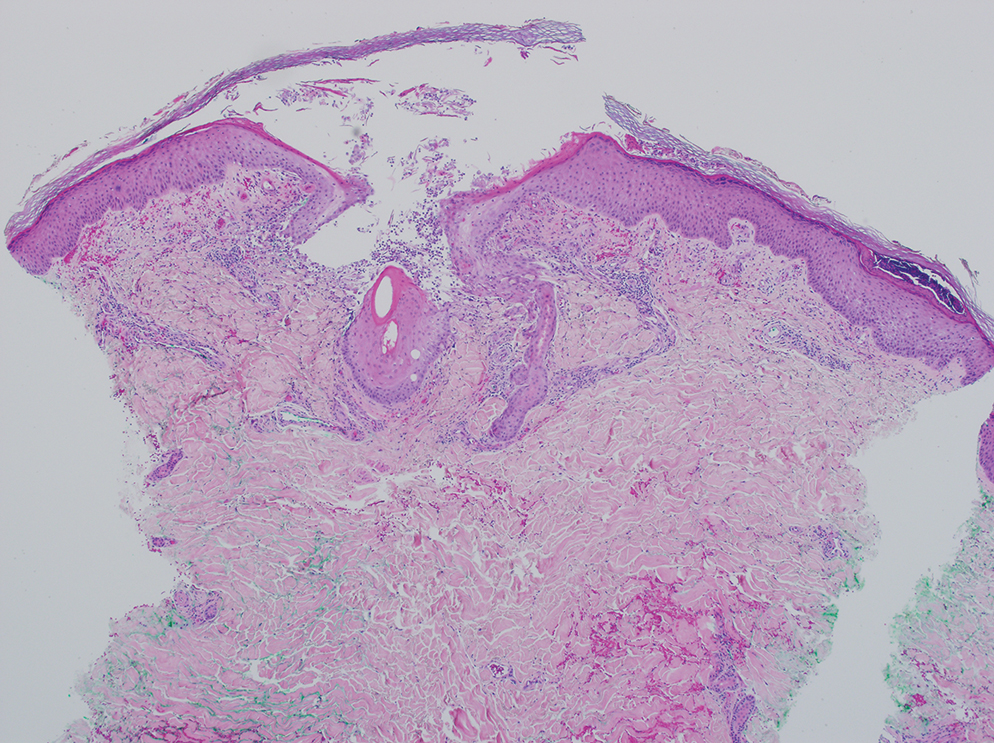
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.


Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.





Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.





Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
PRACTICE POINTS
- Sap from the manchineel tree—found on the coasts of Caribbean islands, the Atlantic coastline of Central and northern South America, and parts of southernmost Florida—can cause severe dermatologic and ophthalmologic injuries. Eating its fruit can lead to oropharyngeal pain and diarrhea.
- Histopathology of manchineel dermatitis reveals a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which is consistent with irritant contact dermatitis.
- There is no specific treatment for manchineel dermatitis. Case reports advocate a thorough cleansing, application of wet compresses, and observation.
Groups With Highest Unmet Need for PrEP Highlighted in Analysis
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2024
Pediatric Myasthenia Gravis: Don’t Treat Children Like Adults
SAVANNAH, GEORGIA — At a pathophysiological level, juvenile myasthenia gravis (MG) seems to be identical to the adult form, neuromuscular specialists learned. But there are still important differences between children and their elders that affect pediatric care.
For example, “we have to think a little bit differently about the side effect profiles of the medications and their toxicity because children may react to medications differently,” said Matthew Ginsberg, MD, a pediatric neurologist based in Akron, Ohio, in a presentation at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
And then there’s the matter of adherence. “It’s hard to get adults to take medication, but a teenager is sometimes an exceptional challenge,” Ginsberg said.
Case In Point: A 13-Year-Old With MG
Pediatric MG is rare. Cases in children are estimated to account for 10% of MG cases diagnosed each year. According to a 2020 report, “the majority will present with ptosis and a variable degree of ophthalmoplegia [paralysis of eye muscles].”
Ginsberg highlighted a case of a 13-year-old girl who’d been healthy but developed fatigable ptosis and mild restriction of extraocular movements. The patient’s acetylcholine receptor antibodies were very elevated, but she didn’t have MuSK antibodies.
“This isn’t a diagnostic conundrum. She has autoimmune myasthenia gravis with ocular manifestations,” Ginsberg said. “For someone like this, whether it’s an adult or a child, many people would start symptomatic treatment with an acetylcholinesterase inhibitor like pyridostigmine.”
The use of the drug in children is similar to that in adults, he said, although weight-based dosing is used. “Usually it’s around 3-7 mg/kg/d, but it’s still very individualized based on patient response.” The timing of symptoms can affect the distribution of doses throughout the day, he said.
“There are extended-release formulations of the medication, and I think some people use them more than I do,” he said. “The side effects are basically similar to adults. Most of the patients I have on it tolerate it really well and don’t have a lot of the muscarinic side effects that you would expect.”
Consider Prescription Eye Drops for Ptosis
Alpha-1A agonists oxymetazoline and apraclonidine in the form of topical eye drops can help with ptosis. “They potentially avoid some of the systemic toxicity of the other medications,” Ginsberg said. “So they might be an option if you’re really just trying to target ptosis as a symptom.”
However, it can be difficult to get insurers to cover these medications, he said.
The 13-year-old patient initially improved but developed difficulty walking. “Her hands began to feel heavy, and she had difficulty chewing and nasal regurgitation. On her exam, she still had fatigable ptosis plus hypernasal speech and generalized weakness. At this point, we’re starting to see that she has generalized myasthenia gravis that may be an impending crisis.”
The Young Patient Worsens. Now What?
The patient was admitted and given intravenous immunoglobulin at 2 g/kg over a couple days. But her symptoms worsened following initial improvement.
Glucocorticoids can play a larger role in treatment at this stage, and the patient was initially on prednisone. But there are reasons for caution, including effects on bone growth and interference with live vaccines.
However, live vaccines aren’t common in children, with the exception of the MMRV vaccine, he said. “It’s worth noting that you can give that second dose as early as 3 months after the initial one, so most patients really should be able to complete a course before they start on immunosuppression,” he said.
Another option is immunotherapy. “There’s a really large menu of options for immunotherapy in myasthenia gravis right now,” Ginsberg said. “It’s great that we have all these options, but it adds to the complexity.”
Rituximab may be considered based on early data, he said. And thymectomy — removal of the thymus gland — should be considered early.
Don’t Neglect Supportive Care
Ginsberg urged colleagues to consider supportive care measures. Advocacy groups such as the Myasthenia Gravis Foundation of America can help with weight management and diet/exercise counseling, especially in patients taking glucocorticoids.
He added that “school accommodations are very important in this age group. They might need a plan, for example, to have modified gym class or an excuse not to carry a book bag between classes.”
How did the 13-year-old do? She underwent thymectomy, and her disease remained stable after 6 months. “Her rituximab was discontinued,” Ginsberg said. “She considered participating in a clinical trial but then started seeing improvements. About a year after the thymectomy, she just stopped her steroids on her own, and she was fine.”
Ginsberg had no disclosures.
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — At a pathophysiological level, juvenile myasthenia gravis (MG) seems to be identical to the adult form, neuromuscular specialists learned. But there are still important differences between children and their elders that affect pediatric care.
For example, “we have to think a little bit differently about the side effect profiles of the medications and their toxicity because children may react to medications differently,” said Matthew Ginsberg, MD, a pediatric neurologist based in Akron, Ohio, in a presentation at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
And then there’s the matter of adherence. “It’s hard to get adults to take medication, but a teenager is sometimes an exceptional challenge,” Ginsberg said.
Case In Point: A 13-Year-Old With MG
Pediatric MG is rare. Cases in children are estimated to account for 10% of MG cases diagnosed each year. According to a 2020 report, “the majority will present with ptosis and a variable degree of ophthalmoplegia [paralysis of eye muscles].”
Ginsberg highlighted a case of a 13-year-old girl who’d been healthy but developed fatigable ptosis and mild restriction of extraocular movements. The patient’s acetylcholine receptor antibodies were very elevated, but she didn’t have MuSK antibodies.
“This isn’t a diagnostic conundrum. She has autoimmune myasthenia gravis with ocular manifestations,” Ginsberg said. “For someone like this, whether it’s an adult or a child, many people would start symptomatic treatment with an acetylcholinesterase inhibitor like pyridostigmine.”
The use of the drug in children is similar to that in adults, he said, although weight-based dosing is used. “Usually it’s around 3-7 mg/kg/d, but it’s still very individualized based on patient response.” The timing of symptoms can affect the distribution of doses throughout the day, he said.
“There are extended-release formulations of the medication, and I think some people use them more than I do,” he said. “The side effects are basically similar to adults. Most of the patients I have on it tolerate it really well and don’t have a lot of the muscarinic side effects that you would expect.”
Consider Prescription Eye Drops for Ptosis
Alpha-1A agonists oxymetazoline and apraclonidine in the form of topical eye drops can help with ptosis. “They potentially avoid some of the systemic toxicity of the other medications,” Ginsberg said. “So they might be an option if you’re really just trying to target ptosis as a symptom.”
However, it can be difficult to get insurers to cover these medications, he said.
The 13-year-old patient initially improved but developed difficulty walking. “Her hands began to feel heavy, and she had difficulty chewing and nasal regurgitation. On her exam, she still had fatigable ptosis plus hypernasal speech and generalized weakness. At this point, we’re starting to see that she has generalized myasthenia gravis that may be an impending crisis.”
The Young Patient Worsens. Now What?
The patient was admitted and given intravenous immunoglobulin at 2 g/kg over a couple days. But her symptoms worsened following initial improvement.
Glucocorticoids can play a larger role in treatment at this stage, and the patient was initially on prednisone. But there are reasons for caution, including effects on bone growth and interference with live vaccines.
However, live vaccines aren’t common in children, with the exception of the MMRV vaccine, he said. “It’s worth noting that you can give that second dose as early as 3 months after the initial one, so most patients really should be able to complete a course before they start on immunosuppression,” he said.
Another option is immunotherapy. “There’s a really large menu of options for immunotherapy in myasthenia gravis right now,” Ginsberg said. “It’s great that we have all these options, but it adds to the complexity.”
Rituximab may be considered based on early data, he said. And thymectomy — removal of the thymus gland — should be considered early.
Don’t Neglect Supportive Care
Ginsberg urged colleagues to consider supportive care measures. Advocacy groups such as the Myasthenia Gravis Foundation of America can help with weight management and diet/exercise counseling, especially in patients taking glucocorticoids.
He added that “school accommodations are very important in this age group. They might need a plan, for example, to have modified gym class or an excuse not to carry a book bag between classes.”
How did the 13-year-old do? She underwent thymectomy, and her disease remained stable after 6 months. “Her rituximab was discontinued,” Ginsberg said. “She considered participating in a clinical trial but then started seeing improvements. About a year after the thymectomy, she just stopped her steroids on her own, and she was fine.”
Ginsberg had no disclosures.
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — At a pathophysiological level, juvenile myasthenia gravis (MG) seems to be identical to the adult form, neuromuscular specialists learned. But there are still important differences between children and their elders that affect pediatric care.
For example, “we have to think a little bit differently about the side effect profiles of the medications and their toxicity because children may react to medications differently,” said Matthew Ginsberg, MD, a pediatric neurologist based in Akron, Ohio, in a presentation at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024.
And then there’s the matter of adherence. “It’s hard to get adults to take medication, but a teenager is sometimes an exceptional challenge,” Ginsberg said.
Case In Point: A 13-Year-Old With MG
Pediatric MG is rare. Cases in children are estimated to account for 10% of MG cases diagnosed each year. According to a 2020 report, “the majority will present with ptosis and a variable degree of ophthalmoplegia [paralysis of eye muscles].”
Ginsberg highlighted a case of a 13-year-old girl who’d been healthy but developed fatigable ptosis and mild restriction of extraocular movements. The patient’s acetylcholine receptor antibodies were very elevated, but she didn’t have MuSK antibodies.
“This isn’t a diagnostic conundrum. She has autoimmune myasthenia gravis with ocular manifestations,” Ginsberg said. “For someone like this, whether it’s an adult or a child, many people would start symptomatic treatment with an acetylcholinesterase inhibitor like pyridostigmine.”
The use of the drug in children is similar to that in adults, he said, although weight-based dosing is used. “Usually it’s around 3-7 mg/kg/d, but it’s still very individualized based on patient response.” The timing of symptoms can affect the distribution of doses throughout the day, he said.
“There are extended-release formulations of the medication, and I think some people use them more than I do,” he said. “The side effects are basically similar to adults. Most of the patients I have on it tolerate it really well and don’t have a lot of the muscarinic side effects that you would expect.”
Consider Prescription Eye Drops for Ptosis
Alpha-1A agonists oxymetazoline and apraclonidine in the form of topical eye drops can help with ptosis. “They potentially avoid some of the systemic toxicity of the other medications,” Ginsberg said. “So they might be an option if you’re really just trying to target ptosis as a symptom.”
However, it can be difficult to get insurers to cover these medications, he said.
The 13-year-old patient initially improved but developed difficulty walking. “Her hands began to feel heavy, and she had difficulty chewing and nasal regurgitation. On her exam, she still had fatigable ptosis plus hypernasal speech and generalized weakness. At this point, we’re starting to see that she has generalized myasthenia gravis that may be an impending crisis.”
The Young Patient Worsens. Now What?
The patient was admitted and given intravenous immunoglobulin at 2 g/kg over a couple days. But her symptoms worsened following initial improvement.
Glucocorticoids can play a larger role in treatment at this stage, and the patient was initially on prednisone. But there are reasons for caution, including effects on bone growth and interference with live vaccines.
However, live vaccines aren’t common in children, with the exception of the MMRV vaccine, he said. “It’s worth noting that you can give that second dose as early as 3 months after the initial one, so most patients really should be able to complete a course before they start on immunosuppression,” he said.
Another option is immunotherapy. “There’s a really large menu of options for immunotherapy in myasthenia gravis right now,” Ginsberg said. “It’s great that we have all these options, but it adds to the complexity.”
Rituximab may be considered based on early data, he said. And thymectomy — removal of the thymus gland — should be considered early.
Don’t Neglect Supportive Care
Ginsberg urged colleagues to consider supportive care measures. Advocacy groups such as the Myasthenia Gravis Foundation of America can help with weight management and diet/exercise counseling, especially in patients taking glucocorticoids.
He added that “school accommodations are very important in this age group. They might need a plan, for example, to have modified gym class or an excuse not to carry a book bag between classes.”
How did the 13-year-old do? She underwent thymectomy, and her disease remained stable after 6 months. “Her rituximab was discontinued,” Ginsberg said. “She considered participating in a clinical trial but then started seeing improvements. About a year after the thymectomy, she just stopped her steroids on her own, and she was fine.”
Ginsberg had no disclosures.
A version of this article appeared on Medscape.com.
FROM AANEM 2024
FDA OKs Novel Levodopa-Based Continuous Sub-Q Regimen for Parkinson’s Disease
Due to the progressive nature of Parkinson’s disease, “oral medications are eventually no longer as effective at motor symptom control and surgical treatment may be required. This new, non-surgical regimen provides continuous delivery of levodopa morning, day, and night,” Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center at the University of South Florida, Tampa, said in a news release.
The FDA approval was supported by results of a 12-week, phase 3 study evaluating the efficacy of continuous subcutaneous infusion foscarbidopa/foslevodopa in adults with advanced Parkinson’s disease compared with oral immediate-release carbidopa/levodopa.
The study showed that patients treated with foscarbidopa/foslevodopa had superior improvement in motor fluctuations, with increased “on” time without troublesome dyskinesia and decreased “off” time, compared with peers receiving oral immediate-release carbidopa/levodopa.
At week 12, the increase in “on” time without troublesome dyskinesia was 2.72 hours for foscarbidopa/foslevodopa continuous infusion versus 0.97 hours for carbidopa/levodopa (P =.0083).
Improvements in “on” time were observed as early as the first week and persisted throughout the 12 weeks.
The approval of foscarbidopa/foslevodopa for advanced Parkinson’s disease was also supported by a 52-week, open-label study which evaluated the long-term safety and efficacy of the drug.
Most adverse reactions with foscarbidopa/foslevodopa were non-serious and mild or moderate in severity. The most frequent adverse reactions were infusion site events, hallucinations, and dyskinesia.
Full prescribing information is available online.
AbbVie said coverage for Medicare patients is expected in the second half of 2025.
A version of this article appeared on Medscape.com.
Due to the progressive nature of Parkinson’s disease, “oral medications are eventually no longer as effective at motor symptom control and surgical treatment may be required. This new, non-surgical regimen provides continuous delivery of levodopa morning, day, and night,” Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center at the University of South Florida, Tampa, said in a news release.
The FDA approval was supported by results of a 12-week, phase 3 study evaluating the efficacy of continuous subcutaneous infusion foscarbidopa/foslevodopa in adults with advanced Parkinson’s disease compared with oral immediate-release carbidopa/levodopa.
The study showed that patients treated with foscarbidopa/foslevodopa had superior improvement in motor fluctuations, with increased “on” time without troublesome dyskinesia and decreased “off” time, compared with peers receiving oral immediate-release carbidopa/levodopa.
At week 12, the increase in “on” time without troublesome dyskinesia was 2.72 hours for foscarbidopa/foslevodopa continuous infusion versus 0.97 hours for carbidopa/levodopa (P =.0083).
Improvements in “on” time were observed as early as the first week and persisted throughout the 12 weeks.
The approval of foscarbidopa/foslevodopa for advanced Parkinson’s disease was also supported by a 52-week, open-label study which evaluated the long-term safety and efficacy of the drug.
Most adverse reactions with foscarbidopa/foslevodopa were non-serious and mild or moderate in severity. The most frequent adverse reactions were infusion site events, hallucinations, and dyskinesia.
Full prescribing information is available online.
AbbVie said coverage for Medicare patients is expected in the second half of 2025.
A version of this article appeared on Medscape.com.
Due to the progressive nature of Parkinson’s disease, “oral medications are eventually no longer as effective at motor symptom control and surgical treatment may be required. This new, non-surgical regimen provides continuous delivery of levodopa morning, day, and night,” Robert A. Hauser, MD, MBA, director of the Parkinson’s and Movement Disorder Center at the University of South Florida, Tampa, said in a news release.
The FDA approval was supported by results of a 12-week, phase 3 study evaluating the efficacy of continuous subcutaneous infusion foscarbidopa/foslevodopa in adults with advanced Parkinson’s disease compared with oral immediate-release carbidopa/levodopa.
The study showed that patients treated with foscarbidopa/foslevodopa had superior improvement in motor fluctuations, with increased “on” time without troublesome dyskinesia and decreased “off” time, compared with peers receiving oral immediate-release carbidopa/levodopa.
At week 12, the increase in “on” time without troublesome dyskinesia was 2.72 hours for foscarbidopa/foslevodopa continuous infusion versus 0.97 hours for carbidopa/levodopa (P =.0083).
Improvements in “on” time were observed as early as the first week and persisted throughout the 12 weeks.
The approval of foscarbidopa/foslevodopa for advanced Parkinson’s disease was also supported by a 52-week, open-label study which evaluated the long-term safety and efficacy of the drug.
Most adverse reactions with foscarbidopa/foslevodopa were non-serious and mild or moderate in severity. The most frequent adverse reactions were infusion site events, hallucinations, and dyskinesia.
Full prescribing information is available online.
AbbVie said coverage for Medicare patients is expected in the second half of 2025.
A version of this article appeared on Medscape.com.
Wrinkles, Dyspigmentation Improve with PDT, in Small Study
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
Adjuvant Chemo Beneficial in TNBC With High Immune Infiltration
TOPLINE:
These “immune-hot” patients had a 5-year DFS rate of 96.9% compared with 79.4% in the control group.
METHODOLOGY:
- In some studies, adding extended capecitabine to standard adjuvant chemotherapy has been shown to improve DFS in patients with early-stage TNBC, and one subset analysis suggested improved outcomes were most strongly associated with high immune infiltration.
- Researchers conducted a retrospective analysis of CBCSG010, a randomized phase 3 clinical trial, to identify the specific population that benefited from adjuvant capecitabine by analyzing the immune infiltration status of the tumors.
- The CBCSGO10 study of 585 patients originally found adjuvant capecitabine improved 5-year survival in patients with TNBC by 5.9%.
- This analysis included 207 patients (capecitabine arm, n = 104; control arm, n = 103) with serial formalin-fixed, paraffin-embedded tumor specimens, of which RNA sequencing data were available from 36 patients (capecitabine, n = 24; control, n = 12).
- Transcriptome data on the tumor microenvironment were validated with immunohistochemical staining of two markers, programmed death-ligand 1 (PD-L1) and CD8, as well as stromal tumor-infiltrating lymphocytes (sTILs); patients with high PD-L1, CD8, and sTIL expression levels were defined as “immune hot.”
TAKEAWAY:
- Patients with TNBC and high immune infiltration treated with capecitabine had a 5-year DFS rate of 96.9% compared with 79.4% in the control group (hazard ratio [HR], 0.13; 95% CI, 0.03-0.52; P = .049).
- In the capecitabine group, the immune-hot patients had a higher 5-year DFS rate (96.9%) compared with immune-cold patients (76.4%; HR, 0.11; 95% CI, 0.04-0.29; P = .028).
- Gene ontology analysis showed greater enrichment of immune-related pathways in patients without recurrence in the capecitabine group, as well as higher expression of TYMP, a key liver enzyme in the metabolism of capecitabine.
- High expression levels of immune biomarkers PD-L1, CD8, and sTILs were associated with significantly improved DFS in the capecitabine group.
IN PRACTICE:
“Our study suggested that immune-hot patients with TNBC are more likely to benefit from adjuvant capecitabine and that combining immunotherapy with chemotherapy may be expected to be more effective in immune-hot patients,” wrote the study authors.
SOURCE:
The study was led by Wenya Wu, MMed, and Yunsong Yang, MD, at the Department of Breast Surgery, Fudan University Shanghai Cancer Center in Shanghai, People’s Republic of China. It was published online October 2024 in JNCCN — Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature of the sample collection limited the availability of RNA sequencing data. External verification was challenging due to limited accessibility of transcriptome data from patients treated with additional adjuvant capecitabine or standard chemotherapy alone. The criteria for identifying immune-hot tumors require further exploration and determination.
DISCLOSURES:
This study was funded by the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Shanghai Science and Technology Development Foundation. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
These “immune-hot” patients had a 5-year DFS rate of 96.9% compared with 79.4% in the control group.
METHODOLOGY:
- In some studies, adding extended capecitabine to standard adjuvant chemotherapy has been shown to improve DFS in patients with early-stage TNBC, and one subset analysis suggested improved outcomes were most strongly associated with high immune infiltration.
- Researchers conducted a retrospective analysis of CBCSG010, a randomized phase 3 clinical trial, to identify the specific population that benefited from adjuvant capecitabine by analyzing the immune infiltration status of the tumors.
- The CBCSGO10 study of 585 patients originally found adjuvant capecitabine improved 5-year survival in patients with TNBC by 5.9%.
- This analysis included 207 patients (capecitabine arm, n = 104; control arm, n = 103) with serial formalin-fixed, paraffin-embedded tumor specimens, of which RNA sequencing data were available from 36 patients (capecitabine, n = 24; control, n = 12).
- Transcriptome data on the tumor microenvironment were validated with immunohistochemical staining of two markers, programmed death-ligand 1 (PD-L1) and CD8, as well as stromal tumor-infiltrating lymphocytes (sTILs); patients with high PD-L1, CD8, and sTIL expression levels were defined as “immune hot.”
TAKEAWAY:
- Patients with TNBC and high immune infiltration treated with capecitabine had a 5-year DFS rate of 96.9% compared with 79.4% in the control group (hazard ratio [HR], 0.13; 95% CI, 0.03-0.52; P = .049).
- In the capecitabine group, the immune-hot patients had a higher 5-year DFS rate (96.9%) compared with immune-cold patients (76.4%; HR, 0.11; 95% CI, 0.04-0.29; P = .028).
- Gene ontology analysis showed greater enrichment of immune-related pathways in patients without recurrence in the capecitabine group, as well as higher expression of TYMP, a key liver enzyme in the metabolism of capecitabine.
- High expression levels of immune biomarkers PD-L1, CD8, and sTILs were associated with significantly improved DFS in the capecitabine group.
IN PRACTICE:
“Our study suggested that immune-hot patients with TNBC are more likely to benefit from adjuvant capecitabine and that combining immunotherapy with chemotherapy may be expected to be more effective in immune-hot patients,” wrote the study authors.
SOURCE:
The study was led by Wenya Wu, MMed, and Yunsong Yang, MD, at the Department of Breast Surgery, Fudan University Shanghai Cancer Center in Shanghai, People’s Republic of China. It was published online October 2024 in JNCCN — Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature of the sample collection limited the availability of RNA sequencing data. External verification was challenging due to limited accessibility of transcriptome data from patients treated with additional adjuvant capecitabine or standard chemotherapy alone. The criteria for identifying immune-hot tumors require further exploration and determination.
DISCLOSURES:
This study was funded by the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Shanghai Science and Technology Development Foundation. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
These “immune-hot” patients had a 5-year DFS rate of 96.9% compared with 79.4% in the control group.
METHODOLOGY:
- In some studies, adding extended capecitabine to standard adjuvant chemotherapy has been shown to improve DFS in patients with early-stage TNBC, and one subset analysis suggested improved outcomes were most strongly associated with high immune infiltration.
- Researchers conducted a retrospective analysis of CBCSG010, a randomized phase 3 clinical trial, to identify the specific population that benefited from adjuvant capecitabine by analyzing the immune infiltration status of the tumors.
- The CBCSGO10 study of 585 patients originally found adjuvant capecitabine improved 5-year survival in patients with TNBC by 5.9%.
- This analysis included 207 patients (capecitabine arm, n = 104; control arm, n = 103) with serial formalin-fixed, paraffin-embedded tumor specimens, of which RNA sequencing data were available from 36 patients (capecitabine, n = 24; control, n = 12).
- Transcriptome data on the tumor microenvironment were validated with immunohistochemical staining of two markers, programmed death-ligand 1 (PD-L1) and CD8, as well as stromal tumor-infiltrating lymphocytes (sTILs); patients with high PD-L1, CD8, and sTIL expression levels were defined as “immune hot.”
TAKEAWAY:
- Patients with TNBC and high immune infiltration treated with capecitabine had a 5-year DFS rate of 96.9% compared with 79.4% in the control group (hazard ratio [HR], 0.13; 95% CI, 0.03-0.52; P = .049).
- In the capecitabine group, the immune-hot patients had a higher 5-year DFS rate (96.9%) compared with immune-cold patients (76.4%; HR, 0.11; 95% CI, 0.04-0.29; P = .028).
- Gene ontology analysis showed greater enrichment of immune-related pathways in patients without recurrence in the capecitabine group, as well as higher expression of TYMP, a key liver enzyme in the metabolism of capecitabine.
- High expression levels of immune biomarkers PD-L1, CD8, and sTILs were associated with significantly improved DFS in the capecitabine group.
IN PRACTICE:
“Our study suggested that immune-hot patients with TNBC are more likely to benefit from adjuvant capecitabine and that combining immunotherapy with chemotherapy may be expected to be more effective in immune-hot patients,” wrote the study authors.
SOURCE:
The study was led by Wenya Wu, MMed, and Yunsong Yang, MD, at the Department of Breast Surgery, Fudan University Shanghai Cancer Center in Shanghai, People’s Republic of China. It was published online October 2024 in JNCCN — Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature of the sample collection limited the availability of RNA sequencing data. External verification was challenging due to limited accessibility of transcriptome data from patients treated with additional adjuvant capecitabine or standard chemotherapy alone. The criteria for identifying immune-hot tumors require further exploration and determination.
DISCLOSURES:
This study was funded by the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Shanghai Science and Technology Development Foundation. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Hospital Diagnostic Errors May Affect 7% of Patients
Diagnostic errors are common in hospitals and are largely preventable, according to a new observational study led by Anuj K. Dalal, MD, from the Division of General Internal Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston, published in BMJ Quality & Safety.
Dalal and his colleagues found that 1 in 14 general medicine patients (7%) suffer harm due to diagnostic errors, and up to 85% of these cases could be prevented.
Few Studies on Diagnostic Errors
The study found that adverse event surveillance in hospital underestimated the prevalence of harmful diagnostic errors.
“It is difficult to quantify and characterize diagnostic errors, which have been studied less than medication errors,” Micaela La Regina, MD, an internist and head of the Clinical Governance and Risk Management Unit at ASL 5 in La Spezia, Italy, told Univadis Italy. “Generally, it is estimated that around 50% of diagnostic errors are preventable, but the authors of this study went beyond simply observing the hospital admission period and followed their sample for 90 days after discharge. Their findings will need to be verified in other studies, but they seem convincing.”
The researchers in Boston selected a random sample of 675 hospital patients from a total of 9147 eligible cases who received general medical care between July 2019 and September 2021, excluding the peak of the COVID-19 pandemic (April-December 2020). They retrospectively reviewed the patients’ electronic health records using a structured method to evaluate the diagnostic process for potential errors and then estimated the impact and severity of any harm.
Cases sampled were those featuring transfer to intensive care more than 24 hours after admission (100% of 130 cases), death within 90 days of hospital admission or after discharge (38.5% of 141 cases), complex clinical problems without transfer to intensive care or death within 90 days of admission (7% of 298 cases), and 2.4% of 106 cases without high-risk criteria.
Each case was reviewed by two experts trained in the use of diagnostic error evaluation and research taxonomy, modified for acute care. Harm was classified as mild, moderate, severe, or fatal. The review assessed whether diagnostic error contributed to the harm and whether it was preventable. Cases with discrepancies or uncertainties regarding the diagnostic error or its impact were further examined by an expert panel.
Most Frequent Situations
Among all the cases examined, diagnostic errors were identified in 160 instances in 154 patients. The most frequent situations with diagnostic errors involved transfer to intensive care (54 cases), death within 90 days (34 cases), and complex clinical problems (52 cases). Diagnostic errors causing harm were found in 84 cases (82 patients), of which 37 (28.5%) occurred in those transferred to intensive care; 18 (13%) among patients who died within 90 days; 23 (8%) among patients with complex clinical issues; and 6 (6%) in low-risk cases.
The severity of harm was categorized as minor in 5 cases (6%), moderate in 36 (43%), major in 25 (30%), and fatal in 18 cases (21.5%). Overall, the researchers estimated that the proportion of harmful, preventable diagnostic errors with serious harm in general medicine patients was slightly more than 7%, 6%, and 1%, respectively.
Most Frequent Diagnoses
The most common diagnoses associated with diagnostic errors in the study included heart failure, acute kidney injury, sepsis, pneumonia, respiratory failure, altered mental state, abdominal pain, and hypoxemia. Dalal and colleagues emphasize the need for more attention to diagnostic error analysis, including the adoption of artificial intelligence–based tools for medical record screening.
“The technological approach, with alert-based systems, can certainly be helpful, but more attention must also be paid to continuous training and the well-being of healthcare workers. It is also crucial to encourage greater listening to caregivers and patients,” said La Regina. She noted that in the past, a focus on error prevention has often led to an increased workload and administrative burden on healthcare workers. However, the well-being of healthcare workers is key to ensuring patient safety.
“Countermeasures to reduce diagnostic errors require a multimodal approach, targeting professionals, the healthcare system, and organizational aspects, because even waiting lists are a critical factor,” she said. As a clinical risk expert, she recently proposed an adaptation of the value-based medicine formula in the International Journal for Quality in Health Care to include healthcare professionals’ care experience as one of the elements that contribute to determining high-value healthcare interventions. “Experiments are already underway to reimburse healthcare costs based on this formula, which also allows the assessment of the value of skills and expertise acquired by healthcare workers,” concluded La Regina.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Diagnostic errors are common in hospitals and are largely preventable, according to a new observational study led by Anuj K. Dalal, MD, from the Division of General Internal Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston, published in BMJ Quality & Safety.
Dalal and his colleagues found that 1 in 14 general medicine patients (7%) suffer harm due to diagnostic errors, and up to 85% of these cases could be prevented.
Few Studies on Diagnostic Errors
The study found that adverse event surveillance in hospital underestimated the prevalence of harmful diagnostic errors.
“It is difficult to quantify and characterize diagnostic errors, which have been studied less than medication errors,” Micaela La Regina, MD, an internist and head of the Clinical Governance and Risk Management Unit at ASL 5 in La Spezia, Italy, told Univadis Italy. “Generally, it is estimated that around 50% of diagnostic errors are preventable, but the authors of this study went beyond simply observing the hospital admission period and followed their sample for 90 days after discharge. Their findings will need to be verified in other studies, but they seem convincing.”
The researchers in Boston selected a random sample of 675 hospital patients from a total of 9147 eligible cases who received general medical care between July 2019 and September 2021, excluding the peak of the COVID-19 pandemic (April-December 2020). They retrospectively reviewed the patients’ electronic health records using a structured method to evaluate the diagnostic process for potential errors and then estimated the impact and severity of any harm.
Cases sampled were those featuring transfer to intensive care more than 24 hours after admission (100% of 130 cases), death within 90 days of hospital admission or after discharge (38.5% of 141 cases), complex clinical problems without transfer to intensive care or death within 90 days of admission (7% of 298 cases), and 2.4% of 106 cases without high-risk criteria.
Each case was reviewed by two experts trained in the use of diagnostic error evaluation and research taxonomy, modified for acute care. Harm was classified as mild, moderate, severe, or fatal. The review assessed whether diagnostic error contributed to the harm and whether it was preventable. Cases with discrepancies or uncertainties regarding the diagnostic error or its impact were further examined by an expert panel.
Most Frequent Situations
Among all the cases examined, diagnostic errors were identified in 160 instances in 154 patients. The most frequent situations with diagnostic errors involved transfer to intensive care (54 cases), death within 90 days (34 cases), and complex clinical problems (52 cases). Diagnostic errors causing harm were found in 84 cases (82 patients), of which 37 (28.5%) occurred in those transferred to intensive care; 18 (13%) among patients who died within 90 days; 23 (8%) among patients with complex clinical issues; and 6 (6%) in low-risk cases.
The severity of harm was categorized as minor in 5 cases (6%), moderate in 36 (43%), major in 25 (30%), and fatal in 18 cases (21.5%). Overall, the researchers estimated that the proportion of harmful, preventable diagnostic errors with serious harm in general medicine patients was slightly more than 7%, 6%, and 1%, respectively.
Most Frequent Diagnoses
The most common diagnoses associated with diagnostic errors in the study included heart failure, acute kidney injury, sepsis, pneumonia, respiratory failure, altered mental state, abdominal pain, and hypoxemia. Dalal and colleagues emphasize the need for more attention to diagnostic error analysis, including the adoption of artificial intelligence–based tools for medical record screening.
“The technological approach, with alert-based systems, can certainly be helpful, but more attention must also be paid to continuous training and the well-being of healthcare workers. It is also crucial to encourage greater listening to caregivers and patients,” said La Regina. She noted that in the past, a focus on error prevention has often led to an increased workload and administrative burden on healthcare workers. However, the well-being of healthcare workers is key to ensuring patient safety.
“Countermeasures to reduce diagnostic errors require a multimodal approach, targeting professionals, the healthcare system, and organizational aspects, because even waiting lists are a critical factor,” she said. As a clinical risk expert, she recently proposed an adaptation of the value-based medicine formula in the International Journal for Quality in Health Care to include healthcare professionals’ care experience as one of the elements that contribute to determining high-value healthcare interventions. “Experiments are already underway to reimburse healthcare costs based on this formula, which also allows the assessment of the value of skills and expertise acquired by healthcare workers,” concluded La Regina.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Diagnostic errors are common in hospitals and are largely preventable, according to a new observational study led by Anuj K. Dalal, MD, from the Division of General Internal Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston, published in BMJ Quality & Safety.
Dalal and his colleagues found that 1 in 14 general medicine patients (7%) suffer harm due to diagnostic errors, and up to 85% of these cases could be prevented.
Few Studies on Diagnostic Errors
The study found that adverse event surveillance in hospital underestimated the prevalence of harmful diagnostic errors.
“It is difficult to quantify and characterize diagnostic errors, which have been studied less than medication errors,” Micaela La Regina, MD, an internist and head of the Clinical Governance and Risk Management Unit at ASL 5 in La Spezia, Italy, told Univadis Italy. “Generally, it is estimated that around 50% of diagnostic errors are preventable, but the authors of this study went beyond simply observing the hospital admission period and followed their sample for 90 days after discharge. Their findings will need to be verified in other studies, but they seem convincing.”
The researchers in Boston selected a random sample of 675 hospital patients from a total of 9147 eligible cases who received general medical care between July 2019 and September 2021, excluding the peak of the COVID-19 pandemic (April-December 2020). They retrospectively reviewed the patients’ electronic health records using a structured method to evaluate the diagnostic process for potential errors and then estimated the impact and severity of any harm.
Cases sampled were those featuring transfer to intensive care more than 24 hours after admission (100% of 130 cases), death within 90 days of hospital admission or after discharge (38.5% of 141 cases), complex clinical problems without transfer to intensive care or death within 90 days of admission (7% of 298 cases), and 2.4% of 106 cases without high-risk criteria.
Each case was reviewed by two experts trained in the use of diagnostic error evaluation and research taxonomy, modified for acute care. Harm was classified as mild, moderate, severe, or fatal. The review assessed whether diagnostic error contributed to the harm and whether it was preventable. Cases with discrepancies or uncertainties regarding the diagnostic error or its impact were further examined by an expert panel.
Most Frequent Situations
Among all the cases examined, diagnostic errors were identified in 160 instances in 154 patients. The most frequent situations with diagnostic errors involved transfer to intensive care (54 cases), death within 90 days (34 cases), and complex clinical problems (52 cases). Diagnostic errors causing harm were found in 84 cases (82 patients), of which 37 (28.5%) occurred in those transferred to intensive care; 18 (13%) among patients who died within 90 days; 23 (8%) among patients with complex clinical issues; and 6 (6%) in low-risk cases.
The severity of harm was categorized as minor in 5 cases (6%), moderate in 36 (43%), major in 25 (30%), and fatal in 18 cases (21.5%). Overall, the researchers estimated that the proportion of harmful, preventable diagnostic errors with serious harm in general medicine patients was slightly more than 7%, 6%, and 1%, respectively.
Most Frequent Diagnoses
The most common diagnoses associated with diagnostic errors in the study included heart failure, acute kidney injury, sepsis, pneumonia, respiratory failure, altered mental state, abdominal pain, and hypoxemia. Dalal and colleagues emphasize the need for more attention to diagnostic error analysis, including the adoption of artificial intelligence–based tools for medical record screening.
“The technological approach, with alert-based systems, can certainly be helpful, but more attention must also be paid to continuous training and the well-being of healthcare workers. It is also crucial to encourage greater listening to caregivers and patients,” said La Regina. She noted that in the past, a focus on error prevention has often led to an increased workload and administrative burden on healthcare workers. However, the well-being of healthcare workers is key to ensuring patient safety.
“Countermeasures to reduce diagnostic errors require a multimodal approach, targeting professionals, the healthcare system, and organizational aspects, because even waiting lists are a critical factor,” she said. As a clinical risk expert, she recently proposed an adaptation of the value-based medicine formula in the International Journal for Quality in Health Care to include healthcare professionals’ care experience as one of the elements that contribute to determining high-value healthcare interventions. “Experiments are already underway to reimburse healthcare costs based on this formula, which also allows the assessment of the value of skills and expertise acquired by healthcare workers,” concluded La Regina.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How Effective Is the High-Dose Flu Vaccine in Older Adults?
How can the immunogenicity and effectiveness of flu vaccines be improved in older adults? Several strategies are available, one being the addition of an adjuvant. For example, the MF59-adjuvanted vaccine has shown superior immunogenicity. However, “we do not have data from controlled and randomized clinical trials showing superior clinical effectiveness versus the standard dose,” Professor Odile Launay, an infectious disease specialist at Cochin Hospital in Paris, France, noted during a press conference. Another option is to increase the antigen dose in the vaccine, creating a high-dose (HD) flu vaccine.
Why is there a need for an HD vaccine? “The elderly population bears the greatest burden from the flu,” explained Launay. “This is due to three factors: An aging immune system, a higher number of comorbidities, and increased frailty.” Standard-dose flu vaccines are seen as offering suboptimal protection for those older than 65 years, which led to the development of a quadrivalent vaccine with four times the antigen dose of standard flu vaccines. This HD vaccine was introduced in France during the 2021/2022 flu season. A real-world cohort study has since been conducted to evaluate its effectiveness in the target population — those aged 65 years or older. The results were recently published in Clinical Microbiology and Infection.
Cohort Study
The study included 405,385 noninstitutionalized people aged 65 years or older matched with 1,621,540 individuals in a 1:4 ratio. The first group received the HD vaccine, while the second group received the standard-dose vaccine. Both the groups had an average age of 77 years, with 56% women, and 51% vaccinated in pharmacies. The majority had been previously vaccinated against flu (91%), and 97% had completed a full COVID-19 vaccination schedule. More than half had at least one chronic illness.
Hospitalization rates for flu — the study’s primary outcome — were 69.5 vs 90.5 per 100,000 person-years in the HD vs standard-dose group. This represented a 23.3% reduction (95% CI, 8.4-35.8; P = .003).
Strengths and Limitations
Among the strengths of the study, Launay highlighted the large number of vaccinated participants older than 65 years — more than 7 million — and the widespread use of polymerase chain reaction flu tests in cases of hospitalization for respiratory infections, which improved flu coding in the database used. Additionally, the results were consistent with those of previous studies.
However, limitations included the retrospective design, which did not randomize participants and introduced potential bias. For example, the HD vaccine may have been prioritized for the oldest people or those with multiple comorbidities. Additionally, the 2021/2022 flu season was atypical, with the simultaneous circulation of the flu virus and SARS-CoV-2, as noted by Launay.
Conclusion
In conclusion, this first evaluation of the HD flu vaccine’s effectiveness in France showed a 25% reduction in hospitalizations, consistent with existing data covering 12 flu seasons. The vaccine has been available for a longer period in the United States and Northern Europe.
“The latest unpublished data from the 2022/23 season show a 27% reduction in hospitalizations with the HD vaccine in people over 65,” added Launay.
Note: Due to a pricing disagreement with the French government, Sanofi’s HD flu vaccine Efluelda, intended for people older than 65 years, will not be available this year. (See: Withdrawal of the Efluelda Influenza Vaccine: The Academy of Medicine Reacts). However, the company has submitted a dossier for a trivalent form for a return in the 2025/2026 season and is working on developing mRNA vaccines. Additionally, a combined flu/COVID-19 vaccine is currently in development.
The study was funded by Sanofi. Several authors are Sanofi employees. Odile Launay reported conflicts of interest with Sanofi, MSD, Pfizer, GSK, and Moderna.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How can the immunogenicity and effectiveness of flu vaccines be improved in older adults? Several strategies are available, one being the addition of an adjuvant. For example, the MF59-adjuvanted vaccine has shown superior immunogenicity. However, “we do not have data from controlled and randomized clinical trials showing superior clinical effectiveness versus the standard dose,” Professor Odile Launay, an infectious disease specialist at Cochin Hospital in Paris, France, noted during a press conference. Another option is to increase the antigen dose in the vaccine, creating a high-dose (HD) flu vaccine.
Why is there a need for an HD vaccine? “The elderly population bears the greatest burden from the flu,” explained Launay. “This is due to three factors: An aging immune system, a higher number of comorbidities, and increased frailty.” Standard-dose flu vaccines are seen as offering suboptimal protection for those older than 65 years, which led to the development of a quadrivalent vaccine with four times the antigen dose of standard flu vaccines. This HD vaccine was introduced in France during the 2021/2022 flu season. A real-world cohort study has since been conducted to evaluate its effectiveness in the target population — those aged 65 years or older. The results were recently published in Clinical Microbiology and Infection.
Cohort Study
The study included 405,385 noninstitutionalized people aged 65 years or older matched with 1,621,540 individuals in a 1:4 ratio. The first group received the HD vaccine, while the second group received the standard-dose vaccine. Both the groups had an average age of 77 years, with 56% women, and 51% vaccinated in pharmacies. The majority had been previously vaccinated against flu (91%), and 97% had completed a full COVID-19 vaccination schedule. More than half had at least one chronic illness.
Hospitalization rates for flu — the study’s primary outcome — were 69.5 vs 90.5 per 100,000 person-years in the HD vs standard-dose group. This represented a 23.3% reduction (95% CI, 8.4-35.8; P = .003).
Strengths and Limitations
Among the strengths of the study, Launay highlighted the large number of vaccinated participants older than 65 years — more than 7 million — and the widespread use of polymerase chain reaction flu tests in cases of hospitalization for respiratory infections, which improved flu coding in the database used. Additionally, the results were consistent with those of previous studies.
However, limitations included the retrospective design, which did not randomize participants and introduced potential bias. For example, the HD vaccine may have been prioritized for the oldest people or those with multiple comorbidities. Additionally, the 2021/2022 flu season was atypical, with the simultaneous circulation of the flu virus and SARS-CoV-2, as noted by Launay.
Conclusion
In conclusion, this first evaluation of the HD flu vaccine’s effectiveness in France showed a 25% reduction in hospitalizations, consistent with existing data covering 12 flu seasons. The vaccine has been available for a longer period in the United States and Northern Europe.
“The latest unpublished data from the 2022/23 season show a 27% reduction in hospitalizations with the HD vaccine in people over 65,” added Launay.
Note: Due to a pricing disagreement with the French government, Sanofi’s HD flu vaccine Efluelda, intended for people older than 65 years, will not be available this year. (See: Withdrawal of the Efluelda Influenza Vaccine: The Academy of Medicine Reacts). However, the company has submitted a dossier for a trivalent form for a return in the 2025/2026 season and is working on developing mRNA vaccines. Additionally, a combined flu/COVID-19 vaccine is currently in development.
The study was funded by Sanofi. Several authors are Sanofi employees. Odile Launay reported conflicts of interest with Sanofi, MSD, Pfizer, GSK, and Moderna.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How can the immunogenicity and effectiveness of flu vaccines be improved in older adults? Several strategies are available, one being the addition of an adjuvant. For example, the MF59-adjuvanted vaccine has shown superior immunogenicity. However, “we do not have data from controlled and randomized clinical trials showing superior clinical effectiveness versus the standard dose,” Professor Odile Launay, an infectious disease specialist at Cochin Hospital in Paris, France, noted during a press conference. Another option is to increase the antigen dose in the vaccine, creating a high-dose (HD) flu vaccine.
Why is there a need for an HD vaccine? “The elderly population bears the greatest burden from the flu,” explained Launay. “This is due to three factors: An aging immune system, a higher number of comorbidities, and increased frailty.” Standard-dose flu vaccines are seen as offering suboptimal protection for those older than 65 years, which led to the development of a quadrivalent vaccine with four times the antigen dose of standard flu vaccines. This HD vaccine was introduced in France during the 2021/2022 flu season. A real-world cohort study has since been conducted to evaluate its effectiveness in the target population — those aged 65 years or older. The results were recently published in Clinical Microbiology and Infection.
Cohort Study
The study included 405,385 noninstitutionalized people aged 65 years or older matched with 1,621,540 individuals in a 1:4 ratio. The first group received the HD vaccine, while the second group received the standard-dose vaccine. Both the groups had an average age of 77 years, with 56% women, and 51% vaccinated in pharmacies. The majority had been previously vaccinated against flu (91%), and 97% had completed a full COVID-19 vaccination schedule. More than half had at least one chronic illness.
Hospitalization rates for flu — the study’s primary outcome — were 69.5 vs 90.5 per 100,000 person-years in the HD vs standard-dose group. This represented a 23.3% reduction (95% CI, 8.4-35.8; P = .003).
Strengths and Limitations
Among the strengths of the study, Launay highlighted the large number of vaccinated participants older than 65 years — more than 7 million — and the widespread use of polymerase chain reaction flu tests in cases of hospitalization for respiratory infections, which improved flu coding in the database used. Additionally, the results were consistent with those of previous studies.
However, limitations included the retrospective design, which did not randomize participants and introduced potential bias. For example, the HD vaccine may have been prioritized for the oldest people or those with multiple comorbidities. Additionally, the 2021/2022 flu season was atypical, with the simultaneous circulation of the flu virus and SARS-CoV-2, as noted by Launay.
Conclusion
In conclusion, this first evaluation of the HD flu vaccine’s effectiveness in France showed a 25% reduction in hospitalizations, consistent with existing data covering 12 flu seasons. The vaccine has been available for a longer period in the United States and Northern Europe.
“The latest unpublished data from the 2022/23 season show a 27% reduction in hospitalizations with the HD vaccine in people over 65,” added Launay.
Note: Due to a pricing disagreement with the French government, Sanofi’s HD flu vaccine Efluelda, intended for people older than 65 years, will not be available this year. (See: Withdrawal of the Efluelda Influenza Vaccine: The Academy of Medicine Reacts). However, the company has submitted a dossier for a trivalent form for a return in the 2025/2026 season and is working on developing mRNA vaccines. Additionally, a combined flu/COVID-19 vaccine is currently in development.
The study was funded by Sanofi. Several authors are Sanofi employees. Odile Launay reported conflicts of interest with Sanofi, MSD, Pfizer, GSK, and Moderna.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Responses Sustained with Ritlecitinib in Patients with Alopecia Through 48 Weeks
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.