User login
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
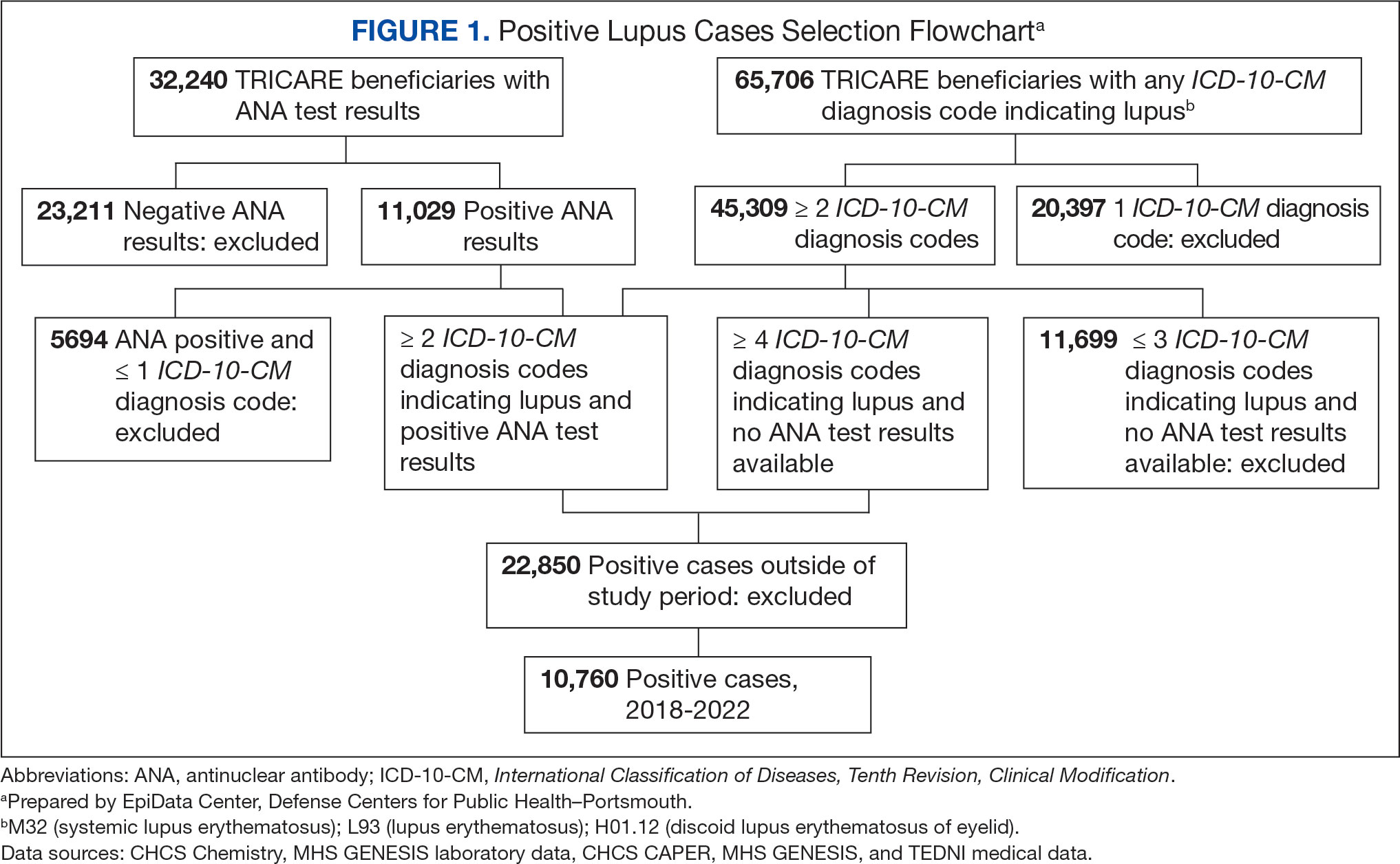
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).
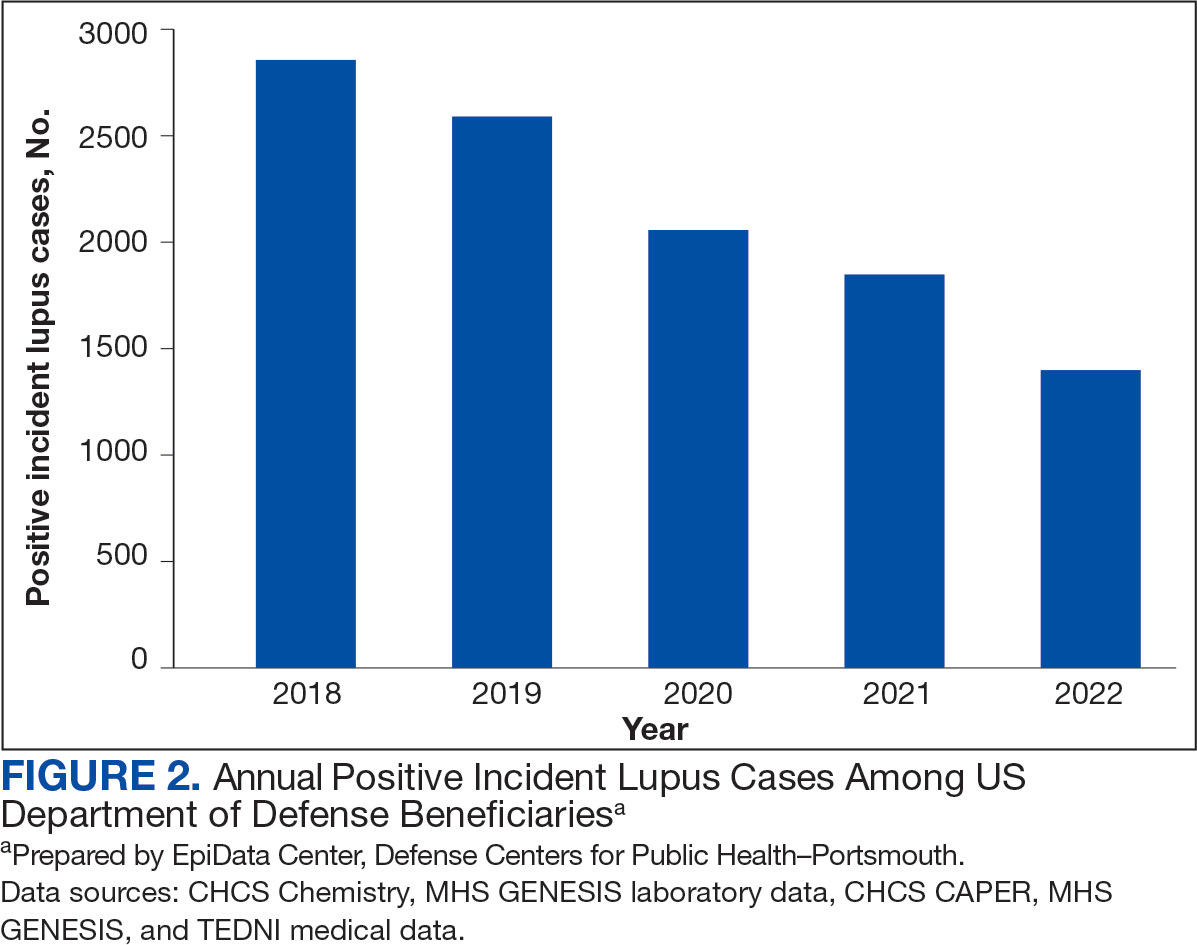
Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.
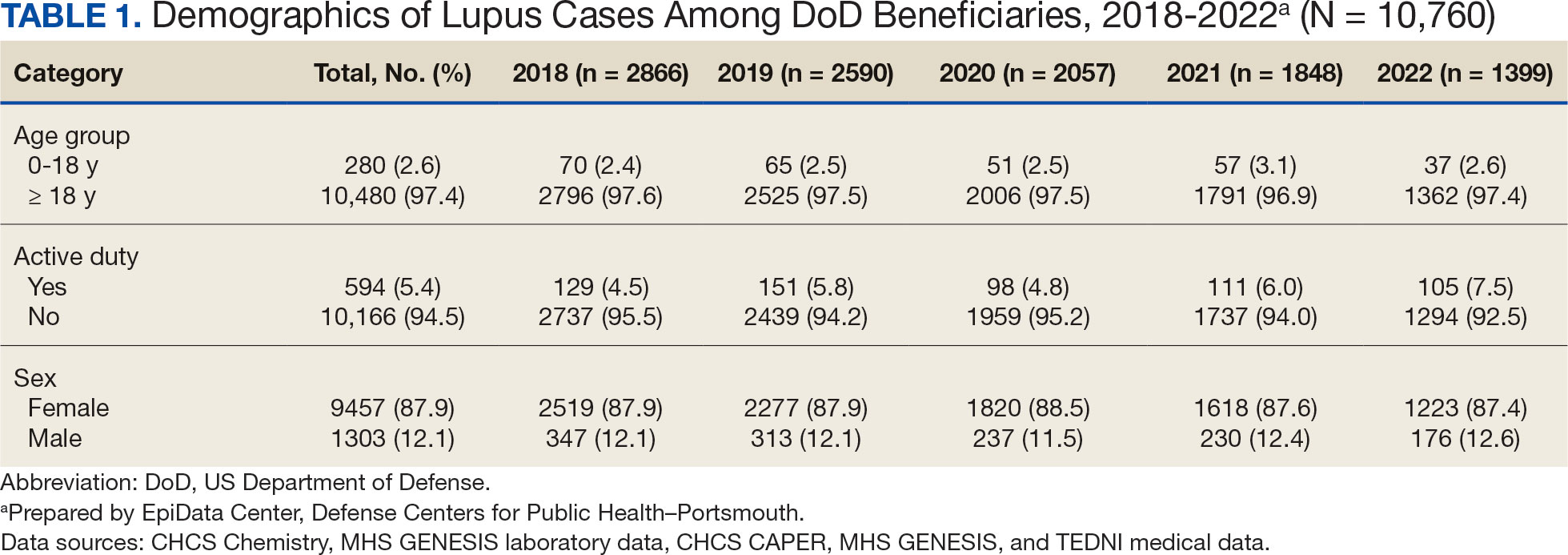
The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.
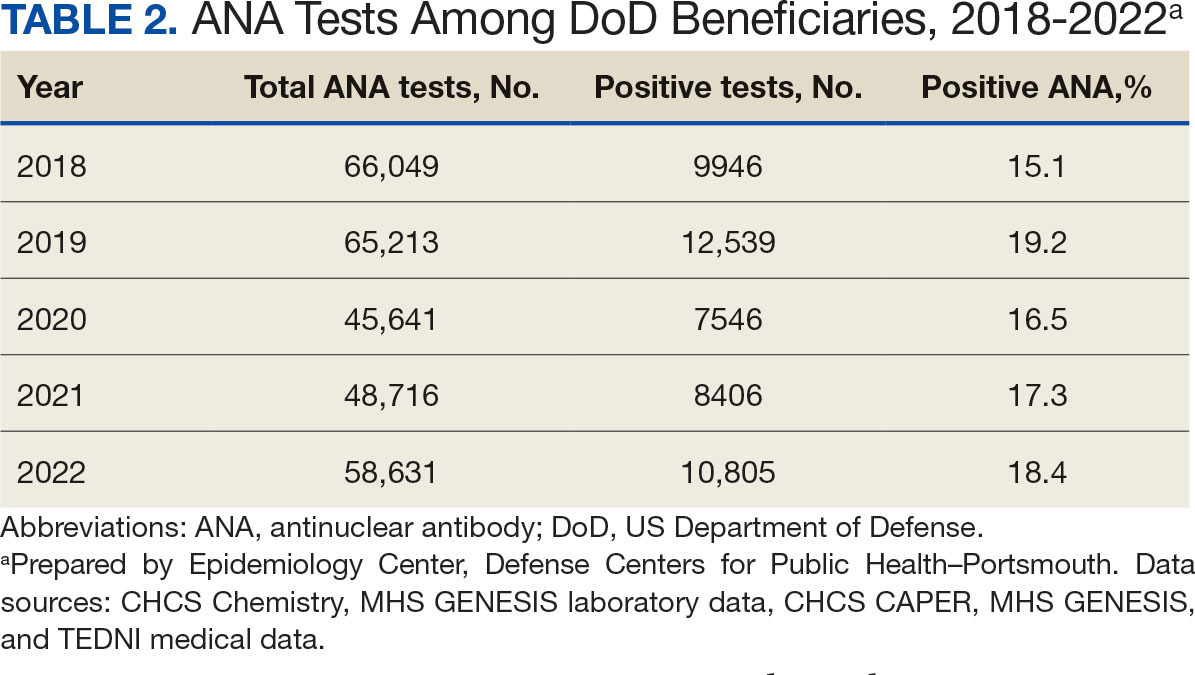
A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.
Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
- Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med. 2021;8(1):e000614. doi:10.1136/lupus-2021-000614
- Centers for Disease Control and Prevention. People with lupus. May 15, 2024. Accessed May 10, 2025. https:// www.cdc.gov/lupus/data-research/index.html
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533. doi:10.1056/nejmoa021933
- Li ZX, Zeng S, Wu HX, Zhou Y. The risk of systemic lupus erythematosus associated with Epstein–Barr virus infection: a systematic review and meta-analysis. Clin Exp Med. 2019;19(1):23-36. doi:10.1007/s10238-018-0535-0
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi:10.1126/science.abd4585
- Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):5417. doi:10.1038/s41467-021-25509-3
- Lee SJ, Yoon T, Ha JW, et al. Prevalence, clinical significance, and persistence of autoantibodies in COVID-19. Virol J. 2023;20(1):236. doi:10.1186/s12985-023-02191-z
- Woodruff MC, Ramonell RP, Haddad NS, et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. 2022;611(7934):139-147. doi:10.1038/s41586-022-05273-0
- Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77(8):2415-2430. doi:10.1111/all.15302
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592 doi:10.3390/cells10123592
- Barnado A, Carroll R, Denny JC, Crofford L. Using IC-10-CM codes to identify patients with systemic lupus erythematosus in the electronic health record [abstract]. Arthritis Rheumatol. 2018;70(suppl 9):abstract 1692. Accessed May 10, 2025. https://acrabstracts.org/abstract/using-icd-10-cm-codes-to-identify-patients-with-systemic-lupus-erythematosus-in-the-electronic-health-record
- Feldman C, Curtis JR, Oates JC, Yazdany J, Izmirly P. Validating claims-based algorithms for a systemic lupus erythematosus diagnosis in Medicare data for informed use of the Lupus Index: a tool for geospatial research. Lupus Sci Med. 2024;11(2):e001329. doi:10.1136/lupus-2024-001329
- Moe SR, Haukeland H, Brunborg C, et al. POS1472: Accuracy of disease-specific ICD-10 code for incident systemic lupus erythematosus; results from a population-based cohort study set in Norway [abstract]. Ann Rheum Dis. 2023;82(suppl 1):1090-1091. doi:10.1136/annrheumdis-2023-eular.1189
- Denagamage P, Mabila SL, McQuistan AA. Trends and disparities in systemic lupus erythematosus incidence among U.S. active component service members, 2000–2022. MSMR. 2023;30(12):2-5.
- Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56:101783. doi:10.1016/j.eclinm.2022.101783
- Tesch F, Ehm F, Vivirito A, et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol. 2023;42(10):2905- 2914. doi:10.1007/s10067-023-06670-0
- Peng K, Li X, Yang D, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine. 2023;63:102154. doi:10.1016/j.eclinm.2023.102154
- US Department of Defense. Coronavirus: DOD response. Updated December 20, 2022. Accessed May 10, 2025. https://www.defense.gov/Spotlights/Coronavirus-DOD-Response/
- Elflein J. Number of cumulative cases of COVID-19 in the United States from January 20, 2020 to November 11, 2022, by week. Statista. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day
- Ye Z, Chen L, Zhong H, Cao L, Fu P, Xu J. Epidemiology and clinical characteristics of Epstein-Barr virus infection among children in Shanghai, China, 2017- 2022. Front Cell Infect Microbiol. 2023;13:1139068. doi:10.3389/fcimb.2023.1139068
- Johnson D, Jiang W. Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962. doi:10.1016/j.jaut.2022.102962
Biomarker Changes in Systemic Sclerosis–Associated Lung Disease Predict Therapy Response
TOPLINE:
Changes in Krebs von den Lungen 6 (KL-6) levels after 12 months of treatment with mycophenolate mofetil (MMF) or cyclophosphamide (CYC) are associated with the development of progressive pulmonary fibrosis (PPF) in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD) in the following year.
METHODOLOGY:
- Despite available treatments, about 25% of patients with SSc-ILD develop PPF, highlighting the need for reliable early treatment response indicators, such as blood biomarkers, which may help predict the risk for PPF.
- Researchers conducted post hoc analyses of a randomized control trial that compared treatment responses to MMF with those to CYC in patients with SSc-ILD. Patients received either oral CYC for 12 months followed by placebo for 12 months or MMF for 24 months.
- A total of 92 patients with complete biomarker measurements at baseline and 12 months were included in the analysis (mean age, 52.2 years; 73.9% women; 68.5% White).
- The analysis included measurement of multiple blood biomarker levels, including C-reactive protein (CRP), interleukin-6, chemokine ligand 4 (CXCL4), CXCL18, and KL-6. Changes in these levels were evaluated from baseline to 12 months.
- The primary outcome was the development of PPF between 12 and 24 months, defined by meeting at least two of these following conditions: Worsening respiratory symptoms, a decline in forced vital capacity ≥ 5% and/or a decline in diffusing capacity for carbon monoxide ≥ 10%, or radiological disease progression.
TAKEAWAY:
- Among 92 patients, 19 developed PPF between 12 and 24 months, with 10 patients in the MMF arm and 9 patients in the CYC arm.
- KL-6 levels increased from baseline to 12 months in patients who developed PPF and decreased in those who did not (mean change, 365.68 vs –207.45 u/mL; P < .001).
- A 0.10-unit increase in KL-6 levels was associated with a 40% increase in the odds of developing PPF in an adjusted analysis (P = .0002).
- In the MMF group, levels of KL-6, CRP, and CXCL4 differed significantly between patients who developed PPF and those who did not (P = .004, P = .04, and P = .038, respectively).
IN PRACTICE:
“Reliable response biomarkers detectable early in the course of SSc-ILD treatment could minimize exposure to toxic therapies that are not conferring benefit and maximize exposure to alternative therapies that do confer benefit,” the authors wrote.
SOURCE:
The study was led by Elizabeth R. Volkmann, MD, MS, University of California, Los Angeles David Geffen School of Medicine. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study population consisted of patients who were treatment-naive to MMF and CYC and had a relatively early disease course, potentially limiting generalizability to patients at later disease stages or with different treatment histories. Additionally, biomarker measurements were conducted at 12 months, when treatment response may be detectable through currently available methods, rather than at earlier timepoints.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Department of Defense. MMF was supplied by Hoffmann–La Roche. Some authors reported having financial relationships with pharmaceutical companies, including Hoffmann–La Roche.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Changes in Krebs von den Lungen 6 (KL-6) levels after 12 months of treatment with mycophenolate mofetil (MMF) or cyclophosphamide (CYC) are associated with the development of progressive pulmonary fibrosis (PPF) in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD) in the following year.
METHODOLOGY:
- Despite available treatments, about 25% of patients with SSc-ILD develop PPF, highlighting the need for reliable early treatment response indicators, such as blood biomarkers, which may help predict the risk for PPF.
- Researchers conducted post hoc analyses of a randomized control trial that compared treatment responses to MMF with those to CYC in patients with SSc-ILD. Patients received either oral CYC for 12 months followed by placebo for 12 months or MMF for 24 months.
- A total of 92 patients with complete biomarker measurements at baseline and 12 months were included in the analysis (mean age, 52.2 years; 73.9% women; 68.5% White).
- The analysis included measurement of multiple blood biomarker levels, including C-reactive protein (CRP), interleukin-6, chemokine ligand 4 (CXCL4), CXCL18, and KL-6. Changes in these levels were evaluated from baseline to 12 months.
- The primary outcome was the development of PPF between 12 and 24 months, defined by meeting at least two of these following conditions: Worsening respiratory symptoms, a decline in forced vital capacity ≥ 5% and/or a decline in diffusing capacity for carbon monoxide ≥ 10%, or radiological disease progression.
TAKEAWAY:
- Among 92 patients, 19 developed PPF between 12 and 24 months, with 10 patients in the MMF arm and 9 patients in the CYC arm.
- KL-6 levels increased from baseline to 12 months in patients who developed PPF and decreased in those who did not (mean change, 365.68 vs –207.45 u/mL; P < .001).
- A 0.10-unit increase in KL-6 levels was associated with a 40% increase in the odds of developing PPF in an adjusted analysis (P = .0002).
- In the MMF group, levels of KL-6, CRP, and CXCL4 differed significantly between patients who developed PPF and those who did not (P = .004, P = .04, and P = .038, respectively).
IN PRACTICE:
“Reliable response biomarkers detectable early in the course of SSc-ILD treatment could minimize exposure to toxic therapies that are not conferring benefit and maximize exposure to alternative therapies that do confer benefit,” the authors wrote.
SOURCE:
The study was led by Elizabeth R. Volkmann, MD, MS, University of California, Los Angeles David Geffen School of Medicine. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study population consisted of patients who were treatment-naive to MMF and CYC and had a relatively early disease course, potentially limiting generalizability to patients at later disease stages or with different treatment histories. Additionally, biomarker measurements were conducted at 12 months, when treatment response may be detectable through currently available methods, rather than at earlier timepoints.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Department of Defense. MMF was supplied by Hoffmann–La Roche. Some authors reported having financial relationships with pharmaceutical companies, including Hoffmann–La Roche.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Changes in Krebs von den Lungen 6 (KL-6) levels after 12 months of treatment with mycophenolate mofetil (MMF) or cyclophosphamide (CYC) are associated with the development of progressive pulmonary fibrosis (PPF) in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD) in the following year.
METHODOLOGY:
- Despite available treatments, about 25% of patients with SSc-ILD develop PPF, highlighting the need for reliable early treatment response indicators, such as blood biomarkers, which may help predict the risk for PPF.
- Researchers conducted post hoc analyses of a randomized control trial that compared treatment responses to MMF with those to CYC in patients with SSc-ILD. Patients received either oral CYC for 12 months followed by placebo for 12 months or MMF for 24 months.
- A total of 92 patients with complete biomarker measurements at baseline and 12 months were included in the analysis (mean age, 52.2 years; 73.9% women; 68.5% White).
- The analysis included measurement of multiple blood biomarker levels, including C-reactive protein (CRP), interleukin-6, chemokine ligand 4 (CXCL4), CXCL18, and KL-6. Changes in these levels were evaluated from baseline to 12 months.
- The primary outcome was the development of PPF between 12 and 24 months, defined by meeting at least two of these following conditions: Worsening respiratory symptoms, a decline in forced vital capacity ≥ 5% and/or a decline in diffusing capacity for carbon monoxide ≥ 10%, or radiological disease progression.
TAKEAWAY:
- Among 92 patients, 19 developed PPF between 12 and 24 months, with 10 patients in the MMF arm and 9 patients in the CYC arm.
- KL-6 levels increased from baseline to 12 months in patients who developed PPF and decreased in those who did not (mean change, 365.68 vs –207.45 u/mL; P < .001).
- A 0.10-unit increase in KL-6 levels was associated with a 40% increase in the odds of developing PPF in an adjusted analysis (P = .0002).
- In the MMF group, levels of KL-6, CRP, and CXCL4 differed significantly between patients who developed PPF and those who did not (P = .004, P = .04, and P = .038, respectively).
IN PRACTICE:
“Reliable response biomarkers detectable early in the course of SSc-ILD treatment could minimize exposure to toxic therapies that are not conferring benefit and maximize exposure to alternative therapies that do confer benefit,” the authors wrote.
SOURCE:
The study was led by Elizabeth R. Volkmann, MD, MS, University of California, Los Angeles David Geffen School of Medicine. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study population consisted of patients who were treatment-naive to MMF and CYC and had a relatively early disease course, potentially limiting generalizability to patients at later disease stages or with different treatment histories. Additionally, biomarker measurements were conducted at 12 months, when treatment response may be detectable through currently available methods, rather than at earlier timepoints.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Department of Defense. MMF was supplied by Hoffmann–La Roche. Some authors reported having financial relationships with pharmaceutical companies, including Hoffmann–La Roche.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Valaciclovir Shows Promise in Preventing Herpes Zoster During Anifrolumab Treatment for Lupus
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Sjögren Subtypes Have Distinct Pathophysiologic Profiles
TOPLINE:
Sjögren disease (SjD) presents three distinct phenotypes ― B-cell active with low symptom burden, high systemic activity with high symptom burden, and low systemic activity with high symptom burden; each phenotype has unique cytokine profiles and interferon (IFN) signatures, with elevated cytokine levels for T and B lymphocyte activation in the first two groups and a prominent IFN signature in the B-cell active group.
METHODOLOGY:
- Researchers conducted this study to assess whether three distinct phenotypes of patients with SjD were associated with distinct pathophysiological pathways and IFN signatures.
- They included 395 patients (median age, 53 years; 94% women) from the Assessment of Systemic Signs and Evolution in Sjögren’s Syndrome (ASSESS) cohort who met the 2002 American-European Consensus Group criteria for SjD.
- A panel of biomarkers including IFN alpha-2, IFN gamma, CXCL10, CXCL13, B-cell activating factor, interleukin (IL) 7, FLT3, CCL19, and tumor necrosis factor receptor II (TNF-RII) was compared between the three phenotypes.
- The IFN signature was assessed using whole blood transcriptomic analysis.
- Analysis compared systemic and symptomatic evolution and assessed the risk for new immunosuppressant prescription and lymphoma development across three clusters on the basis of the IFN signature.
TAKEAWAY:
- Higher levels of CXCL13, IL-7, and TNF-RII cytokines were found in both the B-cell active and high systemic activity groups than in the low systemic activity group (P < .05 for all).
- The low systemic activity cluster with reduced cytokine levels showed less disease progression, with no instances of lymphoma reported in this group.
- A high IFN signature was found in a higher percentage of patients in the B-cell active group (57%) than in the high systemic activity (48%) and low systemic activity (38%) groups.
- In the B-cell active cluster, this high IFN signature was associated with an increased risk for new immunosuppressant prescription, indicating greater disease progression (hazard ratio, 9.38; P = .0032); also, all cases of lymphoma within this group were found in individuals exhibiting a high IFN signature.
IN PRACTICE:
“Our study demonstrated that our stratification, defined by symptoms, systemic clinical signs, and routine biological data, is based on different pathophysiological pathways, particularly B and T lymphocyte activation and the interferon alpha pathway. The latter could help predict the evolution of the BALS cluster, a biological but minimally symptomatic cluster, to consider closer monitoring and/or early treatments to prevent complications,” the authors wrote.
SOURCE:
The study was led by Yann Nguyen, MD, PhD, Department of Rheumatology, Hôpital Bicêtre, Assistance Publique — Hôpitaux de Paris, Université Paris-Saclay, Paris, France, and was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study evaluated only a few cytokines at the time of inclusion in the ASSESS cohort, which may not have captured the full range of biologic markers relevant to SjD. The evolution of systemic activity defined using the European Alliance of Associations for Rheumatology Sjogren’s Syndrome Disease Activity Index showed no difference according to the IFN signature in the B-cell active with low symptom burden cluster, possibly due to treatments received between annual evaluations.
DISCLOSURES:
The ASSESS cohort is supported by research grants from the French Society of Rheumatology. Some authors reported receiving grants, payments, honoraria, consulting fees, and support for attending meetings and having contracts or other ties with pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Sjögren disease (SjD) presents three distinct phenotypes ― B-cell active with low symptom burden, high systemic activity with high symptom burden, and low systemic activity with high symptom burden; each phenotype has unique cytokine profiles and interferon (IFN) signatures, with elevated cytokine levels for T and B lymphocyte activation in the first two groups and a prominent IFN signature in the B-cell active group.
METHODOLOGY:
- Researchers conducted this study to assess whether three distinct phenotypes of patients with SjD were associated with distinct pathophysiological pathways and IFN signatures.
- They included 395 patients (median age, 53 years; 94% women) from the Assessment of Systemic Signs and Evolution in Sjögren’s Syndrome (ASSESS) cohort who met the 2002 American-European Consensus Group criteria for SjD.
- A panel of biomarkers including IFN alpha-2, IFN gamma, CXCL10, CXCL13, B-cell activating factor, interleukin (IL) 7, FLT3, CCL19, and tumor necrosis factor receptor II (TNF-RII) was compared between the three phenotypes.
- The IFN signature was assessed using whole blood transcriptomic analysis.
- Analysis compared systemic and symptomatic evolution and assessed the risk for new immunosuppressant prescription and lymphoma development across three clusters on the basis of the IFN signature.
TAKEAWAY:
- Higher levels of CXCL13, IL-7, and TNF-RII cytokines were found in both the B-cell active and high systemic activity groups than in the low systemic activity group (P < .05 for all).
- The low systemic activity cluster with reduced cytokine levels showed less disease progression, with no instances of lymphoma reported in this group.
- A high IFN signature was found in a higher percentage of patients in the B-cell active group (57%) than in the high systemic activity (48%) and low systemic activity (38%) groups.
- In the B-cell active cluster, this high IFN signature was associated with an increased risk for new immunosuppressant prescription, indicating greater disease progression (hazard ratio, 9.38; P = .0032); also, all cases of lymphoma within this group were found in individuals exhibiting a high IFN signature.
IN PRACTICE:
“Our study demonstrated that our stratification, defined by symptoms, systemic clinical signs, and routine biological data, is based on different pathophysiological pathways, particularly B and T lymphocyte activation and the interferon alpha pathway. The latter could help predict the evolution of the BALS cluster, a biological but minimally symptomatic cluster, to consider closer monitoring and/or early treatments to prevent complications,” the authors wrote.
SOURCE:
The study was led by Yann Nguyen, MD, PhD, Department of Rheumatology, Hôpital Bicêtre, Assistance Publique — Hôpitaux de Paris, Université Paris-Saclay, Paris, France, and was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study evaluated only a few cytokines at the time of inclusion in the ASSESS cohort, which may not have captured the full range of biologic markers relevant to SjD. The evolution of systemic activity defined using the European Alliance of Associations for Rheumatology Sjogren’s Syndrome Disease Activity Index showed no difference according to the IFN signature in the B-cell active with low symptom burden cluster, possibly due to treatments received between annual evaluations.
DISCLOSURES:
The ASSESS cohort is supported by research grants from the French Society of Rheumatology. Some authors reported receiving grants, payments, honoraria, consulting fees, and support for attending meetings and having contracts or other ties with pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Sjögren disease (SjD) presents three distinct phenotypes ― B-cell active with low symptom burden, high systemic activity with high symptom burden, and low systemic activity with high symptom burden; each phenotype has unique cytokine profiles and interferon (IFN) signatures, with elevated cytokine levels for T and B lymphocyte activation in the first two groups and a prominent IFN signature in the B-cell active group.
METHODOLOGY:
- Researchers conducted this study to assess whether three distinct phenotypes of patients with SjD were associated with distinct pathophysiological pathways and IFN signatures.
- They included 395 patients (median age, 53 years; 94% women) from the Assessment of Systemic Signs and Evolution in Sjögren’s Syndrome (ASSESS) cohort who met the 2002 American-European Consensus Group criteria for SjD.
- A panel of biomarkers including IFN alpha-2, IFN gamma, CXCL10, CXCL13, B-cell activating factor, interleukin (IL) 7, FLT3, CCL19, and tumor necrosis factor receptor II (TNF-RII) was compared between the three phenotypes.
- The IFN signature was assessed using whole blood transcriptomic analysis.
- Analysis compared systemic and symptomatic evolution and assessed the risk for new immunosuppressant prescription and lymphoma development across three clusters on the basis of the IFN signature.
TAKEAWAY:
- Higher levels of CXCL13, IL-7, and TNF-RII cytokines were found in both the B-cell active and high systemic activity groups than in the low systemic activity group (P < .05 for all).
- The low systemic activity cluster with reduced cytokine levels showed less disease progression, with no instances of lymphoma reported in this group.
- A high IFN signature was found in a higher percentage of patients in the B-cell active group (57%) than in the high systemic activity (48%) and low systemic activity (38%) groups.
- In the B-cell active cluster, this high IFN signature was associated with an increased risk for new immunosuppressant prescription, indicating greater disease progression (hazard ratio, 9.38; P = .0032); also, all cases of lymphoma within this group were found in individuals exhibiting a high IFN signature.
IN PRACTICE:
“Our study demonstrated that our stratification, defined by symptoms, systemic clinical signs, and routine biological data, is based on different pathophysiological pathways, particularly B and T lymphocyte activation and the interferon alpha pathway. The latter could help predict the evolution of the BALS cluster, a biological but minimally symptomatic cluster, to consider closer monitoring and/or early treatments to prevent complications,” the authors wrote.
SOURCE:
The study was led by Yann Nguyen, MD, PhD, Department of Rheumatology, Hôpital Bicêtre, Assistance Publique — Hôpitaux de Paris, Université Paris-Saclay, Paris, France, and was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study evaluated only a few cytokines at the time of inclusion in the ASSESS cohort, which may not have captured the full range of biologic markers relevant to SjD. The evolution of systemic activity defined using the European Alliance of Associations for Rheumatology Sjogren’s Syndrome Disease Activity Index showed no difference according to the IFN signature in the B-cell active with low symptom burden cluster, possibly due to treatments received between annual evaluations.
DISCLOSURES:
The ASSESS cohort is supported by research grants from the French Society of Rheumatology. Some authors reported receiving grants, payments, honoraria, consulting fees, and support for attending meetings and having contracts or other ties with pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Lupus Ups Atherosclerosis Risk, But Disease Remission Helps
TOPLINE:
Patients with systemic lupus erythematosus (SLE) face more than double the risk for atherosclerotic plaque progression than healthy control individuals without the condition, but management of traditional cardiovascular risk factors and prolonged clinical remission can successfully mitigate it.
METHODOLOGY:
- Researchers performed a prospective study to assess the progression of subclinical atherosclerosis plaques and the development of cardiovascular events in patients with SLE over a 10-year follow-up period.
- They included 111 patients with SLE (mean age, 43 years; 91% women) and 94 matched healthy control individuals without prior atherosclerotic cardiovascular disease (CVD), active malignancy, pregnancy, or diabetes mellitus who underwent carotid ultrasound measurements.
- A total of 738 carotid measurements were analyzed from baseline to 3-, 7-, and 10-year follow-up periods for assessing new carotid plaque development; incident CVD events were also analyzed during follow-up.
- Disease remission was evaluated based on the Definition of Remission in SLE criteria.
- Target for management of cardiovascular risk factors was based on standard recommendations.
TAKEAWAY:
- During the 10-year follow-up, patients with SLE showed a 2.3-fold higher risk for plaque progression than healthy control participants (adjusted incidence rate ratio [aIRR], 2.26; P = .002).
- Achieving risk reduction target for each standard cardiovascular risk factor (blood pressure, lipids, smoking, body weight, and physical activity) was associated with a 32% reduction in the risk for plaque progression (aIRR, 0.68; P = .004).
- Staying in remission for ≥ 75% of the follow-up period was significantly associated with a 43% reduction in the risk for plaque progression (aIRR, 0.57; P = .033).
- Patients with SLE also had a higher incidence of CVD events than healthy control participants (permutation-based log-rank P = .036).
IN PRACTICE:
“These findings support the importance of prioritizing sustained remission rather than a low disease activity state for the prevention of atherosclerosis development and progression in SLE,” the authors wrote.
SOURCE:
The study was led by Nikolaos Papazoglou, MD, First Department of Propaedeutic Internal Medicine, Joint Academic Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens in Greece. It was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had limited statistical power to perform a multivariate analysis of incident CVD events due to low event rates. The cohort consisted solely of White Europeans, possibly limiting the generalizability of the findings to more ethnically diverse populations. Because antiphospholipid antibodies are known to be associated with CVD events in the general population, the lack of testing for antiphospholipid antibody positivity in healthy control participants could be another limitation.
DISCLOSURES:
The study did not receive any funding from public, commercial, or not-for-profit sectors. The authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with systemic lupus erythematosus (SLE) face more than double the risk for atherosclerotic plaque progression than healthy control individuals without the condition, but management of traditional cardiovascular risk factors and prolonged clinical remission can successfully mitigate it.
METHODOLOGY:
- Researchers performed a prospective study to assess the progression of subclinical atherosclerosis plaques and the development of cardiovascular events in patients with SLE over a 10-year follow-up period.
- They included 111 patients with SLE (mean age, 43 years; 91% women) and 94 matched healthy control individuals without prior atherosclerotic cardiovascular disease (CVD), active malignancy, pregnancy, or diabetes mellitus who underwent carotid ultrasound measurements.
- A total of 738 carotid measurements were analyzed from baseline to 3-, 7-, and 10-year follow-up periods for assessing new carotid plaque development; incident CVD events were also analyzed during follow-up.
- Disease remission was evaluated based on the Definition of Remission in SLE criteria.
- Target for management of cardiovascular risk factors was based on standard recommendations.
TAKEAWAY:
- During the 10-year follow-up, patients with SLE showed a 2.3-fold higher risk for plaque progression than healthy control participants (adjusted incidence rate ratio [aIRR], 2.26; P = .002).
- Achieving risk reduction target for each standard cardiovascular risk factor (blood pressure, lipids, smoking, body weight, and physical activity) was associated with a 32% reduction in the risk for plaque progression (aIRR, 0.68; P = .004).
- Staying in remission for ≥ 75% of the follow-up period was significantly associated with a 43% reduction in the risk for plaque progression (aIRR, 0.57; P = .033).
- Patients with SLE also had a higher incidence of CVD events than healthy control participants (permutation-based log-rank P = .036).
IN PRACTICE:
“These findings support the importance of prioritizing sustained remission rather than a low disease activity state for the prevention of atherosclerosis development and progression in SLE,” the authors wrote.
SOURCE:
The study was led by Nikolaos Papazoglou, MD, First Department of Propaedeutic Internal Medicine, Joint Academic Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens in Greece. It was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had limited statistical power to perform a multivariate analysis of incident CVD events due to low event rates. The cohort consisted solely of White Europeans, possibly limiting the generalizability of the findings to more ethnically diverse populations. Because antiphospholipid antibodies are known to be associated with CVD events in the general population, the lack of testing for antiphospholipid antibody positivity in healthy control participants could be another limitation.
DISCLOSURES:
The study did not receive any funding from public, commercial, or not-for-profit sectors. The authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with systemic lupus erythematosus (SLE) face more than double the risk for atherosclerotic plaque progression than healthy control individuals without the condition, but management of traditional cardiovascular risk factors and prolonged clinical remission can successfully mitigate it.
METHODOLOGY:
- Researchers performed a prospective study to assess the progression of subclinical atherosclerosis plaques and the development of cardiovascular events in patients with SLE over a 10-year follow-up period.
- They included 111 patients with SLE (mean age, 43 years; 91% women) and 94 matched healthy control individuals without prior atherosclerotic cardiovascular disease (CVD), active malignancy, pregnancy, or diabetes mellitus who underwent carotid ultrasound measurements.
- A total of 738 carotid measurements were analyzed from baseline to 3-, 7-, and 10-year follow-up periods for assessing new carotid plaque development; incident CVD events were also analyzed during follow-up.
- Disease remission was evaluated based on the Definition of Remission in SLE criteria.
- Target for management of cardiovascular risk factors was based on standard recommendations.
TAKEAWAY:
- During the 10-year follow-up, patients with SLE showed a 2.3-fold higher risk for plaque progression than healthy control participants (adjusted incidence rate ratio [aIRR], 2.26; P = .002).
- Achieving risk reduction target for each standard cardiovascular risk factor (blood pressure, lipids, smoking, body weight, and physical activity) was associated with a 32% reduction in the risk for plaque progression (aIRR, 0.68; P = .004).
- Staying in remission for ≥ 75% of the follow-up period was significantly associated with a 43% reduction in the risk for plaque progression (aIRR, 0.57; P = .033).
- Patients with SLE also had a higher incidence of CVD events than healthy control participants (permutation-based log-rank P = .036).
IN PRACTICE:
“These findings support the importance of prioritizing sustained remission rather than a low disease activity state for the prevention of atherosclerosis development and progression in SLE,” the authors wrote.
SOURCE:
The study was led by Nikolaos Papazoglou, MD, First Department of Propaedeutic Internal Medicine, Joint Academic Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens in Greece. It was published online on December 25, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study had limited statistical power to perform a multivariate analysis of incident CVD events due to low event rates. The cohort consisted solely of White Europeans, possibly limiting the generalizability of the findings to more ethnically diverse populations. Because antiphospholipid antibodies are known to be associated with CVD events in the general population, the lack of testing for antiphospholipid antibody positivity in healthy control participants could be another limitation.
DISCLOSURES:
The study did not receive any funding from public, commercial, or not-for-profit sectors. The authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patients With Refractory Systemic Sclerosis Have Early Success With CAR T-Cell Therapy
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Special Considerations Needed in Applying Lupus Nephritis Guideline to Children
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
WASHINGTON — When the American College of Rheumatology (ACR) released its updated guideline for management of lupus nephritis (LN) at its 2024 Annual Meeting, they included recommendations for managing pediatric LN for the first time.
The pediatric recommendations use the same classification criteria, outcome measures, and treatments as in adults — including the first-line triple therapy recommendation — but there remain important differences between pediatric and adult LN, Mary Beth Son, MD, clinical chief of immunology and section chief of rheumatology at Boston Children’s Hospital in Massachusetts, and an associate professor of pediatrics at Harvard Medical School, also in Boston, told attendees.
“In general, kids and adolescents with lupus are sicker,” Son said. They are more likely to have renal manifestations and neuropsychiatric lupus at diagnosis, compared with adults. Further, “although the disease is the same, it’s happening to kids and adolescents who are undergoing critical periods of growth and development.”
Medication risk profiles also shift for younger patients, Son noted.
“Importantly, they’re at risk for higher cumulative dosing of both glucocorticoids and cyclophosphamide,” Son said. “When we give an adolescent a course of cyclophosphamide, we have to be aware that this might be the first of a few courses over the course of the lifetime disease, and with increasing numbers of cyclophosphamide courses, you have increased risk for infertility and malignancy.”
Son also acknowledged challenges of pediatric literature, including differences in definitions of pediatric lupus, very few randomized controlled trials, and fewer pediatric studies in general, with fewer participants. Given these research gaps, the guideline panels included pediatric rheumatologists and nephrologists, and the patient panel included several patients with childhood-onset disease.
Son also addressed differences in pediatric drug development. Dosing studies also do not always directly translate from adults to children because children have larger drug volume distribution and differences in drug clearance, and they may need different formulations, she said. Children tend to tolerate medications better than adults because they usually have fewer comorbidities, but the assessment of a drug’s safety must take its impact on growth and development into consideration.
During a press conference after the session where the guideline was presented, Linda Hiraki, MD, ScD, a clinician-scientist in rheumatology at the Hospital for Sick Children, Toronto, Ontario, Canada, said the panel took into consideration that pediatric patients receive their diagnosis during a critical time of development, so considerations of medication risks include the fact that children “have much more life to live.”
Triple Therapy Recommended
As with adults, the pediatric LN guideline recommends a triple therapy approach: glucocorticoids plus mycophenolate mofetil and belimumab, in addition to the usual renin-angiotensin-aldosterone system inhibitors and hydroxychloroquine. But Son acknowledged limitations of applying the new guideline to children. For one, voclosporin has not been studied in or approved for pediatric patients, although there exists modest evidence for other calcineurin inhibitors, mainly tacrolimus, in children.
“The other important consideration is that the lower dose of prednisone that’s being offered by the guidelines of 40 mg per day as a starting dose has not been studied in pediatric lupus nephritis patients,” Son said. “However, I would offer that, given that we know that kids get higher doses and longer courses, it’s even more important to consider a lower dose to begin with in the setting of other immunosuppressants.”
Good Practice Statements for Pediatric LN
Son also reviewed three good practice statements for pediatric LN. First, “glucocorticoid regimens should use pediatric-appropriate doses for children, as reduction of human glucocorticoid dosing is critically important given the early age of pediatric lupus onset and attendant comorbidities,” she said.
That statement is based on both common sense and some literature, including awareness that children are more likely to receive higher doses of steroids and that children’s higher damage scores are driven in part by steroid-related toxicity, such as avascular necrosis and cataracts. In addition, glucocorticoids can have profound effects on body mass index, mood, and height attainment.
“This is during a period of emerging self-identity and struggles with appearance; steroids exacerbate that” as well as mood issues already associated with puberty, Son said.
The second good practice statement recommends that clinicians monitor patients “for delayed pubertal onset and decreased growth velocity that can result from disease activity and glucocorticoid treatment and consider referral to pediatric endocrinology if indicated.” The third states that “a structured, intentional transition from pediatric to adult rheumatology care is indicated to avoid poor outcomes during this vulnerable period.”
During the press conference, Hiraki said that pediatric rheumatologists already recognize the need for discussions about transfer to adult care to begin very early, even years before patients are ready to transfer.
“The transition from being a pediatric patient to being an adult patient is very challenging for a number of reasons,” starting with loss of insurance coverage, added Bonnie Bermas, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas, Texas. When adult rheumatologists take on these patients, they may not have had care for 2 or 3 years, she said.
Rebecca Sadun, MD, PhD, an associate professor of pediatrics in rheumatology at Duke University School of Medicine, Durham, North Carolina, and vice-chair of the Systemic Lupus Erythematosus Committee for the Childhood Arthritis and Rheumatology Research Alliance, was not involved in the guideline development process but reviewed the new guideline.
“We appreciate that the ACR took care to involve pediatric rheumatologists, pediatric nephrologists, and patients with childhood-onset lupus in the development of the newest lupus nephritis treatment guidelines,” she said in an interview. She also noted, however, that “the dearth of pediatric-specific clinical trial data means that we continue to wonder when it is appropriate to extrapolate from adult data regarding the efficacy, safety, and dosing of certain medications, including steroids and voclosporin.” She also noted that voclosporin use can increase pill burden and therefore be difficult to use in pediatrics.
“Children, adolescents, and young adults are a unique population with unique challenges, including significant struggles with adherence to complex medication regimens,” she said. Sadun drew attention to two themes from the guideline that she found particularly applicable to management of pediatric LN.
“First, we must remain wary of the serious consequences of long-term, high-dose glucocorticoids, and we should continue to look towards steroid-sparing strategies that will reduce reliance on glucocorticoids,” Sadun said. “Second, we are likely to see better outcomes, including better renal response, when we take advantage of combination immunosuppression earlier in the disease course.”
Son, Bermas, and Sadun had no disclosures. Hiraki has consulted for Janssen. The guideline development did not involve outside funding.
A version of this article first appeared on Medscape.com.
FROM ACR 2024
Cutaneous Lupus Associated with Greater Risk for Atherosclerotic Cardiovascular Disease
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Could a Urinary Biomarker Panel Be a ‘Game Changer’ for Lupus Nephritis Management?
WASHINGTON — An investigational 12-protein panel of urinary biomarkers predicted histologically active lupus nephritis (LN) with 86% accuracy, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
The noninvasive biomarker panel “robustly predicts meaningful and actionable histological findings” in patients with active proliferative LN, Andrea Fava, MD, assistant professor of medicine in the Division of Rheumatology at Johns Hopkins Medicine in Baltimore, told attendees.
“In contrast to proteinuria, which can’t differentiate inflammation from damage, this panel for histological activity includes a set of 12 proteins linked to intrarenal inflammation,” said Fava, director of Lupus Translational Research at Johns Hopkins. A decline in the biomarker score at 3 months predicted a clinical response at 1 year, and persistent elevation of the score at 1 year predicted permanent loss of kidney function, “which makes it tempting as a treatment endpoint,” Fava said. “Upon further validation, this biomarker panel could aid in the diagnosis of lupus nephritis and guide treatment decisions.”
Alfred Kim, MD, PhD, an associate professor of medicine at Washington University in St. Louis, was not involved in the research but noted the potential value of a reliable biomarker panel.
“If we have urinary biomarkers that strongly associate with histologic activity, this would be a game changer in the management of LN,” Kim told Medscape Medical News. “Right now, the gold standard is to perform another kidney biopsy to determine if therapy is working. But this is invasive, and many patients do not want to do another kidney biopsy. Conversely, the easiest way to assess lupus nephritis activity is through a urinalysis, focusing on urinary protein levels,” but relying on proteinuria has limitations as well.
“The most important [limitation] is that proteinuria cannot distinguish treatable inflammation from chronic damage,” Fava said. Persistent histologic activity in patients without proteinuria predicts flares, but tracking histologic activity, as Kim noted, requires repeat biopsies.
“So we need better biomarkers because biomarkers that can reflect tissue biology in real time can guide personalized treatment, and that’s one of the main goals of the Accelerating Medicines Partnership [AMP],” he said. The AMP is a public-private partnership between the National Institutes of Health (NIH), the US Food and Drug Administration (FDA), multiple biopharmaceutical and life science companies, and nonprofit and other organizations. Lupus is one of the AMP’s funded projects.
Kim agreed that “effective biomarkers are a huge unmet need in LN.” Further, he said, “imagine a world where the diagnosis of LN can be made just through urinary biomarkers and obviate the need for biopsy. Both patients and providers will be ecstatic at this possibility.”
Fava described the background for how his research team determined what biomarkers to test. They had previously enrolled 225 patients with LN undergoing a clinically indicated kidney biopsy and collected urine samples from them at baseline and at 12, 24, and 52 weeks after their biopsy.
Of the 225 patients included, 9% with only mesangial LN (class I-II), 25% with pure membranous LN (class V), 24% with mixed LN (class III or IV with or without V), 38% with proliferative LN (class III or IV), and 4% with advanced sclerosis LN (class VI). From these samples, they quantified 1200 proteins and looked at how they correlated with histologic activity.
“What was interesting was that in patients who were classified as responders after 1 year, there were many of these proteins that declined as early as 3 months, suggesting that effective immunosuppression is reducing intrarenal inflammation, and we can capture it in real time,” Fava said.
Biomarker Panel Predicts Histologically Active LN
So they set to determining whether they could develop a urinary biomarker for histologically active LN that could be useful in clinical decision-making. They focused on one that could detect active proliferative LN with an NIH activity index score > 2. Their 179 participants included 47.5% Black, 27.9% White, and 14.5% Asian participants, with 10.1% of other races. The predominantly female (86.6%) cohort had an average age of 37 years. Among the LN classes, about one third (34.6%) had pure proliferative disease, 17.9% had mixed proliferative, 27.9% had pure membranous, 11.7% had class I or II, and 5% had class VI. Just over half the participants (55.7%) had not responded to treatment at 12 months, whereas 25% had a complete response, and 19.3% had a partial response.
However, both the 78 participants with an NIH activity index score > 2 and the 101 with a score ≤ 2 had a median score of 3 on the NIH chronicity index. And the urine protein-to-creatinine ratio — 2.8 in the group with an NIH activity index score > 2 and 2.4 in the other group — was nearly indistinguishable between the two groups, Fava said.
They then trained multiple algorithms on 80% of the data to find the best performing set of proteins (with an area under the curve [AUC] of 90%) for predicting an NIH activity index score > 2. They reduced the number of proteins to maximize practicality and performance of the panel, Fava said, and ultimately identified a 12-protein panel that was highly predictive of an NIH activity index score > 2. Then, they validated that panel using the other 20% of the data. The training set had an AUC of 90%, and the test set was validated with an AUC of 93%.
The 12-protein panel score outperformed anti-dsDNA, C3 complement, and proteinuria, with a sensitivity of 81%, a specificity of 90%, a positive predictive value of 87%, a negative predictive value of 86%, and an accuracy of 86%. The proteins with the greatest relative importance were CD163, cathepsin S, FOLR2, and CEACAM-1.
“In contrast to proteinuria, these proteins were related to inflammatory processes found in the kidneys in patients with lupus nephritis, such as activation of macrophages, neutrophils and monocytes, lymphocytes, and complement,” Fava said.
When they looked at the trajectories of the probabilities from the biomarker panel at 3, 6, and 12 months, the probability of the NIH activity index score remaining > 2 stayed high in the nonresponders over 1 year, but the trajectory declined at 3 months in the responders, indicating a decrease in kidney inflammation (P < .001).
Can the Biomarker Panel Serve as a Treatment Endpoint?
Then, to determine whether the panel could act as a reliable treatment endpoint, the researchers followed the patients for up to 7 years. One third of the patients lost more than 40% of their kidney function during the follow-up. They found that a high urinary biomarker score at 12 months predicted future glomerular filtration rate loss, independent of proteinuria.
This panel was tested specifically for proliferative LN, so “we may need distinct panels for each [LN type] to capture most of these patients,” Kim said. “I think that’s where the gold mine is: A personalized medicine approach where a large biomarker panel identifies which smaller panel that patient best fits, then use that for monitoring.”
Kim did note an important potential limitation in the study regarding how samples are used in biomarker discovery and validation vs in clinical practice. “Most samples in research studies are frozen, then thawed, while urine is assayed within a couple hours after collection in the clinical setting,” he said. “Do sample processing differences create a situation where a biomarker works in a research project but not in the clinical setting?” But more likely, he said, the opposite may be the case, where frozen samples allow for more degradation of proteins and potentially useful LN biomarker candidates are never detected.
Another challenge, Kim added, albeit unrelated to the study findings, is that diagnostic companies are finding it difficult to get payers to cover new tests, so that could become a challenge if the panel undergoes further validation and then FDA qualification.
The research was funded by Exagen. Fava reported disclosures with Arctiva, AstraZeneca, Exagen, Novartis, UCB, Bristol Myers Squibb, Annexon Bio, and Bain Capital. His coauthors reported financial relationships with numerous pharmaceutical and life science companies, including Exagen, and some are employees of Exagen.
Kim reported research agreements with AstraZeneca, Bristol Myers Squibb, Novartis, and CRISPR Therapeutics; receiving royalties from Kypha; and receiving consulting/speaking fees from AbbVie, Amgen, Atara Bio, Aurinia, Cargo Tx, Exagen, GlaxoSmithKline, Hinge Bio, Kypha, and UpToDate.
A version of this article appeared on Medscape.com.
WASHINGTON — An investigational 12-protein panel of urinary biomarkers predicted histologically active lupus nephritis (LN) with 86% accuracy, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
The noninvasive biomarker panel “robustly predicts meaningful and actionable histological findings” in patients with active proliferative LN, Andrea Fava, MD, assistant professor of medicine in the Division of Rheumatology at Johns Hopkins Medicine in Baltimore, told attendees.
“In contrast to proteinuria, which can’t differentiate inflammation from damage, this panel for histological activity includes a set of 12 proteins linked to intrarenal inflammation,” said Fava, director of Lupus Translational Research at Johns Hopkins. A decline in the biomarker score at 3 months predicted a clinical response at 1 year, and persistent elevation of the score at 1 year predicted permanent loss of kidney function, “which makes it tempting as a treatment endpoint,” Fava said. “Upon further validation, this biomarker panel could aid in the diagnosis of lupus nephritis and guide treatment decisions.”
Alfred Kim, MD, PhD, an associate professor of medicine at Washington University in St. Louis, was not involved in the research but noted the potential value of a reliable biomarker panel.
“If we have urinary biomarkers that strongly associate with histologic activity, this would be a game changer in the management of LN,” Kim told Medscape Medical News. “Right now, the gold standard is to perform another kidney biopsy to determine if therapy is working. But this is invasive, and many patients do not want to do another kidney biopsy. Conversely, the easiest way to assess lupus nephritis activity is through a urinalysis, focusing on urinary protein levels,” but relying on proteinuria has limitations as well.
“The most important [limitation] is that proteinuria cannot distinguish treatable inflammation from chronic damage,” Fava said. Persistent histologic activity in patients without proteinuria predicts flares, but tracking histologic activity, as Kim noted, requires repeat biopsies.
“So we need better biomarkers because biomarkers that can reflect tissue biology in real time can guide personalized treatment, and that’s one of the main goals of the Accelerating Medicines Partnership [AMP],” he said. The AMP is a public-private partnership between the National Institutes of Health (NIH), the US Food and Drug Administration (FDA), multiple biopharmaceutical and life science companies, and nonprofit and other organizations. Lupus is one of the AMP’s funded projects.
Kim agreed that “effective biomarkers are a huge unmet need in LN.” Further, he said, “imagine a world where the diagnosis of LN can be made just through urinary biomarkers and obviate the need for biopsy. Both patients and providers will be ecstatic at this possibility.”
Fava described the background for how his research team determined what biomarkers to test. They had previously enrolled 225 patients with LN undergoing a clinically indicated kidney biopsy and collected urine samples from them at baseline and at 12, 24, and 52 weeks after their biopsy.
Of the 225 patients included, 9% with only mesangial LN (class I-II), 25% with pure membranous LN (class V), 24% with mixed LN (class III or IV with or without V), 38% with proliferative LN (class III or IV), and 4% with advanced sclerosis LN (class VI). From these samples, they quantified 1200 proteins and looked at how they correlated with histologic activity.
“What was interesting was that in patients who were classified as responders after 1 year, there were many of these proteins that declined as early as 3 months, suggesting that effective immunosuppression is reducing intrarenal inflammation, and we can capture it in real time,” Fava said.
Biomarker Panel Predicts Histologically Active LN
So they set to determining whether they could develop a urinary biomarker for histologically active LN that could be useful in clinical decision-making. They focused on one that could detect active proliferative LN with an NIH activity index score > 2. Their 179 participants included 47.5% Black, 27.9% White, and 14.5% Asian participants, with 10.1% of other races. The predominantly female (86.6%) cohort had an average age of 37 years. Among the LN classes, about one third (34.6%) had pure proliferative disease, 17.9% had mixed proliferative, 27.9% had pure membranous, 11.7% had class I or II, and 5% had class VI. Just over half the participants (55.7%) had not responded to treatment at 12 months, whereas 25% had a complete response, and 19.3% had a partial response.
However, both the 78 participants with an NIH activity index score > 2 and the 101 with a score ≤ 2 had a median score of 3 on the NIH chronicity index. And the urine protein-to-creatinine ratio — 2.8 in the group with an NIH activity index score > 2 and 2.4 in the other group — was nearly indistinguishable between the two groups, Fava said.
They then trained multiple algorithms on 80% of the data to find the best performing set of proteins (with an area under the curve [AUC] of 90%) for predicting an NIH activity index score > 2. They reduced the number of proteins to maximize practicality and performance of the panel, Fava said, and ultimately identified a 12-protein panel that was highly predictive of an NIH activity index score > 2. Then, they validated that panel using the other 20% of the data. The training set had an AUC of 90%, and the test set was validated with an AUC of 93%.
The 12-protein panel score outperformed anti-dsDNA, C3 complement, and proteinuria, with a sensitivity of 81%, a specificity of 90%, a positive predictive value of 87%, a negative predictive value of 86%, and an accuracy of 86%. The proteins with the greatest relative importance were CD163, cathepsin S, FOLR2, and CEACAM-1.
“In contrast to proteinuria, these proteins were related to inflammatory processes found in the kidneys in patients with lupus nephritis, such as activation of macrophages, neutrophils and monocytes, lymphocytes, and complement,” Fava said.
When they looked at the trajectories of the probabilities from the biomarker panel at 3, 6, and 12 months, the probability of the NIH activity index score remaining > 2 stayed high in the nonresponders over 1 year, but the trajectory declined at 3 months in the responders, indicating a decrease in kidney inflammation (P < .001).
Can the Biomarker Panel Serve as a Treatment Endpoint?
Then, to determine whether the panel could act as a reliable treatment endpoint, the researchers followed the patients for up to 7 years. One third of the patients lost more than 40% of their kidney function during the follow-up. They found that a high urinary biomarker score at 12 months predicted future glomerular filtration rate loss, independent of proteinuria.
This panel was tested specifically for proliferative LN, so “we may need distinct panels for each [LN type] to capture most of these patients,” Kim said. “I think that’s where the gold mine is: A personalized medicine approach where a large biomarker panel identifies which smaller panel that patient best fits, then use that for monitoring.”
Kim did note an important potential limitation in the study regarding how samples are used in biomarker discovery and validation vs in clinical practice. “Most samples in research studies are frozen, then thawed, while urine is assayed within a couple hours after collection in the clinical setting,” he said. “Do sample processing differences create a situation where a biomarker works in a research project but not in the clinical setting?” But more likely, he said, the opposite may be the case, where frozen samples allow for more degradation of proteins and potentially useful LN biomarker candidates are never detected.
Another challenge, Kim added, albeit unrelated to the study findings, is that diagnostic companies are finding it difficult to get payers to cover new tests, so that could become a challenge if the panel undergoes further validation and then FDA qualification.
The research was funded by Exagen. Fava reported disclosures with Arctiva, AstraZeneca, Exagen, Novartis, UCB, Bristol Myers Squibb, Annexon Bio, and Bain Capital. His coauthors reported financial relationships with numerous pharmaceutical and life science companies, including Exagen, and some are employees of Exagen.
Kim reported research agreements with AstraZeneca, Bristol Myers Squibb, Novartis, and CRISPR Therapeutics; receiving royalties from Kypha; and receiving consulting/speaking fees from AbbVie, Amgen, Atara Bio, Aurinia, Cargo Tx, Exagen, GlaxoSmithKline, Hinge Bio, Kypha, and UpToDate.
A version of this article appeared on Medscape.com.
WASHINGTON — An investigational 12-protein panel of urinary biomarkers predicted histologically active lupus nephritis (LN) with 86% accuracy, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
The noninvasive biomarker panel “robustly predicts meaningful and actionable histological findings” in patients with active proliferative LN, Andrea Fava, MD, assistant professor of medicine in the Division of Rheumatology at Johns Hopkins Medicine in Baltimore, told attendees.
“In contrast to proteinuria, which can’t differentiate inflammation from damage, this panel for histological activity includes a set of 12 proteins linked to intrarenal inflammation,” said Fava, director of Lupus Translational Research at Johns Hopkins. A decline in the biomarker score at 3 months predicted a clinical response at 1 year, and persistent elevation of the score at 1 year predicted permanent loss of kidney function, “which makes it tempting as a treatment endpoint,” Fava said. “Upon further validation, this biomarker panel could aid in the diagnosis of lupus nephritis and guide treatment decisions.”
Alfred Kim, MD, PhD, an associate professor of medicine at Washington University in St. Louis, was not involved in the research but noted the potential value of a reliable biomarker panel.
“If we have urinary biomarkers that strongly associate with histologic activity, this would be a game changer in the management of LN,” Kim told Medscape Medical News. “Right now, the gold standard is to perform another kidney biopsy to determine if therapy is working. But this is invasive, and many patients do not want to do another kidney biopsy. Conversely, the easiest way to assess lupus nephritis activity is through a urinalysis, focusing on urinary protein levels,” but relying on proteinuria has limitations as well.
“The most important [limitation] is that proteinuria cannot distinguish treatable inflammation from chronic damage,” Fava said. Persistent histologic activity in patients without proteinuria predicts flares, but tracking histologic activity, as Kim noted, requires repeat biopsies.
“So we need better biomarkers because biomarkers that can reflect tissue biology in real time can guide personalized treatment, and that’s one of the main goals of the Accelerating Medicines Partnership [AMP],” he said. The AMP is a public-private partnership between the National Institutes of Health (NIH), the US Food and Drug Administration (FDA), multiple biopharmaceutical and life science companies, and nonprofit and other organizations. Lupus is one of the AMP’s funded projects.
Kim agreed that “effective biomarkers are a huge unmet need in LN.” Further, he said, “imagine a world where the diagnosis of LN can be made just through urinary biomarkers and obviate the need for biopsy. Both patients and providers will be ecstatic at this possibility.”
Fava described the background for how his research team determined what biomarkers to test. They had previously enrolled 225 patients with LN undergoing a clinically indicated kidney biopsy and collected urine samples from them at baseline and at 12, 24, and 52 weeks after their biopsy.
Of the 225 patients included, 9% with only mesangial LN (class I-II), 25% with pure membranous LN (class V), 24% with mixed LN (class III or IV with or without V), 38% with proliferative LN (class III or IV), and 4% with advanced sclerosis LN (class VI). From these samples, they quantified 1200 proteins and looked at how they correlated with histologic activity.
“What was interesting was that in patients who were classified as responders after 1 year, there were many of these proteins that declined as early as 3 months, suggesting that effective immunosuppression is reducing intrarenal inflammation, and we can capture it in real time,” Fava said.
Biomarker Panel Predicts Histologically Active LN
So they set to determining whether they could develop a urinary biomarker for histologically active LN that could be useful in clinical decision-making. They focused on one that could detect active proliferative LN with an NIH activity index score > 2. Their 179 participants included 47.5% Black, 27.9% White, and 14.5% Asian participants, with 10.1% of other races. The predominantly female (86.6%) cohort had an average age of 37 years. Among the LN classes, about one third (34.6%) had pure proliferative disease, 17.9% had mixed proliferative, 27.9% had pure membranous, 11.7% had class I or II, and 5% had class VI. Just over half the participants (55.7%) had not responded to treatment at 12 months, whereas 25% had a complete response, and 19.3% had a partial response.
However, both the 78 participants with an NIH activity index score > 2 and the 101 with a score ≤ 2 had a median score of 3 on the NIH chronicity index. And the urine protein-to-creatinine ratio — 2.8 in the group with an NIH activity index score > 2 and 2.4 in the other group — was nearly indistinguishable between the two groups, Fava said.
They then trained multiple algorithms on 80% of the data to find the best performing set of proteins (with an area under the curve [AUC] of 90%) for predicting an NIH activity index score > 2. They reduced the number of proteins to maximize practicality and performance of the panel, Fava said, and ultimately identified a 12-protein panel that was highly predictive of an NIH activity index score > 2. Then, they validated that panel using the other 20% of the data. The training set had an AUC of 90%, and the test set was validated with an AUC of 93%.
The 12-protein panel score outperformed anti-dsDNA, C3 complement, and proteinuria, with a sensitivity of 81%, a specificity of 90%, a positive predictive value of 87%, a negative predictive value of 86%, and an accuracy of 86%. The proteins with the greatest relative importance were CD163, cathepsin S, FOLR2, and CEACAM-1.
“In contrast to proteinuria, these proteins were related to inflammatory processes found in the kidneys in patients with lupus nephritis, such as activation of macrophages, neutrophils and monocytes, lymphocytes, and complement,” Fava said.
When they looked at the trajectories of the probabilities from the biomarker panel at 3, 6, and 12 months, the probability of the NIH activity index score remaining > 2 stayed high in the nonresponders over 1 year, but the trajectory declined at 3 months in the responders, indicating a decrease in kidney inflammation (P < .001).
Can the Biomarker Panel Serve as a Treatment Endpoint?
Then, to determine whether the panel could act as a reliable treatment endpoint, the researchers followed the patients for up to 7 years. One third of the patients lost more than 40% of their kidney function during the follow-up. They found that a high urinary biomarker score at 12 months predicted future glomerular filtration rate loss, independent of proteinuria.
This panel was tested specifically for proliferative LN, so “we may need distinct panels for each [LN type] to capture most of these patients,” Kim said. “I think that’s where the gold mine is: A personalized medicine approach where a large biomarker panel identifies which smaller panel that patient best fits, then use that for monitoring.”
Kim did note an important potential limitation in the study regarding how samples are used in biomarker discovery and validation vs in clinical practice. “Most samples in research studies are frozen, then thawed, while urine is assayed within a couple hours after collection in the clinical setting,” he said. “Do sample processing differences create a situation where a biomarker works in a research project but not in the clinical setting?” But more likely, he said, the opposite may be the case, where frozen samples allow for more degradation of proteins and potentially useful LN biomarker candidates are never detected.
Another challenge, Kim added, albeit unrelated to the study findings, is that diagnostic companies are finding it difficult to get payers to cover new tests, so that could become a challenge if the panel undergoes further validation and then FDA qualification.
The research was funded by Exagen. Fava reported disclosures with Arctiva, AstraZeneca, Exagen, Novartis, UCB, Bristol Myers Squibb, Annexon Bio, and Bain Capital. His coauthors reported financial relationships with numerous pharmaceutical and life science companies, including Exagen, and some are employees of Exagen.
Kim reported research agreements with AstraZeneca, Bristol Myers Squibb, Novartis, and CRISPR Therapeutics; receiving royalties from Kypha; and receiving consulting/speaking fees from AbbVie, Amgen, Atara Bio, Aurinia, Cargo Tx, Exagen, GlaxoSmithKline, Hinge Bio, Kypha, and UpToDate.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Real-World Data Question Low-Dose Steroid Use in ANCA Vasculitis
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.