User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
USPSTF recommends against routine herpes screening for asymptomatic teens and adults
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
FROM JAMA
Exercise training reduces liver fat in patients with NAFLD, even without weight loss
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
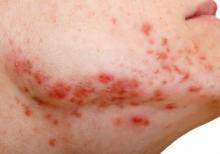
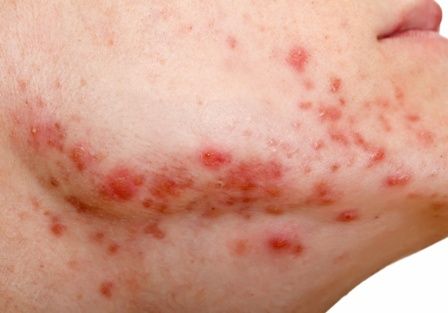
Secukinumab shows benefit for hidradenitis suppurativa out to 52 weeks
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
FROM THE LANCET
More than 97K new cutaneous melanoma diagnoses expected in 2023
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
AT MELANOMA 2023
Notalgia paresthetica: Difelikefalin helps upper-back itch, but with side effects
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Isothiazolinone contact allergy up in North America, down in Europe
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
UnitedHealthcare tried to deny coverage to a chronically ill patient. He fought back, exposing the insurer’s inner workings.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
Vibrating pill can help treat constipation
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
Prehospital COVID therapy effective in rheumatic disease patients
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Embattled iPLEDGE program: Changes ahead?
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.