User login
Primary Sclerosing Cholangitis (PSC) and Its Importance in Clinical Practice
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
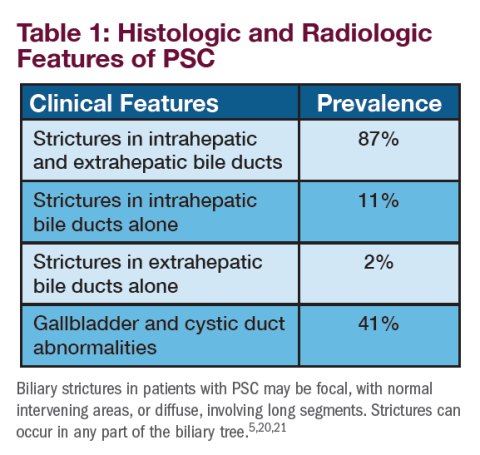
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
1.16 Common Clinical Diagnoses and Conditions: Head and Neck Disorders
Introduction
Disorders of the head and neck, including infectious processes and anatomic abnormalities, are some of the most common encountered by pediatric hospitalists. Upper respiratory tract infections (URIs) are the most common reason for acute pediatric medical care. Children under age six years of age average six to eight URIs per year. Acute illness with an infectious process often exacerbates underlying anatomic abnormalities, such as laryngomalacia, tracheomalacia, subglottic stenosis, and others, but these abnormalities alone can require acute intervention and lead to hospitalization. Bacterial infections have the potential to invade other structures or compromise the airway, rapidly resulting in both immediate and long-term sequelae if not appropriately treated. Pediatric hospitalists frequently encounter patients with disorders of the head and neck and should be able to recognize their signs and symptoms and provide evidence-based and efficient care. In particular, pediatric hospitalists must be able to identify impending airway obstruction, provide immediate care, and arrange for the appropriate subsequent level of care.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the head and neck anatomy of children at different chronological ages, including how abnormalities of airflow in different locations may alter the clinical presentation at different ages.
- Discuss the symptoms of various anatomic abnormalities (such as laryngomalacia, tracheomalacia, subglottic stenosis, and others), including the acute infectious processes which may exacerbate their clinical presentation.
- Discuss the pathophysiology, presenting features, and common pathogens associated with bacterial infections of the head and neck (such as otitis media, otitis externa, retropharyngeal abscess, orbital cellulitis, dental infections, mastoiditis, peritonsillar abscess, and others).
- Describe the differential diagnosis of common presenting symptoms of head and neck disorders, such as shortness of breath, stridor, cough, nasal discharge, neck swelling/pain, dysphagia/drooling, facial swelling, and others.
- Describe the features of upper airway obstruction, such as stertor, stridor, tripod positioning, dysphagia, drooling, trismus, and others.
- Discuss alternate diagnoses that may mimic the presentation of acute upper respiratory infection such as allergic reaction, toxic inhalant exposure, and others.
- List the indications for hospital admission, explain the utility of various monitoring options, and review the indications for emergent and non-emergent subspecialist consultation.
- Describe the signs and symptoms of obstructive sleep apnea (OSA) including snoring, respiratory pauses, and hypoxia, and discuss appropriate evaluation, referral, and management.
- Explain the types of studies available to assess the head and neck (including plain radiographs, fluoroscopy, ultrasonography, computed tomography, magnetic resonance imaging, and direct laryngoscopy) and discuss the risks, benefits, and indications for each.
- Discuss the indications, contraindications, and mechanisms of action of pharmacological agents used to treat various disorders of the head and neck, such as antibiotics, nebulized epinephrine, glucocorticoids, proton pump inhibitors, histamine 2 blockers, and others.
- Compare and contrast the benefits and limitations of various modalities of airway stabilization and respiratory support (including heated humidified high flow nasal cannula, non-invasive positive pressure support, and intubation with mechanical ventilation) in patients with varying degrees of upper airway obstruction.
- Discuss the changes in clinical status that indicate need for escalation of care, such as worsening stridor or work of breathing, decreased air entry, cyanosis, altered mental status, and others.
- Describe the patient characteristics that indicate the need for higher level of care and/or transfer to a referral center in cases requiring pediatric-specific services not available at the local facility.
- Describe criteria, including specific measures of clinical stability, that must be met before discharging patients with head and neck disorders, including oxygenation, hydration, and patient/family education.
Skills
Pediatric hospitalists should be able to:
- Perform an appropriately focused medical history, attending to symptoms of potential airway obstruction.
- Conduct a thorough physical examination directed by signs and symptoms that may indicate the location, etiology, or severity of the disorder.
- Identify patients with comorbidities or underlying anatomic abnormalities that impact the management plan and order appropriate testing, correctly interpreting results.
- Order appropriate monitoring and correctly interpret monitor data.
- Adhere consistently to infection control practices.
- Identify complications and respond with appropriate actions.
- Perform an evidence-based, cost-effective diagnostic evaluation and treatment plan, avoiding unnecessary testing.
- Perform careful reassessments daily and as needed, note changes in clinical status, and respond with appropriate actions and escalation of care as appropriate.
- Stabilize the airway and provide appropriate respiratory support for patients with impending or actual airway obstruction or respiratory failure, including head tilt/chin lift, nasal trumpet, and intubation, or arrange for the appropriate personnel to perform the procedure in an effective and efficient manner.
- Engage consultants (such as otolaryngologists, pulmonologists, surgeons, speech and feeding specialists, dentists, and others) efficiently and effectively when needed.
Attitudes
Pediatric hospitalists should be able to:
- Role model and advocate for strict adherence to infection control practices.
- Realize responsibility for effective communication with patients and the family/caregivers regarding the diagnosis, management plan, and follow-up needs.
- Recognize the value of collaboration with the primary care provider, subspecialists, nursing, the hospital staff, and other outpatient providers to ensure coordinated longitudinal care at the time of discharge.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in the development, implementation, and improvement of cost-effective, safe, evidence-based care within a multidisciplinary team for hospitalized children with head and neck disorders.
- Collaborate with hospital administration and community partners to develop and sustain referral networks between local facilities and tertiary referral centers for hospitalized patients with head and neck disorders.
1. Geddes G, Butterly MM, Patel SM, Marra S. Pediatric neck masses. Pediatr Rev. 2013 Mar;34(3):115-124; quiz 125. https://doi.org/10.1542/pir.34-3-115.
2. Virbalas J, Smith L. Upper airway obstruction. Pediatr Rev. 2015;36(2):62-72. https://doi.org/10.1542/pir.36-2-62.
3. Murray AD. Deep Neck Infections. Medscape. https://emedicine.medscape.com/article/837048-overview. Updated Apr 12, 2018. Accessed August 28, 2019.
Introduction
Disorders of the head and neck, including infectious processes and anatomic abnormalities, are some of the most common encountered by pediatric hospitalists. Upper respiratory tract infections (URIs) are the most common reason for acute pediatric medical care. Children under age six years of age average six to eight URIs per year. Acute illness with an infectious process often exacerbates underlying anatomic abnormalities, such as laryngomalacia, tracheomalacia, subglottic stenosis, and others, but these abnormalities alone can require acute intervention and lead to hospitalization. Bacterial infections have the potential to invade other structures or compromise the airway, rapidly resulting in both immediate and long-term sequelae if not appropriately treated. Pediatric hospitalists frequently encounter patients with disorders of the head and neck and should be able to recognize their signs and symptoms and provide evidence-based and efficient care. In particular, pediatric hospitalists must be able to identify impending airway obstruction, provide immediate care, and arrange for the appropriate subsequent level of care.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the head and neck anatomy of children at different chronological ages, including how abnormalities of airflow in different locations may alter the clinical presentation at different ages.
- Discuss the symptoms of various anatomic abnormalities (such as laryngomalacia, tracheomalacia, subglottic stenosis, and others), including the acute infectious processes which may exacerbate their clinical presentation.
- Discuss the pathophysiology, presenting features, and common pathogens associated with bacterial infections of the head and neck (such as otitis media, otitis externa, retropharyngeal abscess, orbital cellulitis, dental infections, mastoiditis, peritonsillar abscess, and others).
- Describe the differential diagnosis of common presenting symptoms of head and neck disorders, such as shortness of breath, stridor, cough, nasal discharge, neck swelling/pain, dysphagia/drooling, facial swelling, and others.
- Describe the features of upper airway obstruction, such as stertor, stridor, tripod positioning, dysphagia, drooling, trismus, and others.
- Discuss alternate diagnoses that may mimic the presentation of acute upper respiratory infection such as allergic reaction, toxic inhalant exposure, and others.
- List the indications for hospital admission, explain the utility of various monitoring options, and review the indications for emergent and non-emergent subspecialist consultation.
- Describe the signs and symptoms of obstructive sleep apnea (OSA) including snoring, respiratory pauses, and hypoxia, and discuss appropriate evaluation, referral, and management.
- Explain the types of studies available to assess the head and neck (including plain radiographs, fluoroscopy, ultrasonography, computed tomography, magnetic resonance imaging, and direct laryngoscopy) and discuss the risks, benefits, and indications for each.
- Discuss the indications, contraindications, and mechanisms of action of pharmacological agents used to treat various disorders of the head and neck, such as antibiotics, nebulized epinephrine, glucocorticoids, proton pump inhibitors, histamine 2 blockers, and others.
- Compare and contrast the benefits and limitations of various modalities of airway stabilization and respiratory support (including heated humidified high flow nasal cannula, non-invasive positive pressure support, and intubation with mechanical ventilation) in patients with varying degrees of upper airway obstruction.
- Discuss the changes in clinical status that indicate need for escalation of care, such as worsening stridor or work of breathing, decreased air entry, cyanosis, altered mental status, and others.
- Describe the patient characteristics that indicate the need for higher level of care and/or transfer to a referral center in cases requiring pediatric-specific services not available at the local facility.
- Describe criteria, including specific measures of clinical stability, that must be met before discharging patients with head and neck disorders, including oxygenation, hydration, and patient/family education.
Skills
Pediatric hospitalists should be able to:
- Perform an appropriately focused medical history, attending to symptoms of potential airway obstruction.
- Conduct a thorough physical examination directed by signs and symptoms that may indicate the location, etiology, or severity of the disorder.
- Identify patients with comorbidities or underlying anatomic abnormalities that impact the management plan and order appropriate testing, correctly interpreting results.
- Order appropriate monitoring and correctly interpret monitor data.
- Adhere consistently to infection control practices.
- Identify complications and respond with appropriate actions.
- Perform an evidence-based, cost-effective diagnostic evaluation and treatment plan, avoiding unnecessary testing.
- Perform careful reassessments daily and as needed, note changes in clinical status, and respond with appropriate actions and escalation of care as appropriate.
- Stabilize the airway and provide appropriate respiratory support for patients with impending or actual airway obstruction or respiratory failure, including head tilt/chin lift, nasal trumpet, and intubation, or arrange for the appropriate personnel to perform the procedure in an effective and efficient manner.
- Engage consultants (such as otolaryngologists, pulmonologists, surgeons, speech and feeding specialists, dentists, and others) efficiently and effectively when needed.
Attitudes
Pediatric hospitalists should be able to:
- Role model and advocate for strict adherence to infection control practices.
- Realize responsibility for effective communication with patients and the family/caregivers regarding the diagnosis, management plan, and follow-up needs.
- Recognize the value of collaboration with the primary care provider, subspecialists, nursing, the hospital staff, and other outpatient providers to ensure coordinated longitudinal care at the time of discharge.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in the development, implementation, and improvement of cost-effective, safe, evidence-based care within a multidisciplinary team for hospitalized children with head and neck disorders.
- Collaborate with hospital administration and community partners to develop and sustain referral networks between local facilities and tertiary referral centers for hospitalized patients with head and neck disorders.
Introduction
Disorders of the head and neck, including infectious processes and anatomic abnormalities, are some of the most common encountered by pediatric hospitalists. Upper respiratory tract infections (URIs) are the most common reason for acute pediatric medical care. Children under age six years of age average six to eight URIs per year. Acute illness with an infectious process often exacerbates underlying anatomic abnormalities, such as laryngomalacia, tracheomalacia, subglottic stenosis, and others, but these abnormalities alone can require acute intervention and lead to hospitalization. Bacterial infections have the potential to invade other structures or compromise the airway, rapidly resulting in both immediate and long-term sequelae if not appropriately treated. Pediatric hospitalists frequently encounter patients with disorders of the head and neck and should be able to recognize their signs and symptoms and provide evidence-based and efficient care. In particular, pediatric hospitalists must be able to identify impending airway obstruction, provide immediate care, and arrange for the appropriate subsequent level of care.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the head and neck anatomy of children at different chronological ages, including how abnormalities of airflow in different locations may alter the clinical presentation at different ages.
- Discuss the symptoms of various anatomic abnormalities (such as laryngomalacia, tracheomalacia, subglottic stenosis, and others), including the acute infectious processes which may exacerbate their clinical presentation.
- Discuss the pathophysiology, presenting features, and common pathogens associated with bacterial infections of the head and neck (such as otitis media, otitis externa, retropharyngeal abscess, orbital cellulitis, dental infections, mastoiditis, peritonsillar abscess, and others).
- Describe the differential diagnosis of common presenting symptoms of head and neck disorders, such as shortness of breath, stridor, cough, nasal discharge, neck swelling/pain, dysphagia/drooling, facial swelling, and others.
- Describe the features of upper airway obstruction, such as stertor, stridor, tripod positioning, dysphagia, drooling, trismus, and others.
- Discuss alternate diagnoses that may mimic the presentation of acute upper respiratory infection such as allergic reaction, toxic inhalant exposure, and others.
- List the indications for hospital admission, explain the utility of various monitoring options, and review the indications for emergent and non-emergent subspecialist consultation.
- Describe the signs and symptoms of obstructive sleep apnea (OSA) including snoring, respiratory pauses, and hypoxia, and discuss appropriate evaluation, referral, and management.
- Explain the types of studies available to assess the head and neck (including plain radiographs, fluoroscopy, ultrasonography, computed tomography, magnetic resonance imaging, and direct laryngoscopy) and discuss the risks, benefits, and indications for each.
- Discuss the indications, contraindications, and mechanisms of action of pharmacological agents used to treat various disorders of the head and neck, such as antibiotics, nebulized epinephrine, glucocorticoids, proton pump inhibitors, histamine 2 blockers, and others.
- Compare and contrast the benefits and limitations of various modalities of airway stabilization and respiratory support (including heated humidified high flow nasal cannula, non-invasive positive pressure support, and intubation with mechanical ventilation) in patients with varying degrees of upper airway obstruction.
- Discuss the changes in clinical status that indicate need for escalation of care, such as worsening stridor or work of breathing, decreased air entry, cyanosis, altered mental status, and others.
- Describe the patient characteristics that indicate the need for higher level of care and/or transfer to a referral center in cases requiring pediatric-specific services not available at the local facility.
- Describe criteria, including specific measures of clinical stability, that must be met before discharging patients with head and neck disorders, including oxygenation, hydration, and patient/family education.
Skills
Pediatric hospitalists should be able to:
- Perform an appropriately focused medical history, attending to symptoms of potential airway obstruction.
- Conduct a thorough physical examination directed by signs and symptoms that may indicate the location, etiology, or severity of the disorder.
- Identify patients with comorbidities or underlying anatomic abnormalities that impact the management plan and order appropriate testing, correctly interpreting results.
- Order appropriate monitoring and correctly interpret monitor data.
- Adhere consistently to infection control practices.
- Identify complications and respond with appropriate actions.
- Perform an evidence-based, cost-effective diagnostic evaluation and treatment plan, avoiding unnecessary testing.
- Perform careful reassessments daily and as needed, note changes in clinical status, and respond with appropriate actions and escalation of care as appropriate.
- Stabilize the airway and provide appropriate respiratory support for patients with impending or actual airway obstruction or respiratory failure, including head tilt/chin lift, nasal trumpet, and intubation, or arrange for the appropriate personnel to perform the procedure in an effective and efficient manner.
- Engage consultants (such as otolaryngologists, pulmonologists, surgeons, speech and feeding specialists, dentists, and others) efficiently and effectively when needed.
Attitudes
Pediatric hospitalists should be able to:
- Role model and advocate for strict adherence to infection control practices.
- Realize responsibility for effective communication with patients and the family/caregivers regarding the diagnosis, management plan, and follow-up needs.
- Recognize the value of collaboration with the primary care provider, subspecialists, nursing, the hospital staff, and other outpatient providers to ensure coordinated longitudinal care at the time of discharge.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in the development, implementation, and improvement of cost-effective, safe, evidence-based care within a multidisciplinary team for hospitalized children with head and neck disorders.
- Collaborate with hospital administration and community partners to develop and sustain referral networks between local facilities and tertiary referral centers for hospitalized patients with head and neck disorders.
1. Geddes G, Butterly MM, Patel SM, Marra S. Pediatric neck masses. Pediatr Rev. 2013 Mar;34(3):115-124; quiz 125. https://doi.org/10.1542/pir.34-3-115.
2. Virbalas J, Smith L. Upper airway obstruction. Pediatr Rev. 2015;36(2):62-72. https://doi.org/10.1542/pir.36-2-62.
3. Murray AD. Deep Neck Infections. Medscape. https://emedicine.medscape.com/article/837048-overview. Updated Apr 12, 2018. Accessed August 28, 2019.
1. Geddes G, Butterly MM, Patel SM, Marra S. Pediatric neck masses. Pediatr Rev. 2013 Mar;34(3):115-124; quiz 125. https://doi.org/10.1542/pir.34-3-115.
2. Virbalas J, Smith L. Upper airway obstruction. Pediatr Rev. 2015;36(2):62-72. https://doi.org/10.1542/pir.36-2-62.
3. Murray AD. Deep Neck Infections. Medscape. https://emedicine.medscape.com/article/837048-overview. Updated Apr 12, 2018. Accessed August 28, 2019.
Delaying lumbar punctures for a head CT may result in increased mortality in acute bacterial meningitis
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Unexpectedly good results, and no chemotherapy required
Cabozantinib in metastatic prostate cancer1,2
Researchers tested cabozantinib, a tyrosine-kinase inhibitor (TKI) against MET and vascular endothelial growth factor receptor 2 (VEGF), in a large phase 2 randomized discontinuation trial in 9 tumor types. A subset of 171 patients with castrateresistant prostate cancer (CRPC) was reported in this study. Patients were treated on open label for 12 weeks, and then if stable, were randomized to receive the active drug or placebo. The trial was suspended early by the study oversight committee because: a) Cabozantinib was too toxic for the study to continue. b) The prostate-specific antigen (PSA) level fell in most of the treated patients. c) In the initial 121 patients, there was an unexpected improvement in bone scans and decrease in pain in the lead-in stage of the study. d) Unexpected rapid soft tissue progression. Bone scans improved in 78% of patients, and in 12% there was complete remission. After further analysis, the following were true except for: a) Cabozantinib interfered with technetium-99, and thus, the responses were not real, but rather an artifact. b) The PSA did not correlate with improvement in bone pain. c) Markers of bone formation and resorption showed improvement, and there was no correlation with prior bisphosphonate therapy. d) Bone scan improvement correlated with improvement in soft tissue disease.
Key points
The results in patients with prostate cancer were so striking – 72% of patients had regression in soft tissue lesions, and 68% of evaluable patients had improvement on bone scan, including complete resolution in 12% – that the subset analysis was published as a rapid communication.1,2 Because of very high response rates (5% at 12 weeks) and symptomatic improvement in the initial 122 patients who were enrolled, random assignment was discontinued. Bone markers improved in concert with the radiologic and clinical improvement. Answers c, a
Cabozantinib in metastatic prostate cancer1,2
Researchers tested cabozantinib, a tyrosine-kinase inhibitor (TKI) against MET and vascular endothelial growth factor receptor 2 (VEGF), in a large phase 2 randomized discontinuation trial in 9 tumor types. A subset of 171 patients with castrateresistant prostate cancer (CRPC) was reported in this study. Patients were treated on open label for 12 weeks, and then if stable, were randomized to receive the active drug or placebo. The trial was suspended early by the study oversight committee because: a) Cabozantinib was too toxic for the study to continue. b) The prostate-specific antigen (PSA) level fell in most of the treated patients. c) In the initial 121 patients, there was an unexpected improvement in bone scans and decrease in pain in the lead-in stage of the study. d) Unexpected rapid soft tissue progression. Bone scans improved in 78% of patients, and in 12% there was complete remission. After further analysis, the following were true except for: a) Cabozantinib interfered with technetium-99, and thus, the responses were not real, but rather an artifact. b) The PSA did not correlate with improvement in bone pain. c) Markers of bone formation and resorption showed improvement, and there was no correlation with prior bisphosphonate therapy. d) Bone scan improvement correlated with improvement in soft tissue disease.
Key points
The results in patients with prostate cancer were so striking – 72% of patients had regression in soft tissue lesions, and 68% of evaluable patients had improvement on bone scan, including complete resolution in 12% – that the subset analysis was published as a rapid communication.1,2 Because of very high response rates (5% at 12 weeks) and symptomatic improvement in the initial 122 patients who were enrolled, random assignment was discontinued. Bone markers improved in concert with the radiologic and clinical improvement. Answers c, a
Cabozantinib in metastatic prostate cancer1,2
Researchers tested cabozantinib, a tyrosine-kinase inhibitor (TKI) against MET and vascular endothelial growth factor receptor 2 (VEGF), in a large phase 2 randomized discontinuation trial in 9 tumor types. A subset of 171 patients with castrateresistant prostate cancer (CRPC) was reported in this study. Patients were treated on open label for 12 weeks, and then if stable, were randomized to receive the active drug or placebo. The trial was suspended early by the study oversight committee because: a) Cabozantinib was too toxic for the study to continue. b) The prostate-specific antigen (PSA) level fell in most of the treated patients. c) In the initial 121 patients, there was an unexpected improvement in bone scans and decrease in pain in the lead-in stage of the study. d) Unexpected rapid soft tissue progression. Bone scans improved in 78% of patients, and in 12% there was complete remission. After further analysis, the following were true except for: a) Cabozantinib interfered with technetium-99, and thus, the responses were not real, but rather an artifact. b) The PSA did not correlate with improvement in bone pain. c) Markers of bone formation and resorption showed improvement, and there was no correlation with prior bisphosphonate therapy. d) Bone scan improvement correlated with improvement in soft tissue disease.
Key points
The results in patients with prostate cancer were so striking – 72% of patients had regression in soft tissue lesions, and 68% of evaluable patients had improvement on bone scan, including complete resolution in 12% – that the subset analysis was published as a rapid communication.1,2 Because of very high response rates (5% at 12 weeks) and symptomatic improvement in the initial 122 patients who were enrolled, random assignment was discontinued. Bone markers improved in concert with the radiologic and clinical improvement. Answers c, a
What is the best choice for prophylaxis against VTE in medical inpatients?
Case
A 76-year-old gentleman is admitted for progressively worsening dyspnea, cough, and bilateral leg edema. Upon admission, his blood pressure is 150/90 mm/Hg, pulse 90 beats per minute, and respiration is 24 per minute.
Pertinent physical findings include jugular venous distension, bilateral crackles, S3 gallop, and 2+ bilateral lower extremity edema. The chest radiograph shows cardiomegaly and pulmonary edema. He is admitted to the hospital with a diagnosis of acute decompensated heart failure and starts aggressive medical therapy.
Overview
Approximately 2 million cases of deep-vein thrombosis (DVT) occur annually in the United States. Based on studies utilizing ventilation-perfusion scanning, half these patients likely have a silent pulmonary embolism (PE); of these, approximately 250,000 die.
The spectrum of venous thromboembolism (VTE), which includes DVT and PE, can vary from being asymptomatic to sudden death. Autopsy studies suggest a leading cause of sudden death in hospitalized medical patients is often a PE. There also are sequelae, such as chronic pulmonary hypertension, occurring in approximately 5% of PE cases, and post-thrombotic syndrome, occurring in approximately 40% of patients with DVT at two years.1
A recent study suggests DVT occurs three times more commonly in the outpatient setting. However, more than half of these patients were hospitalized in the three months prior.2 This is likely due to inadequate in-hospital prevention because of absence of prophylaxis, use of an unsuitable modality, insufficient dose of the drug, or ineffective duration of therapy. Inadequate and omitted VTE prophylaxis for medical patients was clearly demonstrated in the DVT Free Registry. This registry was created by 183 U.S. hospitals and included 5,451 patients, inpatients, and outpatients with ultrasound-confirmed DVT.
The number of medical inpatients who received prophylaxis in the 30 days prior to diagnosis was 28%—lower than the 48% of surgical patients.3 In a recent international registry, IMPROVE, only approximately 50% of hospitalized patients received prophylaxis.4
Virchow’s triad describes three underlying etiologic factors for thrombosis: stasis of blood flow, endothelial injury, and hypercoagulability. Established VTE risk factors reflect these underlying pathophysiologic processes. Important risk factors for VTE include increasing age, prolonged immobility, malignancy, major surgery, multiple trauma, prior VTE, and chronic heart failure.5
However, the magnitude of risk conferred by these and other risk factors varies (see Table 1, p. 35). It is not known how these factors interact to determine a patient’s individual VTE risk, but there is evidence it increases in proportion to the number of predisposing factors present.
In a recent systematic review of nine studies, including approximately 20,000 patients, prophylaxis reduced the rate of symptomatic VTE in at-risk hospitalized medical patients without increasing major bleeding.6
Multiple healthcare organizations, such as the National Quality Forum (NQF), Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and Agency for Healthcare Research and Quality (AHRQ) have identified VTE as a preventable condition in hospitalized patients. Formal risk assessment must be conducted as a first step, followed by the initiation of timely prophylaxis to improve patient safety.
Review of the Data
Mechanical forms of prophylaxis, such as graduated compression stockings, have been evaluated in patients with stroke and myocardial infarction. Intermittent pneumatic compression stockings and venous foot pumps have not been studied in randomized controlled trials (RCTs) in general medical patients.
Although there is data supporting the efficacy of these devices in surgical patients, the American College of Chest Physicians’ (ACCP) guidelines recommend against the use of mechanical forms of prophylaxis in medical patients unless there is a contraindication to pharmacologic prophylaxis.7
The ideal prophylactic agent is cost-effective and has no side effects. Available pharmacologic options for prevention of VTE in medical patients include unfractionated heparin (UFH), low molecular weight heparins (LMWHs), and the synthetic pentasaccharide, fondaparinux. Oral anticoagulants, (e.g., vitamin K antagonists [VKA]), have not been adequately studied in medical inpatients. Since VKA take several days to achieve therapeutic anticoagulation, we do not recommend using them de novo as VTE prophylaxis.
However, patients taking an oral VKA in the outpatient setting who have a therapeutic international normalized ratio (INR) during hospitalization probably are adequately protected from VTE and do not need additional pharmacologic prophylaxis. Newer anticoagulants in phase III testing for prevention of VTE in medically ill patients include oral direct thrombin inhibitors and anti-Xa inhibitors. ACCP guidelines recommend either low-dose UFH or LMWH as first-line agents for VTE prevention in medical inpatients.
Unfractionated heparin: UFH is a heterogeneous mixture of repeating polysaccharide chains of varying sizes, averaging about 15,000 Daltons. It binds anti-thrombin III (AT-III) and facilitates AT-III-mediated inactivation of factors IIa, IXa, Xa, and XIIa; of these, IIa and Xa are most responsive to inhibition.
Due to its large size, UFH only is partially absorbed from subcutaneous (SC) tissue, and it has a variable anticoagulant response due to interactions with plasma proteins, macrophages, and endothelial cells.8 However, in prophylactic SC doses (5,000 units two or three times daily), monitoring of the activated partial thromboplastin time (aPTT) is not required. In some cases, (e.g., frail or elderly patients), prophylactic SC doses may slightly prolong the aPTT.
UFH also binds to platelets and platelet factor 4 (PF4), and may precipitate heparin-induced thrombocytopenia (HIT). At least three clinical trials have compared the efficacy of SC UFH with a placebo and found prophylactic doses of UFH decrease the relative risk of DVT as detected by fibrinogen uptake test by about 70% without increasing the risk of bleeding.9-11
Low molecular weight heparins: LMWHs are derived from UFH through a chemical depolymerization, or fractionation, process. They are about one-third the size of UFH, with a molecular weight of approximately 5,000 Daltons.
These smaller molecules are readily absorbed from the SC tissue, eliciting a more predictable anticoagulant response than UFH. Unlike UFH, LMWHs have only minimal nonspecific binding to plasma proteins, endothelial cells, and monocytes, resulting in a predictable dose response. This obviates the need for lab monitoring, even when used in full, therapeutic dosing.
Compared with UFH, these agents have a longer plasma half-life, allowing them to be dosed SC once or twice daily. Also, they don’t bind platelets as readily as UFH resulting in a lower risk of HIT. Because they’re smaller, LMWHs tend to preferentially inhibit factor Xa, whereas UFH tends to inhibit factors Xa and IIa equally.
LMWHs have been evaluated in two large, placebo-controlled clinical trials for the prevention of VTE in medical inpatients.
In the first trial, MEDENOX, almost half the patients were older than 75 (mean age approximately 73). Inclusion criteria were NYHA class 3 or 4 heart failure, acute respiratory failure without mechanical ventilation, acute infection without septic shock, acute rheumatic disease, or inflammatory bowel disease. The primary outcome was assessed in 866 patients. Enoxaparin 40 mg SC once daily decreased the rate of VTE by two-thirds, from 15% to 5% (p=0.0002), without increased bleeding or thrombocytopenia compared with placebo.12 Enoxaparin 40 mg SC once daily is approved by the Food and Drug Administration (FDA) for VTE prophylaxis in medically ill patients.
PREVENT was an international, multicenter, randomized, double-blind, placebo-controlled trial evaluating dalteparin’s efficacy and safety. The inclusion criteria in this trial were acute congestive heart failure, non-ventilator-requiring acute respiratory failure, infection without septic shock, acute rheumatologic disorders, or inflammatory bowel disease. It studied 2,991 patients, and the primary outcome was VTE incidence and sudden death at day 21.
Dalteparin at 5,000 units decreased the rate of VTE, as detected by compression ultrasound, from 5% in the placebo group to 2.8%, a relative risk reduction of 45% (p=0.0015). The authors concluded the use of dalteparin reduced the incidence of VTE without increased risk of bleeding.13 Dalteparin is FDA approved for VTE prophylaxis in medical inpatients.
At least two meta-analyses have evaluated LMWHs compared with UFH. In the first, nine trials comparing LMWH to UFH (4,669 patients) were included. No significant effect was observed on DVT, clinical PE, or mortality. However, LMWH reduced the risk of major hemorrhage by 52% (p=0.049).14
In a more recent meta-analysis, 36 studies were included comparing placebos with UFH and LMWH. Both agents were associated with a reduced risk of VTE. A UFH dosage of 5,000 units three times daily was more effective in preventing DVT than a dosage of 5,000 units twice daily when compared with the control (risk ratio [RR], 0.27; 95% confidence interval [CI], 0.20-0.36; vs. RR, 0.52; 95% CI, 0.28-0.96). Neither UFH nor LMWH reduced mortality. When directly compared with UFH, LMWH was associated with a lower risk of DVT (RR, 0.68; 95% CI, 0.52-0.88) and injection-site hematoma (RR, 0.47; 95% CI, 0.36-0.62), but no difference was seen between the two agents in the risk of bleeding or thrombocytopenia.
This contemporary meta-analysis clearly illustrates that UFH and LMWH reduce VTE risk in hospitalized medical patients, but neither agent alters mortality. When directly compared, LMWH is more effective in preventing DVT.15
Despite the higher drug acquisition costs, LMWHs are more cost-effective than UFH for prophylaxis in medical patients because of their lower complication rates of HIT. LMWH reduces incremental costs by $13.88 per day compared with UFH.16, 17
Synthetic pentasaccharide: Fondaparinux is a synthetic analogue of the unique pentasaccharide sequence that mediates the interaction of heparin with antithrombin. It inhibits both free and platelet-bound factor Xa. It binds antithrombin with high affinity, has close to 100% bioavailability, and has a plasma half-life of 17 hours that permits once-daily administration.
The drug is excreted unchanged in the urine and therefore contraindicated in patients with severe renal impairment (e.g., creatinine clearance less than 30 mL/min). It does not bind PF4 in vitro and thus should not cause HIT.
Fondaparinux has been evaluated in medical inpatients in a randomized placebo controlled trial, ARTEMIS. Fondaparinux 2.5 mg SC once daily decreased the rate of venographically-confirmed DVT from 10.5% to 5.6% (p=0.029); there was also a decrease in fatal PE from 1.7% to 0.7% (p=0.029). A reduction in overall mortality from 6% to 3.3% (P=NS) was observed. Major bleeding occurred in 0.2% of patients in both groups. The drug is not FDA approved to prevent VTE in medical inpatients.18
Duration of therapy: Most clinical trials have used prophylaxis for seven to 14 days. The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial evaluated duration of therapy, the results of which were presented recently at the 21st Congress of the International Society on Thrombosis and Haemostasis (ISTH).
Of the 5,105 patients who met inclusion criteria, 5,049, or 99%, received open-label prophylaxis with enoxaparin (10 ± four days); 2,013 patients subsequently received extended-duration enoxaparin; and 2,027 received placebo (each for 28 ± four days). Subjects were at least 40 years old, had been recently immobilized (for at least 3 days) and had a predefined acute medical illness. Mobility was defined as level 1 (total bed rest or sedentary) or level 2 (bathroom privileges). After a planned blinded interim analysis, the trial’s data safety monitoring board recommended an amendment to the inclusion criteria, changing level 2 mobility to include age >75 years, and/or prior VTE, and/or diagnosed cancer.
When compared with placebo, extended-duration enoxaparin following the open-label, standard-duration enoxaparin reduced the relative risk of VTE by 44% (2.8% vs. 4.9%; p=0.0011). There was major bleeding in 12 subjects who received extended-duration enoxaparin and three subjects receiving placebo (0.6% vs. 0.1%; p=0.0192).
There was no difference in all-cause mortality between the extended-duration enoxaparin and placebo groups at six months (10.1% vs. 8.9%, p=0.179). The authors concluded 38 days of enoxaparin 40 mg SC once daily significantly reduced the overall incidence of VTE compared with a 10-day regimen in acutely ill medical patients with reduced mobility. This reduction in overall risk for VTE was consistent with a reduction in risk for asymptomatic proximal DVT and symptomatic VTE.
Based on this trial, we would recommend five weeks of prophylaxis for those older than 75 years with prior history of VTE or with active cancer. In the absence of these criteria, we recommend up to 14 days of therapy.
For many patients, this will mean prophylaxis after discharge from the hospital. While this represents a change in practice for many and may significantly complicate some discharge care plans we believe the significant burden of post-hospital VTE mandates a more aggressive approach to peri-hospitalization prophylaxis.
Back to the Case
Our patient has acute decompensated heart failure and likely will be limited in his ability to ambulate. All the clinical trials discussed above included similar patients and support the use of pharmacological prophylaxis. All hospitalized medical patients should undergo VTE risk assessment and implementation of pharmacologic prophylaxis in the absence of contraindications. TH
Dr. Lenchus is an assistant professor of medicine at the University of Miami School of Medicine. Dr. Jaffer is an associate professor of medicine at the University of Miami School of Medicine, where he serves as the chief of the division of hospital medicine.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996 Jun 15;93(12):2212-2245.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007 Jul 23;167(14):1471-1475.
- Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004 Jan 15;93(2):259-262.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. Sep 2007;132(3):936-945.
- Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I9-16.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007 Feb 20;146(4):278-288.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S-400S.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):188S-203S.
- Cade JF, Andrews JT, Stubbs AE. Comparison of sodium and calcium heparin in prevention of venous thromboembolism. Aust N Z J Med. 1982 Oct;12(5):501-504.
- Gallus AS, Hirsh J, Tutle RJ, et al. Small subcutaneous doses of heparin in prevention of venous thrombosis. N Engl J Med. 1973 Mar 15;288(11):545-551.
- Belch JJ, Lowe GD, Ward AG, Forbes CD, Prentice CR. Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott Med J. 1981 Apr;26(2):115-117.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999 Sep 9;341(11):793-800.
- Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004 Aug 17;110(7):874-879.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost. Jan 2000;83(1):14-19.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Jul 23;167(14):1476-1486.
- McGarry LJ, Thompson D, Weinstein MC, Goldhaber SZ. Cost effectiveness of thromboprophylaxis with a low-molecular-weight heparin versus unfractionated heparin in acutely ill medical inpatients. Am J Manag Care. 2004 Sep;10(9):632-642.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy. 2006 Oct;26(10):1438-1445.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006 Feb 11;332(7537):325-329.
Case
A 76-year-old gentleman is admitted for progressively worsening dyspnea, cough, and bilateral leg edema. Upon admission, his blood pressure is 150/90 mm/Hg, pulse 90 beats per minute, and respiration is 24 per minute.
Pertinent physical findings include jugular venous distension, bilateral crackles, S3 gallop, and 2+ bilateral lower extremity edema. The chest radiograph shows cardiomegaly and pulmonary edema. He is admitted to the hospital with a diagnosis of acute decompensated heart failure and starts aggressive medical therapy.
Overview
Approximately 2 million cases of deep-vein thrombosis (DVT) occur annually in the United States. Based on studies utilizing ventilation-perfusion scanning, half these patients likely have a silent pulmonary embolism (PE); of these, approximately 250,000 die.
The spectrum of venous thromboembolism (VTE), which includes DVT and PE, can vary from being asymptomatic to sudden death. Autopsy studies suggest a leading cause of sudden death in hospitalized medical patients is often a PE. There also are sequelae, such as chronic pulmonary hypertension, occurring in approximately 5% of PE cases, and post-thrombotic syndrome, occurring in approximately 40% of patients with DVT at two years.1
A recent study suggests DVT occurs three times more commonly in the outpatient setting. However, more than half of these patients were hospitalized in the three months prior.2 This is likely due to inadequate in-hospital prevention because of absence of prophylaxis, use of an unsuitable modality, insufficient dose of the drug, or ineffective duration of therapy. Inadequate and omitted VTE prophylaxis for medical patients was clearly demonstrated in the DVT Free Registry. This registry was created by 183 U.S. hospitals and included 5,451 patients, inpatients, and outpatients with ultrasound-confirmed DVT.
The number of medical inpatients who received prophylaxis in the 30 days prior to diagnosis was 28%—lower than the 48% of surgical patients.3 In a recent international registry, IMPROVE, only approximately 50% of hospitalized patients received prophylaxis.4
Virchow’s triad describes three underlying etiologic factors for thrombosis: stasis of blood flow, endothelial injury, and hypercoagulability. Established VTE risk factors reflect these underlying pathophysiologic processes. Important risk factors for VTE include increasing age, prolonged immobility, malignancy, major surgery, multiple trauma, prior VTE, and chronic heart failure.5
However, the magnitude of risk conferred by these and other risk factors varies (see Table 1, p. 35). It is not known how these factors interact to determine a patient’s individual VTE risk, but there is evidence it increases in proportion to the number of predisposing factors present.
In a recent systematic review of nine studies, including approximately 20,000 patients, prophylaxis reduced the rate of symptomatic VTE in at-risk hospitalized medical patients without increasing major bleeding.6
Multiple healthcare organizations, such as the National Quality Forum (NQF), Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and Agency for Healthcare Research and Quality (AHRQ) have identified VTE as a preventable condition in hospitalized patients. Formal risk assessment must be conducted as a first step, followed by the initiation of timely prophylaxis to improve patient safety.
Review of the Data
Mechanical forms of prophylaxis, such as graduated compression stockings, have been evaluated in patients with stroke and myocardial infarction. Intermittent pneumatic compression stockings and venous foot pumps have not been studied in randomized controlled trials (RCTs) in general medical patients.
Although there is data supporting the efficacy of these devices in surgical patients, the American College of Chest Physicians’ (ACCP) guidelines recommend against the use of mechanical forms of prophylaxis in medical patients unless there is a contraindication to pharmacologic prophylaxis.7
The ideal prophylactic agent is cost-effective and has no side effects. Available pharmacologic options for prevention of VTE in medical patients include unfractionated heparin (UFH), low molecular weight heparins (LMWHs), and the synthetic pentasaccharide, fondaparinux. Oral anticoagulants, (e.g., vitamin K antagonists [VKA]), have not been adequately studied in medical inpatients. Since VKA take several days to achieve therapeutic anticoagulation, we do not recommend using them de novo as VTE prophylaxis.
However, patients taking an oral VKA in the outpatient setting who have a therapeutic international normalized ratio (INR) during hospitalization probably are adequately protected from VTE and do not need additional pharmacologic prophylaxis. Newer anticoagulants in phase III testing for prevention of VTE in medically ill patients include oral direct thrombin inhibitors and anti-Xa inhibitors. ACCP guidelines recommend either low-dose UFH or LMWH as first-line agents for VTE prevention in medical inpatients.
Unfractionated heparin: UFH is a heterogeneous mixture of repeating polysaccharide chains of varying sizes, averaging about 15,000 Daltons. It binds anti-thrombin III (AT-III) and facilitates AT-III-mediated inactivation of factors IIa, IXa, Xa, and XIIa; of these, IIa and Xa are most responsive to inhibition.
Due to its large size, UFH only is partially absorbed from subcutaneous (SC) tissue, and it has a variable anticoagulant response due to interactions with plasma proteins, macrophages, and endothelial cells.8 However, in prophylactic SC doses (5,000 units two or three times daily), monitoring of the activated partial thromboplastin time (aPTT) is not required. In some cases, (e.g., frail or elderly patients), prophylactic SC doses may slightly prolong the aPTT.
UFH also binds to platelets and platelet factor 4 (PF4), and may precipitate heparin-induced thrombocytopenia (HIT). At least three clinical trials have compared the efficacy of SC UFH with a placebo and found prophylactic doses of UFH decrease the relative risk of DVT as detected by fibrinogen uptake test by about 70% without increasing the risk of bleeding.9-11
Low molecular weight heparins: LMWHs are derived from UFH through a chemical depolymerization, or fractionation, process. They are about one-third the size of UFH, with a molecular weight of approximately 5,000 Daltons.
These smaller molecules are readily absorbed from the SC tissue, eliciting a more predictable anticoagulant response than UFH. Unlike UFH, LMWHs have only minimal nonspecific binding to plasma proteins, endothelial cells, and monocytes, resulting in a predictable dose response. This obviates the need for lab monitoring, even when used in full, therapeutic dosing.
Compared with UFH, these agents have a longer plasma half-life, allowing them to be dosed SC once or twice daily. Also, they don’t bind platelets as readily as UFH resulting in a lower risk of HIT. Because they’re smaller, LMWHs tend to preferentially inhibit factor Xa, whereas UFH tends to inhibit factors Xa and IIa equally.
LMWHs have been evaluated in two large, placebo-controlled clinical trials for the prevention of VTE in medical inpatients.
In the first trial, MEDENOX, almost half the patients were older than 75 (mean age approximately 73). Inclusion criteria were NYHA class 3 or 4 heart failure, acute respiratory failure without mechanical ventilation, acute infection without septic shock, acute rheumatic disease, or inflammatory bowel disease. The primary outcome was assessed in 866 patients. Enoxaparin 40 mg SC once daily decreased the rate of VTE by two-thirds, from 15% to 5% (p=0.0002), without increased bleeding or thrombocytopenia compared with placebo.12 Enoxaparin 40 mg SC once daily is approved by the Food and Drug Administration (FDA) for VTE prophylaxis in medically ill patients.
PREVENT was an international, multicenter, randomized, double-blind, placebo-controlled trial evaluating dalteparin’s efficacy and safety. The inclusion criteria in this trial were acute congestive heart failure, non-ventilator-requiring acute respiratory failure, infection without septic shock, acute rheumatologic disorders, or inflammatory bowel disease. It studied 2,991 patients, and the primary outcome was VTE incidence and sudden death at day 21.
Dalteparin at 5,000 units decreased the rate of VTE, as detected by compression ultrasound, from 5% in the placebo group to 2.8%, a relative risk reduction of 45% (p=0.0015). The authors concluded the use of dalteparin reduced the incidence of VTE without increased risk of bleeding.13 Dalteparin is FDA approved for VTE prophylaxis in medical inpatients.
At least two meta-analyses have evaluated LMWHs compared with UFH. In the first, nine trials comparing LMWH to UFH (4,669 patients) were included. No significant effect was observed on DVT, clinical PE, or mortality. However, LMWH reduced the risk of major hemorrhage by 52% (p=0.049).14
In a more recent meta-analysis, 36 studies were included comparing placebos with UFH and LMWH. Both agents were associated with a reduced risk of VTE. A UFH dosage of 5,000 units three times daily was more effective in preventing DVT than a dosage of 5,000 units twice daily when compared with the control (risk ratio [RR], 0.27; 95% confidence interval [CI], 0.20-0.36; vs. RR, 0.52; 95% CI, 0.28-0.96). Neither UFH nor LMWH reduced mortality. When directly compared with UFH, LMWH was associated with a lower risk of DVT (RR, 0.68; 95% CI, 0.52-0.88) and injection-site hematoma (RR, 0.47; 95% CI, 0.36-0.62), but no difference was seen between the two agents in the risk of bleeding or thrombocytopenia.
This contemporary meta-analysis clearly illustrates that UFH and LMWH reduce VTE risk in hospitalized medical patients, but neither agent alters mortality. When directly compared, LMWH is more effective in preventing DVT.15
Despite the higher drug acquisition costs, LMWHs are more cost-effective than UFH for prophylaxis in medical patients because of their lower complication rates of HIT. LMWH reduces incremental costs by $13.88 per day compared with UFH.16, 17
Synthetic pentasaccharide: Fondaparinux is a synthetic analogue of the unique pentasaccharide sequence that mediates the interaction of heparin with antithrombin. It inhibits both free and platelet-bound factor Xa. It binds antithrombin with high affinity, has close to 100% bioavailability, and has a plasma half-life of 17 hours that permits once-daily administration.
The drug is excreted unchanged in the urine and therefore contraindicated in patients with severe renal impairment (e.g., creatinine clearance less than 30 mL/min). It does not bind PF4 in vitro and thus should not cause HIT.
Fondaparinux has been evaluated in medical inpatients in a randomized placebo controlled trial, ARTEMIS. Fondaparinux 2.5 mg SC once daily decreased the rate of venographically-confirmed DVT from 10.5% to 5.6% (p=0.029); there was also a decrease in fatal PE from 1.7% to 0.7% (p=0.029). A reduction in overall mortality from 6% to 3.3% (P=NS) was observed. Major bleeding occurred in 0.2% of patients in both groups. The drug is not FDA approved to prevent VTE in medical inpatients.18
Duration of therapy: Most clinical trials have used prophylaxis for seven to 14 days. The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial evaluated duration of therapy, the results of which were presented recently at the 21st Congress of the International Society on Thrombosis and Haemostasis (ISTH).
Of the 5,105 patients who met inclusion criteria, 5,049, or 99%, received open-label prophylaxis with enoxaparin (10 ± four days); 2,013 patients subsequently received extended-duration enoxaparin; and 2,027 received placebo (each for 28 ± four days). Subjects were at least 40 years old, had been recently immobilized (for at least 3 days) and had a predefined acute medical illness. Mobility was defined as level 1 (total bed rest or sedentary) or level 2 (bathroom privileges). After a planned blinded interim analysis, the trial’s data safety monitoring board recommended an amendment to the inclusion criteria, changing level 2 mobility to include age >75 years, and/or prior VTE, and/or diagnosed cancer.
When compared with placebo, extended-duration enoxaparin following the open-label, standard-duration enoxaparin reduced the relative risk of VTE by 44% (2.8% vs. 4.9%; p=0.0011). There was major bleeding in 12 subjects who received extended-duration enoxaparin and three subjects receiving placebo (0.6% vs. 0.1%; p=0.0192).
There was no difference in all-cause mortality between the extended-duration enoxaparin and placebo groups at six months (10.1% vs. 8.9%, p=0.179). The authors concluded 38 days of enoxaparin 40 mg SC once daily significantly reduced the overall incidence of VTE compared with a 10-day regimen in acutely ill medical patients with reduced mobility. This reduction in overall risk for VTE was consistent with a reduction in risk for asymptomatic proximal DVT and symptomatic VTE.
Based on this trial, we would recommend five weeks of prophylaxis for those older than 75 years with prior history of VTE or with active cancer. In the absence of these criteria, we recommend up to 14 days of therapy.
For many patients, this will mean prophylaxis after discharge from the hospital. While this represents a change in practice for many and may significantly complicate some discharge care plans we believe the significant burden of post-hospital VTE mandates a more aggressive approach to peri-hospitalization prophylaxis.
Back to the Case
Our patient has acute decompensated heart failure and likely will be limited in his ability to ambulate. All the clinical trials discussed above included similar patients and support the use of pharmacological prophylaxis. All hospitalized medical patients should undergo VTE risk assessment and implementation of pharmacologic prophylaxis in the absence of contraindications. TH
Dr. Lenchus is an assistant professor of medicine at the University of Miami School of Medicine. Dr. Jaffer is an associate professor of medicine at the University of Miami School of Medicine, where he serves as the chief of the division of hospital medicine.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996 Jun 15;93(12):2212-2245.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007 Jul 23;167(14):1471-1475.
- Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004 Jan 15;93(2):259-262.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. Sep 2007;132(3):936-945.
- Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I9-16.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007 Feb 20;146(4):278-288.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S-400S.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):188S-203S.
- Cade JF, Andrews JT, Stubbs AE. Comparison of sodium and calcium heparin in prevention of venous thromboembolism. Aust N Z J Med. 1982 Oct;12(5):501-504.
- Gallus AS, Hirsh J, Tutle RJ, et al. Small subcutaneous doses of heparin in prevention of venous thrombosis. N Engl J Med. 1973 Mar 15;288(11):545-551.
- Belch JJ, Lowe GD, Ward AG, Forbes CD, Prentice CR. Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott Med J. 1981 Apr;26(2):115-117.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999 Sep 9;341(11):793-800.
- Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004 Aug 17;110(7):874-879.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost. Jan 2000;83(1):14-19.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Jul 23;167(14):1476-1486.
- McGarry LJ, Thompson D, Weinstein MC, Goldhaber SZ. Cost effectiveness of thromboprophylaxis with a low-molecular-weight heparin versus unfractionated heparin in acutely ill medical inpatients. Am J Manag Care. 2004 Sep;10(9):632-642.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy. 2006 Oct;26(10):1438-1445.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006 Feb 11;332(7537):325-329.
Case
A 76-year-old gentleman is admitted for progressively worsening dyspnea, cough, and bilateral leg edema. Upon admission, his blood pressure is 150/90 mm/Hg, pulse 90 beats per minute, and respiration is 24 per minute.
Pertinent physical findings include jugular venous distension, bilateral crackles, S3 gallop, and 2+ bilateral lower extremity edema. The chest radiograph shows cardiomegaly and pulmonary edema. He is admitted to the hospital with a diagnosis of acute decompensated heart failure and starts aggressive medical therapy.
Overview
Approximately 2 million cases of deep-vein thrombosis (DVT) occur annually in the United States. Based on studies utilizing ventilation-perfusion scanning, half these patients likely have a silent pulmonary embolism (PE); of these, approximately 250,000 die.
The spectrum of venous thromboembolism (VTE), which includes DVT and PE, can vary from being asymptomatic to sudden death. Autopsy studies suggest a leading cause of sudden death in hospitalized medical patients is often a PE. There also are sequelae, such as chronic pulmonary hypertension, occurring in approximately 5% of PE cases, and post-thrombotic syndrome, occurring in approximately 40% of patients with DVT at two years.1
A recent study suggests DVT occurs three times more commonly in the outpatient setting. However, more than half of these patients were hospitalized in the three months prior.2 This is likely due to inadequate in-hospital prevention because of absence of prophylaxis, use of an unsuitable modality, insufficient dose of the drug, or ineffective duration of therapy. Inadequate and omitted VTE prophylaxis for medical patients was clearly demonstrated in the DVT Free Registry. This registry was created by 183 U.S. hospitals and included 5,451 patients, inpatients, and outpatients with ultrasound-confirmed DVT.
The number of medical inpatients who received prophylaxis in the 30 days prior to diagnosis was 28%—lower than the 48% of surgical patients.3 In a recent international registry, IMPROVE, only approximately 50% of hospitalized patients received prophylaxis.4
Virchow’s triad describes three underlying etiologic factors for thrombosis: stasis of blood flow, endothelial injury, and hypercoagulability. Established VTE risk factors reflect these underlying pathophysiologic processes. Important risk factors for VTE include increasing age, prolonged immobility, malignancy, major surgery, multiple trauma, prior VTE, and chronic heart failure.5
However, the magnitude of risk conferred by these and other risk factors varies (see Table 1, p. 35). It is not known how these factors interact to determine a patient’s individual VTE risk, but there is evidence it increases in proportion to the number of predisposing factors present.
In a recent systematic review of nine studies, including approximately 20,000 patients, prophylaxis reduced the rate of symptomatic VTE in at-risk hospitalized medical patients without increasing major bleeding.6
Multiple healthcare organizations, such as the National Quality Forum (NQF), Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and Agency for Healthcare Research and Quality (AHRQ) have identified VTE as a preventable condition in hospitalized patients. Formal risk assessment must be conducted as a first step, followed by the initiation of timely prophylaxis to improve patient safety.
Review of the Data
Mechanical forms of prophylaxis, such as graduated compression stockings, have been evaluated in patients with stroke and myocardial infarction. Intermittent pneumatic compression stockings and venous foot pumps have not been studied in randomized controlled trials (RCTs) in general medical patients.
Although there is data supporting the efficacy of these devices in surgical patients, the American College of Chest Physicians’ (ACCP) guidelines recommend against the use of mechanical forms of prophylaxis in medical patients unless there is a contraindication to pharmacologic prophylaxis.7
The ideal prophylactic agent is cost-effective and has no side effects. Available pharmacologic options for prevention of VTE in medical patients include unfractionated heparin (UFH), low molecular weight heparins (LMWHs), and the synthetic pentasaccharide, fondaparinux. Oral anticoagulants, (e.g., vitamin K antagonists [VKA]), have not been adequately studied in medical inpatients. Since VKA take several days to achieve therapeutic anticoagulation, we do not recommend using them de novo as VTE prophylaxis.
However, patients taking an oral VKA in the outpatient setting who have a therapeutic international normalized ratio (INR) during hospitalization probably are adequately protected from VTE and do not need additional pharmacologic prophylaxis. Newer anticoagulants in phase III testing for prevention of VTE in medically ill patients include oral direct thrombin inhibitors and anti-Xa inhibitors. ACCP guidelines recommend either low-dose UFH or LMWH as first-line agents for VTE prevention in medical inpatients.
Unfractionated heparin: UFH is a heterogeneous mixture of repeating polysaccharide chains of varying sizes, averaging about 15,000 Daltons. It binds anti-thrombin III (AT-III) and facilitates AT-III-mediated inactivation of factors IIa, IXa, Xa, and XIIa; of these, IIa and Xa are most responsive to inhibition.
Due to its large size, UFH only is partially absorbed from subcutaneous (SC) tissue, and it has a variable anticoagulant response due to interactions with plasma proteins, macrophages, and endothelial cells.8 However, in prophylactic SC doses (5,000 units two or three times daily), monitoring of the activated partial thromboplastin time (aPTT) is not required. In some cases, (e.g., frail or elderly patients), prophylactic SC doses may slightly prolong the aPTT.
UFH also binds to platelets and platelet factor 4 (PF4), and may precipitate heparin-induced thrombocytopenia (HIT). At least three clinical trials have compared the efficacy of SC UFH with a placebo and found prophylactic doses of UFH decrease the relative risk of DVT as detected by fibrinogen uptake test by about 70% without increasing the risk of bleeding.9-11
Low molecular weight heparins: LMWHs are derived from UFH through a chemical depolymerization, or fractionation, process. They are about one-third the size of UFH, with a molecular weight of approximately 5,000 Daltons.
These smaller molecules are readily absorbed from the SC tissue, eliciting a more predictable anticoagulant response than UFH. Unlike UFH, LMWHs have only minimal nonspecific binding to plasma proteins, endothelial cells, and monocytes, resulting in a predictable dose response. This obviates the need for lab monitoring, even when used in full, therapeutic dosing.
Compared with UFH, these agents have a longer plasma half-life, allowing them to be dosed SC once or twice daily. Also, they don’t bind platelets as readily as UFH resulting in a lower risk of HIT. Because they’re smaller, LMWHs tend to preferentially inhibit factor Xa, whereas UFH tends to inhibit factors Xa and IIa equally.
LMWHs have been evaluated in two large, placebo-controlled clinical trials for the prevention of VTE in medical inpatients.
In the first trial, MEDENOX, almost half the patients were older than 75 (mean age approximately 73). Inclusion criteria were NYHA class 3 or 4 heart failure, acute respiratory failure without mechanical ventilation, acute infection without septic shock, acute rheumatic disease, or inflammatory bowel disease. The primary outcome was assessed in 866 patients. Enoxaparin 40 mg SC once daily decreased the rate of VTE by two-thirds, from 15% to 5% (p=0.0002), without increased bleeding or thrombocytopenia compared with placebo.12 Enoxaparin 40 mg SC once daily is approved by the Food and Drug Administration (FDA) for VTE prophylaxis in medically ill patients.
PREVENT was an international, multicenter, randomized, double-blind, placebo-controlled trial evaluating dalteparin’s efficacy and safety. The inclusion criteria in this trial were acute congestive heart failure, non-ventilator-requiring acute respiratory failure, infection without septic shock, acute rheumatologic disorders, or inflammatory bowel disease. It studied 2,991 patients, and the primary outcome was VTE incidence and sudden death at day 21.
Dalteparin at 5,000 units decreased the rate of VTE, as detected by compression ultrasound, from 5% in the placebo group to 2.8%, a relative risk reduction of 45% (p=0.0015). The authors concluded the use of dalteparin reduced the incidence of VTE without increased risk of bleeding.13 Dalteparin is FDA approved for VTE prophylaxis in medical inpatients.
At least two meta-analyses have evaluated LMWHs compared with UFH. In the first, nine trials comparing LMWH to UFH (4,669 patients) were included. No significant effect was observed on DVT, clinical PE, or mortality. However, LMWH reduced the risk of major hemorrhage by 52% (p=0.049).14
In a more recent meta-analysis, 36 studies were included comparing placebos with UFH and LMWH. Both agents were associated with a reduced risk of VTE. A UFH dosage of 5,000 units three times daily was more effective in preventing DVT than a dosage of 5,000 units twice daily when compared with the control (risk ratio [RR], 0.27; 95% confidence interval [CI], 0.20-0.36; vs. RR, 0.52; 95% CI, 0.28-0.96). Neither UFH nor LMWH reduced mortality. When directly compared with UFH, LMWH was associated with a lower risk of DVT (RR, 0.68; 95% CI, 0.52-0.88) and injection-site hematoma (RR, 0.47; 95% CI, 0.36-0.62), but no difference was seen between the two agents in the risk of bleeding or thrombocytopenia.
This contemporary meta-analysis clearly illustrates that UFH and LMWH reduce VTE risk in hospitalized medical patients, but neither agent alters mortality. When directly compared, LMWH is more effective in preventing DVT.15
Despite the higher drug acquisition costs, LMWHs are more cost-effective than UFH for prophylaxis in medical patients because of their lower complication rates of HIT. LMWH reduces incremental costs by $13.88 per day compared with UFH.16, 17
Synthetic pentasaccharide: Fondaparinux is a synthetic analogue of the unique pentasaccharide sequence that mediates the interaction of heparin with antithrombin. It inhibits both free and platelet-bound factor Xa. It binds antithrombin with high affinity, has close to 100% bioavailability, and has a plasma half-life of 17 hours that permits once-daily administration.
The drug is excreted unchanged in the urine and therefore contraindicated in patients with severe renal impairment (e.g., creatinine clearance less than 30 mL/min). It does not bind PF4 in vitro and thus should not cause HIT.
Fondaparinux has been evaluated in medical inpatients in a randomized placebo controlled trial, ARTEMIS. Fondaparinux 2.5 mg SC once daily decreased the rate of venographically-confirmed DVT from 10.5% to 5.6% (p=0.029); there was also a decrease in fatal PE from 1.7% to 0.7% (p=0.029). A reduction in overall mortality from 6% to 3.3% (P=NS) was observed. Major bleeding occurred in 0.2% of patients in both groups. The drug is not FDA approved to prevent VTE in medical inpatients.18
Duration of therapy: Most clinical trials have used prophylaxis for seven to 14 days. The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial evaluated duration of therapy, the results of which were presented recently at the 21st Congress of the International Society on Thrombosis and Haemostasis (ISTH).
Of the 5,105 patients who met inclusion criteria, 5,049, or 99%, received open-label prophylaxis with enoxaparin (10 ± four days); 2,013 patients subsequently received extended-duration enoxaparin; and 2,027 received placebo (each for 28 ± four days). Subjects were at least 40 years old, had been recently immobilized (for at least 3 days) and had a predefined acute medical illness. Mobility was defined as level 1 (total bed rest or sedentary) or level 2 (bathroom privileges). After a planned blinded interim analysis, the trial’s data safety monitoring board recommended an amendment to the inclusion criteria, changing level 2 mobility to include age >75 years, and/or prior VTE, and/or diagnosed cancer.
When compared with placebo, extended-duration enoxaparin following the open-label, standard-duration enoxaparin reduced the relative risk of VTE by 44% (2.8% vs. 4.9%; p=0.0011). There was major bleeding in 12 subjects who received extended-duration enoxaparin and three subjects receiving placebo (0.6% vs. 0.1%; p=0.0192).
There was no difference in all-cause mortality between the extended-duration enoxaparin and placebo groups at six months (10.1% vs. 8.9%, p=0.179). The authors concluded 38 days of enoxaparin 40 mg SC once daily significantly reduced the overall incidence of VTE compared with a 10-day regimen in acutely ill medical patients with reduced mobility. This reduction in overall risk for VTE was consistent with a reduction in risk for asymptomatic proximal DVT and symptomatic VTE.
Based on this trial, we would recommend five weeks of prophylaxis for those older than 75 years with prior history of VTE or with active cancer. In the absence of these criteria, we recommend up to 14 days of therapy.
For many patients, this will mean prophylaxis after discharge from the hospital. While this represents a change in practice for many and may significantly complicate some discharge care plans we believe the significant burden of post-hospital VTE mandates a more aggressive approach to peri-hospitalization prophylaxis.
Back to the Case
Our patient has acute decompensated heart failure and likely will be limited in his ability to ambulate. All the clinical trials discussed above included similar patients and support the use of pharmacological prophylaxis. All hospitalized medical patients should undergo VTE risk assessment and implementation of pharmacologic prophylaxis in the absence of contraindications. TH
Dr. Lenchus is an assistant professor of medicine at the University of Miami School of Medicine. Dr. Jaffer is an associate professor of medicine at the University of Miami School of Medicine, where he serves as the chief of the division of hospital medicine.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996 Jun 15;93(12):2212-2245.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007 Jul 23;167(14):1471-1475.
- Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004 Jan 15;93(2):259-262.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. Sep 2007;132(3):936-945.
- Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I9-16.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007 Feb 20;146(4):278-288.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S-400S.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):188S-203S.
- Cade JF, Andrews JT, Stubbs AE. Comparison of sodium and calcium heparin in prevention of venous thromboembolism. Aust N Z J Med. 1982 Oct;12(5):501-504.
- Gallus AS, Hirsh J, Tutle RJ, et al. Small subcutaneous doses of heparin in prevention of venous thrombosis. N Engl J Med. 1973 Mar 15;288(11):545-551.
- Belch JJ, Lowe GD, Ward AG, Forbes CD, Prentice CR. Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott Med J. 1981 Apr;26(2):115-117.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999 Sep 9;341(11):793-800.
- Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004 Aug 17;110(7):874-879.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost. Jan 2000;83(1):14-19.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Jul 23;167(14):1476-1486.
- McGarry LJ, Thompson D, Weinstein MC, Goldhaber SZ. Cost effectiveness of thromboprophylaxis with a low-molecular-weight heparin versus unfractionated heparin in acutely ill medical inpatients. Am J Manag Care. 2004 Sep;10(9):632-642.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy. 2006 Oct;26(10):1438-1445.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006 Feb 11;332(7537):325-329.