User login
MD-IQ only
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).
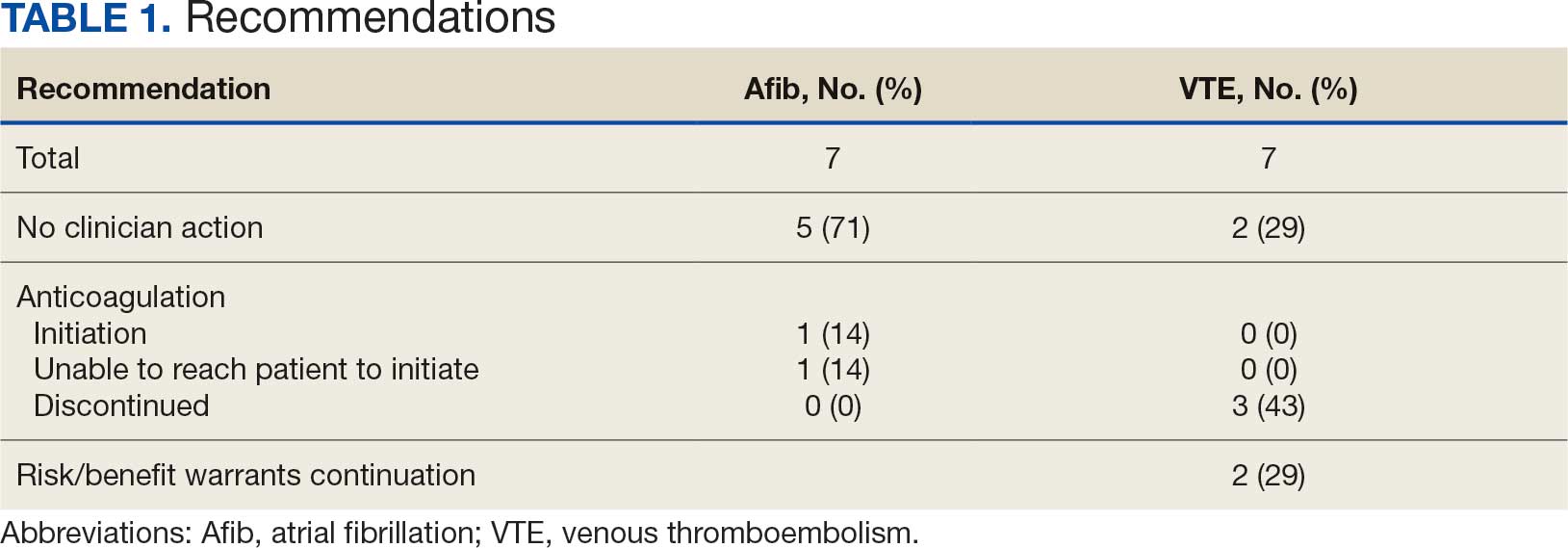
In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
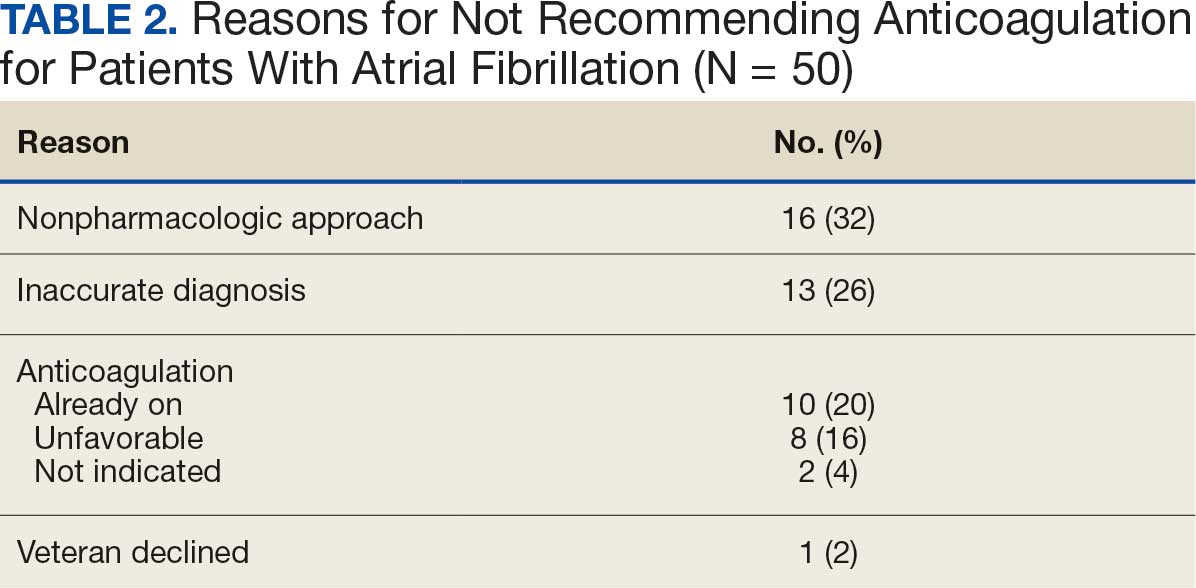
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).
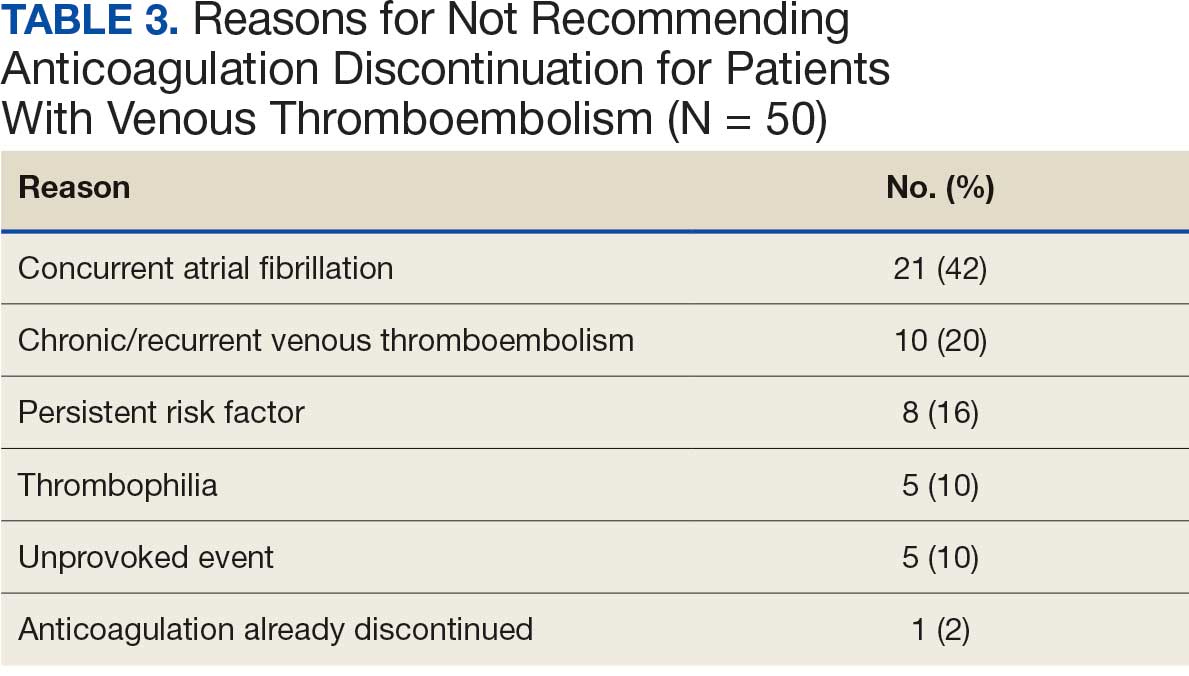
In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).
Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Targeted Syncope Workup in Hypercoagulable Patients
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Cardiac Risks of Newer Psoriasis Biologics vs. TNF Inhibitors Compared
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New Investigation Casts Doubt on Landmark Ticagrelor Trial
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
FROM THE BMJ
In IBD Patients, No Increased Risk for MACE Seen for JAK Inhibitors vs Anti-TNF
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In particular, 1.76% of patients taking JAKi and 1.94% of patients taking anti-TNF developed MACE. There also weren’t significant differences when comparing ulcerative colitis with Crohn’s disease, upadacitinib with tofacitinib, or JAKi with infliximab.
“IBD is associated with an increased risk of cardiovascular diseases, and with the emergence of JAK inhibitors and anti-TNF therapies, there is a concern about the increased risk of MACE,” said lead author Saqr Alsakarneh, MD, an internal medicine resident at the University of Missouri–Kansas City School of Medicine.
Previous randomized controlled trials have indicated increased risks of MACE with JAKi and anti-TNF agents, compared with placebo, but researchers haven’t conducted a head-to-head comparison, he said.
“A potential explanation for previous associations could be linked to immune modulation and inflammation that can increase coagulation risk, as well as fluctuation in disease severity while patients are on the medications, which can impact cardiovascular risk factors,” he added.
Alsakarneh and colleagues conducted a retrospective cohort study using the TriNetX database to identify adult patients with IBD who were treated with JAKi or anti-TNF therapy after diagnosis. After matching patients in the JAKi cohort with patients in the anti-TNF cohort, the research team looked for MACE and VTE within a year of medication initiation, as well as associations by age, sex, and IBD type.
Overall, 3740 patients in the JAKi cohort had a mean age of 43.1 and were 48.9% women and 75.3% White individuals, while 3,740 patients in the anti-TNF cohort had a mean age of 43 and were 48.9% women and 75.3% White individuals.
After excluding those with a history of a prior cardiovascular event, 57 patients (1.76%) in the JAKi cohort developed MACE, compared with 63 patients (1.94%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (adjusted hazard ratio [aHR], 0.99) or VTE (aHR, 0.9).
Among patients aged ≥ 65, 25 patients (5.3%) in the JAKi cohort developed MACE, as compared with 30 patients (6.4%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (aHR, 0.83) or VTE (aHR, 0.77).
In addition, there were no differences when comparing Crohn’s disease with ulcerative colitis for MACE (aHR, 1.69) or VTE (aHR, 0.85); upadacitinib with tofacitinib for MACE (aHR, 1.1) or VTE (aHR, 1.13); or JAKi medications with infliximab for MACE (aHR, 0.85) or VTE (aHR, 0.8).
Patients in the JAKi group were more likely to undergo intestinal resection surgery (aHR, 1.32), but there wasn’t a statistically significant difference in systematic corticosteroid use (aHR, 0.99).
The study limitations included the inability to assess for disease severity, dose-dependent risk for MACE or VTE, or long-term outcomes among the two cohorts, Alsakarneh said. Prospective controlled trials are needed to confirm findings.
“This is a wonderful study and nice to see. We presented the same thing at Digestive Disease Week that’s being confirmed in this data,” said Miguel Regueiro, MD, AGAF, chief of Cleveland Clinic’s Digestive Disease Institute in Ohio. Regueiro, who wasn’t involved with the study, attended the conference session.
“Looking ahead, all of us are wondering if the regulatory guidance by the FDA [Food and Drug Administration] is going to change the label so we don’t need to step through a TNF,” he said. “I think we’re seeing study after study showing safety or at least not an increased risk with JAK.”
The study was awarded an ACG Noteworthy Abstract. Alsakarneh and Regueiro reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In particular, 1.76% of patients taking JAKi and 1.94% of patients taking anti-TNF developed MACE. There also weren’t significant differences when comparing ulcerative colitis with Crohn’s disease, upadacitinib with tofacitinib, or JAKi with infliximab.
“IBD is associated with an increased risk of cardiovascular diseases, and with the emergence of JAK inhibitors and anti-TNF therapies, there is a concern about the increased risk of MACE,” said lead author Saqr Alsakarneh, MD, an internal medicine resident at the University of Missouri–Kansas City School of Medicine.
Previous randomized controlled trials have indicated increased risks of MACE with JAKi and anti-TNF agents, compared with placebo, but researchers haven’t conducted a head-to-head comparison, he said.
“A potential explanation for previous associations could be linked to immune modulation and inflammation that can increase coagulation risk, as well as fluctuation in disease severity while patients are on the medications, which can impact cardiovascular risk factors,” he added.
Alsakarneh and colleagues conducted a retrospective cohort study using the TriNetX database to identify adult patients with IBD who were treated with JAKi or anti-TNF therapy after diagnosis. After matching patients in the JAKi cohort with patients in the anti-TNF cohort, the research team looked for MACE and VTE within a year of medication initiation, as well as associations by age, sex, and IBD type.
Overall, 3740 patients in the JAKi cohort had a mean age of 43.1 and were 48.9% women and 75.3% White individuals, while 3,740 patients in the anti-TNF cohort had a mean age of 43 and were 48.9% women and 75.3% White individuals.
After excluding those with a history of a prior cardiovascular event, 57 patients (1.76%) in the JAKi cohort developed MACE, compared with 63 patients (1.94%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (adjusted hazard ratio [aHR], 0.99) or VTE (aHR, 0.9).
Among patients aged ≥ 65, 25 patients (5.3%) in the JAKi cohort developed MACE, as compared with 30 patients (6.4%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (aHR, 0.83) or VTE (aHR, 0.77).
In addition, there were no differences when comparing Crohn’s disease with ulcerative colitis for MACE (aHR, 1.69) or VTE (aHR, 0.85); upadacitinib with tofacitinib for MACE (aHR, 1.1) or VTE (aHR, 1.13); or JAKi medications with infliximab for MACE (aHR, 0.85) or VTE (aHR, 0.8).
Patients in the JAKi group were more likely to undergo intestinal resection surgery (aHR, 1.32), but there wasn’t a statistically significant difference in systematic corticosteroid use (aHR, 0.99).
The study limitations included the inability to assess for disease severity, dose-dependent risk for MACE or VTE, or long-term outcomes among the two cohorts, Alsakarneh said. Prospective controlled trials are needed to confirm findings.
“This is a wonderful study and nice to see. We presented the same thing at Digestive Disease Week that’s being confirmed in this data,” said Miguel Regueiro, MD, AGAF, chief of Cleveland Clinic’s Digestive Disease Institute in Ohio. Regueiro, who wasn’t involved with the study, attended the conference session.
“Looking ahead, all of us are wondering if the regulatory guidance by the FDA [Food and Drug Administration] is going to change the label so we don’t need to step through a TNF,” he said. “I think we’re seeing study after study showing safety or at least not an increased risk with JAK.”
The study was awarded an ACG Noteworthy Abstract. Alsakarneh and Regueiro reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In particular, 1.76% of patients taking JAKi and 1.94% of patients taking anti-TNF developed MACE. There also weren’t significant differences when comparing ulcerative colitis with Crohn’s disease, upadacitinib with tofacitinib, or JAKi with infliximab.
“IBD is associated with an increased risk of cardiovascular diseases, and with the emergence of JAK inhibitors and anti-TNF therapies, there is a concern about the increased risk of MACE,” said lead author Saqr Alsakarneh, MD, an internal medicine resident at the University of Missouri–Kansas City School of Medicine.
Previous randomized controlled trials have indicated increased risks of MACE with JAKi and anti-TNF agents, compared with placebo, but researchers haven’t conducted a head-to-head comparison, he said.
“A potential explanation for previous associations could be linked to immune modulation and inflammation that can increase coagulation risk, as well as fluctuation in disease severity while patients are on the medications, which can impact cardiovascular risk factors,” he added.
Alsakarneh and colleagues conducted a retrospective cohort study using the TriNetX database to identify adult patients with IBD who were treated with JAKi or anti-TNF therapy after diagnosis. After matching patients in the JAKi cohort with patients in the anti-TNF cohort, the research team looked for MACE and VTE within a year of medication initiation, as well as associations by age, sex, and IBD type.
Overall, 3740 patients in the JAKi cohort had a mean age of 43.1 and were 48.9% women and 75.3% White individuals, while 3,740 patients in the anti-TNF cohort had a mean age of 43 and were 48.9% women and 75.3% White individuals.
After excluding those with a history of a prior cardiovascular event, 57 patients (1.76%) in the JAKi cohort developed MACE, compared with 63 patients (1.94%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (adjusted hazard ratio [aHR], 0.99) or VTE (aHR, 0.9).
Among patients aged ≥ 65, 25 patients (5.3%) in the JAKi cohort developed MACE, as compared with 30 patients (6.4%) in the anti-TNF cohort. There weren’t significant differences between the groups in MACE (aHR, 0.83) or VTE (aHR, 0.77).
In addition, there were no differences when comparing Crohn’s disease with ulcerative colitis for MACE (aHR, 1.69) or VTE (aHR, 0.85); upadacitinib with tofacitinib for MACE (aHR, 1.1) or VTE (aHR, 1.13); or JAKi medications with infliximab for MACE (aHR, 0.85) or VTE (aHR, 0.8).
Patients in the JAKi group were more likely to undergo intestinal resection surgery (aHR, 1.32), but there wasn’t a statistically significant difference in systematic corticosteroid use (aHR, 0.99).
The study limitations included the inability to assess for disease severity, dose-dependent risk for MACE or VTE, or long-term outcomes among the two cohorts, Alsakarneh said. Prospective controlled trials are needed to confirm findings.
“This is a wonderful study and nice to see. We presented the same thing at Digestive Disease Week that’s being confirmed in this data,” said Miguel Regueiro, MD, AGAF, chief of Cleveland Clinic’s Digestive Disease Institute in Ohio. Regueiro, who wasn’t involved with the study, attended the conference session.
“Looking ahead, all of us are wondering if the regulatory guidance by the FDA [Food and Drug Administration] is going to change the label so we don’t need to step through a TNF,” he said. “I think we’re seeing study after study showing safety or at least not an increased risk with JAK.”
The study was awarded an ACG Noteworthy Abstract. Alsakarneh and Regueiro reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Study Finds No Increased MACE Risk for JAK Inhibitors in Patients With Atopic Dermatitis
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
, suggested the results of a large, US-based, retrospective cohort study.
This holds true even in individuals aged 50 years or older, whose age puts them at increased cardiovascular (CV) risk, said Amina El Ayadi, PhD, of the University of Texas Medical Branch at Galveston. He presented the findings at the recent European Academy of Dermatology and Venereology (EADV) 2024 Congress.
Specifically, the analysis looked at treatment with the oral JAK1 inhibitors upadacitinib (Rinvoq) and abrocitinib (Cibinqo), both approved for treating AD in the United States, and found that the relative risk for MACE, such as acute myocardial infarction, cardiac arrest, stroke, or acute deep vein thrombosis, was ≤ 1.0 compared with those not treated with a JAKi.
Similarly, the relative risk for other CV safety endpoints, such as having an abnormal ECG or pericardial effusion, was also around 1.0. There was a slight increase in the relative risk for arrhythmias, peripheral edema, angina pectoris, or heart failure, but no value went > 1.6 and CIs spanned 1.0, indicating the results lack statistical significance.
Reassurance for Dermatologists?
“This suggests that oral administration of these drugs to the patient with atopic dermatitis does not increase the risk of major adverse cardiac events, and dermatologists, based on our data, can safely consider JAK inhibitors for treating moderate to severe dermatitis, even in patients with high risk for these diseases,” El Ayadi said during a late-breaking news session at the meeting.
Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Department at Miguel Servet University Hospital in Zaragoza, Spain, who was one of the chairs for the session, said that this was “very good news for us.”
Gilaberte Calzada, president of the Spanish Academy of Dermatology and Venereology, asked if there were any data on the duration of treatment with the two JAKis included in the analysis. El Ayadi said that this was something that would be looked at in future data analyses.
Gilaberte Calzada also observed that because the CIs were wide, with more time, “we will have more defined data.”
Analyses Overview
For the two analyses — one in the overall population of patients with AD and the other in those aged 50 years or older — electronic medical record (EMR) data from the TriNetX Research Network were used. This is a global, federated health research network that contains EMRs for more than 275 million patients from over 120 healthcare organizations, El Ayadi explained.
To perform the analyses, the research team queried the TriNetX database to find all patients diagnosed with AD via the International Classification of Diseases, Tenth Revision code L20. They then determined if patients had been treated with JAKi or not, and specifically, with upadacitinib or abrocitinib. Those who had not received any JAKi treatment were the control population.
For the first analysis, no age-specific filter was applied. The investigators identified 1674 people with AD who had been treated with the JAKis and around 1.2 million who had not. Propensity score matching, based on age at diagnosis, biologic sex, and CV comorbidities, was performed to give a total of 1674 patients who had and 1674 who had not been treated with these medications.
In the second analysis, only those aged 50 years or older were considered; 875 patients who had received JAKi treatment were identified and around 250,000 who had not. Propensity score matching based on the same variables gave two groups of 875 people who had or had not taken a JAKi.
Queried over the age cutoff used, El Ayadi noted, “We did an analysis looking at patients 65 and older. However, we came up with lower patient numbers. … We do have this data, and we did not see any significant risk.”
The study was independently supported. El Ayadi and Gilaberte Calzada reported no conflicts of interest in relation to the presented findings.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
On Second Thought: Aspirin for Primary Prevention — What We Really Know
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Older Patients With COPD at Increased Risk for PE-Associated Death
BOSTON — Patients with COPD are at an increased risk for fatal pulmonary embolism (PE) and may require personalized, targeted thromboprophylaxis.
The data suggest that “maybe we should start thinking about if we are admitting a patient with COPD in that specific age group, higher thromboprophylaxis for PE,” said Marwa Oudah, MD, a pulmonary hypertension fellow at the University of Pennsylvania, Philadelphia. She presented her group’s findings in a rapid-fire oral abstract session at the CHEST Annual Meeting.
Known Risk Factor
COPD is a known risk factor for PE. To estimate how the obstructive lung disease may contribute to PE-related deaths among patients of varying ages, Oudah and colleagues drew data on deaths due to an underlying cause of PE from 1999 to 2020 from the Centers for Disease Control and Prevention’s WONDER database.
They stratified the patients into two groups — those with or without COPD — whose data were included in the Multiple Causes of Death dataset, according to age groups ranging from 35 years to over 100 years. The investigators calculated proportional mortality ratios in the non-COPD group and applied these to the COPD-positive group among different age ranges to estimate the observed vs expected number of deaths.
A total of 10,434 persons who died from PE and had COPD listed among causes of death were identified. The sample was evenly divided by sex. The peak range of deaths was among those aged 75-84 years.
The authors saw an increase in PE-related mortality among patients with COPD aged 65-85 years (P < .001).
The ratios of observed-to-expected deaths among patients in this age range were “substantially greater than 1” said Oudah, with patients aged 75-79 years at highest risk for PE-related death, with an observed-to-expected ratio of 1.443.
In contrast, the rate of observed deaths among patients aged 85-89 years was similar to the expected rate, suggesting that the COPD-PE interaction may wane among older patients, she said.
Among patients aged 35-64 years, the risk for death from PE was not significantly higher for any of the 5-year age categories.
The investigators emphasized that “given the observed trend, individualized patient assessments are imperative to optimize preventable measures against PE in the aging COPD population.”
Confounding Comorbidities
In an interview, a pulmonary specialist who was not involved in the study commented that older persons with COPD tend to have multiple comorbidities that may contribute to the risk for PE.
“Older patients have so many comorbidities, and their risk for pulmonary embolism and thromboembolic disease is pretty high, so I’m not surprised that 75 to 79 years olds are having a higher mortality from PE, but it’s a little difficult to say whether that’s due to COPD,” said Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, who moderated the session.
The authors did not report a study funding source. Oudah and Sundar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Patients with COPD are at an increased risk for fatal pulmonary embolism (PE) and may require personalized, targeted thromboprophylaxis.
The data suggest that “maybe we should start thinking about if we are admitting a patient with COPD in that specific age group, higher thromboprophylaxis for PE,” said Marwa Oudah, MD, a pulmonary hypertension fellow at the University of Pennsylvania, Philadelphia. She presented her group’s findings in a rapid-fire oral abstract session at the CHEST Annual Meeting.
Known Risk Factor
COPD is a known risk factor for PE. To estimate how the obstructive lung disease may contribute to PE-related deaths among patients of varying ages, Oudah and colleagues drew data on deaths due to an underlying cause of PE from 1999 to 2020 from the Centers for Disease Control and Prevention’s WONDER database.
They stratified the patients into two groups — those with or without COPD — whose data were included in the Multiple Causes of Death dataset, according to age groups ranging from 35 years to over 100 years. The investigators calculated proportional mortality ratios in the non-COPD group and applied these to the COPD-positive group among different age ranges to estimate the observed vs expected number of deaths.
A total of 10,434 persons who died from PE and had COPD listed among causes of death were identified. The sample was evenly divided by sex. The peak range of deaths was among those aged 75-84 years.
The authors saw an increase in PE-related mortality among patients with COPD aged 65-85 years (P < .001).
The ratios of observed-to-expected deaths among patients in this age range were “substantially greater than 1” said Oudah, with patients aged 75-79 years at highest risk for PE-related death, with an observed-to-expected ratio of 1.443.
In contrast, the rate of observed deaths among patients aged 85-89 years was similar to the expected rate, suggesting that the COPD-PE interaction may wane among older patients, she said.
Among patients aged 35-64 years, the risk for death from PE was not significantly higher for any of the 5-year age categories.
The investigators emphasized that “given the observed trend, individualized patient assessments are imperative to optimize preventable measures against PE in the aging COPD population.”
Confounding Comorbidities
In an interview, a pulmonary specialist who was not involved in the study commented that older persons with COPD tend to have multiple comorbidities that may contribute to the risk for PE.
“Older patients have so many comorbidities, and their risk for pulmonary embolism and thromboembolic disease is pretty high, so I’m not surprised that 75 to 79 years olds are having a higher mortality from PE, but it’s a little difficult to say whether that’s due to COPD,” said Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, who moderated the session.
The authors did not report a study funding source. Oudah and Sundar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Patients with COPD are at an increased risk for fatal pulmonary embolism (PE) and may require personalized, targeted thromboprophylaxis.
The data suggest that “maybe we should start thinking about if we are admitting a patient with COPD in that specific age group, higher thromboprophylaxis for PE,” said Marwa Oudah, MD, a pulmonary hypertension fellow at the University of Pennsylvania, Philadelphia. She presented her group’s findings in a rapid-fire oral abstract session at the CHEST Annual Meeting.
Known Risk Factor
COPD is a known risk factor for PE. To estimate how the obstructive lung disease may contribute to PE-related deaths among patients of varying ages, Oudah and colleagues drew data on deaths due to an underlying cause of PE from 1999 to 2020 from the Centers for Disease Control and Prevention’s WONDER database.
They stratified the patients into two groups — those with or without COPD — whose data were included in the Multiple Causes of Death dataset, according to age groups ranging from 35 years to over 100 years. The investigators calculated proportional mortality ratios in the non-COPD group and applied these to the COPD-positive group among different age ranges to estimate the observed vs expected number of deaths.
A total of 10,434 persons who died from PE and had COPD listed among causes of death were identified. The sample was evenly divided by sex. The peak range of deaths was among those aged 75-84 years.
The authors saw an increase in PE-related mortality among patients with COPD aged 65-85 years (P < .001).
The ratios of observed-to-expected deaths among patients in this age range were “substantially greater than 1” said Oudah, with patients aged 75-79 years at highest risk for PE-related death, with an observed-to-expected ratio of 1.443.
In contrast, the rate of observed deaths among patients aged 85-89 years was similar to the expected rate, suggesting that the COPD-PE interaction may wane among older patients, she said.
Among patients aged 35-64 years, the risk for death from PE was not significantly higher for any of the 5-year age categories.
The investigators emphasized that “given the observed trend, individualized patient assessments are imperative to optimize preventable measures against PE in the aging COPD population.”
Confounding Comorbidities
In an interview, a pulmonary specialist who was not involved in the study commented that older persons with COPD tend to have multiple comorbidities that may contribute to the risk for PE.
“Older patients have so many comorbidities, and their risk for pulmonary embolism and thromboembolic disease is pretty high, so I’m not surprised that 75 to 79 years olds are having a higher mortality from PE, but it’s a little difficult to say whether that’s due to COPD,” said Krishna Sundar, MBBS, MD, FCCP, a pulmonary, sleep medicine, and critical care medicine specialist at St. John’s Medical Center in Jackson, Wyoming, who moderated the session.
The authors did not report a study funding source. Oudah and Sundar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST 2024
‘Door-to-Thrombectomy’ Time for Acute PE Linked to Better Outcomes
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In the article "‘Door-to-thrombectomy ’ time linked to better acute PE outcomes" in the November 2024 issue of CHEST Physician, Dr. Patel was quoted as stating, "Mechanical thrombectomy in the FLASH registry showed mortality benefit," and, "There's a mortality benefit in any case whether the patient is high risk or intermediate-high." Dr. Patel was referring to the FLASH registry as a whole and not to the registry data study described in this article. CHEST Physician regrets the error and any confusion this may have caused.
BOSTON —
Among nearly 800 patients with acute PE whose data are recorded in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH), a prospective multicenter registry of individuals treated with mechanical thrombectomy using the FlowTriever system (Inari Medical), shorter time from admission to mechanical thrombectomy was associated with significantly greater reductions in intraprocedural mean and systolic pulmonary artery pressures (PAP), greater reductions in the right ventricular/left ventricular (RV/LV) ratio, and longer 6-minute walk times at 6 months, reported Krunal H. Patel, MD, a pulmonary and critical care fellow at the Lewis Katz School of Medicine at Temple University Hospital in Philadelphia.
“Mechanical thrombectomy in the FLASH registry showed a mortality benefit. I think as time progresses and mechanical thrombectomy becomes more popular, we’re just going to need to figure out what is the ideal time for intervention,” he said during an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
“There’s mortality benefit in any case whether the patient is high-risk or intermediate-high. This is a thought-provoking retrospective analysis that says that early intervention is probably better than doing it late, but regardless, the FLASH registry trial showed that early thrombectomy or thrombectomy in general shows positive mortality benefit,” Patel said in an interview.
He likened the challenge for pulmonary and critical care specialists to that of interventional cardiologists, who have determined that the ideal window for starting percutaneous coronary interventions is within 90 minutes of the patient’s arrival at the facility.
“I think we have to get our ‘door-to-balloon’ time for PE care,” he said.
Study Details
Patel and colleague Parth M. Rali, MD, FCCP, associate professor of thoracic medicine at Temple, conducted a retrospective review of data on 787 US patients in the FLASH registry for whom time to mechanical thrombectomy data were available. They stratified the patients into short and long time to mechanical thrombectomy groups, with “short” defined as ≤ 12 hours of presentation and “long” as > 12 hours.
They found that the median time to thrombectomy was 19.68 hours. In all, 242 patients (31%) were treated within the short window, and the remaining 545 patients (69%) were treated after at least 12 hours had passed.
Comparing clinical characteristics between the groups, the investigators noted that significantly more patients in the short time group vs long time group were categorized as high-risk (11.2% vs 6.2%; P = .0026). This difference is likely due to the need for greater urgency among high-risk patients, Patel said.
Patients in the short time group also had significantly higher baseline RV/LV ratios and lactate levels, but baseline dyspnea scores and pre-procedure median and systolic PAP were similar between the groups.
The mean time to thrombectomy was 6.08 hours in the short time group vs 34.04 hours in the long time group. Their respective median times were 6.01 and 24.73 hours.
The procedural time was similar between the groups, at 45 and 42 minutes, respectively.
The location of the treated thrombus was central only in 35.1% and 26.5% patients in the short and long time groups, respectively. Lobar-only thrombi were treated in 7.9% and 14.3%, respectively, and both central and lobar thrombi were treated in 57.0% and 59.2%, respectively.
Both 48-hour and 30-day all-cause mortality rates were similar between the groups (0.4%/0.2% and 0.5%/1.0%).
Patients in the short time group had slightly but significantly longer post-procedure hospital and intensive care unit stays, but 30-day readmission rates — whether for PE- or non-PE–related causes — were similar.
Where the differences between the groups really showed, however, were PAP reductions over baseline, with decline in median pressures of −8.7 mm Hg in the short group vs −7.2 mm Hg in the long group (P = .0008), and drops in systolic PAP of −14.4 vs −12.1 mm Hg, respectively (P = .0011).
In addition, reductions in RV/LV ratios from baseline were also significantly greater among patients whose thrombectomies had been expedited at the 48-hour, 30-day, and 6-month follow-up periods.
At 6 months, patients who had received mechanical thrombectomy within 12 hours also had significantly longer 6-minute walk distances (442.2 vs 390.5 m; P = .0032).
Low Thrombolysis Rate
Following his presentation, session co-moderator Galina Glazman-Kuczaj, MD, from the Division of Pulmonary and Critical Care Medicine at Albany Med Health System, Albany, New York, asked Patel what percentage of patients, if any, had received thrombolytic therapy before the thrombectomy procedure.
He noted that only 1% or 2% patients in the FLASH registry received thrombolysis.
In an interview, Glazman-Kuczaj said that “it was reassuring for [Patel] to report that it was only a small population of patients who got thrombolysis beforehand in either group because you would expect that maybe people in the group that took longer to have a thrombectomy got some thrombolysis beforehand and that perhaps they were more stable, but it seems like thrombectomy was the first-line treatment in both groups.”
The FLASH Registry is funded by Inari Medical. Patel and Glazman-Kuczaj reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CHEST 2024
ILD Linked to Poorer Outcomes in Pulmonary Embolism
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON — Patients with pulmonary embolism (PE) who also present with interstitial lung disease (ILD) have worse outcomes with respect to in-hospital mortality, length of hospital stay, hospital cost, and all-cause readmission, according to results from a new retrospective analysis.
Unfortunately, there’s not a whole lot of evidence out there to really demonstrate it,” Leah Yuan, MD, said during a presentation of the results at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
The question is complicated by the nebulous nature of ILD, which includes a diverse set of diseases and etiologies, and different levels of inflammation and fibrosis. It has been employed in the Pulmonary Embolism Severity Index but counts for only 10 points out of 210. “If you look at ILD and PE outcomes, there’s nothing really out there [in the literature],” Yuan said in an interview. She is a resident physician at Cook County Health and Hospitals System.
The new study suggested that ILD could have an important influence and perhaps should have greater weight in risk stratification of patients with PE, she said. “We looked at all-cause readmissions and we looked at in-hospital mortality, [both] of which are significant for increased odds ratio. One thing that I’m very curious to see is whether there is increased PE readmissions [associated with ILD], which is something that we couldn’t find to be significant in our study,” said Yuan.
The researchers used data from hospitalizations for PE drawn from the Nationwide Readmissions Database in 2019, using International Classification of Diseases, Tenth Revision, codes to identify admissions. Among a total of 105,133 patients admitted for PE, 158 patients also had ILD. The mean age was 63.6 years for those without ILD (SD, 0.1) and 66.5 years for those with ILD (SD, 1.3).
Admission with ILD was associated with all-cause readmission (odds ratio [OR], 4.12; P < .01), in-hospital mortality (OR, 2.17; P = .01), a longer length of stay (+2.07 days; P < .01), and higher hospitalization charges (+$22,627; P < .01).
In the Q&A period after the presentation, Parth Rali, MD, professor of thoracic medicine and surgery at Temple University, Philadelphia, suggested phenotyping patients to better understand the location of the PE in relation to the ILD. “It may not fall into your classic PE classification. It may just depend on where the clot is in relationship to the interstitial lung disease. I think that’s where the field is going to evolve,” he later said in an interview.
“What is interesting is that patients with interstitial lung disease have a lot of fibrotic disease, and they do not need to have a large clot burden to make them sick. An example [is someone] who has undergone a lung transplant evaluation, and if their right lung is completely diseased from interstitial lung disease and if they get a big blood clot on the right side, it doesn’t affect them because the lung is already fibrotic, so the clot doesn’t matter. If they get a small clot [in the left lung], even though if you look at the standard PE classification they may qualify as a low-risk PE or even as an intermediate-low-risk PE, they are much sicker because that’s the functioning part of the lung,” said Rali.
He advised physicians to pay close attention to the location of PEs in relation to fibrotic tissue in patients with ILD. A PE in healthy lung tissue could have an outsized effect on hemodynamics, whereas a PE in fibrotic tissue may be clinically insignificant and not require treatment. “So it goes both ways: You don’t overtreat and you don’t undertreat,” Rali said.
Yuan and Rali disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
CHEST 2024