User login
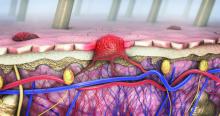
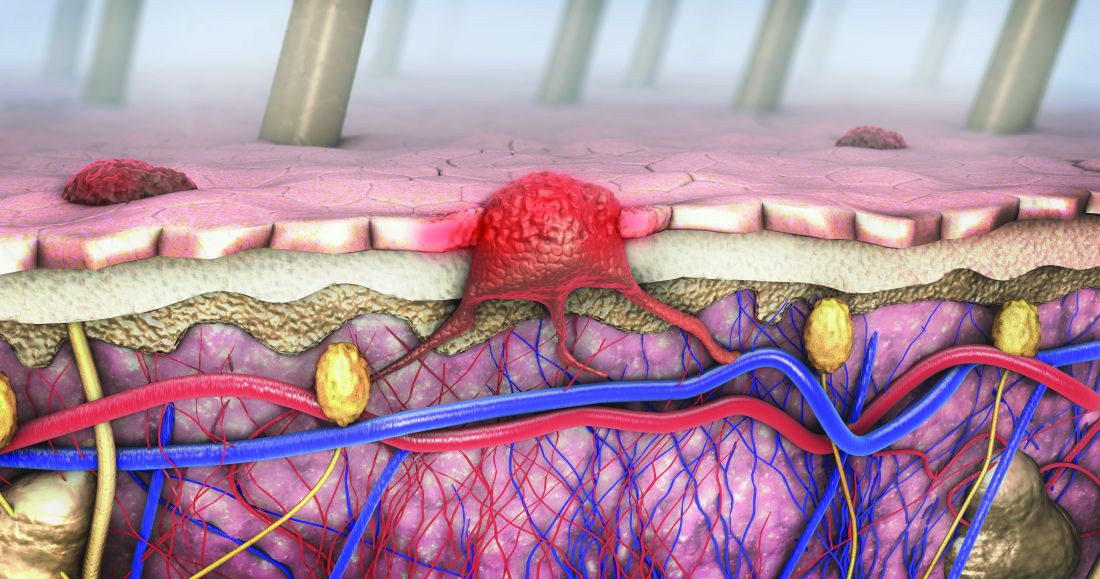
Skin reactions from melanoma targeted and immune therapies range from pruritus to SJS
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
AT MELANOMA 2023
Expert discusses pros, cons of molecular tests for melanoma
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
AT MELANOMA 2023
How prevalent is pediatric melanoma?
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
AT MELANOMA 2023
Optimal management of dysplastic nevi continues to evolve
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
AT MELANOMA 2023
More than 97K new cutaneous melanoma diagnoses expected in 2023
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
AT MELANOMA 2023
Dermoscopy, other modalities for improving melanoma diagnoses reviewed
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
AT MELANOMA 2023
Dermatopathologist reflects on the early history of melanoma
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
AT MELANOMA 2023
How should PRAME be used to evaluate melanocytic lesions?
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
AT MELANOMA 2023