User login
PPI-Responsive Disease a Subtype of EoE Rather Than GERD
, according to comparative proteomic analyses.
Notably, after PPI therapy, the protein profiles of responsive patients reverted and appeared similar to non-EoE patients, whereas the profiles of nonresponsive patients remained largely unchanged.
“Identifying protein biomarkers associated with PPI response may help distinguish EoE phenotypes and guide therapy selections,” said senior author Walter Chan, MD, AGAF, associate professor of medicine in the Division of Gastroenterology, Hepatology, and Endoscopy at Harvard Medical School and director of the center for gastrointestinal motility at Brigham and Women’s Hospital, Boston.
“These findings may provide the framework for developing protein biomarkers to assess response to therapy and monitor disease activity,” he added.
The study was published online in Gastroenterology.
Comparative Proteomic Analyses
Chan and colleagues conducted a prospective exploratory pilot study to identify the differences in esophageal protein profiles among PPI-responsive-EoE (PPI-R-EoE), PPI-nonresponsive-EoE (PPI-NR-EoE), and non-EoE controls using SOMAscan, a proteomics platform that allows simultaneous detection of 1305 human proteins.
The research team prospectively enrolled patients undergoing endoscopy for esophageal symptoms or for EoE follow-up, obtaining clinically indicated biopsies as well as extra samples from the midesophagus.
Patients who were diagnosed with EoE (at 15 or greater eosinophils per high-power field, or eos/hpf) were treated with 20 mg of omeprazole twice daily for 8 weeks, followed by repeat biopsies to assess treatment response.
Patients with histologic remission (fewer than 15 eos/hpf) were classified as PPI-R-EoE, whereas those with persistently active disease were classified as PPI-NR-EoE. Patients without EoE served as controls and were categorized as having erosive esophagitis (EE) or no esophagitis.
Overall, the study enrolled 32 patients, including 15 with PPI-R-EoE, eight with PPI-NR-EoE, three with EE, and six with no esophagitis. The demographics, symptoms, and endoscopic findings were similar between the PPI-R-EoE and PPI-NR-EoE patients.
At the index endoscopy, the PPI-R-EoE and PPI-NR-EoE patients had similar esophageal protein profiles, with only 20 proteins differentially expressed at a relaxed cutoff of P < .1. An analysis of the 20 proteins predicted lower expression of six proteins that may be associated with gastrointestinal inflammation in nonresponsive patients, including STAT1, STAT3, CFB, interleukin (IL)-17RA, TNFRSF1A, and SERPINA3.
In addition, 136 proteins — including 15 with corrected P < .05 — clearly discriminated PPI-R-EoE patients from non-EoE controls, and 255 proteins — including 249 with P < .05 — discriminated PPI-NR-EoE patients from controls. Both types of EoE patients had proteins associated with enhanced inflammation and vasculogenesis, as well as down-regulation of CRISP3 and DSG1 and upregulation of TNFAIP6.
The comparative analyses also showed that the follow-up biopsies of PPI-R-EoE patients had protein profiles that resembled non-EoE controls after PPI therapy.
“This further supports the hypothesis that despite the PPI response, PPI-R-EoE represents a subtype of EoE rather than gastroesophageal reflux disease (GERD),” Chan said.
Future EoE Considerations
Although most expressed proteins appeared similar between PPI-responsive and nonresponsive patients before treatment, a few proteins differed related to gastrointestinal inflammation, the study authors wrote, including some previously implicated in IL4 and IL13 inflammatory pathways.
“Further study of these proteins may provide insights into the EoE pathogenic pathway, explore their potential to predict PPI response at diagnosis, and identify possible therapeutic targets,” they wrote.
The authors pointed to the small study size as the primary limitation, noting that the pilot study was intended to explore the feasibility of using SomaScan to assess esophageal protein profiles in different EoE phenotypes. In the future, larger studies with more expansive candidate proteins could help characterize the differences and better identify specific proteins and pathways in EoE, they wrote.
“The takeaway is that PPI responsiveness does not distinguish EoE from GERD but rather PPI is a primary therapy for EoE independent of GERD,” said Marc Rothenberg, MD, director of allergy and immunology and director of the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Rothenberg, who wasn’t involved with this study, has conducted transcriptome analyses of PPI-R-EoE, which showed PPI-reversible allergic inflammation.
“PPI-R-EoE and PPI-NR-EoE look the same at the molecular level,” he said. “After therapy, PPI-R-EoE normalizes, as per its definition.”
This study was supported by the Campaign Urging Research for Eosinophilic Disease Foundation Grant, the Kenneth and Louise Goldberg Junior Faculty Award, and a National Institutes of Health award. Chan declared advisory board positions with several pharmaceutical companies and Rothenberg reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to comparative proteomic analyses.
Notably, after PPI therapy, the protein profiles of responsive patients reverted and appeared similar to non-EoE patients, whereas the profiles of nonresponsive patients remained largely unchanged.
“Identifying protein biomarkers associated with PPI response may help distinguish EoE phenotypes and guide therapy selections,” said senior author Walter Chan, MD, AGAF, associate professor of medicine in the Division of Gastroenterology, Hepatology, and Endoscopy at Harvard Medical School and director of the center for gastrointestinal motility at Brigham and Women’s Hospital, Boston.
“These findings may provide the framework for developing protein biomarkers to assess response to therapy and monitor disease activity,” he added.
The study was published online in Gastroenterology.
Comparative Proteomic Analyses
Chan and colleagues conducted a prospective exploratory pilot study to identify the differences in esophageal protein profiles among PPI-responsive-EoE (PPI-R-EoE), PPI-nonresponsive-EoE (PPI-NR-EoE), and non-EoE controls using SOMAscan, a proteomics platform that allows simultaneous detection of 1305 human proteins.
The research team prospectively enrolled patients undergoing endoscopy for esophageal symptoms or for EoE follow-up, obtaining clinically indicated biopsies as well as extra samples from the midesophagus.
Patients who were diagnosed with EoE (at 15 or greater eosinophils per high-power field, or eos/hpf) were treated with 20 mg of omeprazole twice daily for 8 weeks, followed by repeat biopsies to assess treatment response.
Patients with histologic remission (fewer than 15 eos/hpf) were classified as PPI-R-EoE, whereas those with persistently active disease were classified as PPI-NR-EoE. Patients without EoE served as controls and were categorized as having erosive esophagitis (EE) or no esophagitis.
Overall, the study enrolled 32 patients, including 15 with PPI-R-EoE, eight with PPI-NR-EoE, three with EE, and six with no esophagitis. The demographics, symptoms, and endoscopic findings were similar between the PPI-R-EoE and PPI-NR-EoE patients.
At the index endoscopy, the PPI-R-EoE and PPI-NR-EoE patients had similar esophageal protein profiles, with only 20 proteins differentially expressed at a relaxed cutoff of P < .1. An analysis of the 20 proteins predicted lower expression of six proteins that may be associated with gastrointestinal inflammation in nonresponsive patients, including STAT1, STAT3, CFB, interleukin (IL)-17RA, TNFRSF1A, and SERPINA3.
In addition, 136 proteins — including 15 with corrected P < .05 — clearly discriminated PPI-R-EoE patients from non-EoE controls, and 255 proteins — including 249 with P < .05 — discriminated PPI-NR-EoE patients from controls. Both types of EoE patients had proteins associated with enhanced inflammation and vasculogenesis, as well as down-regulation of CRISP3 and DSG1 and upregulation of TNFAIP6.
The comparative analyses also showed that the follow-up biopsies of PPI-R-EoE patients had protein profiles that resembled non-EoE controls after PPI therapy.
“This further supports the hypothesis that despite the PPI response, PPI-R-EoE represents a subtype of EoE rather than gastroesophageal reflux disease (GERD),” Chan said.
Future EoE Considerations
Although most expressed proteins appeared similar between PPI-responsive and nonresponsive patients before treatment, a few proteins differed related to gastrointestinal inflammation, the study authors wrote, including some previously implicated in IL4 and IL13 inflammatory pathways.
“Further study of these proteins may provide insights into the EoE pathogenic pathway, explore their potential to predict PPI response at diagnosis, and identify possible therapeutic targets,” they wrote.
The authors pointed to the small study size as the primary limitation, noting that the pilot study was intended to explore the feasibility of using SomaScan to assess esophageal protein profiles in different EoE phenotypes. In the future, larger studies with more expansive candidate proteins could help characterize the differences and better identify specific proteins and pathways in EoE, they wrote.
“The takeaway is that PPI responsiveness does not distinguish EoE from GERD but rather PPI is a primary therapy for EoE independent of GERD,” said Marc Rothenberg, MD, director of allergy and immunology and director of the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Rothenberg, who wasn’t involved with this study, has conducted transcriptome analyses of PPI-R-EoE, which showed PPI-reversible allergic inflammation.
“PPI-R-EoE and PPI-NR-EoE look the same at the molecular level,” he said. “After therapy, PPI-R-EoE normalizes, as per its definition.”
This study was supported by the Campaign Urging Research for Eosinophilic Disease Foundation Grant, the Kenneth and Louise Goldberg Junior Faculty Award, and a National Institutes of Health award. Chan declared advisory board positions with several pharmaceutical companies and Rothenberg reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to comparative proteomic analyses.
Notably, after PPI therapy, the protein profiles of responsive patients reverted and appeared similar to non-EoE patients, whereas the profiles of nonresponsive patients remained largely unchanged.
“Identifying protein biomarkers associated with PPI response may help distinguish EoE phenotypes and guide therapy selections,” said senior author Walter Chan, MD, AGAF, associate professor of medicine in the Division of Gastroenterology, Hepatology, and Endoscopy at Harvard Medical School and director of the center for gastrointestinal motility at Brigham and Women’s Hospital, Boston.
“These findings may provide the framework for developing protein biomarkers to assess response to therapy and monitor disease activity,” he added.
The study was published online in Gastroenterology.
Comparative Proteomic Analyses
Chan and colleagues conducted a prospective exploratory pilot study to identify the differences in esophageal protein profiles among PPI-responsive-EoE (PPI-R-EoE), PPI-nonresponsive-EoE (PPI-NR-EoE), and non-EoE controls using SOMAscan, a proteomics platform that allows simultaneous detection of 1305 human proteins.
The research team prospectively enrolled patients undergoing endoscopy for esophageal symptoms or for EoE follow-up, obtaining clinically indicated biopsies as well as extra samples from the midesophagus.
Patients who were diagnosed with EoE (at 15 or greater eosinophils per high-power field, or eos/hpf) were treated with 20 mg of omeprazole twice daily for 8 weeks, followed by repeat biopsies to assess treatment response.
Patients with histologic remission (fewer than 15 eos/hpf) were classified as PPI-R-EoE, whereas those with persistently active disease were classified as PPI-NR-EoE. Patients without EoE served as controls and were categorized as having erosive esophagitis (EE) or no esophagitis.
Overall, the study enrolled 32 patients, including 15 with PPI-R-EoE, eight with PPI-NR-EoE, three with EE, and six with no esophagitis. The demographics, symptoms, and endoscopic findings were similar between the PPI-R-EoE and PPI-NR-EoE patients.
At the index endoscopy, the PPI-R-EoE and PPI-NR-EoE patients had similar esophageal protein profiles, with only 20 proteins differentially expressed at a relaxed cutoff of P < .1. An analysis of the 20 proteins predicted lower expression of six proteins that may be associated with gastrointestinal inflammation in nonresponsive patients, including STAT1, STAT3, CFB, interleukin (IL)-17RA, TNFRSF1A, and SERPINA3.
In addition, 136 proteins — including 15 with corrected P < .05 — clearly discriminated PPI-R-EoE patients from non-EoE controls, and 255 proteins — including 249 with P < .05 — discriminated PPI-NR-EoE patients from controls. Both types of EoE patients had proteins associated with enhanced inflammation and vasculogenesis, as well as down-regulation of CRISP3 and DSG1 and upregulation of TNFAIP6.
The comparative analyses also showed that the follow-up biopsies of PPI-R-EoE patients had protein profiles that resembled non-EoE controls after PPI therapy.
“This further supports the hypothesis that despite the PPI response, PPI-R-EoE represents a subtype of EoE rather than gastroesophageal reflux disease (GERD),” Chan said.
Future EoE Considerations
Although most expressed proteins appeared similar between PPI-responsive and nonresponsive patients before treatment, a few proteins differed related to gastrointestinal inflammation, the study authors wrote, including some previously implicated in IL4 and IL13 inflammatory pathways.
“Further study of these proteins may provide insights into the EoE pathogenic pathway, explore their potential to predict PPI response at diagnosis, and identify possible therapeutic targets,” they wrote.
The authors pointed to the small study size as the primary limitation, noting that the pilot study was intended to explore the feasibility of using SomaScan to assess esophageal protein profiles in different EoE phenotypes. In the future, larger studies with more expansive candidate proteins could help characterize the differences and better identify specific proteins and pathways in EoE, they wrote.
“The takeaway is that PPI responsiveness does not distinguish EoE from GERD but rather PPI is a primary therapy for EoE independent of GERD,” said Marc Rothenberg, MD, director of allergy and immunology and director of the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Rothenberg, who wasn’t involved with this study, has conducted transcriptome analyses of PPI-R-EoE, which showed PPI-reversible allergic inflammation.
“PPI-R-EoE and PPI-NR-EoE look the same at the molecular level,” he said. “After therapy, PPI-R-EoE normalizes, as per its definition.”
This study was supported by the Campaign Urging Research for Eosinophilic Disease Foundation Grant, the Kenneth and Louise Goldberg Junior Faculty Award, and a National Institutes of Health award. Chan declared advisory board positions with several pharmaceutical companies and Rothenberg reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Tapering Corticosteroids in Severe Alcohol-Associated Hepatitis Appears Safe
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
FROM AASLD 2024
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
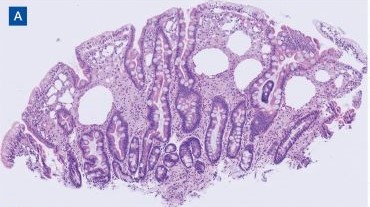
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
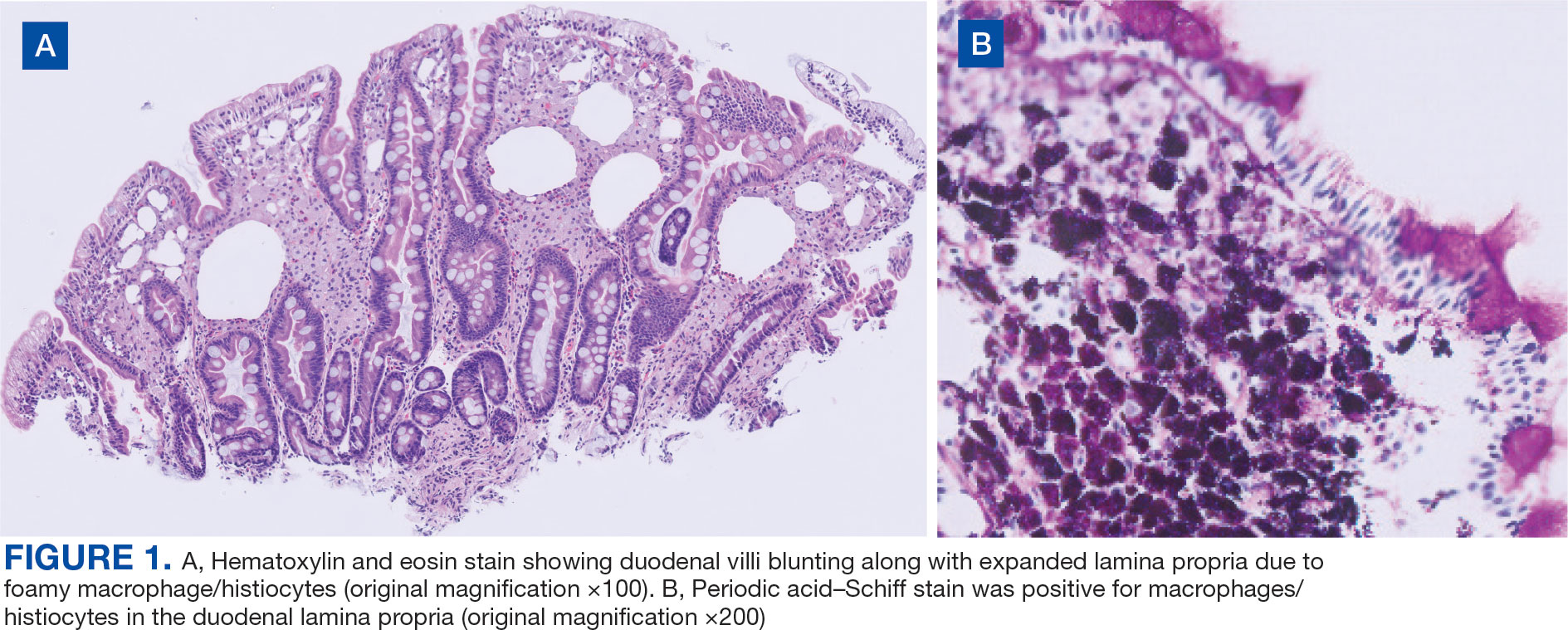
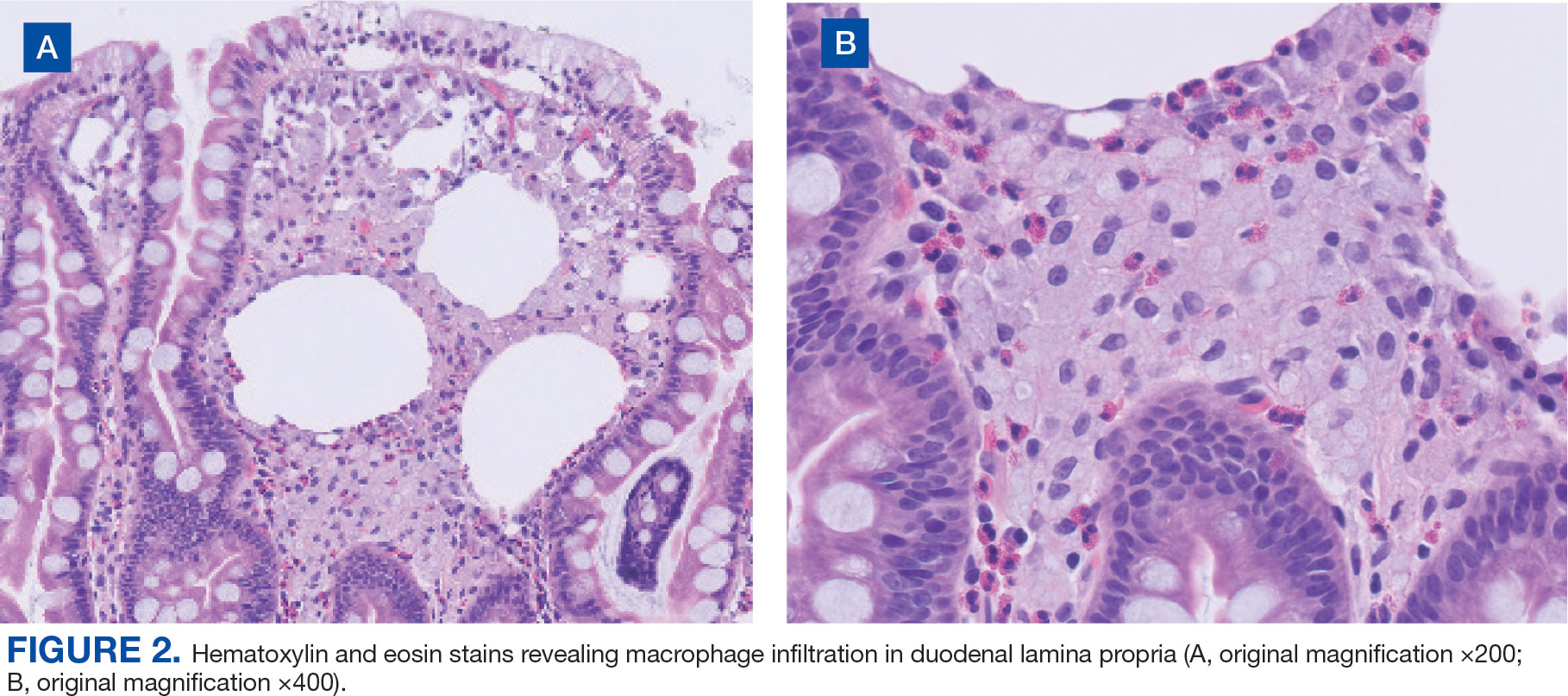
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10
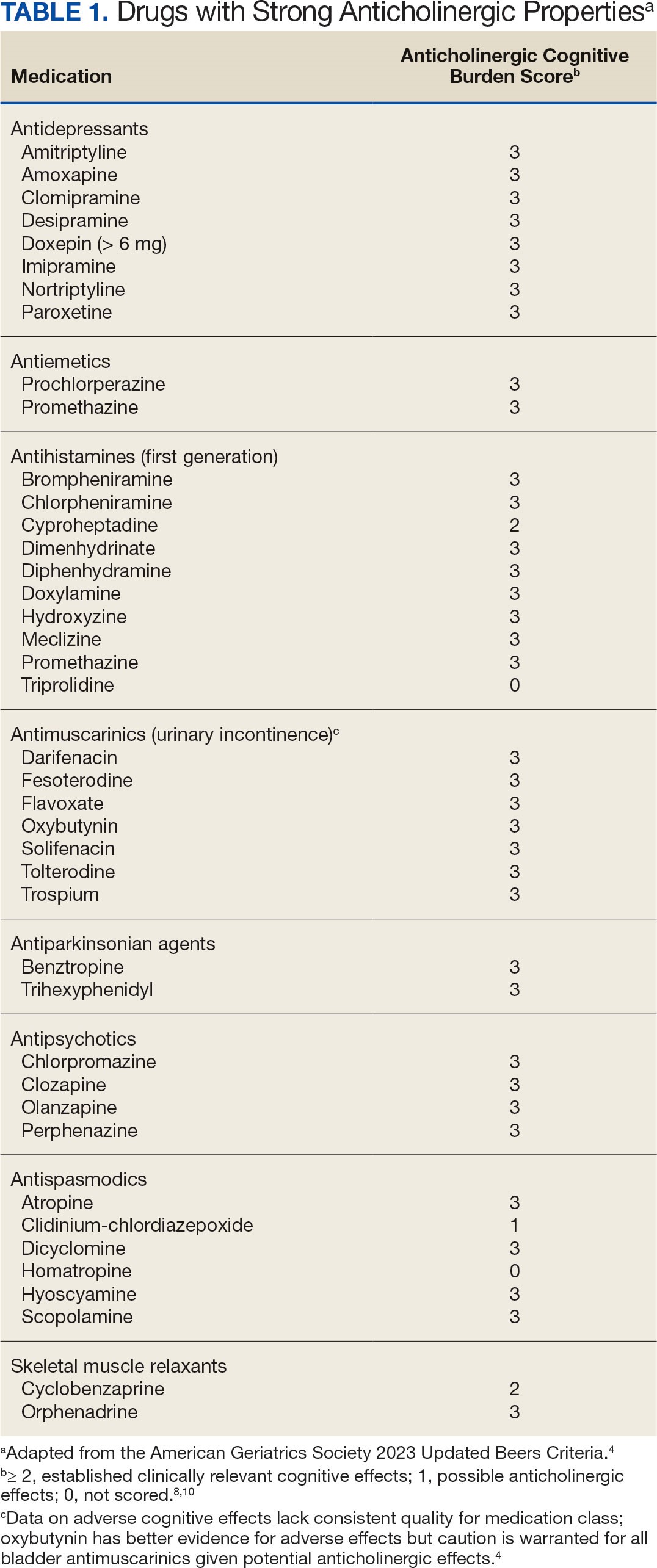
Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.
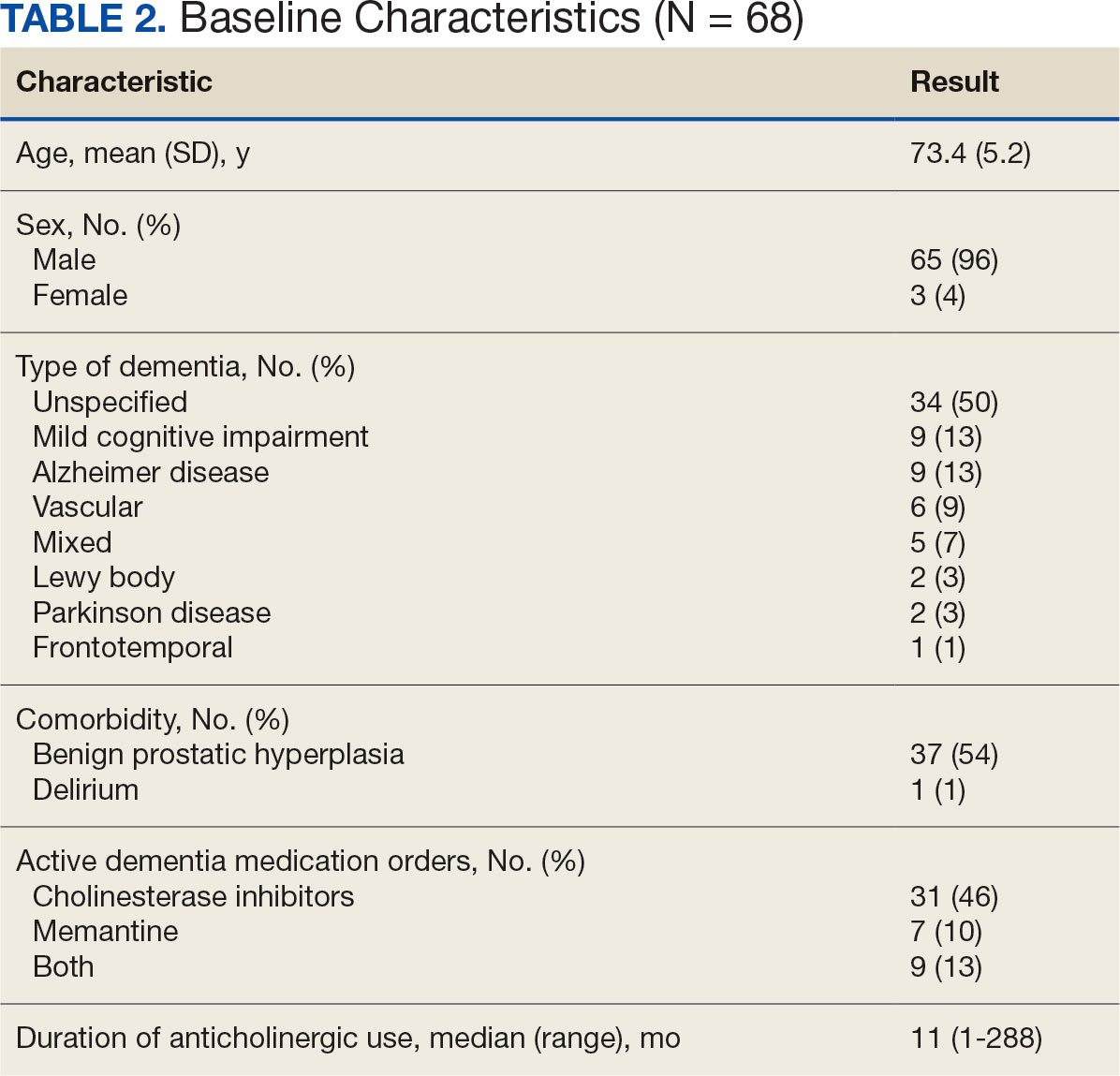
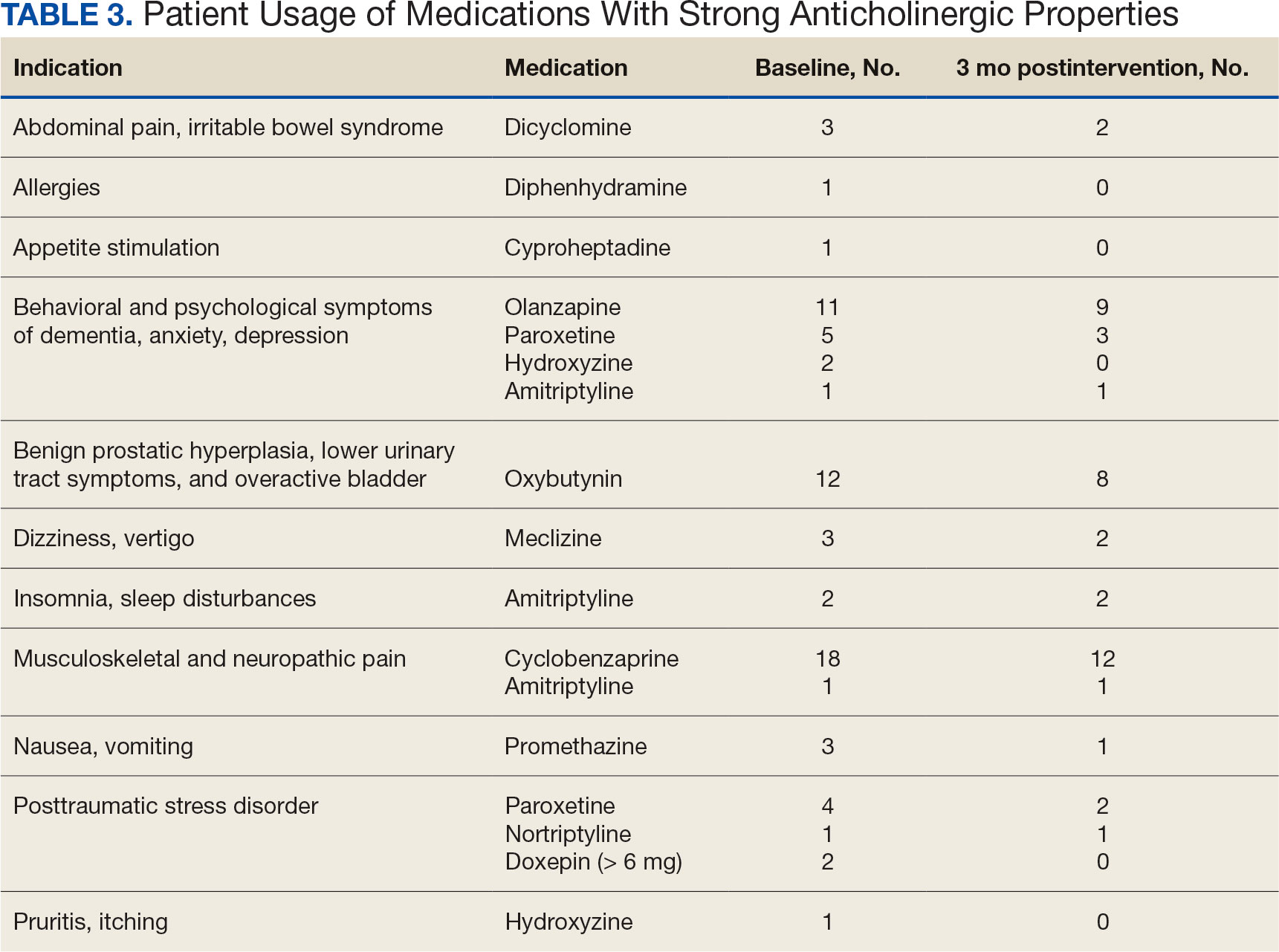
Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.
Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Biomarkers Predict Villous Atrophy in Potential Celiac Disease Patients
according to investigators.
Given that PCD patients present with positive serology and intact duodenal architecture, these findings may provide a much-needed tool for identifying patients who are more likely to benefit from early dietary interventions, lead author Renata Auricchio, MD, PhD, of the University of Naples Federico II, Italy, and colleagues reported.
“PCD offers the unique opportunity to observe the progression of gluten-induced tissue damage in celiac disease,” the investigators wrote in Gastroenterology. “These patients recognize gluten and produce specific autoantibodies, but have not developed intestinal damage.”
The study included 31 children with asymptomatic PCD who were eating a gluten-containing diet. Serum samples from each child were analyzed for the relative abundance of 92 inflammation-linked proteins using a proximity extension immunoassay. Statistical analyses, including partial least squares discriminant and linear discriminant analyses, were then applied to identify which proteins were associated with the development of VA.
After a mean follow-up period of 5.85 years, 14 participants developed VA (ie, celiac disease), while the remaining 17 remained asymptomatic.
Panel analysis revealed that specific inflammatory proteins, including interleukin (IL)–20, IL-2, sirtuin 2 (SIRT2), leukemia inhibitory factor (LIF), IL-22 receptor subunit a1, cystatin D (CST5), IL-17 receptor A, IL-15 receptor subunit a (RA), CUB domain–containing protein 1 (CDCP1), and IL-14, were 1.23- to 1.76-fold higher in children who developed VA. Among these, seven proteins — CDCP1, IL-2, LIF, IL10RA, SIRT2, CST5, and IL-4 — were able to significantly distinguish between symptomatic and asymptomatic cases in a linear discriminant model. This panel of seven proteins achieved a predictive accuracy of 96.8% in identifying children at risk of VA.
Additionally, bioinformatics pathway analysis confirmed that the broader set of proteins is involved in the positive regulation of JAK-STAT signaling (involving IL-22 receptor subunit a1, IL-4, IL-20, IL10RA, LIF, and IL-2), inflammatory responses (IL-4, IL-20, LIF, and IL-2), and processes such as tyrosine phosphorylation, leukocyte differentiation, IgG isotype switching, and protein phosphorylation regulation. These findings suggest that gluten-induced inflammation may already be active in early stages of the disease, including the initial phases of leukocyte differentiation, according to the investigators.
“Over a long follow-up on a gluten-containing diet, only 40% of these patients progressed to VA,” Dr. Auricchio and colleagues wrote. “Notably, 25%-30% of children with PCD even stop producing anti–tissue transglutaminase antibodies, and the others keep on producing autoantibodies but preserve a normal intestinal mucosa. Considering these data, the decision to address a patient with PCD on a gluten-free diet at time of diagnosis is quite critical.”
The researchers noted that this new model, with accuracy exceeding 95%, is well suited for routine use because of its practicality and reliability.
“Our previous model, based mainly on small intestinal mucosa features, moved a step toward the prediction of outcome but still required a mucosal biopsy, and the accuracy of prediction was not greater than 80%, which is somewhat uncertain for a lifelong clinical decision,” they wrote. In contrast, the present model “appears to be sufficient to immediately suggest a gluten-free diet in children with PCD, who are almost certainly committed to developing VA.”
The investigators called for long-term studies to validate their findings in other cohorts, including adult populations.This study was supported by the TIMID project and Inflammation in Human Early Life: Targeting Impacts on Life Course Health (INITIALISE) by the Horizon Europe Program of the European Union. The investigators disclosed no conflicts of interest.
Patients with positive celiac serologies but normal villous architecture on biopsy are considered to have potential celiac disease (PCD). While the prevalence of PCD is not well-established, it is estimated to be around 1%. This study by Auricchio and colleagues investigates seven serum proteomic biomarkers that could help predict whether asymptomatic patients with PCD are at risk of developing villous atrophy (VA).
The study also identifies specific inflammatory proteins present in PCD patients who are likely to develop VA. These biomarkers provide valuable insights into the pathogenesis of celiac disease and the development of VA in genetically predisposed individuals.
As celiac disease is increasingly diagnosed without biopsies, serum proteomic biomarkers could be crucial in identifying patients who may benefit from starting a gluten-free diet (GFD) earlier, potentially preventing complications. According to the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) guidelines, children can be diagnosed with celiac disease if their tissue transglutaminase IgA level is 10 times the upper limit of normal, confirmed by a positive endomysial antibody test. However, this approach may lead to many patients committing to a lifelong GFD despite having only PCD, as biopsies may not have been performed. In this study, 60% of patients with PCD did not progress to VA, suggesting that biomarkers could help prevent unnecessary long-term GFD commitments.
Stephanie M. Moleski, MD, is the director of the Jefferson Celiac Center and associate professor in the division of gastroenterology at Thomas Jefferson University Hospital in Philadelphia. She reported no conflicts of interest.
Patients with positive celiac serologies but normal villous architecture on biopsy are considered to have potential celiac disease (PCD). While the prevalence of PCD is not well-established, it is estimated to be around 1%. This study by Auricchio and colleagues investigates seven serum proteomic biomarkers that could help predict whether asymptomatic patients with PCD are at risk of developing villous atrophy (VA).
The study also identifies specific inflammatory proteins present in PCD patients who are likely to develop VA. These biomarkers provide valuable insights into the pathogenesis of celiac disease and the development of VA in genetically predisposed individuals.
As celiac disease is increasingly diagnosed without biopsies, serum proteomic biomarkers could be crucial in identifying patients who may benefit from starting a gluten-free diet (GFD) earlier, potentially preventing complications. According to the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) guidelines, children can be diagnosed with celiac disease if their tissue transglutaminase IgA level is 10 times the upper limit of normal, confirmed by a positive endomysial antibody test. However, this approach may lead to many patients committing to a lifelong GFD despite having only PCD, as biopsies may not have been performed. In this study, 60% of patients with PCD did not progress to VA, suggesting that biomarkers could help prevent unnecessary long-term GFD commitments.
Stephanie M. Moleski, MD, is the director of the Jefferson Celiac Center and associate professor in the division of gastroenterology at Thomas Jefferson University Hospital in Philadelphia. She reported no conflicts of interest.
Patients with positive celiac serologies but normal villous architecture on biopsy are considered to have potential celiac disease (PCD). While the prevalence of PCD is not well-established, it is estimated to be around 1%. This study by Auricchio and colleagues investigates seven serum proteomic biomarkers that could help predict whether asymptomatic patients with PCD are at risk of developing villous atrophy (VA).
The study also identifies specific inflammatory proteins present in PCD patients who are likely to develop VA. These biomarkers provide valuable insights into the pathogenesis of celiac disease and the development of VA in genetically predisposed individuals.
As celiac disease is increasingly diagnosed without biopsies, serum proteomic biomarkers could be crucial in identifying patients who may benefit from starting a gluten-free diet (GFD) earlier, potentially preventing complications. According to the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) guidelines, children can be diagnosed with celiac disease if their tissue transglutaminase IgA level is 10 times the upper limit of normal, confirmed by a positive endomysial antibody test. However, this approach may lead to many patients committing to a lifelong GFD despite having only PCD, as biopsies may not have been performed. In this study, 60% of patients with PCD did not progress to VA, suggesting that biomarkers could help prevent unnecessary long-term GFD commitments.
Stephanie M. Moleski, MD, is the director of the Jefferson Celiac Center and associate professor in the division of gastroenterology at Thomas Jefferson University Hospital in Philadelphia. She reported no conflicts of interest.
according to investigators.
Given that PCD patients present with positive serology and intact duodenal architecture, these findings may provide a much-needed tool for identifying patients who are more likely to benefit from early dietary interventions, lead author Renata Auricchio, MD, PhD, of the University of Naples Federico II, Italy, and colleagues reported.
“PCD offers the unique opportunity to observe the progression of gluten-induced tissue damage in celiac disease,” the investigators wrote in Gastroenterology. “These patients recognize gluten and produce specific autoantibodies, but have not developed intestinal damage.”
The study included 31 children with asymptomatic PCD who were eating a gluten-containing diet. Serum samples from each child were analyzed for the relative abundance of 92 inflammation-linked proteins using a proximity extension immunoassay. Statistical analyses, including partial least squares discriminant and linear discriminant analyses, were then applied to identify which proteins were associated with the development of VA.
After a mean follow-up period of 5.85 years, 14 participants developed VA (ie, celiac disease), while the remaining 17 remained asymptomatic.
Panel analysis revealed that specific inflammatory proteins, including interleukin (IL)–20, IL-2, sirtuin 2 (SIRT2), leukemia inhibitory factor (LIF), IL-22 receptor subunit a1, cystatin D (CST5), IL-17 receptor A, IL-15 receptor subunit a (RA), CUB domain–containing protein 1 (CDCP1), and IL-14, were 1.23- to 1.76-fold higher in children who developed VA. Among these, seven proteins — CDCP1, IL-2, LIF, IL10RA, SIRT2, CST5, and IL-4 — were able to significantly distinguish between symptomatic and asymptomatic cases in a linear discriminant model. This panel of seven proteins achieved a predictive accuracy of 96.8% in identifying children at risk of VA.
Additionally, bioinformatics pathway analysis confirmed that the broader set of proteins is involved in the positive regulation of JAK-STAT signaling (involving IL-22 receptor subunit a1, IL-4, IL-20, IL10RA, LIF, and IL-2), inflammatory responses (IL-4, IL-20, LIF, and IL-2), and processes such as tyrosine phosphorylation, leukocyte differentiation, IgG isotype switching, and protein phosphorylation regulation. These findings suggest that gluten-induced inflammation may already be active in early stages of the disease, including the initial phases of leukocyte differentiation, according to the investigators.
“Over a long follow-up on a gluten-containing diet, only 40% of these patients progressed to VA,” Dr. Auricchio and colleagues wrote. “Notably, 25%-30% of children with PCD even stop producing anti–tissue transglutaminase antibodies, and the others keep on producing autoantibodies but preserve a normal intestinal mucosa. Considering these data, the decision to address a patient with PCD on a gluten-free diet at time of diagnosis is quite critical.”
The researchers noted that this new model, with accuracy exceeding 95%, is well suited for routine use because of its practicality and reliability.
“Our previous model, based mainly on small intestinal mucosa features, moved a step toward the prediction of outcome but still required a mucosal biopsy, and the accuracy of prediction was not greater than 80%, which is somewhat uncertain for a lifelong clinical decision,” they wrote. In contrast, the present model “appears to be sufficient to immediately suggest a gluten-free diet in children with PCD, who are almost certainly committed to developing VA.”
The investigators called for long-term studies to validate their findings in other cohorts, including adult populations.This study was supported by the TIMID project and Inflammation in Human Early Life: Targeting Impacts on Life Course Health (INITIALISE) by the Horizon Europe Program of the European Union. The investigators disclosed no conflicts of interest.
according to investigators.
Given that PCD patients present with positive serology and intact duodenal architecture, these findings may provide a much-needed tool for identifying patients who are more likely to benefit from early dietary interventions, lead author Renata Auricchio, MD, PhD, of the University of Naples Federico II, Italy, and colleagues reported.
“PCD offers the unique opportunity to observe the progression of gluten-induced tissue damage in celiac disease,” the investigators wrote in Gastroenterology. “These patients recognize gluten and produce specific autoantibodies, but have not developed intestinal damage.”
The study included 31 children with asymptomatic PCD who were eating a gluten-containing diet. Serum samples from each child were analyzed for the relative abundance of 92 inflammation-linked proteins using a proximity extension immunoassay. Statistical analyses, including partial least squares discriminant and linear discriminant analyses, were then applied to identify which proteins were associated with the development of VA.
After a mean follow-up period of 5.85 years, 14 participants developed VA (ie, celiac disease), while the remaining 17 remained asymptomatic.
Panel analysis revealed that specific inflammatory proteins, including interleukin (IL)–20, IL-2, sirtuin 2 (SIRT2), leukemia inhibitory factor (LIF), IL-22 receptor subunit a1, cystatin D (CST5), IL-17 receptor A, IL-15 receptor subunit a (RA), CUB domain–containing protein 1 (CDCP1), and IL-14, were 1.23- to 1.76-fold higher in children who developed VA. Among these, seven proteins — CDCP1, IL-2, LIF, IL10RA, SIRT2, CST5, and IL-4 — were able to significantly distinguish between symptomatic and asymptomatic cases in a linear discriminant model. This panel of seven proteins achieved a predictive accuracy of 96.8% in identifying children at risk of VA.
Additionally, bioinformatics pathway analysis confirmed that the broader set of proteins is involved in the positive regulation of JAK-STAT signaling (involving IL-22 receptor subunit a1, IL-4, IL-20, IL10RA, LIF, and IL-2), inflammatory responses (IL-4, IL-20, LIF, and IL-2), and processes such as tyrosine phosphorylation, leukocyte differentiation, IgG isotype switching, and protein phosphorylation regulation. These findings suggest that gluten-induced inflammation may already be active in early stages of the disease, including the initial phases of leukocyte differentiation, according to the investigators.
“Over a long follow-up on a gluten-containing diet, only 40% of these patients progressed to VA,” Dr. Auricchio and colleagues wrote. “Notably, 25%-30% of children with PCD even stop producing anti–tissue transglutaminase antibodies, and the others keep on producing autoantibodies but preserve a normal intestinal mucosa. Considering these data, the decision to address a patient with PCD on a gluten-free diet at time of diagnosis is quite critical.”
The researchers noted that this new model, with accuracy exceeding 95%, is well suited for routine use because of its practicality and reliability.
“Our previous model, based mainly on small intestinal mucosa features, moved a step toward the prediction of outcome but still required a mucosal biopsy, and the accuracy of prediction was not greater than 80%, which is somewhat uncertain for a lifelong clinical decision,” they wrote. In contrast, the present model “appears to be sufficient to immediately suggest a gluten-free diet in children with PCD, who are almost certainly committed to developing VA.”
The investigators called for long-term studies to validate their findings in other cohorts, including adult populations.This study was supported by the TIMID project and Inflammation in Human Early Life: Targeting Impacts on Life Course Health (INITIALISE) by the Horizon Europe Program of the European Union. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Clinical Edge Journal Scan: Migraine ICYMI December 2024
Primary Sclerosing Cholangitis (PSC) and Its Importance in Clinical Practice
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.