User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
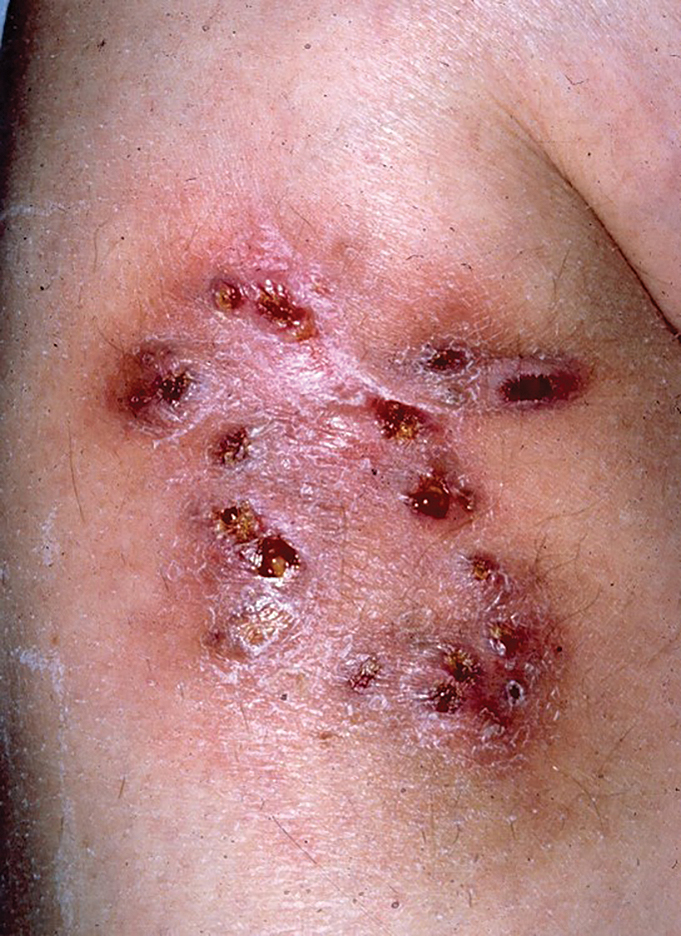
Topical Treatment Provides a Noninvasive Option for Pyogenic Granuloma in Children
HUNTINGTON BEACH, CALIF. — Mounting according to Julie Dhossche, MD.
A PG is a common, benign vascular tumor that often occurs in children under 5 years of age, “usually in a very inconvenient spot, like the cheek,” Dr. Dhossche, a pediatric dermatologist at Oregon Health & Science University (OHSU), Portland, said at the annual meeting of the Pacific Dermatologic Association. “It can bleed a lot. Often, parents take their child to the emergency department for unstoppable bleeding. Our first-line treatment is often surgical: shave removal, electrocautery, or excision.”
Several case reports about the use of the topical form of timolol, a nonselective beta-adrenergic antagonist, for PG have been published in the medical literature including a case series of seven patients (six were treated with topical timolol). The authors of the case series hypothesized that a beta-blocker may be effective for PGs by causing vasoconstriction that stops bleeding.
In addition, Dr. Dhossche and colleagues retrospectively evaluated 92 children with a mean age of 4.5 years who were treated with topical timolol for PG at OHSU from 2010 to 2020. The results were presented in an abstract at the 2022 Pediatric Dermatology Research Alliance annual conference.
At the initial visit, 80 of 92 (87%) children were treated with timolol only, 6 of 92 (6.5%) underwent a procedure, and 6 of 92 (6.5%) were treated with timolol and a procedure. The researchers observed that of the 80 patients who received timolol monotherapy, 42 (52.5%) were spared a procedural intervention. “So, we have had some success with this,” she said. “It can also help with bleeding episodes if you are waiting for a procedure.”
Surgery May Still Be Needed
For PGs, she applies one drop of timolol to the lesion under occlusion with DuoDERM or a similar dressing, which is repeated every 1-3 days depending on how long the dressing stays on. “It may take 3-4 months of this treatment to clear,” she said.
If topical timolol doesn’t stop the PG from bleeding, or if parents elect for surgical removal, “some tears [during removal of the lesion] may be inevitable,” Dr. Dhossche said. “My goal is to make it as good of an experience as it can be, by being very confident and offering lots of smiles, pretreatment with topical lidocaine for 20-30 minutes, icing, and formulating an alliance with parents” to help calm nerves, “knowing if that doesn’t work, I might need help from my colleagues in pediatric sedation.”
Choice of language matters when describing to children what to expect during a procedure, she continued. For example, instead of saying, “it will feel like a bee sting,” say, “some kids say it is uncomfortable like a pinch and some kids say it’s not so bad.” And, when describing the size of a needle or an incision, instead of saying, “it’s as big as ...” say, “it’s as small as ...”
As described in a 2020 paper published in Pediatric Dermatology, proper comfort positioning of children during in-office dermatologic procedures is also key, which can include having the parent or caregiver hug a child during removal of a PG, Dr. Dhossche said. “You want to optimize distractions for the patient while you do the procedure. This is the time to bring out your iPhone, iPad, or enlist help from a certified child life specialist if you have one at your institution.”
When she administers injections to children, “I don’t lie about the shot, but I do hide the actual needle from sight, if possible,” she said. “I’ll say, ‘you’ll feel a pinch.’ Vibration tools can help while you’re injecting.” She showed an image of a vibrating light-up children’s toothbrush she found on Amazon for $10 “that has served me well. It’s also kind of a tension diffuser.”
Dr. Dhossche reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — Mounting according to Julie Dhossche, MD.
A PG is a common, benign vascular tumor that often occurs in children under 5 years of age, “usually in a very inconvenient spot, like the cheek,” Dr. Dhossche, a pediatric dermatologist at Oregon Health & Science University (OHSU), Portland, said at the annual meeting of the Pacific Dermatologic Association. “It can bleed a lot. Often, parents take their child to the emergency department for unstoppable bleeding. Our first-line treatment is often surgical: shave removal, electrocautery, or excision.”
Several case reports about the use of the topical form of timolol, a nonselective beta-adrenergic antagonist, for PG have been published in the medical literature including a case series of seven patients (six were treated with topical timolol). The authors of the case series hypothesized that a beta-blocker may be effective for PGs by causing vasoconstriction that stops bleeding.
In addition, Dr. Dhossche and colleagues retrospectively evaluated 92 children with a mean age of 4.5 years who were treated with topical timolol for PG at OHSU from 2010 to 2020. The results were presented in an abstract at the 2022 Pediatric Dermatology Research Alliance annual conference.
At the initial visit, 80 of 92 (87%) children were treated with timolol only, 6 of 92 (6.5%) underwent a procedure, and 6 of 92 (6.5%) were treated with timolol and a procedure. The researchers observed that of the 80 patients who received timolol monotherapy, 42 (52.5%) were spared a procedural intervention. “So, we have had some success with this,” she said. “It can also help with bleeding episodes if you are waiting for a procedure.”
Surgery May Still Be Needed
For PGs, she applies one drop of timolol to the lesion under occlusion with DuoDERM or a similar dressing, which is repeated every 1-3 days depending on how long the dressing stays on. “It may take 3-4 months of this treatment to clear,” she said.
If topical timolol doesn’t stop the PG from bleeding, or if parents elect for surgical removal, “some tears [during removal of the lesion] may be inevitable,” Dr. Dhossche said. “My goal is to make it as good of an experience as it can be, by being very confident and offering lots of smiles, pretreatment with topical lidocaine for 20-30 minutes, icing, and formulating an alliance with parents” to help calm nerves, “knowing if that doesn’t work, I might need help from my colleagues in pediatric sedation.”
Choice of language matters when describing to children what to expect during a procedure, she continued. For example, instead of saying, “it will feel like a bee sting,” say, “some kids say it is uncomfortable like a pinch and some kids say it’s not so bad.” And, when describing the size of a needle or an incision, instead of saying, “it’s as big as ...” say, “it’s as small as ...”
As described in a 2020 paper published in Pediatric Dermatology, proper comfort positioning of children during in-office dermatologic procedures is also key, which can include having the parent or caregiver hug a child during removal of a PG, Dr. Dhossche said. “You want to optimize distractions for the patient while you do the procedure. This is the time to bring out your iPhone, iPad, or enlist help from a certified child life specialist if you have one at your institution.”
When she administers injections to children, “I don’t lie about the shot, but I do hide the actual needle from sight, if possible,” she said. “I’ll say, ‘you’ll feel a pinch.’ Vibration tools can help while you’re injecting.” She showed an image of a vibrating light-up children’s toothbrush she found on Amazon for $10 “that has served me well. It’s also kind of a tension diffuser.”
Dr. Dhossche reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — Mounting according to Julie Dhossche, MD.
A PG is a common, benign vascular tumor that often occurs in children under 5 years of age, “usually in a very inconvenient spot, like the cheek,” Dr. Dhossche, a pediatric dermatologist at Oregon Health & Science University (OHSU), Portland, said at the annual meeting of the Pacific Dermatologic Association. “It can bleed a lot. Often, parents take their child to the emergency department for unstoppable bleeding. Our first-line treatment is often surgical: shave removal, electrocautery, or excision.”
Several case reports about the use of the topical form of timolol, a nonselective beta-adrenergic antagonist, for PG have been published in the medical literature including a case series of seven patients (six were treated with topical timolol). The authors of the case series hypothesized that a beta-blocker may be effective for PGs by causing vasoconstriction that stops bleeding.
In addition, Dr. Dhossche and colleagues retrospectively evaluated 92 children with a mean age of 4.5 years who were treated with topical timolol for PG at OHSU from 2010 to 2020. The results were presented in an abstract at the 2022 Pediatric Dermatology Research Alliance annual conference.
At the initial visit, 80 of 92 (87%) children were treated with timolol only, 6 of 92 (6.5%) underwent a procedure, and 6 of 92 (6.5%) were treated with timolol and a procedure. The researchers observed that of the 80 patients who received timolol monotherapy, 42 (52.5%) were spared a procedural intervention. “So, we have had some success with this,” she said. “It can also help with bleeding episodes if you are waiting for a procedure.”
Surgery May Still Be Needed
For PGs, she applies one drop of timolol to the lesion under occlusion with DuoDERM or a similar dressing, which is repeated every 1-3 days depending on how long the dressing stays on. “It may take 3-4 months of this treatment to clear,” she said.
If topical timolol doesn’t stop the PG from bleeding, or if parents elect for surgical removal, “some tears [during removal of the lesion] may be inevitable,” Dr. Dhossche said. “My goal is to make it as good of an experience as it can be, by being very confident and offering lots of smiles, pretreatment with topical lidocaine for 20-30 minutes, icing, and formulating an alliance with parents” to help calm nerves, “knowing if that doesn’t work, I might need help from my colleagues in pediatric sedation.”
Choice of language matters when describing to children what to expect during a procedure, she continued. For example, instead of saying, “it will feel like a bee sting,” say, “some kids say it is uncomfortable like a pinch and some kids say it’s not so bad.” And, when describing the size of a needle or an incision, instead of saying, “it’s as big as ...” say, “it’s as small as ...”
As described in a 2020 paper published in Pediatric Dermatology, proper comfort positioning of children during in-office dermatologic procedures is also key, which can include having the parent or caregiver hug a child during removal of a PG, Dr. Dhossche said. “You want to optimize distractions for the patient while you do the procedure. This is the time to bring out your iPhone, iPad, or enlist help from a certified child life specialist if you have one at your institution.”
When she administers injections to children, “I don’t lie about the shot, but I do hide the actual needle from sight, if possible,” she said. “I’ll say, ‘you’ll feel a pinch.’ Vibration tools can help while you’re injecting.” She showed an image of a vibrating light-up children’s toothbrush she found on Amazon for $10 “that has served me well. It’s also kind of a tension diffuser.”
Dr. Dhossche reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Managing Vitiligo: Combination Therapies, New Treatments
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Metformin Led to Improvements in Women with Central Centrifugal Cicatricial Alopecia
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Analysis of Colchicine’s Drug-Drug Interactions Finds Little Risk
TOPLINE:
The presence of an operational classification of drug interactions (ORCA) class 3 or 4 drug-drug interactions (DDIs) did not increase the risk for colchicine-related gastrointestinal adverse events or modify the effect of colchicine on death or hospitalization caused by COVID-19 infection in ambulatory patients.
METHODOLOGY:
- This secondary analysis of the COLCORONA trial aimed to evaluate if a potential DDI of colchicine was associated with changes in its pharmacokinetics or modified its clinical safety and efficacy in patients with COVID-19.
- Overall, 4432 ambulatory patients with COVID-19 (median age, 54 years; 54% women) were randomly assigned to receive colchicine 0.5 mg twice daily for 3 days and then 0.5 mg once daily for 27 days (n = 2205) or a placebo (n = 2227).
- All the participants had at least one high-risk criterion such as age ≥ 70 years, diabetes, heart failure, systolic blood pressure ≥ 150 mm Hg, respiratory disease, coronary disease, body temperature ≥ 38.4 °C within the last 48 hours, dyspnea, bicytopenia, pancytopenia, or high neutrophil count with low lymphocyte count.
- The medications that could interact with colchicine were determined and categorized under ORCA classes 1 (contraindicated), 2 (provisionally contraindicated), 3 (conditional use), or 4 (minimal risk).
- The primary outcome was any gastrointestinal adverse event assessed over a 30-day follow-up period.
TAKEAWAY:
- Among all the participants, 1% received medications with an ORCA class 2 interaction, 14% with a class 3 interaction, and 13% with a class 4 interaction; rosuvastatin (12%) and atorvastatin (10%) were the most common interacting medications.
- The odds of any gastrointestinal adverse event were 1.80 times and 1.68 times higher in the colchicine arm than in the placebo arm among those without and with a DDI, respectively, with the effect of colchicine being consistent regardless of the presence of drug interactions (P = .69 for interaction).
- Similarly, DDIs did not influence the effect of colchicine on combined risk for COVID-19 hospitalization or mortality (P = .80 for interaction).
IN PRACTICE:
“Once potential DDIs have been identified through screening, they must be tested,” Hemalkumar B. Mehta, PhD, and G. Caleb Alexander, MD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, wrote in an invited commentary published online in JAMA Network Open. “Theoretical DDIs may not translate into real-world harms,” they added.
SOURCE:
The study was led by Lama S. Alfehaid, PharmD, of Brigham and Women’s Hospital, Boston. It was published online in JAMA Network Open.
LIMITATIONS:
This study focused on the medications used by participants at baseline, which may not have captured all potential DDIs. The findings did not provide information on rare adverse events, such as rhabdomyolysis, which usually occur months after initiating drug therapy. Furthermore, all the study participants had confirmed SARS-CoV-2 infection, which may have increased their susceptibility to adverse reactions associated with the use of colchicine.
DISCLOSURES:
Some authors were supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute, American Heart Association, and other sources. The authors also declared serving on advisory boards or on the board of directors; receiving personal fees, grants, research support, or speaking fees; or having other ties with many pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The presence of an operational classification of drug interactions (ORCA) class 3 or 4 drug-drug interactions (DDIs) did not increase the risk for colchicine-related gastrointestinal adverse events or modify the effect of colchicine on death or hospitalization caused by COVID-19 infection in ambulatory patients.
METHODOLOGY:
- This secondary analysis of the COLCORONA trial aimed to evaluate if a potential DDI of colchicine was associated with changes in its pharmacokinetics or modified its clinical safety and efficacy in patients with COVID-19.
- Overall, 4432 ambulatory patients with COVID-19 (median age, 54 years; 54% women) were randomly assigned to receive colchicine 0.5 mg twice daily for 3 days and then 0.5 mg once daily for 27 days (n = 2205) or a placebo (n = 2227).
- All the participants had at least one high-risk criterion such as age ≥ 70 years, diabetes, heart failure, systolic blood pressure ≥ 150 mm Hg, respiratory disease, coronary disease, body temperature ≥ 38.4 °C within the last 48 hours, dyspnea, bicytopenia, pancytopenia, or high neutrophil count with low lymphocyte count.
- The medications that could interact with colchicine were determined and categorized under ORCA classes 1 (contraindicated), 2 (provisionally contraindicated), 3 (conditional use), or 4 (minimal risk).
- The primary outcome was any gastrointestinal adverse event assessed over a 30-day follow-up period.
TAKEAWAY:
- Among all the participants, 1% received medications with an ORCA class 2 interaction, 14% with a class 3 interaction, and 13% with a class 4 interaction; rosuvastatin (12%) and atorvastatin (10%) were the most common interacting medications.
- The odds of any gastrointestinal adverse event were 1.80 times and 1.68 times higher in the colchicine arm than in the placebo arm among those without and with a DDI, respectively, with the effect of colchicine being consistent regardless of the presence of drug interactions (P = .69 for interaction).
- Similarly, DDIs did not influence the effect of colchicine on combined risk for COVID-19 hospitalization or mortality (P = .80 for interaction).
IN PRACTICE:
“Once potential DDIs have been identified through screening, they must be tested,” Hemalkumar B. Mehta, PhD, and G. Caleb Alexander, MD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, wrote in an invited commentary published online in JAMA Network Open. “Theoretical DDIs may not translate into real-world harms,” they added.
SOURCE:
The study was led by Lama S. Alfehaid, PharmD, of Brigham and Women’s Hospital, Boston. It was published online in JAMA Network Open.
LIMITATIONS:
This study focused on the medications used by participants at baseline, which may not have captured all potential DDIs. The findings did not provide information on rare adverse events, such as rhabdomyolysis, which usually occur months after initiating drug therapy. Furthermore, all the study participants had confirmed SARS-CoV-2 infection, which may have increased their susceptibility to adverse reactions associated with the use of colchicine.
DISCLOSURES:
Some authors were supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute, American Heart Association, and other sources. The authors also declared serving on advisory boards or on the board of directors; receiving personal fees, grants, research support, or speaking fees; or having other ties with many pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The presence of an operational classification of drug interactions (ORCA) class 3 or 4 drug-drug interactions (DDIs) did not increase the risk for colchicine-related gastrointestinal adverse events or modify the effect of colchicine on death or hospitalization caused by COVID-19 infection in ambulatory patients.
METHODOLOGY:
- This secondary analysis of the COLCORONA trial aimed to evaluate if a potential DDI of colchicine was associated with changes in its pharmacokinetics or modified its clinical safety and efficacy in patients with COVID-19.
- Overall, 4432 ambulatory patients with COVID-19 (median age, 54 years; 54% women) were randomly assigned to receive colchicine 0.5 mg twice daily for 3 days and then 0.5 mg once daily for 27 days (n = 2205) or a placebo (n = 2227).
- All the participants had at least one high-risk criterion such as age ≥ 70 years, diabetes, heart failure, systolic blood pressure ≥ 150 mm Hg, respiratory disease, coronary disease, body temperature ≥ 38.4 °C within the last 48 hours, dyspnea, bicytopenia, pancytopenia, or high neutrophil count with low lymphocyte count.
- The medications that could interact with colchicine were determined and categorized under ORCA classes 1 (contraindicated), 2 (provisionally contraindicated), 3 (conditional use), or 4 (minimal risk).
- The primary outcome was any gastrointestinal adverse event assessed over a 30-day follow-up period.
TAKEAWAY:
- Among all the participants, 1% received medications with an ORCA class 2 interaction, 14% with a class 3 interaction, and 13% with a class 4 interaction; rosuvastatin (12%) and atorvastatin (10%) were the most common interacting medications.
- The odds of any gastrointestinal adverse event were 1.80 times and 1.68 times higher in the colchicine arm than in the placebo arm among those without and with a DDI, respectively, with the effect of colchicine being consistent regardless of the presence of drug interactions (P = .69 for interaction).
- Similarly, DDIs did not influence the effect of colchicine on combined risk for COVID-19 hospitalization or mortality (P = .80 for interaction).
IN PRACTICE:
“Once potential DDIs have been identified through screening, they must be tested,” Hemalkumar B. Mehta, PhD, and G. Caleb Alexander, MD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, wrote in an invited commentary published online in JAMA Network Open. “Theoretical DDIs may not translate into real-world harms,” they added.
SOURCE:
The study was led by Lama S. Alfehaid, PharmD, of Brigham and Women’s Hospital, Boston. It was published online in JAMA Network Open.
LIMITATIONS:
This study focused on the medications used by participants at baseline, which may not have captured all potential DDIs. The findings did not provide information on rare adverse events, such as rhabdomyolysis, which usually occur months after initiating drug therapy. Furthermore, all the study participants had confirmed SARS-CoV-2 infection, which may have increased their susceptibility to adverse reactions associated with the use of colchicine.
DISCLOSURES:
Some authors were supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute, American Heart Association, and other sources. The authors also declared serving on advisory boards or on the board of directors; receiving personal fees, grants, research support, or speaking fees; or having other ties with many pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
What’s Causing Raynaud Phenomenon Severity to Rise With High Temperatures?
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Focusing on Value in Social Media Posts
CARLSBAD, CALIFORNIA — Posting on social media may not be your cup of tea, but in the opinion of Jessica G. Labadie, MD,
“Over the past 2 decades, there has been a surge in social media use,” Dr. Labadie, a dermatologist who practices in Chestnut Hill, Massachusetts, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “Most of our patients use social media to find their doctors, and it plays a role in how our patients form their decision about whether to have a cosmetic procedure or not. Doctors, especially dermatologists, continue to actively participate in this ‘skinfluencer’ trend.”
According to a review of social media’s impact on aesthetic medicine, use of social media by American adults increased from 5% in 2005 to 72% in 2020, and 77% of patients search for a physician online. The review’s authors cited YouTube as the most popular platform among adults and noted that social media ranks as the sixth top factor for a patient deciding whether to have a laser procedure.
Dr. Labadie, who is also an assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said several factors should be considered when establishing and maintaining a social media presence, starting with personal ones. “Your followers are not your patients yet, and just because you may have thousands of followers does not necessarily mean that you’re busier in the clinic,” she said. “Be careful if you combine professional and personal accounts; be careful of those parasocial relationships that can form. Your followers tend to learn a lot about you. Posting can take a lot of time; it can take away from your clinical duties. Do you want to make your account private or public? There are pros and cons to both.”
Ethics also play a role. “Be transparent in your disclosure forms, especially if you’re posting ‘before’ and ‘after’ images of patients,” advised Dr. Labadie, who described herself as a social media minimalist. “Stay true to yourself in your posts, and always prioritize safety over posting.”
Don’t forget legal obligations. “Social media can facilitate a passive income, but make sure this isn’t impacting any conflicts of interest, and make sure that you meticulously follow any Health Insurance Portability and Accountability Act regulations,” she said. She also cautioned against violating intellectual property rights and making false claims about a product or procedure.
Deciding which platforms to use and what voice or tone to adopt requires some soul-searching. “What is your brand?” Dr. Labadie asked. “How do you want to portray yourself? Does your social media brand match your office brand? Does it match who you are as a provider and the type of patient you wish to attract? Would you prefer to have one collective social media presence as an office or multiple provider accounts?”
Being mindful of how your patients perceive and use social media in relation to their dermatologic concerns is also important. “What are your patients viewing on social media, and how is it affecting their decisions?” Dr. Labadie asked. “Are they coming in asking for something that is not right for what they need? At the end of the day, you are their doctor, and it’s your duty to treat the patients and not the trend.”
She encouraged dermatologists to “aim for high value and accurate posts coupled with high popularity and reach.” She added that “this is really the future of getting our research out there to the public. Academic notoriety is not enough. Our professional societies and skinfluencer colleagues need to get involved to help promote our expert research.”
Dr. Labadie reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — Posting on social media may not be your cup of tea, but in the opinion of Jessica G. Labadie, MD,
“Over the past 2 decades, there has been a surge in social media use,” Dr. Labadie, a dermatologist who practices in Chestnut Hill, Massachusetts, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “Most of our patients use social media to find their doctors, and it plays a role in how our patients form their decision about whether to have a cosmetic procedure or not. Doctors, especially dermatologists, continue to actively participate in this ‘skinfluencer’ trend.”
According to a review of social media’s impact on aesthetic medicine, use of social media by American adults increased from 5% in 2005 to 72% in 2020, and 77% of patients search for a physician online. The review’s authors cited YouTube as the most popular platform among adults and noted that social media ranks as the sixth top factor for a patient deciding whether to have a laser procedure.
Dr. Labadie, who is also an assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said several factors should be considered when establishing and maintaining a social media presence, starting with personal ones. “Your followers are not your patients yet, and just because you may have thousands of followers does not necessarily mean that you’re busier in the clinic,” she said. “Be careful if you combine professional and personal accounts; be careful of those parasocial relationships that can form. Your followers tend to learn a lot about you. Posting can take a lot of time; it can take away from your clinical duties. Do you want to make your account private or public? There are pros and cons to both.”
Ethics also play a role. “Be transparent in your disclosure forms, especially if you’re posting ‘before’ and ‘after’ images of patients,” advised Dr. Labadie, who described herself as a social media minimalist. “Stay true to yourself in your posts, and always prioritize safety over posting.”
Don’t forget legal obligations. “Social media can facilitate a passive income, but make sure this isn’t impacting any conflicts of interest, and make sure that you meticulously follow any Health Insurance Portability and Accountability Act regulations,” she said. She also cautioned against violating intellectual property rights and making false claims about a product or procedure.
Deciding which platforms to use and what voice or tone to adopt requires some soul-searching. “What is your brand?” Dr. Labadie asked. “How do you want to portray yourself? Does your social media brand match your office brand? Does it match who you are as a provider and the type of patient you wish to attract? Would you prefer to have one collective social media presence as an office or multiple provider accounts?”
Being mindful of how your patients perceive and use social media in relation to their dermatologic concerns is also important. “What are your patients viewing on social media, and how is it affecting their decisions?” Dr. Labadie asked. “Are they coming in asking for something that is not right for what they need? At the end of the day, you are their doctor, and it’s your duty to treat the patients and not the trend.”
She encouraged dermatologists to “aim for high value and accurate posts coupled with high popularity and reach.” She added that “this is really the future of getting our research out there to the public. Academic notoriety is not enough. Our professional societies and skinfluencer colleagues need to get involved to help promote our expert research.”
Dr. Labadie reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — Posting on social media may not be your cup of tea, but in the opinion of Jessica G. Labadie, MD,
“Over the past 2 decades, there has been a surge in social media use,” Dr. Labadie, a dermatologist who practices in Chestnut Hill, Massachusetts, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “Most of our patients use social media to find their doctors, and it plays a role in how our patients form their decision about whether to have a cosmetic procedure or not. Doctors, especially dermatologists, continue to actively participate in this ‘skinfluencer’ trend.”
According to a review of social media’s impact on aesthetic medicine, use of social media by American adults increased from 5% in 2005 to 72% in 2020, and 77% of patients search for a physician online. The review’s authors cited YouTube as the most popular platform among adults and noted that social media ranks as the sixth top factor for a patient deciding whether to have a laser procedure.
Dr. Labadie, who is also an assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said several factors should be considered when establishing and maintaining a social media presence, starting with personal ones. “Your followers are not your patients yet, and just because you may have thousands of followers does not necessarily mean that you’re busier in the clinic,” she said. “Be careful if you combine professional and personal accounts; be careful of those parasocial relationships that can form. Your followers tend to learn a lot about you. Posting can take a lot of time; it can take away from your clinical duties. Do you want to make your account private or public? There are pros and cons to both.”
Ethics also play a role. “Be transparent in your disclosure forms, especially if you’re posting ‘before’ and ‘after’ images of patients,” advised Dr. Labadie, who described herself as a social media minimalist. “Stay true to yourself in your posts, and always prioritize safety over posting.”
Don’t forget legal obligations. “Social media can facilitate a passive income, but make sure this isn’t impacting any conflicts of interest, and make sure that you meticulously follow any Health Insurance Portability and Accountability Act regulations,” she said. She also cautioned against violating intellectual property rights and making false claims about a product or procedure.
Deciding which platforms to use and what voice or tone to adopt requires some soul-searching. “What is your brand?” Dr. Labadie asked. “How do you want to portray yourself? Does your social media brand match your office brand? Does it match who you are as a provider and the type of patient you wish to attract? Would you prefer to have one collective social media presence as an office or multiple provider accounts?”
Being mindful of how your patients perceive and use social media in relation to their dermatologic concerns is also important. “What are your patients viewing on social media, and how is it affecting their decisions?” Dr. Labadie asked. “Are they coming in asking for something that is not right for what they need? At the end of the day, you are their doctor, and it’s your duty to treat the patients and not the trend.”
She encouraged dermatologists to “aim for high value and accurate posts coupled with high popularity and reach.” She added that “this is really the future of getting our research out there to the public. Academic notoriety is not enough. Our professional societies and skinfluencer colleagues need to get involved to help promote our expert research.”
Dr. Labadie reported having no financial disclosures.
A version of this article appeared on Medscape.com.
Do Clonal Hematopoiesis and Mosaic Chromosomal Alterations Increase Solid Tumor Risk?
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
FROM CANCER
Study Indicates Skin Cancer Risk Elevated Among Veterans
TOPLINE:
METHODOLOGY:
- Researchers analyzed the prevalence and likelihood of skin cancer and other dermatologic conditions between veterans and nonveterans using national representative NHANES data collected over two decades (1999-2018).
- They included 61,307 participants, with 54,554 nonveterans (42.76% men; 65.78% non-Hispanic White individuals) and 6753 veterans (92.74% men; 80.42% non-Hispanic White individuals).
- A total of 54,991 participants (48,278 nonveterans and 6713 veterans) answered questions about their cancer history.
TAKEAWAY:
- Veterans had a higher prevalence of any skin cancer than nonveterans (9% vs 2.9%; P < .001). Specifically, the prevalence of melanoma (2.2% vs 0.6%), nonmelanoma skin cancer (5.1% vs 1.6%), and skin cancer of unknown subtype (2.2% vs 0.8%) was significantly higher in veterans (P < .001, for all).
- Veterans showed elevated risks for any skin cancer (odds ratio [OR], 1.72; 95% CI, 1.23-2.40), melanoma (OR, 2.27; 95% CI, 1.17-4.39), and nonmelanoma skin cancer (OR, 1.80; 95% CI, 1.17-2.78) after adjusting for demographic factors.
- Veterans also had a higher risk for psoriasis (OR, 1.61; 95% CI, 1.05-2.46), but not for eczema/dermatitis/inflamed rash in the previous 30 days anywhere on the body, although risk was significantly increased when localized to the arms.
- Veterans were more likely to spend time outdoors on workdays (OR, 1.22; 95% CI, 1.04-2.25) but their status did not differ significantly from that of nonveterans in sunscreen use or other sun protection behaviors. However, veterans had a 44%-45% (P < .05) increased risk for severe sunburn after brief sun exposure.
IN PRACTICE:
“Public health measures seeking to address veteran healthcare differences could emphasize primary preventive strategies to mitigate risk and early detection of dermatologic conditions through regular skin examinations,” the study authors concluded. An accompanying editorial noted that “dermatologists should be aware that veterans face higher skin cancer risks even after adjusting for demographic differences, potentially due at least in part, to occupational exposures.” In addition, the editorial authors wrote, “additional research is needed to identify and quantify the effects of UV and military toxic exposures on skin cancer risk among active duty service members.”
SOURCE:
The study was led by Shawheen J. Rezaei, MPhil, from the Department of Dermatology, Stanford University School of Medicine, Stanford, California, and was published online in JAMA Dermatology. The authors of the editorial are from the Departments of Dermatology at Brigham and Women’s Hospital, Boston, and Vanderbilt University, Nashville, Tennessee.
LIMITATIONS:
Skin cancer, psoriasis, and eczema/dermatitis were self-reported, and the predominance of older White men limited the generalizability of the findings.
DISCLOSURES:
The study was supported by Veterans Affairs (VA) Palo Alto Health Care System, Palo Alto, California, and Providence VA Medical Center, Providence, Rhode Island. The authors had no disclosures. The authors of the editorial disclosed receiving grants from the VA; one author’s disclosures included receiving personal fees from and being a scientific officer for Evereden, receiving grants and research funding from DermaSensor, and consulting for Oasis Pharmaceuticals and Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed the prevalence and likelihood of skin cancer and other dermatologic conditions between veterans and nonveterans using national representative NHANES data collected over two decades (1999-2018).
- They included 61,307 participants, with 54,554 nonveterans (42.76% men; 65.78% non-Hispanic White individuals) and 6753 veterans (92.74% men; 80.42% non-Hispanic White individuals).
- A total of 54,991 participants (48,278 nonveterans and 6713 veterans) answered questions about their cancer history.
TAKEAWAY:
- Veterans had a higher prevalence of any skin cancer than nonveterans (9% vs 2.9%; P < .001). Specifically, the prevalence of melanoma (2.2% vs 0.6%), nonmelanoma skin cancer (5.1% vs 1.6%), and skin cancer of unknown subtype (2.2% vs 0.8%) was significantly higher in veterans (P < .001, for all).
- Veterans showed elevated risks for any skin cancer (odds ratio [OR], 1.72; 95% CI, 1.23-2.40), melanoma (OR, 2.27; 95% CI, 1.17-4.39), and nonmelanoma skin cancer (OR, 1.80; 95% CI, 1.17-2.78) after adjusting for demographic factors.
- Veterans also had a higher risk for psoriasis (OR, 1.61; 95% CI, 1.05-2.46), but not for eczema/dermatitis/inflamed rash in the previous 30 days anywhere on the body, although risk was significantly increased when localized to the arms.
- Veterans were more likely to spend time outdoors on workdays (OR, 1.22; 95% CI, 1.04-2.25) but their status did not differ significantly from that of nonveterans in sunscreen use or other sun protection behaviors. However, veterans had a 44%-45% (P < .05) increased risk for severe sunburn after brief sun exposure.
IN PRACTICE:
“Public health measures seeking to address veteran healthcare differences could emphasize primary preventive strategies to mitigate risk and early detection of dermatologic conditions through regular skin examinations,” the study authors concluded. An accompanying editorial noted that “dermatologists should be aware that veterans face higher skin cancer risks even after adjusting for demographic differences, potentially due at least in part, to occupational exposures.” In addition, the editorial authors wrote, “additional research is needed to identify and quantify the effects of UV and military toxic exposures on skin cancer risk among active duty service members.”
SOURCE:
The study was led by Shawheen J. Rezaei, MPhil, from the Department of Dermatology, Stanford University School of Medicine, Stanford, California, and was published online in JAMA Dermatology. The authors of the editorial are from the Departments of Dermatology at Brigham and Women’s Hospital, Boston, and Vanderbilt University, Nashville, Tennessee.
LIMITATIONS:
Skin cancer, psoriasis, and eczema/dermatitis were self-reported, and the predominance of older White men limited the generalizability of the findings.
DISCLOSURES:
The study was supported by Veterans Affairs (VA) Palo Alto Health Care System, Palo Alto, California, and Providence VA Medical Center, Providence, Rhode Island. The authors had no disclosures. The authors of the editorial disclosed receiving grants from the VA; one author’s disclosures included receiving personal fees from and being a scientific officer for Evereden, receiving grants and research funding from DermaSensor, and consulting for Oasis Pharmaceuticals and Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed the prevalence and likelihood of skin cancer and other dermatologic conditions between veterans and nonveterans using national representative NHANES data collected over two decades (1999-2018).
- They included 61,307 participants, with 54,554 nonveterans (42.76% men; 65.78% non-Hispanic White individuals) and 6753 veterans (92.74% men; 80.42% non-Hispanic White individuals).
- A total of 54,991 participants (48,278 nonveterans and 6713 veterans) answered questions about their cancer history.
TAKEAWAY:
- Veterans had a higher prevalence of any skin cancer than nonveterans (9% vs 2.9%; P < .001). Specifically, the prevalence of melanoma (2.2% vs 0.6%), nonmelanoma skin cancer (5.1% vs 1.6%), and skin cancer of unknown subtype (2.2% vs 0.8%) was significantly higher in veterans (P < .001, for all).
- Veterans showed elevated risks for any skin cancer (odds ratio [OR], 1.72; 95% CI, 1.23-2.40), melanoma (OR, 2.27; 95% CI, 1.17-4.39), and nonmelanoma skin cancer (OR, 1.80; 95% CI, 1.17-2.78) after adjusting for demographic factors.
- Veterans also had a higher risk for psoriasis (OR, 1.61; 95% CI, 1.05-2.46), but not for eczema/dermatitis/inflamed rash in the previous 30 days anywhere on the body, although risk was significantly increased when localized to the arms.
- Veterans were more likely to spend time outdoors on workdays (OR, 1.22; 95% CI, 1.04-2.25) but their status did not differ significantly from that of nonveterans in sunscreen use or other sun protection behaviors. However, veterans had a 44%-45% (P < .05) increased risk for severe sunburn after brief sun exposure.
IN PRACTICE:
“Public health measures seeking to address veteran healthcare differences could emphasize primary preventive strategies to mitigate risk and early detection of dermatologic conditions through regular skin examinations,” the study authors concluded. An accompanying editorial noted that “dermatologists should be aware that veterans face higher skin cancer risks even after adjusting for demographic differences, potentially due at least in part, to occupational exposures.” In addition, the editorial authors wrote, “additional research is needed to identify and quantify the effects of UV and military toxic exposures on skin cancer risk among active duty service members.”
SOURCE:
The study was led by Shawheen J. Rezaei, MPhil, from the Department of Dermatology, Stanford University School of Medicine, Stanford, California, and was published online in JAMA Dermatology. The authors of the editorial are from the Departments of Dermatology at Brigham and Women’s Hospital, Boston, and Vanderbilt University, Nashville, Tennessee.
LIMITATIONS:
Skin cancer, psoriasis, and eczema/dermatitis were self-reported, and the predominance of older White men limited the generalizability of the findings.
DISCLOSURES:
The study was supported by Veterans Affairs (VA) Palo Alto Health Care System, Palo Alto, California, and Providence VA Medical Center, Providence, Rhode Island. The authors had no disclosures. The authors of the editorial disclosed receiving grants from the VA; one author’s disclosures included receiving personal fees from and being a scientific officer for Evereden, receiving grants and research funding from DermaSensor, and consulting for Oasis Pharmaceuticals and Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
The Wellness Industry: Financially Toxic, Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Multiple Draining Sinus Tracts on the Thigh
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
A 40-year-old woman presented with multiple draining sinus tracts on the right thigh following an injury sustained weeks earlier while mowing wet grass.