User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Screen high-risk individuals for NAFLD, urges guidance
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM HEPATOLOGY
Treatment of several nail disorders reviewed
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
AT ODAC 2023
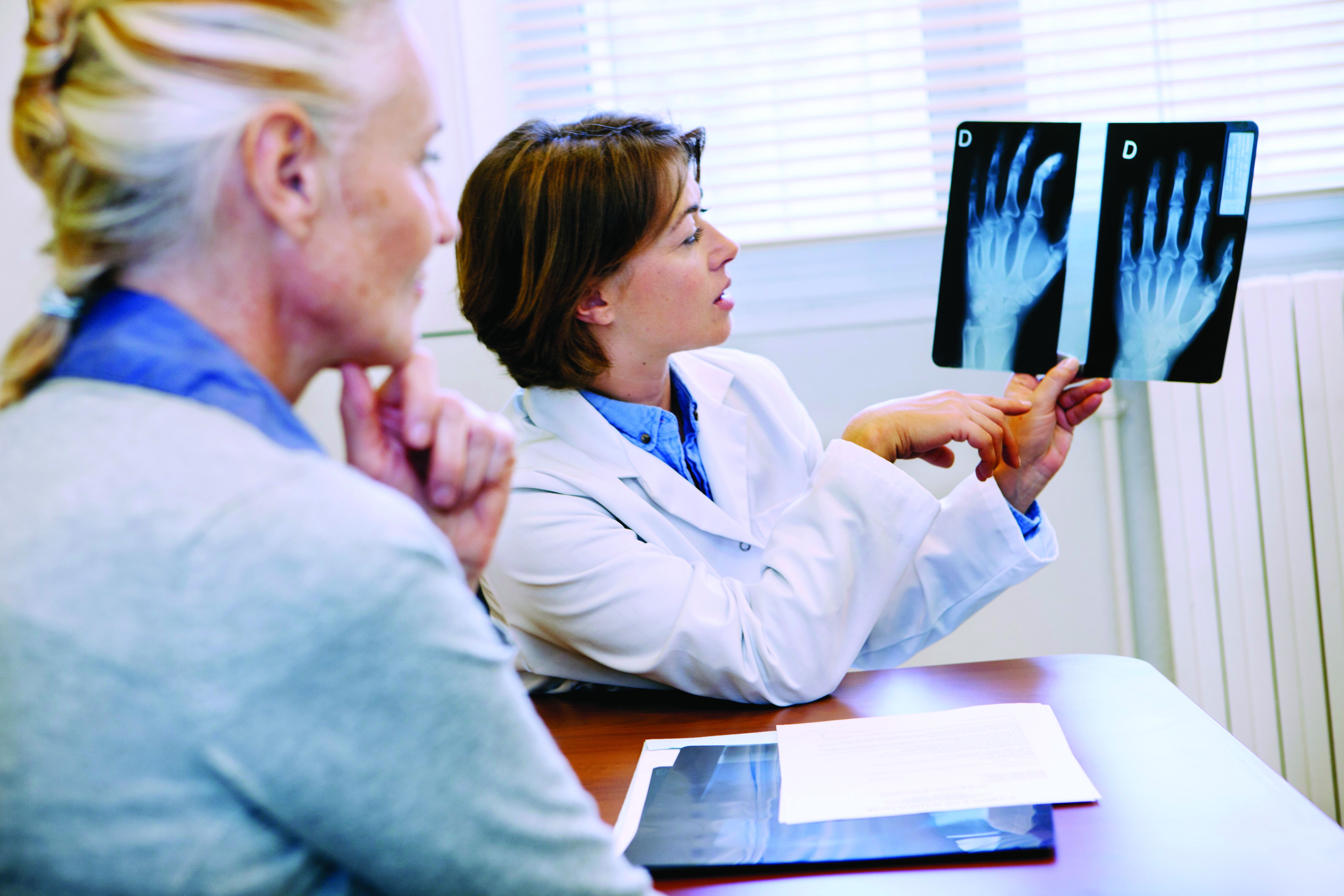
Are repeat radiographs necessary in rheumatoid and psoriatic arthritis?
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
FROM RWCS 2023
Novel celery seed–derived drug may improve stroke outcomes
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
New tool better estimates cardiovascular risk in people with lupus
Current risk estimators are inaccurate
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
Current risk estimators are inaccurate
Current risk estimators are inaccurate
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
FROM CRA 2023
PsA prediction tool approaches clinical utility
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
Easily collected variables establish risk
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
FROM CRA 2023
Eye drops delay, may even prevent, nearsightedness
Dilating eye drops may delay – and perhaps even prevent – the onset of myopia in children, according to findings from a study published in JAMA. The study was conducted by researchers in Hong Kong.
Myopia is irreversible once it takes root and can contribute to other vision problems, such as macular degeneration, retinal detachment, glaucoma, and cataracts. The incidence of nearsightedness has nearly doubled since the 1970s, rising from 25% of the U.S. population to nearly 42%. Children have been particularly affected by the increase; reasons may include spending more time indoors looking at screens, experts say.
“Myopia is an ongoing and growing worldwide concern. This is of particular importance because of the change in children’s lifestyle, such as decreased outdoor time and increased screen time during and after the COVID-19 pandemic,” Jason C. Yam, MPH, of the department of ophthalmology and visual services at the Chinese University of Hong Kong, said in an interview.
Mr. Yam said that, while encouraging children to engage more in outdoor activity and spend less time using screens would help delay myopia, pharmaceutical interventions are needed, given myopia’s potential lifelong effects.
Putting eye drops to the test
In 2020, Mr. Yam and colleagues reported results of the Low-Concentration Atropine for Myopia Progression (LAMP) study, which showed that eye drops containing a solution of 0.05% atropine worked best at slowing the progression of myopia in 4- to 12-year-olds who already had the condition. Atropine relaxes eye muscles, causing dilation.
In that study, Mr. Yam and colleagues measured the rate of change in the eye’s ability to see at a great distance using a unit of measure known as the diopter. The higher the diopter, the more myopic a person’s vision. The 0.05% atropine solution was better at slowing this decline than placebo or solutions that contained a lower concentration of the substance.
The new study enrolled 474 children who were evenly divided by sex. None of the children had myopia when the trial began. Of that starting group, 353 children (age, 4-9 years) completed the study, which involved receiving eye drops once nightly in both eyes for 2 years.
Some children (n = 116) received 0.05% atropine, others (n = 122) received 0.01% atropine, and the rest (n = 115) received placebo drops. Mr. Yam and colleagues assessed how many children in each group had myopia after 2 years, as measured by a decline of at least a half diopter in one eye.
At the 2-year mark, more than half of children who received the placebo drops (61/115) had developed myopia, as had nearly half of those given 0.01% atropine (56/122). But fewer than one-third of children (33/116, 28.4%) who had received the drops with 0.05% atropine developed myopia during that period, the researchers reported.
The percentage of children with myopia in the placebo group (39/128, 30.5%) was larger by the end of the first year of the study than was the share of children in the 0.05% atropine group by the end of the trial. (Between 12 months and 24 months, 13 children in the placebo group left the study.) The main adverse event, in all treatment groups, was discomfort when exposed to bright light, according to the researchers.
“We are continuing the current study with the total intended follow-up duration of at least 6years,” added Mr. Yam, who hopes to determine whether the 0.05% atropine solution not only delays myopia but also prevents it altogether. While myopia is a concern worldwide, the condition is particularly prevalent in East Asia.
Mark A. Bullimore, MCOptom, PhD, FAAO, an adjunct professor at the University of Houston, and a consultant to ophthalmologic companies, called the trial “a landmark study. Finding children who are eligible and parents who are willing to deal with 2 years of drops is no small feat.
“The 0.05% atropine, on average, delays onset of myopia by a year,” Dr. Bullimore noted. He pointed to the similar percentages of myopia with placebo at 12 months, compared with 0.05% atropine 1 year later. He added that few clinicians in the United States use higher than 0.05% atropine for control of myopia because doing so can lead to excessive dilation and difficulty focusing.
While preventing myopia altogether would be ideal, simply delaying its onset can also be of tangible benefit, Bullimore said. In an article published in January, Dr. Bullimore and Noel A. Brennan of Johnson & Johnson Vision showed that delaying the onset of myopia reduces its severity.
“Optometrists prescribe low-concentration atropine already for myopia control, and there’s no reason now – in the light of this study – that they wouldn’t also do it to delay onset,” Dr. Bullimore said.
But in an editorial accompanying the journal article David A. Berntsen, OD, PhD, and Jeffrey J. Walline, OD, PhD, both of the University of Houston, wrote that a change in practice would be premature.
“The evidence presented does not yet warrant a change in the standard care of children because we do not yet know the long-term effects of delaying the onset of myopia with low-concentration atropine,” they wrote.
Identifying which children to consider for treatment is “a challenge,” they noted, because those who are not nearsighted typically do not undergo routine examination unless they have failed a vision test.
“Ultimately, the implementation of vision screenings that include determining a child’s prescription will likely be needed to identify children most likely to become myopic who may benefit from low-concentration atropine,” Dr. Berntsen and Dr. Walline wrote.
Mr. Yam and coinvestigators have applied for a patent on a 0.05% atropine solution. Dr. Bullimore reported relationships with Alcon Research,CooperVision, CorneaGen, EssilorLuxottica, Eyenovia, Genentech, Johnson & Johnson Vision, Lentechs, Novartis, and Vyluma, and is the sole owner of Ridgevue Publishing and Ridgevue Vision.
A version of this article first appeared on Medscape.com.
Dilating eye drops may delay – and perhaps even prevent – the onset of myopia in children, according to findings from a study published in JAMA. The study was conducted by researchers in Hong Kong.
Myopia is irreversible once it takes root and can contribute to other vision problems, such as macular degeneration, retinal detachment, glaucoma, and cataracts. The incidence of nearsightedness has nearly doubled since the 1970s, rising from 25% of the U.S. population to nearly 42%. Children have been particularly affected by the increase; reasons may include spending more time indoors looking at screens, experts say.
“Myopia is an ongoing and growing worldwide concern. This is of particular importance because of the change in children’s lifestyle, such as decreased outdoor time and increased screen time during and after the COVID-19 pandemic,” Jason C. Yam, MPH, of the department of ophthalmology and visual services at the Chinese University of Hong Kong, said in an interview.
Mr. Yam said that, while encouraging children to engage more in outdoor activity and spend less time using screens would help delay myopia, pharmaceutical interventions are needed, given myopia’s potential lifelong effects.
Putting eye drops to the test
In 2020, Mr. Yam and colleagues reported results of the Low-Concentration Atropine for Myopia Progression (LAMP) study, which showed that eye drops containing a solution of 0.05% atropine worked best at slowing the progression of myopia in 4- to 12-year-olds who already had the condition. Atropine relaxes eye muscles, causing dilation.
In that study, Mr. Yam and colleagues measured the rate of change in the eye’s ability to see at a great distance using a unit of measure known as the diopter. The higher the diopter, the more myopic a person’s vision. The 0.05% atropine solution was better at slowing this decline than placebo or solutions that contained a lower concentration of the substance.
The new study enrolled 474 children who were evenly divided by sex. None of the children had myopia when the trial began. Of that starting group, 353 children (age, 4-9 years) completed the study, which involved receiving eye drops once nightly in both eyes for 2 years.
Some children (n = 116) received 0.05% atropine, others (n = 122) received 0.01% atropine, and the rest (n = 115) received placebo drops. Mr. Yam and colleagues assessed how many children in each group had myopia after 2 years, as measured by a decline of at least a half diopter in one eye.
At the 2-year mark, more than half of children who received the placebo drops (61/115) had developed myopia, as had nearly half of those given 0.01% atropine (56/122). But fewer than one-third of children (33/116, 28.4%) who had received the drops with 0.05% atropine developed myopia during that period, the researchers reported.
The percentage of children with myopia in the placebo group (39/128, 30.5%) was larger by the end of the first year of the study than was the share of children in the 0.05% atropine group by the end of the trial. (Between 12 months and 24 months, 13 children in the placebo group left the study.) The main adverse event, in all treatment groups, was discomfort when exposed to bright light, according to the researchers.
“We are continuing the current study with the total intended follow-up duration of at least 6years,” added Mr. Yam, who hopes to determine whether the 0.05% atropine solution not only delays myopia but also prevents it altogether. While myopia is a concern worldwide, the condition is particularly prevalent in East Asia.
Mark A. Bullimore, MCOptom, PhD, FAAO, an adjunct professor at the University of Houston, and a consultant to ophthalmologic companies, called the trial “a landmark study. Finding children who are eligible and parents who are willing to deal with 2 years of drops is no small feat.
“The 0.05% atropine, on average, delays onset of myopia by a year,” Dr. Bullimore noted. He pointed to the similar percentages of myopia with placebo at 12 months, compared with 0.05% atropine 1 year later. He added that few clinicians in the United States use higher than 0.05% atropine for control of myopia because doing so can lead to excessive dilation and difficulty focusing.
While preventing myopia altogether would be ideal, simply delaying its onset can also be of tangible benefit, Bullimore said. In an article published in January, Dr. Bullimore and Noel A. Brennan of Johnson & Johnson Vision showed that delaying the onset of myopia reduces its severity.
“Optometrists prescribe low-concentration atropine already for myopia control, and there’s no reason now – in the light of this study – that they wouldn’t also do it to delay onset,” Dr. Bullimore said.
But in an editorial accompanying the journal article David A. Berntsen, OD, PhD, and Jeffrey J. Walline, OD, PhD, both of the University of Houston, wrote that a change in practice would be premature.
“The evidence presented does not yet warrant a change in the standard care of children because we do not yet know the long-term effects of delaying the onset of myopia with low-concentration atropine,” they wrote.
Identifying which children to consider for treatment is “a challenge,” they noted, because those who are not nearsighted typically do not undergo routine examination unless they have failed a vision test.
“Ultimately, the implementation of vision screenings that include determining a child’s prescription will likely be needed to identify children most likely to become myopic who may benefit from low-concentration atropine,” Dr. Berntsen and Dr. Walline wrote.
Mr. Yam and coinvestigators have applied for a patent on a 0.05% atropine solution. Dr. Bullimore reported relationships with Alcon Research,CooperVision, CorneaGen, EssilorLuxottica, Eyenovia, Genentech, Johnson & Johnson Vision, Lentechs, Novartis, and Vyluma, and is the sole owner of Ridgevue Publishing and Ridgevue Vision.
A version of this article first appeared on Medscape.com.
Dilating eye drops may delay – and perhaps even prevent – the onset of myopia in children, according to findings from a study published in JAMA. The study was conducted by researchers in Hong Kong.
Myopia is irreversible once it takes root and can contribute to other vision problems, such as macular degeneration, retinal detachment, glaucoma, and cataracts. The incidence of nearsightedness has nearly doubled since the 1970s, rising from 25% of the U.S. population to nearly 42%. Children have been particularly affected by the increase; reasons may include spending more time indoors looking at screens, experts say.
“Myopia is an ongoing and growing worldwide concern. This is of particular importance because of the change in children’s lifestyle, such as decreased outdoor time and increased screen time during and after the COVID-19 pandemic,” Jason C. Yam, MPH, of the department of ophthalmology and visual services at the Chinese University of Hong Kong, said in an interview.
Mr. Yam said that, while encouraging children to engage more in outdoor activity and spend less time using screens would help delay myopia, pharmaceutical interventions are needed, given myopia’s potential lifelong effects.
Putting eye drops to the test
In 2020, Mr. Yam and colleagues reported results of the Low-Concentration Atropine for Myopia Progression (LAMP) study, which showed that eye drops containing a solution of 0.05% atropine worked best at slowing the progression of myopia in 4- to 12-year-olds who already had the condition. Atropine relaxes eye muscles, causing dilation.
In that study, Mr. Yam and colleagues measured the rate of change in the eye’s ability to see at a great distance using a unit of measure known as the diopter. The higher the diopter, the more myopic a person’s vision. The 0.05% atropine solution was better at slowing this decline than placebo or solutions that contained a lower concentration of the substance.
The new study enrolled 474 children who were evenly divided by sex. None of the children had myopia when the trial began. Of that starting group, 353 children (age, 4-9 years) completed the study, which involved receiving eye drops once nightly in both eyes for 2 years.
Some children (n = 116) received 0.05% atropine, others (n = 122) received 0.01% atropine, and the rest (n = 115) received placebo drops. Mr. Yam and colleagues assessed how many children in each group had myopia after 2 years, as measured by a decline of at least a half diopter in one eye.
At the 2-year mark, more than half of children who received the placebo drops (61/115) had developed myopia, as had nearly half of those given 0.01% atropine (56/122). But fewer than one-third of children (33/116, 28.4%) who had received the drops with 0.05% atropine developed myopia during that period, the researchers reported.
The percentage of children with myopia in the placebo group (39/128, 30.5%) was larger by the end of the first year of the study than was the share of children in the 0.05% atropine group by the end of the trial. (Between 12 months and 24 months, 13 children in the placebo group left the study.) The main adverse event, in all treatment groups, was discomfort when exposed to bright light, according to the researchers.
“We are continuing the current study with the total intended follow-up duration of at least 6years,” added Mr. Yam, who hopes to determine whether the 0.05% atropine solution not only delays myopia but also prevents it altogether. While myopia is a concern worldwide, the condition is particularly prevalent in East Asia.
Mark A. Bullimore, MCOptom, PhD, FAAO, an adjunct professor at the University of Houston, and a consultant to ophthalmologic companies, called the trial “a landmark study. Finding children who are eligible and parents who are willing to deal with 2 years of drops is no small feat.
“The 0.05% atropine, on average, delays onset of myopia by a year,” Dr. Bullimore noted. He pointed to the similar percentages of myopia with placebo at 12 months, compared with 0.05% atropine 1 year later. He added that few clinicians in the United States use higher than 0.05% atropine for control of myopia because doing so can lead to excessive dilation and difficulty focusing.
While preventing myopia altogether would be ideal, simply delaying its onset can also be of tangible benefit, Bullimore said. In an article published in January, Dr. Bullimore and Noel A. Brennan of Johnson & Johnson Vision showed that delaying the onset of myopia reduces its severity.
“Optometrists prescribe low-concentration atropine already for myopia control, and there’s no reason now – in the light of this study – that they wouldn’t also do it to delay onset,” Dr. Bullimore said.
But in an editorial accompanying the journal article David A. Berntsen, OD, PhD, and Jeffrey J. Walline, OD, PhD, both of the University of Houston, wrote that a change in practice would be premature.
“The evidence presented does not yet warrant a change in the standard care of children because we do not yet know the long-term effects of delaying the onset of myopia with low-concentration atropine,” they wrote.
Identifying which children to consider for treatment is “a challenge,” they noted, because those who are not nearsighted typically do not undergo routine examination unless they have failed a vision test.
“Ultimately, the implementation of vision screenings that include determining a child’s prescription will likely be needed to identify children most likely to become myopic who may benefit from low-concentration atropine,” Dr. Berntsen and Dr. Walline wrote.
Mr. Yam and coinvestigators have applied for a patent on a 0.05% atropine solution. Dr. Bullimore reported relationships with Alcon Research,CooperVision, CorneaGen, EssilorLuxottica, Eyenovia, Genentech, Johnson & Johnson Vision, Lentechs, Novartis, and Vyluma, and is the sole owner of Ridgevue Publishing and Ridgevue Vision.
A version of this article first appeared on Medscape.com.
FROM JAMA
COVID-19 shot appears to reduce diabetes risk, even after Omicron
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
new data suggest.
The findings, from more than 20,000 patients in the Cedars-Sinai Health System in Los Angeles, suggest that “continued efforts to prevent COVID-19 infection may be beneficial to patient health until we develop better understanding of the effects of potential long-term effects of COVID-19,” lead author Alan C. Kwan, MD, of the department of cardiology at Cedars Sinai’s Smidt Heart Institute, said in an interview.
Several studies conducted early in the pandemic suggested increased risks for both new-onset diabetes and cardiometabolic diseases following COVID-19 infection, possibly because of persistent inflammation contributing to insulin resistance.
However, it hasn’t been clear if those risks have persisted with the more recent predominance of the less-virulent Omicron variant or whether the COVID-19 vaccine influences the risk. This new study suggests that both are the case.
“Our results verify that the risk of developing type 2 diabetes after a COVID-19 infection was not just an early observation but, in fact, a real risk that has, unfortunately, persisted through the Omicron era,” Dr. Kwan noted.
“While the level of evidence by our study and others may not reach the degree needed to affect formal guidelines at this time, we believe it is reasonable to have increased clinical suspicion for diabetes after COVID-19 infection and a lower threshold for testing,” he added.
Moreover, “we believe that our study and others suggest the potential role of COVID-19 to affect cardiovascular risk, and so both prevention of COVID-19 infection, through reasonable personal practices and vaccination, and an increased attention to cardiovascular health after COVID-19 infection is warranted.”
The findings were published online in JAMA Network Open.
Dr. Kwan and colleagues analyzed data for a total of 23,709 patients treated (inpatient and outpatient) for at least one COVID-19 infection between March 2020 and June 2022.
Rates of new-onset diabetes (using ICD-10 codes, primarily type 2 diabetes), hypertension, and hyperlipidemia were all elevated in the 90 days following COVID-19 infection compared with the 90 days prior. The same was true of two diagnoses unrelated to COVID-19, urinary tract infection and gastroesophageal reflux, used as benchmarks of health care engagement.
The highest odds for post versus preinfection were for diabetes (odds ratio, 2.35; P < .001), followed by hypertension (OR, 1.54; P < .001), the benchmark diagnoses (OR, 1.42; P < .001), and hyperlipidemia (OR, 1.22; P = .03).
Following adjustments, the risk versus the benchmark conditions for new-onset diabetes before versus after COVID-19 was significantly elevated (OR, 1.58; P < .001), while the risks for hypertension and hyperlipidemia versus benchmark diagnoses were not (OR, 1.06; P = .52 and 0.91, P = .43, respectively).
The diabetes risk after versus before COVID-19 infection was higher among those who had not been vaccinated (OR, 1.78; P < .001), compared with those who had received the vaccine (OR, 1.07; P = .80).
However, there was no significant interaction between vaccination and diabetes diagnosis (P = .08). “For this reason, we believe our data are suggestive of a protective effect in the population who received vaccination prior to infection, but [this is] not definitive,” Dr. Kwan said.
There were no apparent interactions by age, sex, or pre-existing cardiovascular risk factors, including hypertension or hyperlipidemia. Age, sex, and timing of index infection regarding the Omicron variant were not associated with an increased risk of a new cardiometabolic diagnosis before or after COVID-19 infection in any of the models.
Dr. Kwan said in an interview: “We have continued to be surprised by the evolving understanding of the SARS-CoV-2 virus and the effects on human health. In the beginning of the pandemic it was framed as a purely respiratory virus, which we now know to be a severely limited description of all of its potential effects on the human body. We believe that our research and others raise a concern for increased cardiometabolic risk after COVID infection.”
He added that, “while knowledge is incomplete on this topic, we believe that clinical providers may wish to have a higher degree of suspicion for both diabetes and risk of future cardiac events in patients after COVID infection, and that continued efforts to prevent COVID infection may be beneficial to patient health until we develop better understanding of the potential long-term effects of COVID.”
This study was funded by the Erika J. Glazer Family Foundation, the Doris Duke Charitable Foundation, and grants from the National Institutes of Health. Dr. Kwan reported receiving grants from the Doris Duke Charitable Foundation during the conduct of the study.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Physicians don’t feel safe with some patients: Here’s how to reduce the danger
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.