User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Gastroparesis referrals often based on misdiagnosis
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Meta-analysis throws more shade aspirin’s way
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
FROM JACC: ADVANCES
Longitudinal arm lesion
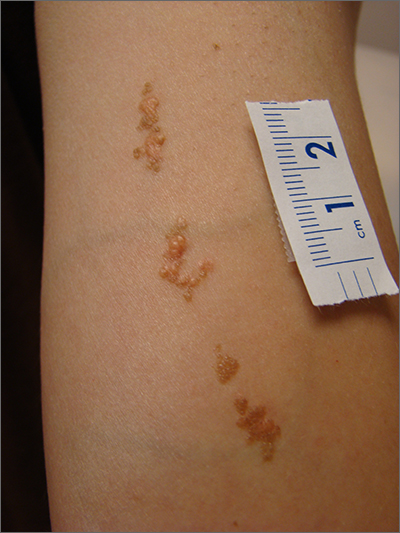
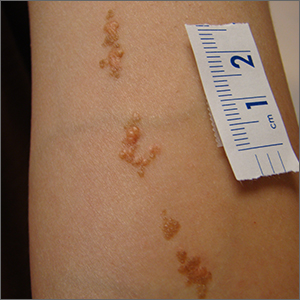
This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001

This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This linear pattern of hyper-pigmented, often verrucous tissue oriented along Blaschko skin lines is typical for linear epidermal nevi (LEN). In some cases, lesions are not in a linear pattern and are actually in more of a localized or whorled pattern (called epidermal nevi).
LEN are usually present at birth, as in this individual. They are frequently seen on the head and neck region and are often asymptomatic. LEN are considered a birthmark that develops because of a genetic abnormality that typically affects keratinocytes. This genetic mutation only affects a portion of the body (mosaicism) without affecting the overall genetics of the individual. This is important to note because LEN do not typically have a hereditary component or implications for offspring. While usually asymptomatic and localized, LEN can be associated with extracutaneous and neurologic difficulties. In these situations, it is called epidermal nevus syndrome, and is more common if the LEN occur on the face or head.1
Since LEN are usually asymptomatic, treatment is not required unless the lesions affect the function of adjacent structures, such as the eyes, lips, or nose. Due to their frequent presence on the face or other visible areas, some patients may choose to get these lesions treated for cosmetic purposes. In the past, full-thickness excision was recommended. Topical medications are ineffective, and superficial shave excision usually leads to recurrence. More recently, destructive laser treatments have been used, with success, to reduce the appearance of the lesions.2
This patient was not concerned about the appearance of the asymptomatic lesions and chose not to have any treatment.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001
1. Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi: 10.1111/pde.13273
2. Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-8. doi: 10.1016/j.adengl.2012.10.001
Uptick in natriuretic peptides with long-term serial testing predicts new heart failure
A jump in natriuretic peptide levels over several years in middle-aged adults points to worsened long-term risks for incident heart failure (HF) and death. But their predicted long-term survival improves if serial testing shows a drop in those levels, suggests a new analysis based on a well-known longitudinal study cohort.
The findings support the risk-stratification potential of serial natriuretic peptide testing, which may improve on individual assays for predicting future HF. Such serial assays might also be useful for guiding therapy aimed at preventing, for example, progression to clinical HF, researchers speculate on the basis of the current study,
The analysis of almost 1,000 members of the ARIC (Atherosclerosis Risk in Community) cohort had been free of clinical HF at the first of two NT-proBNP assays, which were performed 6 years apart. Their 20-year clinical risk was linked to the trajectory of NT-proBNP levels across the two earlier assays.
For example, adjusted risk of incident HF more than doubled for participants with NT-proBNP levels exceeding 125 pg/mL on both assays, compared with levels that stayed under the cut point at both assays. Their mortality risk climbed by about two-thirds.
Risk for incident HF and of death climbed 86% and 32%, respectively, if NT-proBNP levels rose over the 6 years from less than to greater than 125 pg/mL. But long-term survival improved if serial assays showed a drop from the higher to the lower level.
Rising NT-proBNP levels over several years probably reflect ongoing exposure to risk factors such as hypertension or diabetes. Conversely, decreasing NT-proBNP levels likely reflect some success at keeping such risk factors under control, propose the authors of the analysis published in JAMA Cardiology. The study was led by Xiaoming Jia, MD, Baylor College of Medicine, Houston.
The findings raise the possibility that reducing NT-proBNP levels through risk-factor modification, tracked by serial assays, may potentially improve long-term risk for death or incident HF.
Such therapy, guided by natriuretic peptides, might prove especially useful in asymptomatic adults with modifiable HF risk factors but without known NT-proBNP elevation or cardiac structural changes, so-called stage A HF, senior author Vijay Nambi, MD, PhD, also of Baylor, observed for this news organization.
The best populations for serial NT-proBNP assays to guide therapy, Dr. Nambi said, should become clear “as more data emerges.” But the threshold for ordering such tests would probably be lower for people in stage A whose rising NT-proBNP levels later reclassify them as stage B, also called pre-HF.
In such cases, he speculated, intensified therapy of HF risk factors such as uncontrolled hypertension or diabetes – prompted by greater NT-proBNP levels at serial testing – might possibly avert progression to clinical HF.
“These investigators have nicely demonstrated that one measurement of the biomarker may not be sufficient, that maybe it undercaptures the true burden of people who eventually will develop heart failure,” Muthiah Vaduganathan, MD, MPH, told this news organization.
The study raises the possibility “that the serial natriuretic peptide strategy may be more efficient and more comprehensive in identifying those who will eventually progress,” said Dr. Vaduganathan, of Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not associated with the ARIC analysis.
An open question, he added, is whether the predicted risk is modifiable. “If you are able to provide the biomarker information to treating clinicians, can they do something to attenuate the risk?”
The outlook is hopeful, given contemporary therapies “that can slow and even prevent heart failure in at-risk populations,” Dr. Vaduganathan said. For example, “The selective allocation of SGLT2 inhibitors to those with elevated natriuretic peptide levels, perhaps as captured in serial measurements, would be of great interest.”
The analysis included 9,776 adults (56.5% women, 21.3% Black) without HF who underwent NT-proBNP testing at the second and – about 6 years later – the fourth scheduled clinical visits in the ARIC study, which had enrolled persons aged 45-64 from four diverse communities from across the United States.
Adjusted hazard ratios for incident HF according to NT-proBNP changes from the first to second assays relative to 125 pg/mL were as follows:
- 1.86 (95% confidence interval, 1.60-2.16) when levels rose to higher than the cut point.
- 2.40 (95% CI, 2.00-2.88) when both levels exceeded the cut point.
The corresponding adjusted HRs for death from any cause were as follows:
- 1.32 (95%CI, 1.19-1.47) when levels rose to higher than 125 mg/mL.
- 1.68 (95% CI, 1.47-1.91) when both levels were above the cut point.
The risks for incident HF and for death rose significantly by 6% and 5%, respectively, per standard deviation NT-proBNP increase from the first to second assay.
Risks for HF and mortality for participants whose NT-proBNP levels declined from greater than to less than 125 pg/mL were similar to those whose levels remained low at both assays.
Cost-effectiveness would be another issue when implementing a strategy that calls for multiple biomarker assays, Dr. Vaduganathan observed.
“Surely, we would want to demonstrate that the laboratory measurement costs are offset by downstream prevention of heart failure events that could be averted by use of effective medical therapy, such SGLT2 inhibitors.”
ARIC has been funded by the National Institutes of Health and Department of Health and Human Services. Dr. Nambi discloses receiving grants from the National Institutes of Health during the conduct of the study; support from Amgen; and stocks from Abbott Laboratories. Disclosures for the other authors are in the report. Dr. Vaduganathan has disclosed receiving grants or serving on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; speaking for AstraZeneca, Novartis, and Roche Diagnostics; and serving on trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article originally appeared on Medscape.com.
A jump in natriuretic peptide levels over several years in middle-aged adults points to worsened long-term risks for incident heart failure (HF) and death. But their predicted long-term survival improves if serial testing shows a drop in those levels, suggests a new analysis based on a well-known longitudinal study cohort.
The findings support the risk-stratification potential of serial natriuretic peptide testing, which may improve on individual assays for predicting future HF. Such serial assays might also be useful for guiding therapy aimed at preventing, for example, progression to clinical HF, researchers speculate on the basis of the current study,
The analysis of almost 1,000 members of the ARIC (Atherosclerosis Risk in Community) cohort had been free of clinical HF at the first of two NT-proBNP assays, which were performed 6 years apart. Their 20-year clinical risk was linked to the trajectory of NT-proBNP levels across the two earlier assays.
For example, adjusted risk of incident HF more than doubled for participants with NT-proBNP levels exceeding 125 pg/mL on both assays, compared with levels that stayed under the cut point at both assays. Their mortality risk climbed by about two-thirds.
Risk for incident HF and of death climbed 86% and 32%, respectively, if NT-proBNP levels rose over the 6 years from less than to greater than 125 pg/mL. But long-term survival improved if serial assays showed a drop from the higher to the lower level.
Rising NT-proBNP levels over several years probably reflect ongoing exposure to risk factors such as hypertension or diabetes. Conversely, decreasing NT-proBNP levels likely reflect some success at keeping such risk factors under control, propose the authors of the analysis published in JAMA Cardiology. The study was led by Xiaoming Jia, MD, Baylor College of Medicine, Houston.
The findings raise the possibility that reducing NT-proBNP levels through risk-factor modification, tracked by serial assays, may potentially improve long-term risk for death or incident HF.
Such therapy, guided by natriuretic peptides, might prove especially useful in asymptomatic adults with modifiable HF risk factors but without known NT-proBNP elevation or cardiac structural changes, so-called stage A HF, senior author Vijay Nambi, MD, PhD, also of Baylor, observed for this news organization.
The best populations for serial NT-proBNP assays to guide therapy, Dr. Nambi said, should become clear “as more data emerges.” But the threshold for ordering such tests would probably be lower for people in stage A whose rising NT-proBNP levels later reclassify them as stage B, also called pre-HF.
In such cases, he speculated, intensified therapy of HF risk factors such as uncontrolled hypertension or diabetes – prompted by greater NT-proBNP levels at serial testing – might possibly avert progression to clinical HF.
“These investigators have nicely demonstrated that one measurement of the biomarker may not be sufficient, that maybe it undercaptures the true burden of people who eventually will develop heart failure,” Muthiah Vaduganathan, MD, MPH, told this news organization.
The study raises the possibility “that the serial natriuretic peptide strategy may be more efficient and more comprehensive in identifying those who will eventually progress,” said Dr. Vaduganathan, of Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not associated with the ARIC analysis.
An open question, he added, is whether the predicted risk is modifiable. “If you are able to provide the biomarker information to treating clinicians, can they do something to attenuate the risk?”
The outlook is hopeful, given contemporary therapies “that can slow and even prevent heart failure in at-risk populations,” Dr. Vaduganathan said. For example, “The selective allocation of SGLT2 inhibitors to those with elevated natriuretic peptide levels, perhaps as captured in serial measurements, would be of great interest.”
The analysis included 9,776 adults (56.5% women, 21.3% Black) without HF who underwent NT-proBNP testing at the second and – about 6 years later – the fourth scheduled clinical visits in the ARIC study, which had enrolled persons aged 45-64 from four diverse communities from across the United States.
Adjusted hazard ratios for incident HF according to NT-proBNP changes from the first to second assays relative to 125 pg/mL were as follows:
- 1.86 (95% confidence interval, 1.60-2.16) when levels rose to higher than the cut point.
- 2.40 (95% CI, 2.00-2.88) when both levels exceeded the cut point.
The corresponding adjusted HRs for death from any cause were as follows:
- 1.32 (95%CI, 1.19-1.47) when levels rose to higher than 125 mg/mL.
- 1.68 (95% CI, 1.47-1.91) when both levels were above the cut point.
The risks for incident HF and for death rose significantly by 6% and 5%, respectively, per standard deviation NT-proBNP increase from the first to second assay.
Risks for HF and mortality for participants whose NT-proBNP levels declined from greater than to less than 125 pg/mL were similar to those whose levels remained low at both assays.
Cost-effectiveness would be another issue when implementing a strategy that calls for multiple biomarker assays, Dr. Vaduganathan observed.
“Surely, we would want to demonstrate that the laboratory measurement costs are offset by downstream prevention of heart failure events that could be averted by use of effective medical therapy, such SGLT2 inhibitors.”
ARIC has been funded by the National Institutes of Health and Department of Health and Human Services. Dr. Nambi discloses receiving grants from the National Institutes of Health during the conduct of the study; support from Amgen; and stocks from Abbott Laboratories. Disclosures for the other authors are in the report. Dr. Vaduganathan has disclosed receiving grants or serving on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; speaking for AstraZeneca, Novartis, and Roche Diagnostics; and serving on trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article originally appeared on Medscape.com.
A jump in natriuretic peptide levels over several years in middle-aged adults points to worsened long-term risks for incident heart failure (HF) and death. But their predicted long-term survival improves if serial testing shows a drop in those levels, suggests a new analysis based on a well-known longitudinal study cohort.
The findings support the risk-stratification potential of serial natriuretic peptide testing, which may improve on individual assays for predicting future HF. Such serial assays might also be useful for guiding therapy aimed at preventing, for example, progression to clinical HF, researchers speculate on the basis of the current study,
The analysis of almost 1,000 members of the ARIC (Atherosclerosis Risk in Community) cohort had been free of clinical HF at the first of two NT-proBNP assays, which were performed 6 years apart. Their 20-year clinical risk was linked to the trajectory of NT-proBNP levels across the two earlier assays.
For example, adjusted risk of incident HF more than doubled for participants with NT-proBNP levels exceeding 125 pg/mL on both assays, compared with levels that stayed under the cut point at both assays. Their mortality risk climbed by about two-thirds.
Risk for incident HF and of death climbed 86% and 32%, respectively, if NT-proBNP levels rose over the 6 years from less than to greater than 125 pg/mL. But long-term survival improved if serial assays showed a drop from the higher to the lower level.
Rising NT-proBNP levels over several years probably reflect ongoing exposure to risk factors such as hypertension or diabetes. Conversely, decreasing NT-proBNP levels likely reflect some success at keeping such risk factors under control, propose the authors of the analysis published in JAMA Cardiology. The study was led by Xiaoming Jia, MD, Baylor College of Medicine, Houston.
The findings raise the possibility that reducing NT-proBNP levels through risk-factor modification, tracked by serial assays, may potentially improve long-term risk for death or incident HF.
Such therapy, guided by natriuretic peptides, might prove especially useful in asymptomatic adults with modifiable HF risk factors but without known NT-proBNP elevation or cardiac structural changes, so-called stage A HF, senior author Vijay Nambi, MD, PhD, also of Baylor, observed for this news organization.
The best populations for serial NT-proBNP assays to guide therapy, Dr. Nambi said, should become clear “as more data emerges.” But the threshold for ordering such tests would probably be lower for people in stage A whose rising NT-proBNP levels later reclassify them as stage B, also called pre-HF.
In such cases, he speculated, intensified therapy of HF risk factors such as uncontrolled hypertension or diabetes – prompted by greater NT-proBNP levels at serial testing – might possibly avert progression to clinical HF.
“These investigators have nicely demonstrated that one measurement of the biomarker may not be sufficient, that maybe it undercaptures the true burden of people who eventually will develop heart failure,” Muthiah Vaduganathan, MD, MPH, told this news organization.
The study raises the possibility “that the serial natriuretic peptide strategy may be more efficient and more comprehensive in identifying those who will eventually progress,” said Dr. Vaduganathan, of Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not associated with the ARIC analysis.
An open question, he added, is whether the predicted risk is modifiable. “If you are able to provide the biomarker information to treating clinicians, can they do something to attenuate the risk?”
The outlook is hopeful, given contemporary therapies “that can slow and even prevent heart failure in at-risk populations,” Dr. Vaduganathan said. For example, “The selective allocation of SGLT2 inhibitors to those with elevated natriuretic peptide levels, perhaps as captured in serial measurements, would be of great interest.”
The analysis included 9,776 adults (56.5% women, 21.3% Black) without HF who underwent NT-proBNP testing at the second and – about 6 years later – the fourth scheduled clinical visits in the ARIC study, which had enrolled persons aged 45-64 from four diverse communities from across the United States.
Adjusted hazard ratios for incident HF according to NT-proBNP changes from the first to second assays relative to 125 pg/mL were as follows:
- 1.86 (95% confidence interval, 1.60-2.16) when levels rose to higher than the cut point.
- 2.40 (95% CI, 2.00-2.88) when both levels exceeded the cut point.
The corresponding adjusted HRs for death from any cause were as follows:
- 1.32 (95%CI, 1.19-1.47) when levels rose to higher than 125 mg/mL.
- 1.68 (95% CI, 1.47-1.91) when both levels were above the cut point.
The risks for incident HF and for death rose significantly by 6% and 5%, respectively, per standard deviation NT-proBNP increase from the first to second assay.
Risks for HF and mortality for participants whose NT-proBNP levels declined from greater than to less than 125 pg/mL were similar to those whose levels remained low at both assays.
Cost-effectiveness would be another issue when implementing a strategy that calls for multiple biomarker assays, Dr. Vaduganathan observed.
“Surely, we would want to demonstrate that the laboratory measurement costs are offset by downstream prevention of heart failure events that could be averted by use of effective medical therapy, such SGLT2 inhibitors.”
ARIC has been funded by the National Institutes of Health and Department of Health and Human Services. Dr. Nambi discloses receiving grants from the National Institutes of Health during the conduct of the study; support from Amgen; and stocks from Abbott Laboratories. Disclosures for the other authors are in the report. Dr. Vaduganathan has disclosed receiving grants or serving on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; speaking for AstraZeneca, Novartis, and Roche Diagnostics; and serving on trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article originally appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Cardiac issues twice as likely with COVID plus high troponin
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Type 1 diabetes no longer a disease of the thin: Lifestyle advice needed
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
About two-thirds of people with type 1 diabetes in the United States have overweight or obesity, nearly the same proportion as Americans without diabetes, new nationwide survey data suggest.
What’s more, among people with overweight or obesity, those with type 1 diabetes are less likely to receive lifestyle recommendations from health care professionals than those with type 2 diabetes, and are less likely to actually engage in lifestyle weight management activities than others with overweight or obesity, with or without type 2 diabetes.
“Among U.S. adults with type 1 diabetes, the burden of overweight and obesity is substantial and remains poorly managed,” write Michael Fang, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues.
Their data, from the National Health Interview Survey (NHIS), were published online in Annals of Internal Medicine.
The need for insulin complicates weight management in people with type 1 diabetes because changes in diet and physical activity typically require adjustments to insulin timing and dosage to prevent hypoglycemia. There is little evidence to guide this for weight management, Dr. Fang and colleagues explain.
Consequently, “the lack of evidence for safe, effective methods of diet- and exercise-based weight control in people with type 1 diabetes may be keeping doctors from recommending such methods,” Dr. Fang said in a statement.
“Large clinical trials have been done in type 2 diabetes patients to establish guidelines for diet- and exercise-based weight management, and we now need something similar for type 1 diabetes patients.”
Asked to comment, M. Sue Kirkman, MD, told this news organization: “The days when we could teach simple concepts about diabetes type like ‘those with type 1 are lean and those with type 2 are overweight’ are long gone. … Of concern, fewer adults with type 1 diabetes and overweight/obesity report that they are engaging in physical activity or caloric restriction than those without diabetes or those with type 2 diabetes.”
There are several likely reasons for the low rates of obesity/overweight lifestyle modification advice and implementation for those with type 1 diabetes, noted Dr. Kirkman, of the University of North Carolina at Chapel Hill, who coauthored joint American/European guidance on type 1 diabetes management.
“Medical visits are often primarily focused on glycemic management and complications screening, and we know that physicians in general are not very knowledgeable about how to counsel people – even those without diabetes – on weight loss. When you add in potential worries, real or not, about hypoglycemia, ketosis with carbohydrate restriction … it’s no wonder that this may not be addressed in busy visits.”
She also observed, “In years of going to diabetes meetings, I’ve noticed occasional sessions on managing ‘elite athletes’ with type 1 diabetes, but rarely are there sessions on how to counsel people about everyday healthy living.”
Many with type 1 diabetes have overweight/obesity
Dr. Fang and colleagues analyzed NHIS data for the years 2016, 2017, 2019, 2020, and 2021, when diabetes subtype data were available, for 128,571 adults. Diabetes type and height/weight data were self-reported. In the 2016, 2017, and 2020 surveys, participants were asked whether their physicians had recommended increasing physical activity and/or reducing calorie or fat consumption, and whether they were currently engaging in those activities.
The study population comprised 733 people with type 1 diabetes, 12,397 with type 2 diabetes, and 115,441 without diabetes. The proportions with overweight (body mass index, 25 to < 30 kg/m2) or obesity (≥ 30 kg/m2) were 62% among those with type 1 diabetes and 64% among those without diabetes, compared with 86% among those with type 2 diabetes.
Among those with overweight or obesity, the proportions who reported having received lifestyle recommendations were greatest among those with type 2 diabetes and least among those without diabetes, with the type 1 diabetes group in the middle.
After adjustment for age, sex, and race/ethnicity, the adjusted prevalence of receiving a provider recommendation to increase physical activity was 60% for those with type 2 diabetes, 54% for type 1 diabetes, and 44% for those without diabetes. Proportions for receiving recommendations for reducing fat/caloric intake were similar, at 60%, 51%, and 41%, respectively.
The proportions who reported actually engaging in lifestyle activities for weight management were lowest among those with type 1 diabetes, with 52% and 56% of them reporting having increased their physical activity and reducing fat/calories, respectively, compared with proportions ranging from 56% to 63% among the other two groups.
Regarding those findings, Dr. Kirkman commented, “In addition to the factors regarding physician interactions, people with type 1 diabetes may see this as a lower-priority health issue after years of being told that glucose control is the main priority.”
“I also wonder if the many, many tasks people with type 1 diabetes must do every day to manage their diabetes – along with other life issues all adults face – mean that there is just too much on the plate to add more lifestyle changes,” she added.
Asked about the potential for off-label use of glucagonlike peptide–1 agonists for weight management for people with type 1 diabetes, Dr. Kirkman said they could probably help some patients. However, she also pointed to two clinical trials in which liraglutide added to insulin therapy helped with glycemic control and weight reduction, but also increased the risk for hypoglycemia and diabetic ketoacidosis.
“It’s really important that researchers engage with adults with type 1 diabetes to better understand the unique priorities and barriers they face in addressing body weight,” Dr. Kirkman said.
Senior study author Elizabeth Selvin, PhD, professor of epidemiology at the Bloomberg School, said in the statement: “Our study busts the myth that people with type 1 diabetes are not being affected by the global obesity epidemic. … These findings should be a wake-up call that we need to be aggressive in addressing the obesity epidemic in persons with type 1 diabetes.”
The study was funded by the U.S. National Institutes of Health. Dr. Fang and Dr. Kirkman have reported no relevant financial relationships. Dr. Selvin has reported receiving royalty payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate. She also reports receiving honoraria for editorial work on journals published by the American Diabetes Association and European Association for the Study of Diabetes.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Could ChatGPT write this column?
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
New challenge for docs: End of COVID federal public health emergency
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
The 5-year survival rate for pancreatic cancer is increasing
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
John Whyte, MD: Hello, I’m Dr. John Whyte, the Chief Medical Officer of WebMD. One of those cancers was pancreatic cancer, which historically has had a very low survival rate. What’s going on here? Are we doing better with diagnosis, treatment, a combination?
Joining me today is Dr. Lynn Matrisian. She is PanCAN’s chief science officer. Dr. Matrisian, thanks for joining me today. It’s great to see you.
Lynn Matrisian, PhD, MBA: Great to be here. Thank you.
Dr. Whyte: Well, tell me what your first reaction was when you saw the recent data from the American Cancer Society. What one word would you use?
Dr. Matrisian: Hopeful. I think hopeful in general that survival rates are increasing, not for all cancers, but for many cancers. We continue to make progress. Research is making a difference. And we’re making progress against cancer in general.
Dr. Whyte: You’re passionate, as our viewers know, about pancreatic cancer. And that’s been one of the hardest cancers to treat, and one of the lowest survival rates. But there’s some encouraging news that we saw, didn’t we?
Dr. Matrisian: Yes. So the 5-year survival rate for pancreatic cancer went up a whole percentage. It’s at 12% now. And what’s really good is it was at 11% last year. It was at 10% the year before. So that’s 2 years in a row that we’ve had an increase in the 5-year survival rate for pancreatic cancer. So we’re hopeful that’s a trajectory that we can really capitalize on is how fast we’re making progress in this disease.
Dr. Whyte: I want to put it into context, Lynn. Because some people might be thinking, 1%? Like you’re excited about 1%? That doesn’t seem that much. But correct me if I’m wrong. A one percentage point increase means 641 more loved ones will enjoy life’s moments, as you put it, 5 years after their diagnosis that otherwise wouldn’t have. What does that practically mean to viewers?
Dr. Matrisian: That means that more than 600 people in the United States will hug a loved one 5 years after that diagnosis of pancreatic cancer. It is a very deadly disease. But we’re going to, by continuing to make progress, it gives those moments to those people. And it means that we’re making progress against the disease in general.
Dr. Whyte: So even 1%, and 1% each year, does have value.
Dr. Matrisian: It has a lot of value.
Dr. Whyte: What’s driving this improvement? Is it better screening? And we’re not so great still in screening a pancreatic cancer. Is it the innovation in cancer treatments? What do you think is accounting for what we hope is this trajectory of increases in 5-year survival?
Dr. Matrisian: Right, so the nice thing the reason that we like looking at 5-year survival rates is because it takes into account all of those things. And we have actually made progress in all of those things. So by looking at those that are diagnosed with pancreatic cancer in general as a whole, and looking at their survival, we are looking at better treatments. People who are getting pancreatic cancer later are living longer as a result of better treatments.
But it’s not just that. It’s also, if you’re diagnosed earlier, your 5-year survival rate is higher. More people who are diagnosed early live to five years than those that are diagnosed later. So within that statistic, there are more people who are diagnosed earlier. And those people also live longer. So it takes into account all of those things, which is why we really like to look at that five-year survival rate for a disease like pancreatic cancer.
Dr. Whyte: Where are we on screening? Because we always want to catch people early. That gives them that greatest chance of survival. Have we made much improvements there? And if we have, what are they?
Dr. Matrisian: Well we have made improvements there are more people that are now diagnosed with localized disease than there were 20 years ago. So that is increasing. And we’re still doing it really by being aware of the symptoms right now. Being aware that kind of chronic indigestion, lower back pain that won’t go away, these are signs and symptoms. And especially things like jaundice ...
Dr. Whyte: That yellow color that they might see.
Dr. Matrisian: Yes, that yellow colors in your eye, that’s a really important symptom that would certainly send people to the doctor in order to look at this. So some of it is being more aware and finding the disease earlier. But what we’re really hoping for is some sort of blood test or some sort of other way of looking through medical records and identifying those people that need to go and be checked.
Dr. Whyte: Now we chatted about that almost two years ago. So tell me the progress that we’ve made. How are we doing?
Dr. Matrisian: Yeah, well there’s a number of companies now that have blood tests that are available. They still need more work. They still need more studies to really understand how good they are at finding pancreatic cancer early. But we didn’t have them a couple of years ago. And so it’s really a very exciting time in the field, that there’s companies that were taking advantage of research for many years and actually turning it into a commercial product that is available for people to check.
Dr. Whyte: And then what about treatments? More treatment options today than there were just a few years ago, but still a lot of progress to be made. So when we talk about even 12% 5-year survival, we’d love to see it much more. And you talk about, I don’t want to misquote, so correct me if I’m wrong. Your goal is 20%. Five-year survival by 2030. That’s not too far. So, Lynn, how are we going to get there?
Dr. Matrisian: Okay, well this is our mission. And that’s exactly our goal, 20% by 2030. So we’ve got some work to do. And we are working at both fronts. You’re right, we need better treatments. And so we’ve set up a clinical trial platform where we can look at a lot of different treatments much more efficiently, much faster, kind of taking advantage of an infrastructure to do that. And that’s called Precision Promise. And we’re excited about that as a way to get new treatments for advanced pancreatic cancer.
And then we’re also working on the early detection end. We think an important symptom of pancreatic cancer that isn’t often recognized is new onset diabetes, sudden diabetes in those over 50 where that person did not have diabetes before. So it’s new, looks like type 2 diabetes, but it’s actually caused by pancreatic cancer.
And so we have an initiative, The Early Detection Initiative, that is taking advantage of that. And seeing if we image people right away based on that symptom, can we find pancreatic cancer early? So we think it’s important to look both at trying to diagnose it earlier, as well as trying to treat it better for advanced disease.
Dr. Whyte: Yeah. You know, at WebMD we’re always trying to empower people with better information so they can also become advocates for their health. You’re an expert in advocacy on pancreatic cancer. So what’s your advice to listeners as to how they become good advocates for themselves or advocates in general for loved ones who have pancreatic cancer?
Dr. Matrisian: Yeah. Yeah. Well certainly, knowledge is power. And so the real thing to do is to call the Pancreatic Cancer Action Network. This is what we do. We stay up on the most current information. We have very experienced case managers who can help navigate the complexities of pancreatic cancer at every stage of the journey.
Or if you have questions about pancreatic cancer, call PanCAN. Go to PanCAN.org and give us a call. Because it’s really that knowledge, knowing what it is that you need to get more knowledge about, how to advocate for yourself is very important in a disease, in any disease, but in particular a disease like pancreatic cancer.
Dr. Whyte: And I don’t want to dismiss the progress that we’ve made, that you’ve just referenced in terms of the increased survival. But there’s still a long way to go. We need a lot more dollars for research. We need a lot more clinical trials to take place. What’s your message to a viewer who’s been diagnosed with pancreatic cancer or a loved one? What’s your message, Lynn, today for them?
Dr. Matrisian: Well, first, get as much knowledge as you can. Call PanCAN, and let us help you help your loved one. But then help us. Let’s do research. Let’s do more research. Let’s understand this disease better so we can make those kinds of progress in both treatment and early detection.
And PanCAN works very hard at understanding the disease and setting up research programs that are going to make a difference, that are going to get us to that aggressive goal of 20% survival by 2030. So there is a lot of things that can be done, raise awareness to your friends and neighbors about the disease, lots of things that will help this whole field.
Dr. Whyte: What’s your feeling on second opinions? Given that this can be a difficult cancer to treat, given that there’s emerging therapies that are always developing, when you have a diagnosis of pancreatic cancer, is it important to consider getting a second opinion?
Dr. Matrisian: Yes. Yes, it is. And our case managers will help with that process. We do think it’s important.
Dr. Whyte: Because sometimes, Lynn, people just want to get started, right? Get it out of me. Get treatment. And sometimes getting a second opinion, doing some genomic testing can take time. So what’s your response to that?
Dr. Matrisian: Yeah. Yeah. Well we say, your care team is very important. Who is on your care team, and it may take a little time to find the right people on your care team. But that is an incredibly important step. Sometimes it’s not just one person. Sometimes you need more than one doctor, more than one nurse, more than one type of specialty to help you deal with this. And taking the time to do that is incredibly important.
Yes, you need to – you do need to act. But act smart. And do it with knowledge. Do it really understanding what your options are, and advocate for yourself.
Dr. Whyte: And surround yourself as you reference with that right care team for you, because that’s the most important thing when you have any type of cancer diagnosis. Dr. Lynn Matrisian, I want to thank you for taking time today.
Dr. Matrisian: Thank you so much, John.
A version of this article first appeared on Medscape.com.
New report says suicide rates rising among young Black people
Significant increases in suicide occurred among Native American, Black and Hispanic people, with a startling rise among young Black people. Meanwhile, the rate of suicide among older people declined between 2018 and 2021, the Centers for Disease Control and Prevention has reported.
In 2021, 48,183 people died by suicide in the United States, which equates to a suicide rate of 14.1 per 100,000 people. That level equals the 2018 suicide rate, which had seen a peak that was followed by declines associated with the pandemic.
Experts said rebounding suicide rates are common following times of crisis, such as the COVID-19 pandemic. Suicide declines have also occurred during times of war and natural disaster, when psychological resilience tends to increase and people work together to overcome shared adversity.
“That will wane, and then you will see rebounding in suicide rates. That is, in fact, what we feared would happen. And it has happened, at least in 2021,” Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, told the New York Times.
The new CDC report found that the largest increase was among Black people aged 10-24 years, who experienced a 36.6% increase in suicide rate between 2018 and 2021. While Black people experience mental illness at the same rates as that of the general population, historically they have disproportionately limited access to mental health care, according to the American Psychiatric Association.
CDC report authors noted that some of the biggest increases in suicide rates occurred among groups most affected by the pandemic.
From 2018 to 2021, the suicide rate for people aged 25-44 increased among Native Americans by 33.7% and among Black people by 22.9%. Suicide increased among multiracial people by 20.6% and among Hispanic or Latinx people by 19.4%. Among White people of all ages, the suicide rate declined or remained steady.
“As the nation continues to respond to the short- and long-term impacts of the COVID-19 pandemic, remaining vigilant in prevention efforts is critical, especially among disproportionately affected populations where longer-term impacts might compound preexisting inequities in suicide risk,” the CDC researchers wrote.
A version of this article first appeared on WebMD.com.
Significant increases in suicide occurred among Native American, Black and Hispanic people, with a startling rise among young Black people. Meanwhile, the rate of suicide among older people declined between 2018 and 2021, the Centers for Disease Control and Prevention has reported.
In 2021, 48,183 people died by suicide in the United States, which equates to a suicide rate of 14.1 per 100,000 people. That level equals the 2018 suicide rate, which had seen a peak that was followed by declines associated with the pandemic.
Experts said rebounding suicide rates are common following times of crisis, such as the COVID-19 pandemic. Suicide declines have also occurred during times of war and natural disaster, when psychological resilience tends to increase and people work together to overcome shared adversity.
“That will wane, and then you will see rebounding in suicide rates. That is, in fact, what we feared would happen. And it has happened, at least in 2021,” Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, told the New York Times.
The new CDC report found that the largest increase was among Black people aged 10-24 years, who experienced a 36.6% increase in suicide rate between 2018 and 2021. While Black people experience mental illness at the same rates as that of the general population, historically they have disproportionately limited access to mental health care, according to the American Psychiatric Association.
CDC report authors noted that some of the biggest increases in suicide rates occurred among groups most affected by the pandemic.
From 2018 to 2021, the suicide rate for people aged 25-44 increased among Native Americans by 33.7% and among Black people by 22.9%. Suicide increased among multiracial people by 20.6% and among Hispanic or Latinx people by 19.4%. Among White people of all ages, the suicide rate declined or remained steady.
“As the nation continues to respond to the short- and long-term impacts of the COVID-19 pandemic, remaining vigilant in prevention efforts is critical, especially among disproportionately affected populations where longer-term impacts might compound preexisting inequities in suicide risk,” the CDC researchers wrote.
A version of this article first appeared on WebMD.com.
Significant increases in suicide occurred among Native American, Black and Hispanic people, with a startling rise among young Black people. Meanwhile, the rate of suicide among older people declined between 2018 and 2021, the Centers for Disease Control and Prevention has reported.
In 2021, 48,183 people died by suicide in the United States, which equates to a suicide rate of 14.1 per 100,000 people. That level equals the 2018 suicide rate, which had seen a peak that was followed by declines associated with the pandemic.
Experts said rebounding suicide rates are common following times of crisis, such as the COVID-19 pandemic. Suicide declines have also occurred during times of war and natural disaster, when psychological resilience tends to increase and people work together to overcome shared adversity.
“That will wane, and then you will see rebounding in suicide rates. That is, in fact, what we feared would happen. And it has happened, at least in 2021,” Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, told the New York Times.
The new CDC report found that the largest increase was among Black people aged 10-24 years, who experienced a 36.6% increase in suicide rate between 2018 and 2021. While Black people experience mental illness at the same rates as that of the general population, historically they have disproportionately limited access to mental health care, according to the American Psychiatric Association.
CDC report authors noted that some of the biggest increases in suicide rates occurred among groups most affected by the pandemic.
From 2018 to 2021, the suicide rate for people aged 25-44 increased among Native Americans by 33.7% and among Black people by 22.9%. Suicide increased among multiracial people by 20.6% and among Hispanic or Latinx people by 19.4%. Among White people of all ages, the suicide rate declined or remained steady.
“As the nation continues to respond to the short- and long-term impacts of the COVID-19 pandemic, remaining vigilant in prevention efforts is critical, especially among disproportionately affected populations where longer-term impacts might compound preexisting inequities in suicide risk,” the CDC researchers wrote.
A version of this article first appeared on WebMD.com.