User login
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
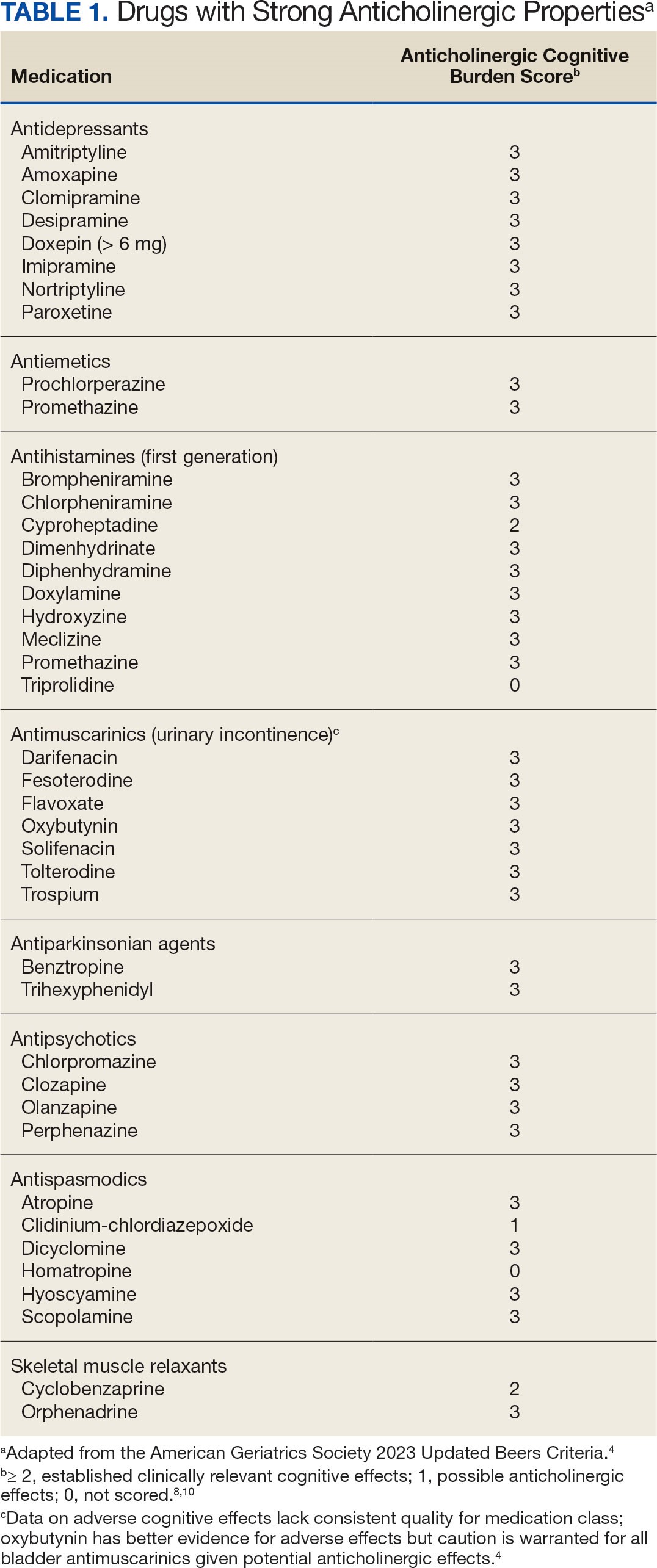
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10
Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
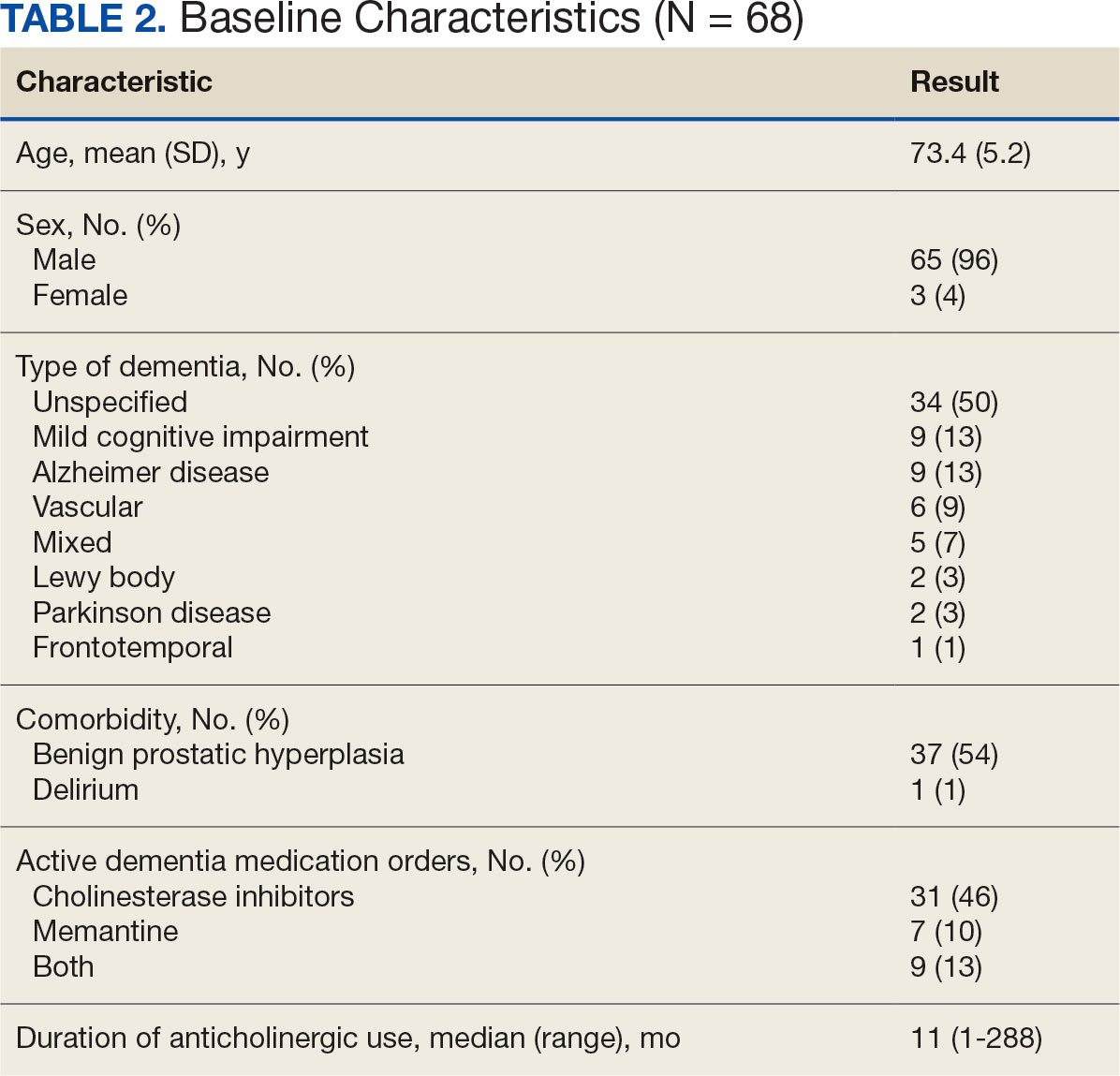
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.
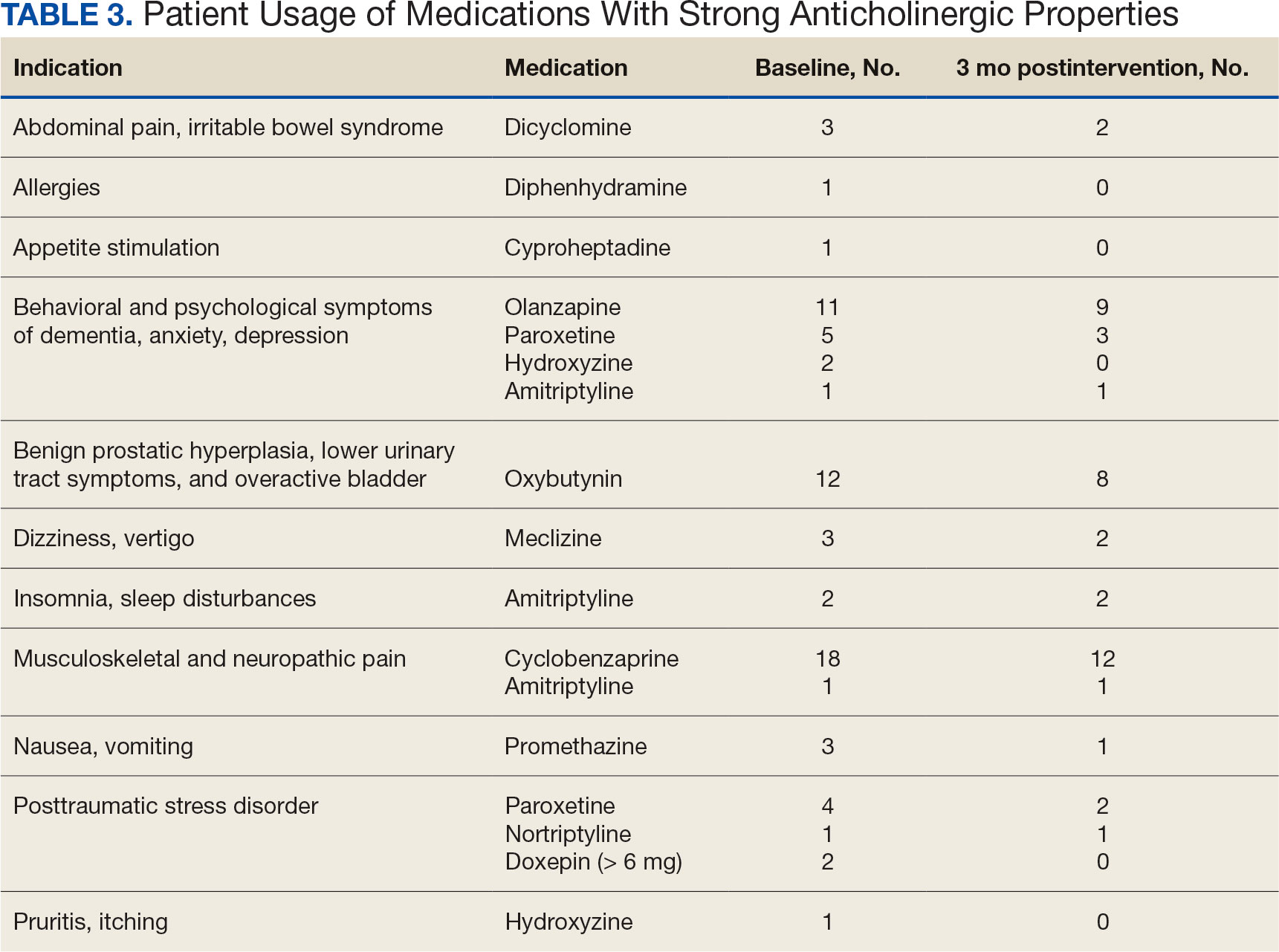
Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.
Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
