User login
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.
Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
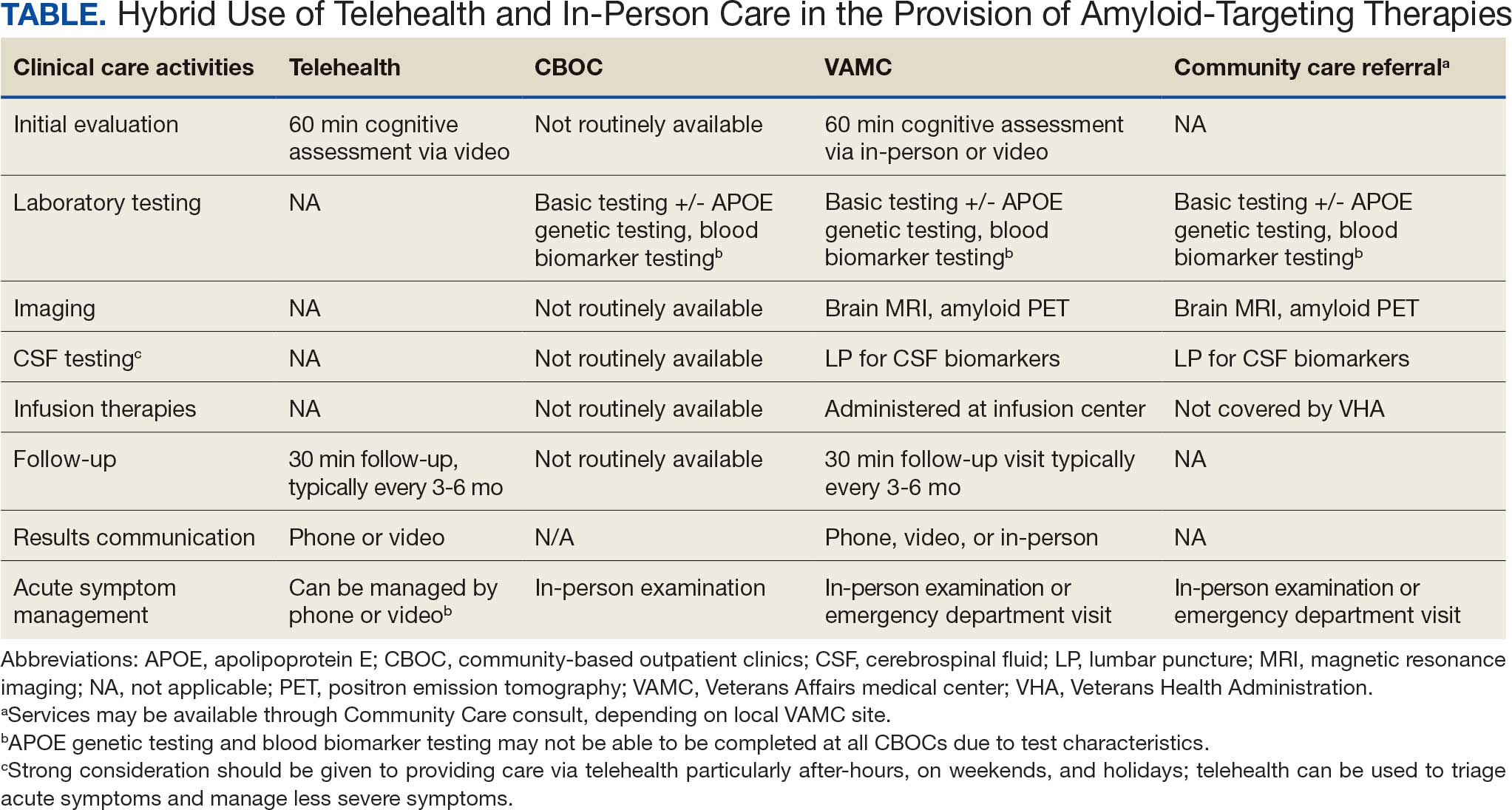
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.
The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Veterans With Dementia Face Extended Time Away From Home After Emergency Department Care
Veterans With Dementia Face Extended Time Away From Home After Emergency Department Care
TOPLINE:
Veterans with dementia experienced significant reductions in time spent at home following emergency department (ED) visits, with a mean of 21.7 days away from home within 180 days of the index visit. ED admission was the strongest predictor of extended time away from home, followed by high frailty, an unmarried status, and lack of housing.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Department of Veterans Affairs (VA) and Centers for Medicare & Medicaid Services administrative data of 51,707 veterans with dementia (mean age, 79.9 years; 97.6% men; 52.2% married individuals; 73% White individuals) who had an eligible Veterans Health Administration ED visit between October 2016 and September 2018.
- The primary outcome was home time, defined as days alive and not spent in institutional care settings during the 180 days following the index ED visit; secondary outcomes included ED revisits within 30 days of the index visit and 30-day mortality.
TAKEAWAY:
- Veterans experienced a mean of 21.7 days away from home within 180 days after the ED visit; 4.5% never returned home, and 18.2% spent the entire 180-day follow-up period at home. Patients admitted from the ED spent a mean of 34.2 days away from home within 180 days, whereas those discharged directly spent a mean of 13.6 days.
- ED admission had the strongest association with increased days away from home (rate ratio [RR], 3.18), followed by patient factors such as unhoused status (RR, 1.50), very high frailty (RR, 1.27), unmarried status — never married (RR, 1.24) or divorced, separated, or widowed (RR, 1.24) — and depression (RR, 1.13).
- Compared with the overall cohort, veterans with psychiatric concerns had the highest risk for extended time away from home (RR, 1.31), followed by those with nonspecific concerns and geriatric syndromes.
- Among all participants, 27.6% had a 30-day ED revisit, and 4% died within 30 days of the index visit. An admission was associated with a lower likelihood of a 30-day ED revisit (hazard ratio [HR], 0.75) but an increased likelihood of 30-day mortality (HR, 4.87).
IN PRACTICE:
"Home time offers a promising, patient-centered measure to align emergency care with patients' and care partners' goals and preferences to remain at home," the authors wrote. However, they emphasized that "refining its application — particularly in accounting for index hospitalizations and long-term care transitions — is critical to accurately capturing quality of care and long-term well-being."
SOURCE:
The study was led by Justine Seidenfeld, MD, MHs, Durham Veterans Affairs Health Care System, Durham, North Carolina. It was published online on December 29, 2025, in JAMA Network Open.
LIMITATIONS:
The study population of veterans aged 65-66 years may have had incomplete dementia confirmation as Medicare data were limited, and the predominantly male cohort limited generalizability. Marriage status served as an imperfect proxy for social and care partner support. The varying severity of dementia among participants could not be fully assessed using VA administrative data. Additionally, some highly emergent ED visits may have been inadvertently included if patients were not properly triaged, and very low-acuity visits could not be reliably identified due to the lack of validated approaches.
DISCLOSURES:
The study was supported by the National Institute on Aging-Veterans Affairs Mentored Physician and Clinical Psychologist Scientist Award in Alzheimer's Disease (AD) and AD-Related Dementias, a project grant from the National Institute on Aging, and a grant from the Veterans Affairs Office of Health Systems Research, Center of Innovation to Accelerate Discovery and Practice Transformation at the Durham VA Health Care System. Several authors reported receiving grants, personal fees, and payments for literature reviews from or serving as consultants for various organizations. Detailed disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans with dementia experienced significant reductions in time spent at home following emergency department (ED) visits, with a mean of 21.7 days away from home within 180 days of the index visit. ED admission was the strongest predictor of extended time away from home, followed by high frailty, an unmarried status, and lack of housing.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Department of Veterans Affairs (VA) and Centers for Medicare & Medicaid Services administrative data of 51,707 veterans with dementia (mean age, 79.9 years; 97.6% men; 52.2% married individuals; 73% White individuals) who had an eligible Veterans Health Administration ED visit between October 2016 and September 2018.
- The primary outcome was home time, defined as days alive and not spent in institutional care settings during the 180 days following the index ED visit; secondary outcomes included ED revisits within 30 days of the index visit and 30-day mortality.
TAKEAWAY:
- Veterans experienced a mean of 21.7 days away from home within 180 days after the ED visit; 4.5% never returned home, and 18.2% spent the entire 180-day follow-up period at home. Patients admitted from the ED spent a mean of 34.2 days away from home within 180 days, whereas those discharged directly spent a mean of 13.6 days.
- ED admission had the strongest association with increased days away from home (rate ratio [RR], 3.18), followed by patient factors such as unhoused status (RR, 1.50), very high frailty (RR, 1.27), unmarried status — never married (RR, 1.24) or divorced, separated, or widowed (RR, 1.24) — and depression (RR, 1.13).
- Compared with the overall cohort, veterans with psychiatric concerns had the highest risk for extended time away from home (RR, 1.31), followed by those with nonspecific concerns and geriatric syndromes.
- Among all participants, 27.6% had a 30-day ED revisit, and 4% died within 30 days of the index visit. An admission was associated with a lower likelihood of a 30-day ED revisit (hazard ratio [HR], 0.75) but an increased likelihood of 30-day mortality (HR, 4.87).
IN PRACTICE:
"Home time offers a promising, patient-centered measure to align emergency care with patients' and care partners' goals and preferences to remain at home," the authors wrote. However, they emphasized that "refining its application — particularly in accounting for index hospitalizations and long-term care transitions — is critical to accurately capturing quality of care and long-term well-being."
SOURCE:
The study was led by Justine Seidenfeld, MD, MHs, Durham Veterans Affairs Health Care System, Durham, North Carolina. It was published online on December 29, 2025, in JAMA Network Open.
LIMITATIONS:
The study population of veterans aged 65-66 years may have had incomplete dementia confirmation as Medicare data were limited, and the predominantly male cohort limited generalizability. Marriage status served as an imperfect proxy for social and care partner support. The varying severity of dementia among participants could not be fully assessed using VA administrative data. Additionally, some highly emergent ED visits may have been inadvertently included if patients were not properly triaged, and very low-acuity visits could not be reliably identified due to the lack of validated approaches.
DISCLOSURES:
The study was supported by the National Institute on Aging-Veterans Affairs Mentored Physician and Clinical Psychologist Scientist Award in Alzheimer's Disease (AD) and AD-Related Dementias, a project grant from the National Institute on Aging, and a grant from the Veterans Affairs Office of Health Systems Research, Center of Innovation to Accelerate Discovery and Practice Transformation at the Durham VA Health Care System. Several authors reported receiving grants, personal fees, and payments for literature reviews from or serving as consultants for various organizations. Detailed disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans with dementia experienced significant reductions in time spent at home following emergency department (ED) visits, with a mean of 21.7 days away from home within 180 days of the index visit. ED admission was the strongest predictor of extended time away from home, followed by high frailty, an unmarried status, and lack of housing.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Department of Veterans Affairs (VA) and Centers for Medicare & Medicaid Services administrative data of 51,707 veterans with dementia (mean age, 79.9 years; 97.6% men; 52.2% married individuals; 73% White individuals) who had an eligible Veterans Health Administration ED visit between October 2016 and September 2018.
- The primary outcome was home time, defined as days alive and not spent in institutional care settings during the 180 days following the index ED visit; secondary outcomes included ED revisits within 30 days of the index visit and 30-day mortality.
TAKEAWAY:
- Veterans experienced a mean of 21.7 days away from home within 180 days after the ED visit; 4.5% never returned home, and 18.2% spent the entire 180-day follow-up period at home. Patients admitted from the ED spent a mean of 34.2 days away from home within 180 days, whereas those discharged directly spent a mean of 13.6 days.
- ED admission had the strongest association with increased days away from home (rate ratio [RR], 3.18), followed by patient factors such as unhoused status (RR, 1.50), very high frailty (RR, 1.27), unmarried status — never married (RR, 1.24) or divorced, separated, or widowed (RR, 1.24) — and depression (RR, 1.13).
- Compared with the overall cohort, veterans with psychiatric concerns had the highest risk for extended time away from home (RR, 1.31), followed by those with nonspecific concerns and geriatric syndromes.
- Among all participants, 27.6% had a 30-day ED revisit, and 4% died within 30 days of the index visit. An admission was associated with a lower likelihood of a 30-day ED revisit (hazard ratio [HR], 0.75) but an increased likelihood of 30-day mortality (HR, 4.87).
IN PRACTICE:
"Home time offers a promising, patient-centered measure to align emergency care with patients' and care partners' goals and preferences to remain at home," the authors wrote. However, they emphasized that "refining its application — particularly in accounting for index hospitalizations and long-term care transitions — is critical to accurately capturing quality of care and long-term well-being."
SOURCE:
The study was led by Justine Seidenfeld, MD, MHs, Durham Veterans Affairs Health Care System, Durham, North Carolina. It was published online on December 29, 2025, in JAMA Network Open.
LIMITATIONS:
The study population of veterans aged 65-66 years may have had incomplete dementia confirmation as Medicare data were limited, and the predominantly male cohort limited generalizability. Marriage status served as an imperfect proxy for social and care partner support. The varying severity of dementia among participants could not be fully assessed using VA administrative data. Additionally, some highly emergent ED visits may have been inadvertently included if patients were not properly triaged, and very low-acuity visits could not be reliably identified due to the lack of validated approaches.
DISCLOSURES:
The study was supported by the National Institute on Aging-Veterans Affairs Mentored Physician and Clinical Psychologist Scientist Award in Alzheimer's Disease (AD) and AD-Related Dementias, a project grant from the National Institute on Aging, and a grant from the Veterans Affairs Office of Health Systems Research, Center of Innovation to Accelerate Discovery and Practice Transformation at the Durham VA Health Care System. Several authors reported receiving grants, personal fees, and payments for literature reviews from or serving as consultants for various organizations. Detailed disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Veterans With Dementia Face Extended Time Away From Home After Emergency Department Care
Veterans With Dementia Face Extended Time Away From Home After Emergency Department Care
Agitation and emotional lability
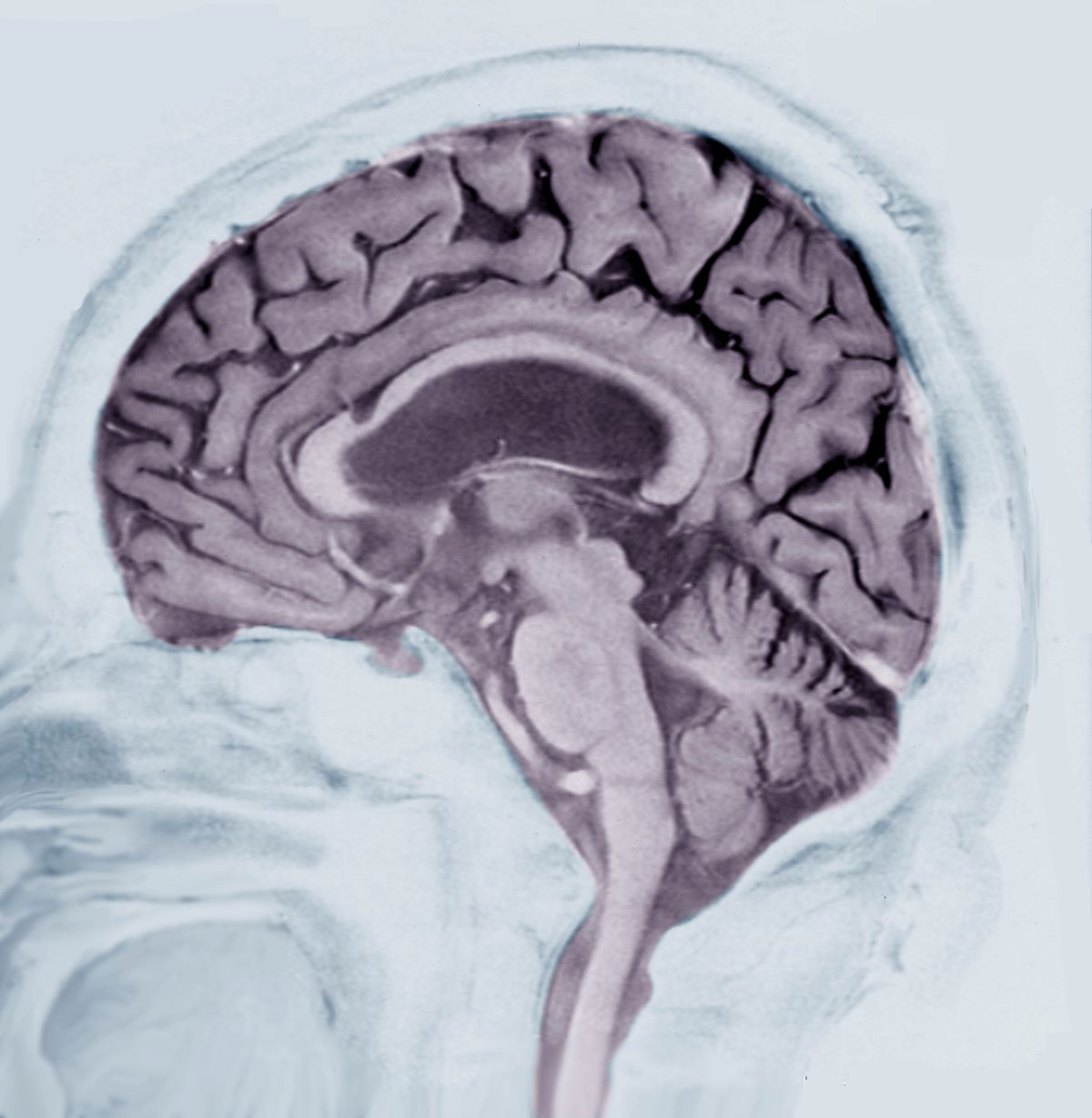
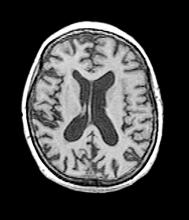
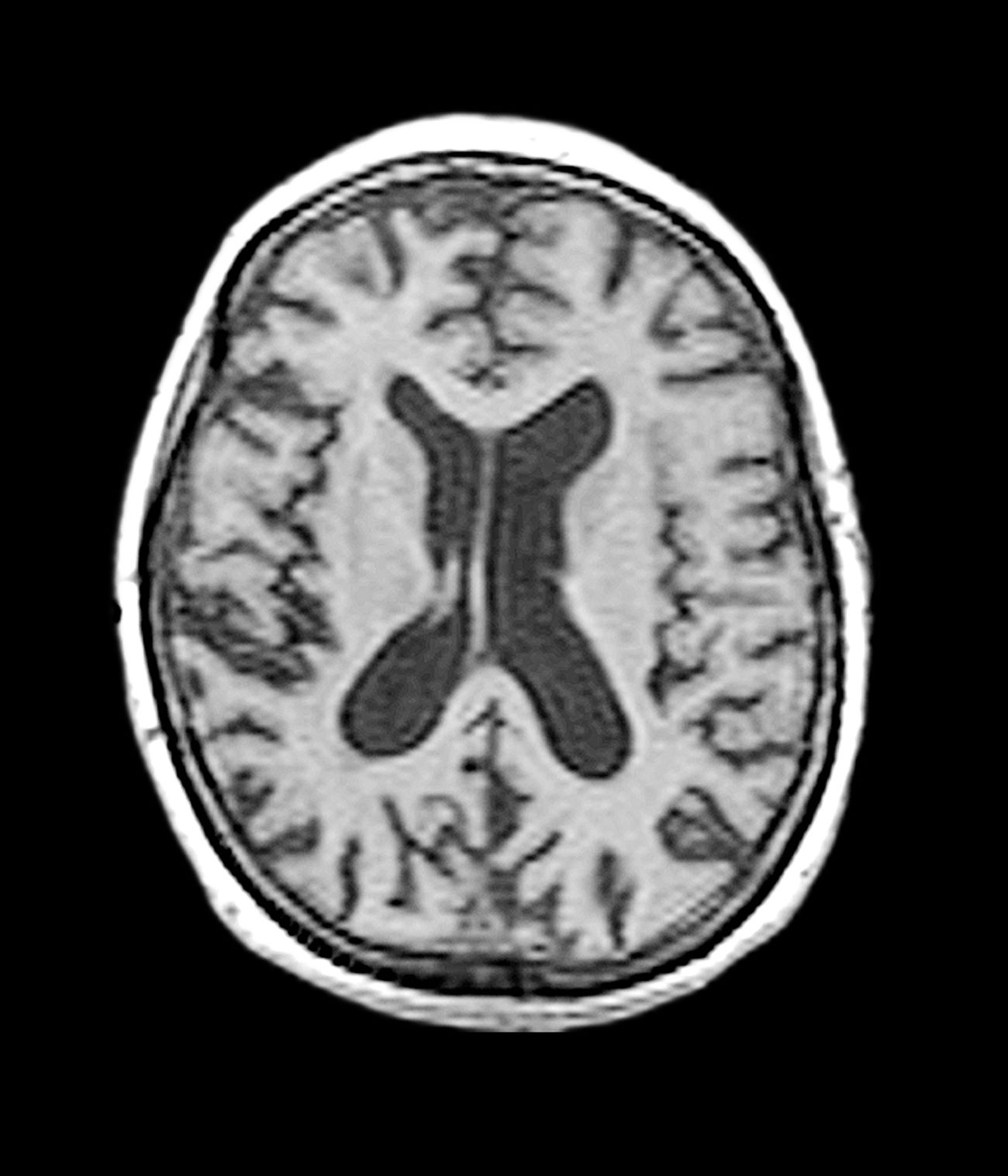
This patient’s presentation is indicative of moderate-stage Alzheimer’s disease (AD), which is confirmed by physical exam and testing; most notably, her MMSE score of 18 (a score of ≥ 25 is considered normal) and MRI results showing distinct cortical atrophy. At this stage of disease, signs and symptoms become more pronounced and widespread to include not only language deficiencies, prominent memory loss, and sensory processing, but also motor deficits and behavioral issues, all of which are clearly present in this patient.
A very important clinical consideration is a possible delay in diagnosis. It is atypical for an initial diagnosis of AD to be made when a patient is in the moderate stage of disease. This patient’s history over 5 years before her diagnosis included complaints of forgetfulness and low-level dyspraxia, which were not pursued. Reasons for this can vary. Broadly, patient interactions in a primary care setting tend to be brief, thus, many patients are not engaged in their care. Early symptoms — eg, memory impairment — can be missed during routine office visits.
There are also significant racial disparities in the diagnosis of dementia. According to National Institute of Aging-funded studies that were conducted in 39 AD Research Centers, White patients > 65 years old had a significantly higher prevalence of dementia diagnoses at baseline visits than Black patients in the same age group. Black patients, especially Black women, tend not to be diagnosed with AD until it has progressed. Conversely, Black patients had more risk factors for AD, greater cognitive impairment, and more severe neuropsychiatric symptoms (delusions and hallucinations) than those of other races and ethnicities.
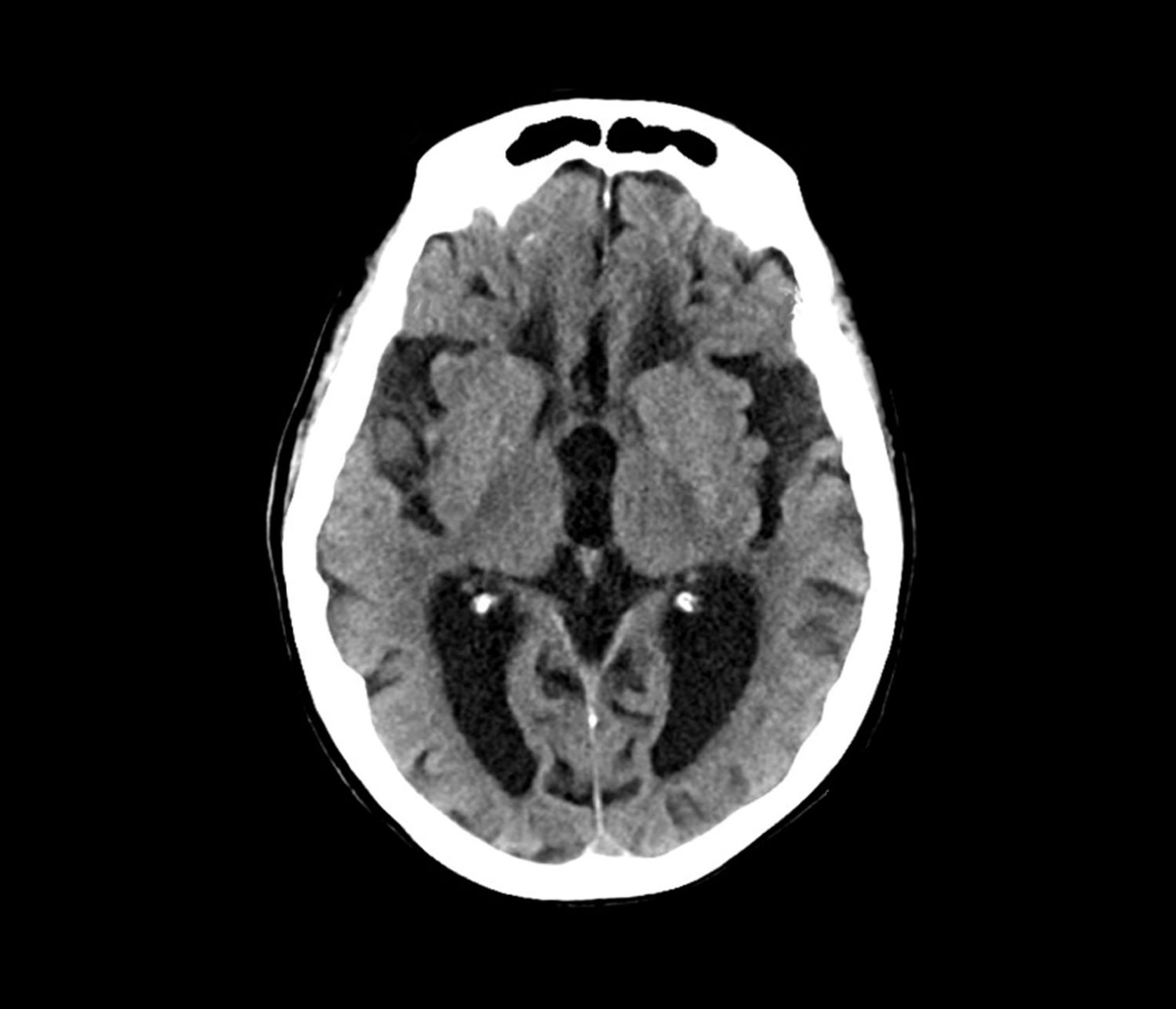
Another barrier to timely diagnosis in Black patients is disparity in access to neuroimaging. In a study conducted by Wibecan and colleagues at Boston Medical Center, researchers found that among neuroimaging assessments conducted at the facility, Black patients who received MRI or CT scan for the diagnosis of cognitive impairment were older than White patients (72.5 years vs 67 years). Additionally, Black patients were significantly less likely to undergo MRI (the gold standard of care for dementia diagnosis) than CT scan.
Hypothyroidism is an endocrine disorder that occurs because of a deficiency in thyroid hormone. Symptoms tend to be subtle and non-specific but vary greatly. Some of the hallmark symptoms are fatigue, weight gain, cold intolerance, dry skin, and hair loss. Additionally, emotional lability and depressed mood with mental impairment, slowed speech, and movement, as evident in this patient. However, hypothyroidism was ruled out when her laboratory results returned with all values within normal range.
Vascular dementia is the second-most prevalent form of dementia after AD. It is characterized as cognitive impairment that occurs after one, or a series of, neurologic events and does not refer to a single disease but to a variety of vascular disorders. Patients with vascular dementia often exhibit mood and behavioral changes, deficits in executive function, and severe memory loss, all of which are present in this patient. However, as there were no (known) neurologic events in this patient — and no evidence thereof on imaging — and her hypertension is relatively well controlled, this is not a diagnostic consideration for this patient.
Normal pressure hydrocephalus (NPH) is caused by the build-up of cerebrospinal fluid in the brain. It is characterized by abnormal gait, dementia, and urinary incontinence. Patients with NPH experience decreased attention, significant memory loss, bradyphrenia, bradykinesia, and broad-based gait, all of which feature in this patient. However, brain MRI was negative for structural abnormalities of this type, and there was no indication of NPH, which rules it out as a potential diagnosis.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient’s presentation is indicative of moderate-stage Alzheimer’s disease (AD), which is confirmed by physical exam and testing; most notably, her MMSE score of 18 (a score of ≥ 25 is considered normal) and MRI results showing distinct cortical atrophy. At this stage of disease, signs and symptoms become more pronounced and widespread to include not only language deficiencies, prominent memory loss, and sensory processing, but also motor deficits and behavioral issues, all of which are clearly present in this patient.
A very important clinical consideration is a possible delay in diagnosis. It is atypical for an initial diagnosis of AD to be made when a patient is in the moderate stage of disease. This patient’s history over 5 years before her diagnosis included complaints of forgetfulness and low-level dyspraxia, which were not pursued. Reasons for this can vary. Broadly, patient interactions in a primary care setting tend to be brief, thus, many patients are not engaged in their care. Early symptoms — eg, memory impairment — can be missed during routine office visits.
There are also significant racial disparities in the diagnosis of dementia. According to National Institute of Aging-funded studies that were conducted in 39 AD Research Centers, White patients > 65 years old had a significantly higher prevalence of dementia diagnoses at baseline visits than Black patients in the same age group. Black patients, especially Black women, tend not to be diagnosed with AD until it has progressed. Conversely, Black patients had more risk factors for AD, greater cognitive impairment, and more severe neuropsychiatric symptoms (delusions and hallucinations) than those of other races and ethnicities.
Another barrier to timely diagnosis in Black patients is disparity in access to neuroimaging. In a study conducted by Wibecan and colleagues at Boston Medical Center, researchers found that among neuroimaging assessments conducted at the facility, Black patients who received MRI or CT scan for the diagnosis of cognitive impairment were older than White patients (72.5 years vs 67 years). Additionally, Black patients were significantly less likely to undergo MRI (the gold standard of care for dementia diagnosis) than CT scan.
Hypothyroidism is an endocrine disorder that occurs because of a deficiency in thyroid hormone. Symptoms tend to be subtle and non-specific but vary greatly. Some of the hallmark symptoms are fatigue, weight gain, cold intolerance, dry skin, and hair loss. Additionally, emotional lability and depressed mood with mental impairment, slowed speech, and movement, as evident in this patient. However, hypothyroidism was ruled out when her laboratory results returned with all values within normal range.
Vascular dementia is the second-most prevalent form of dementia after AD. It is characterized as cognitive impairment that occurs after one, or a series of, neurologic events and does not refer to a single disease but to a variety of vascular disorders. Patients with vascular dementia often exhibit mood and behavioral changes, deficits in executive function, and severe memory loss, all of which are present in this patient. However, as there were no (known) neurologic events in this patient — and no evidence thereof on imaging — and her hypertension is relatively well controlled, this is not a diagnostic consideration for this patient.
Normal pressure hydrocephalus (NPH) is caused by the build-up of cerebrospinal fluid in the brain. It is characterized by abnormal gait, dementia, and urinary incontinence. Patients with NPH experience decreased attention, significant memory loss, bradyphrenia, bradykinesia, and broad-based gait, all of which feature in this patient. However, brain MRI was negative for structural abnormalities of this type, and there was no indication of NPH, which rules it out as a potential diagnosis.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient’s presentation is indicative of moderate-stage Alzheimer’s disease (AD), which is confirmed by physical exam and testing; most notably, her MMSE score of 18 (a score of ≥ 25 is considered normal) and MRI results showing distinct cortical atrophy. At this stage of disease, signs and symptoms become more pronounced and widespread to include not only language deficiencies, prominent memory loss, and sensory processing, but also motor deficits and behavioral issues, all of which are clearly present in this patient.
A very important clinical consideration is a possible delay in diagnosis. It is atypical for an initial diagnosis of AD to be made when a patient is in the moderate stage of disease. This patient’s history over 5 years before her diagnosis included complaints of forgetfulness and low-level dyspraxia, which were not pursued. Reasons for this can vary. Broadly, patient interactions in a primary care setting tend to be brief, thus, many patients are not engaged in their care. Early symptoms — eg, memory impairment — can be missed during routine office visits.
There are also significant racial disparities in the diagnosis of dementia. According to National Institute of Aging-funded studies that were conducted in 39 AD Research Centers, White patients > 65 years old had a significantly higher prevalence of dementia diagnoses at baseline visits than Black patients in the same age group. Black patients, especially Black women, tend not to be diagnosed with AD until it has progressed. Conversely, Black patients had more risk factors for AD, greater cognitive impairment, and more severe neuropsychiatric symptoms (delusions and hallucinations) than those of other races and ethnicities.
Another barrier to timely diagnosis in Black patients is disparity in access to neuroimaging. In a study conducted by Wibecan and colleagues at Boston Medical Center, researchers found that among neuroimaging assessments conducted at the facility, Black patients who received MRI or CT scan for the diagnosis of cognitive impairment were older than White patients (72.5 years vs 67 years). Additionally, Black patients were significantly less likely to undergo MRI (the gold standard of care for dementia diagnosis) than CT scan.
Hypothyroidism is an endocrine disorder that occurs because of a deficiency in thyroid hormone. Symptoms tend to be subtle and non-specific but vary greatly. Some of the hallmark symptoms are fatigue, weight gain, cold intolerance, dry skin, and hair loss. Additionally, emotional lability and depressed mood with mental impairment, slowed speech, and movement, as evident in this patient. However, hypothyroidism was ruled out when her laboratory results returned with all values within normal range.
Vascular dementia is the second-most prevalent form of dementia after AD. It is characterized as cognitive impairment that occurs after one, or a series of, neurologic events and does not refer to a single disease but to a variety of vascular disorders. Patients with vascular dementia often exhibit mood and behavioral changes, deficits in executive function, and severe memory loss, all of which are present in this patient. However, as there were no (known) neurologic events in this patient — and no evidence thereof on imaging — and her hypertension is relatively well controlled, this is not a diagnostic consideration for this patient.
Normal pressure hydrocephalus (NPH) is caused by the build-up of cerebrospinal fluid in the brain. It is characterized by abnormal gait, dementia, and urinary incontinence. Patients with NPH experience decreased attention, significant memory loss, bradyphrenia, bradykinesia, and broad-based gait, all of which feature in this patient. However, brain MRI was negative for structural abnormalities of this type, and there was no indication of NPH, which rules it out as a potential diagnosis.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 76-year-old Black woman presents to her physician. She is accompanied by her daughter who reports that, over the last 9 months, her mother has exhibited worsening memory loss, confusion, impaired judgment, agitation, and emotional lability. She is often unaware of where she is or how she got there. She sometimes does not recognize her family members or people who are familiar to her. Her appetite has been variable, and her sleep schedule is altered so that she often sleeps during the day and is awake at night. Sometimes, she is so irritable that she becomes aggressive and insists on situations that don’t exist. Her executive function is low. Her daughter reports that the patient had a fall 9 months ago and again 4 months ago, after which her symptoms became progressively worse.
The patient has complained about being forgetful and clumsy for much of the past 5 years, which she has attributed to old age. Until the last year or so, these have not greatly impaired her daily function and were not of great concern to her family or providers. She has a history of hypertension and diabetes, both of which are pharmacologically managed with mixed results due to variable adherence.
Physical exam confirms her daughter’s report. The patient appears thin, fatigued, and anxious. She has lost 20 lb since her last visit. She has great difficulty maintaining focus on what is being asked of her and in following the conversation. When she does speak, her speech is slow. She exhibits both motor deficits — in balance and coordination — and a bradykinetic gait.
Laboratory testing is performed: complete blood count w/diff, comprehensive metabolic panel, thyroid panel, cobalamin level, vitamin D screening. All results are within normal range for this patient. She was unable to complete the Geriatric Depression Scale on her own; direct questioning about whether she was feeling depressed is negative. Mini-Mental State Examination (MMSE) score is 18. MRI is performed; sagittal view reveals cortical atrophy.
Alzheimer's Disease Signs and Symptoms
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Fatigue and brain fog
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
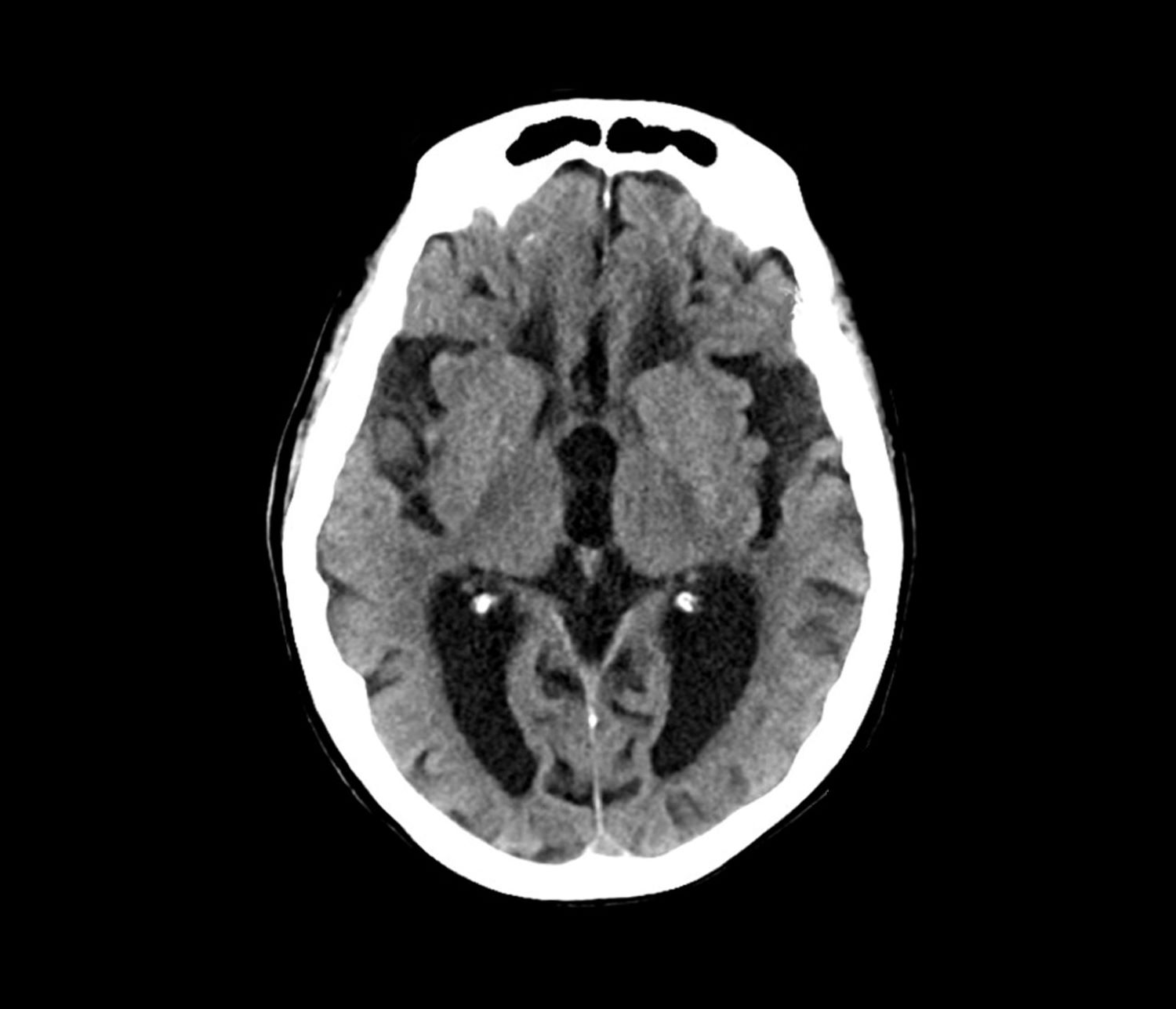
A 52-year-old female presents complaining of fatigue, brain fog, and headaches. She reports that, over the last year, she feels increasingly more irritable, anxious, overwhelmed, and sometimes disoriented. When prompted, she admits to having trouble keeping track of things — dates, important deadlines, objects like her keys, and things she uses each day. She needs multiple reminder events or tasks and must write everything down. She also reports that she often loses her train of thought and sometimes struggles to find words. She works in a medical office, is married, and has two children in college. She helps with caring for her in-laws on the weekend by doing their bills and grocery shopping. She admits to being moody and does not want to socialize, which she attributes to fatigue and having a busy life. She also complains of muscle stiffness and general clumsiness. Eight months ago, the patient was in an automobile accident in which her car was totaled, but she did not sustain serious injury and opted not to seek medical evaluation.
Physical exam reveals slight visuospatial dysfunction, with issues with both coordination and balance. The patient appears fatigued and has some trouble maintaining her attention during the conversation. Further questioning reveals mild anomic aphasia and some lapses in recent memory. Her reflexes are otherwise normal, as are heart, lung, and breath sounds. All systemic lymph nodes are normal as are liver and spleen on palpation. The patient recently discovered that her estranged father had been diagnosed with Alzheimer’s disease (AD) at 58 years of age and passed away 5 years later.
Laboratory testing is performed and reveals nothing remarkable. Considering the car accident and new cognitive/behavioral changes experienced by the patient, a CT of the brain is ordered. Results are negative for concussion and cerebral hemorrhage but show cortical atrophy of the temporal territories and a large increase in the size of the subarachnoid spaces predominantly affecting the parietal region.
Alzheimer's Disease and Athletes
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Progressive cognitive decline
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 40-year-old man with Down syndrome (DS) presented with progressive cognitive decline over 2 years, characterized by memory impairment, difficulty performing familiar tasks, and increasing disorientation. His caregivers noted mood changes, including irritability and withdrawal, and occasional episodes of agitation. Clinical history revealed congenital heart disease (surgically repaired in childhood) and hypothyroidism, which was well controlled with levothyroxine. Physical examination showed no focal neurologic deficits, but he exhibited mild hypotonia, a characteristic feature of DS, and a shuffling gait. Routine blood tests revealed normal thyroid function, no evidence of vitamin B12 deficiency, and unremarkable metabolic panels. An MRI scan (as shown in the image) demonstrated marked ventricular enlargement and generalized cortical atrophy.
Alzheimer's Disease & Down Syndrome
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Updated Alzheimer’s Guidelines Chart the Full Diagnostic Journey
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Losing Your Mind Trying to Understand the BP-Dementia Link
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.