User login
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
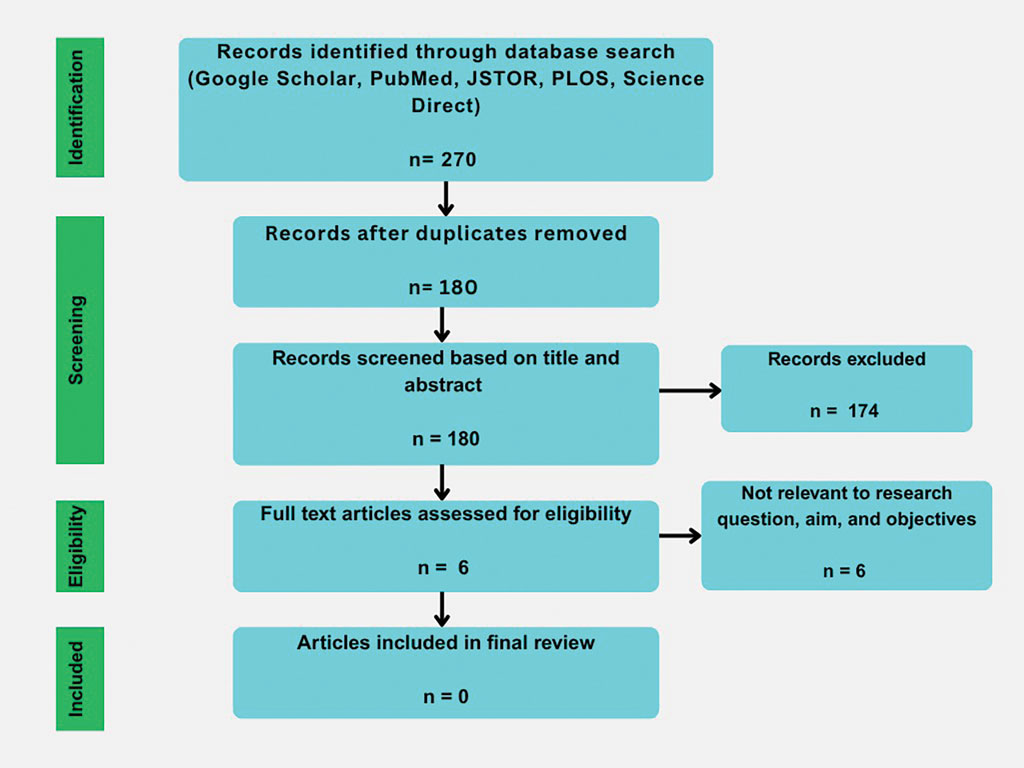
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).
Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Practice Points
- Synthetic hair extensions are made from materials that can expose patients to high levels of carcinogens beginning in early childhood.
- The apple cider vinegar rinse method is an anecdotal remedy lacking data validating its ability to mitigate adverse reactions and complications associated with synthetic hair extensions, including carcinogenic exposure to materials they comprise.
- Dermatologists should inform patients of the potential exposure risks when using synthetic hair extensions to help patients make informed decisions regarding future styling habits and hair care choices.
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
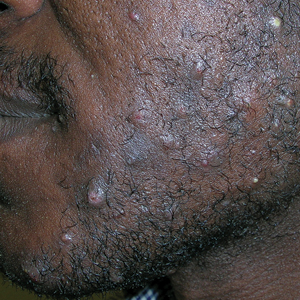
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).
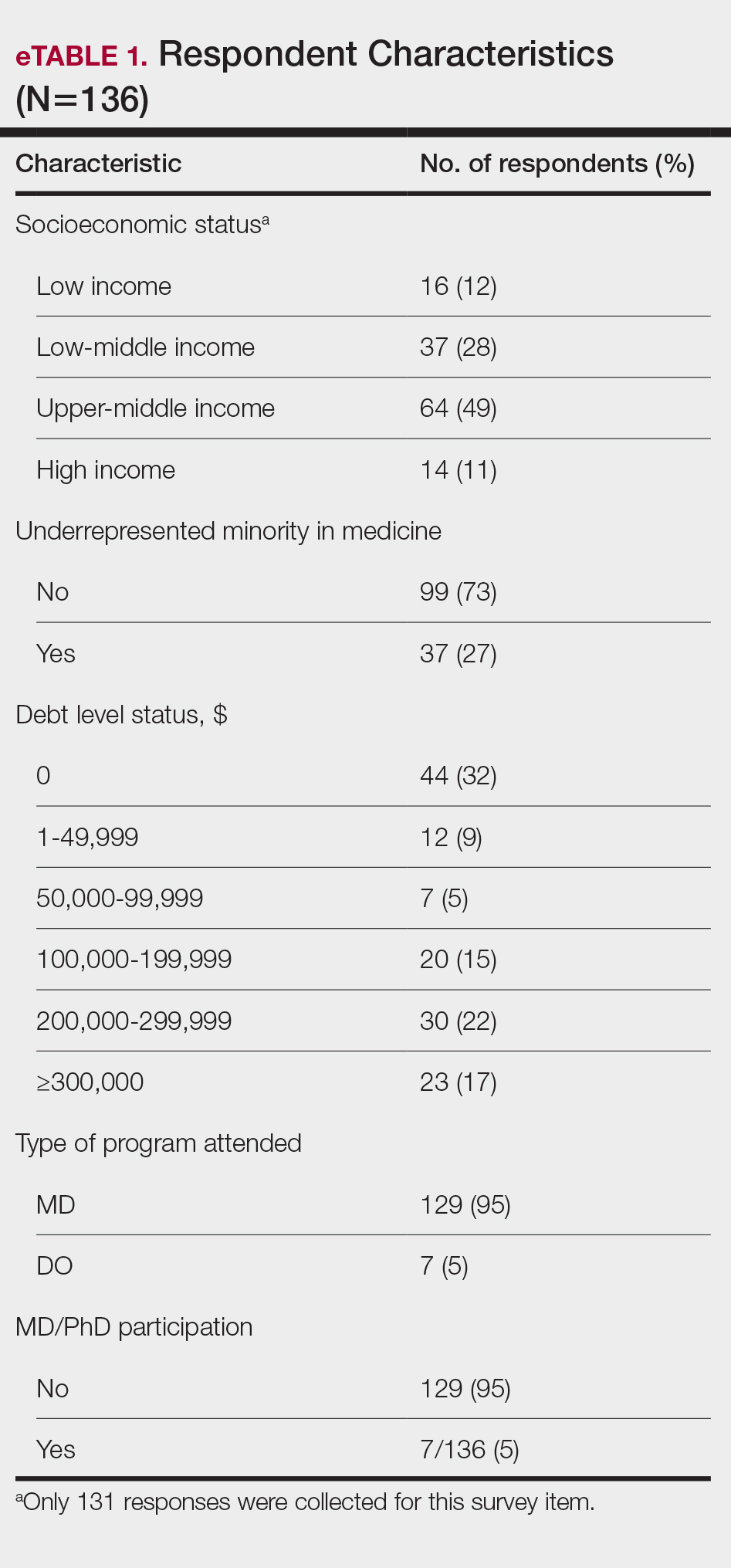
Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).
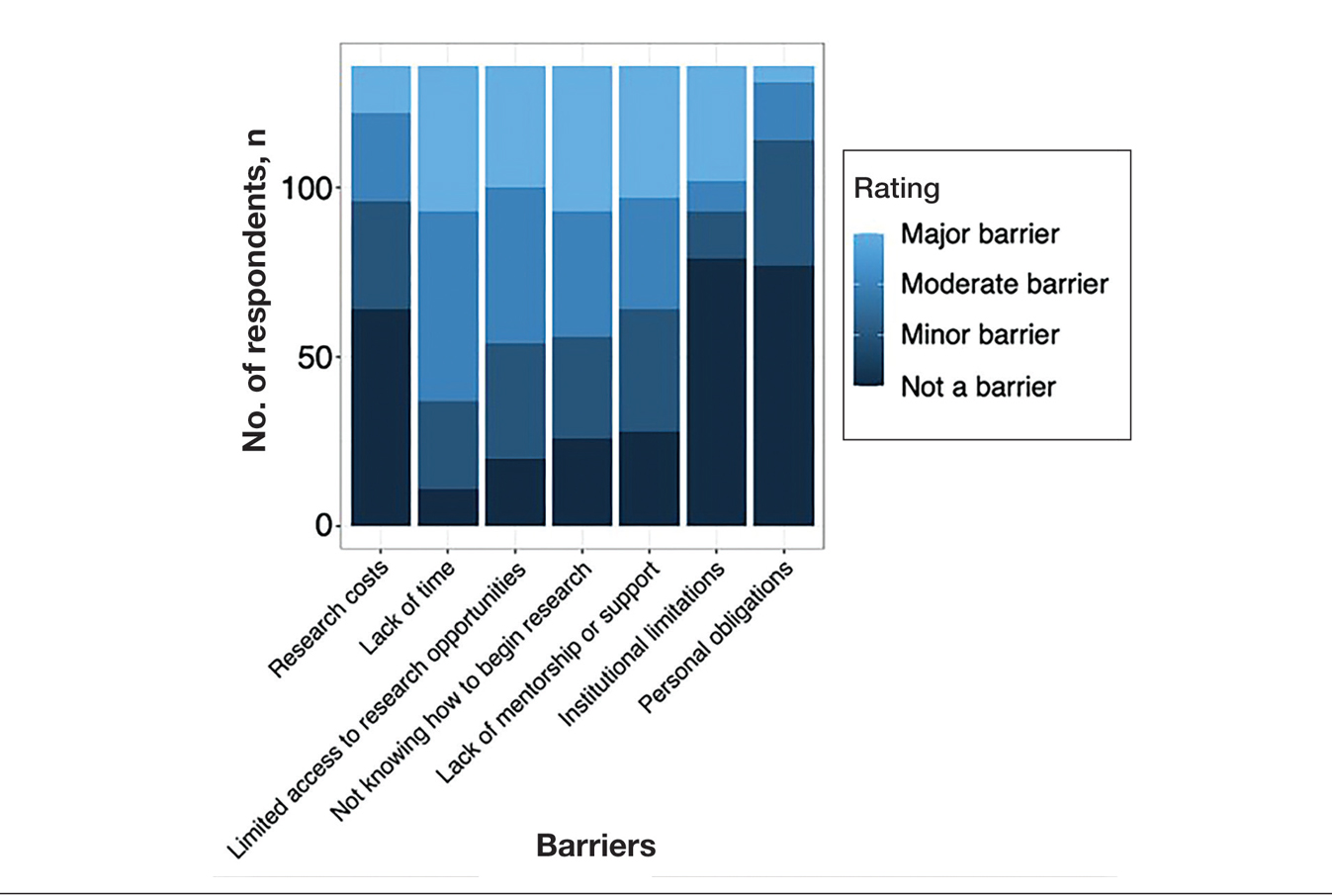
Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).
The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).
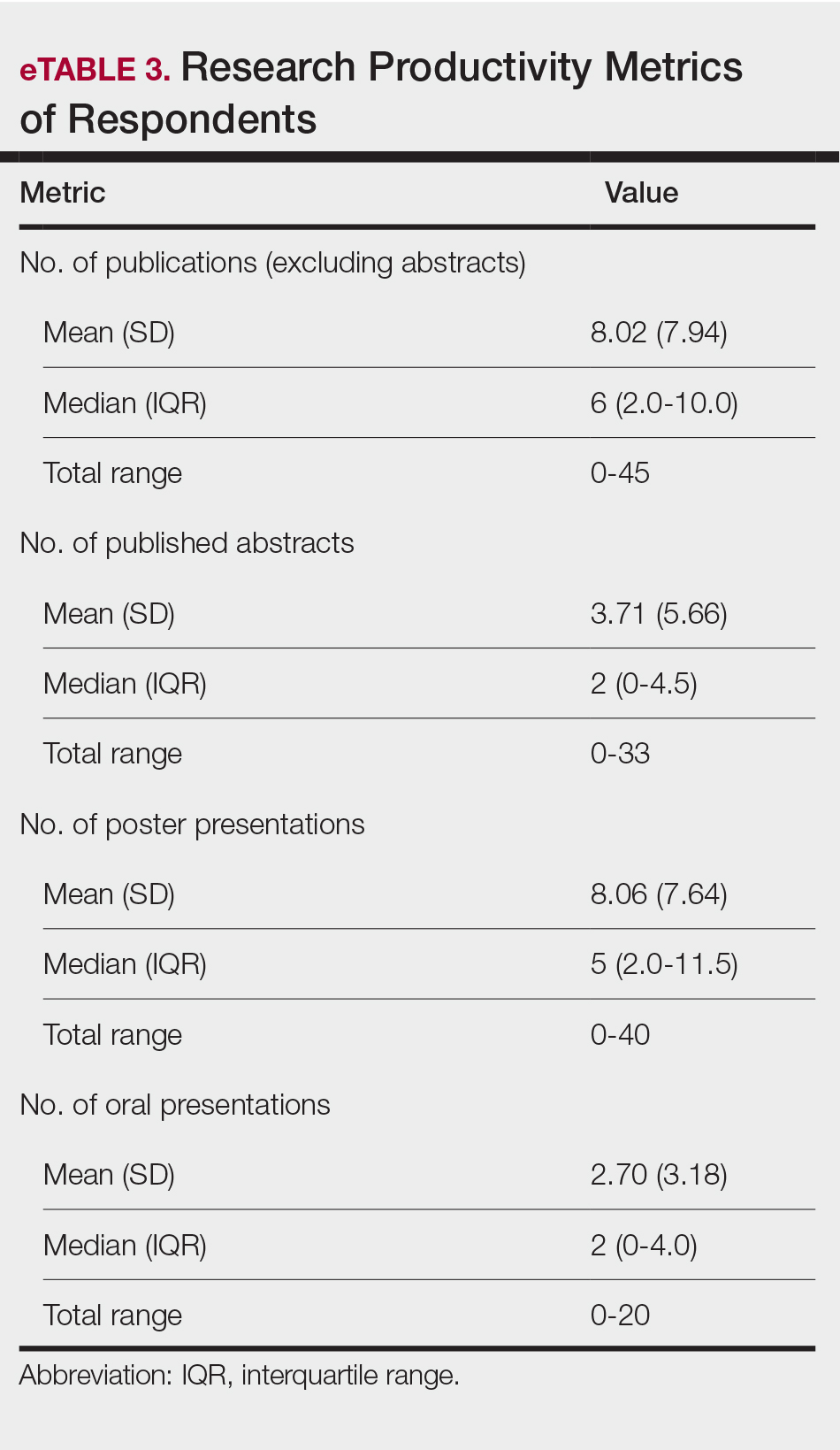
Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).
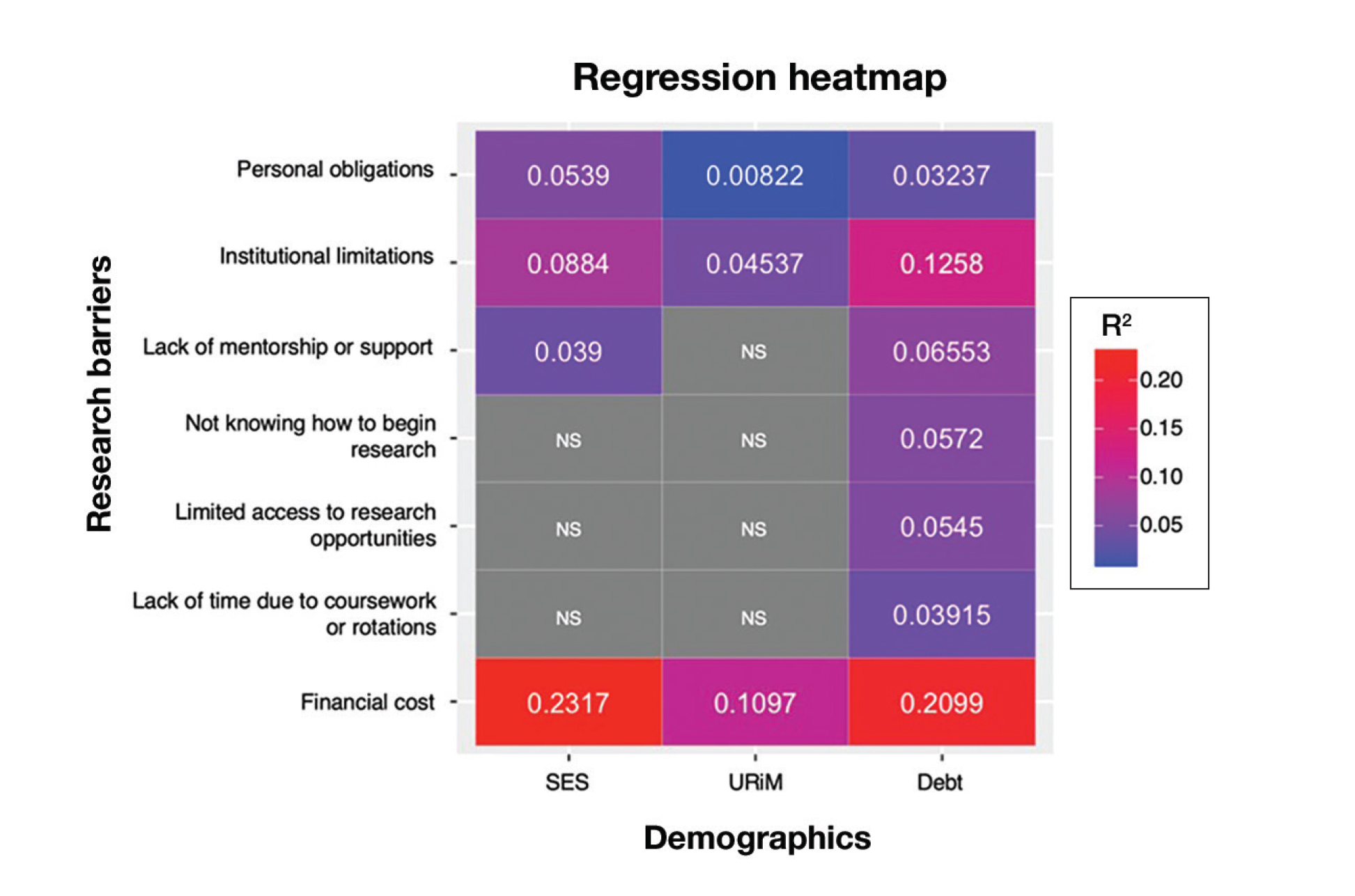
Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).
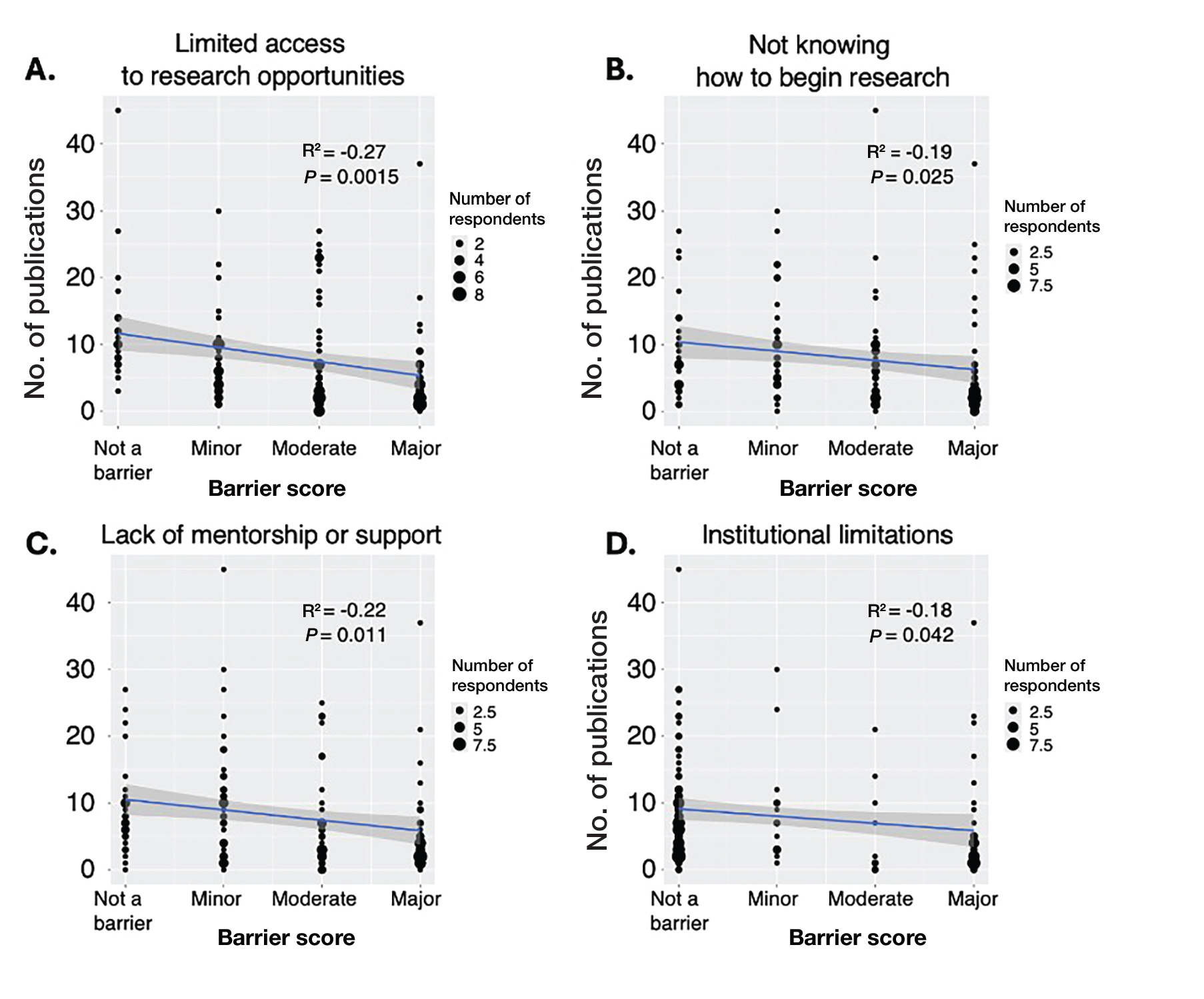
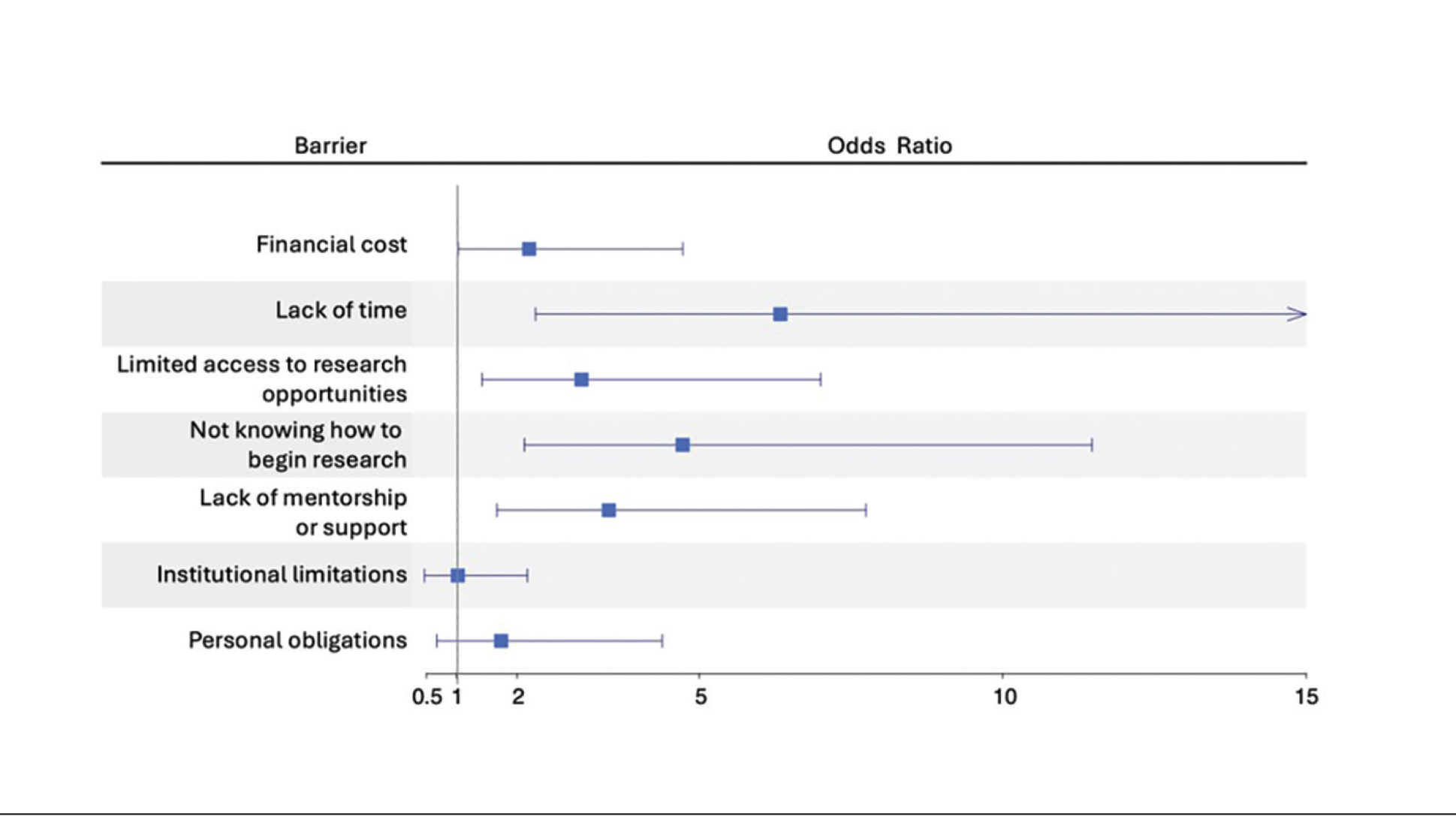
Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).
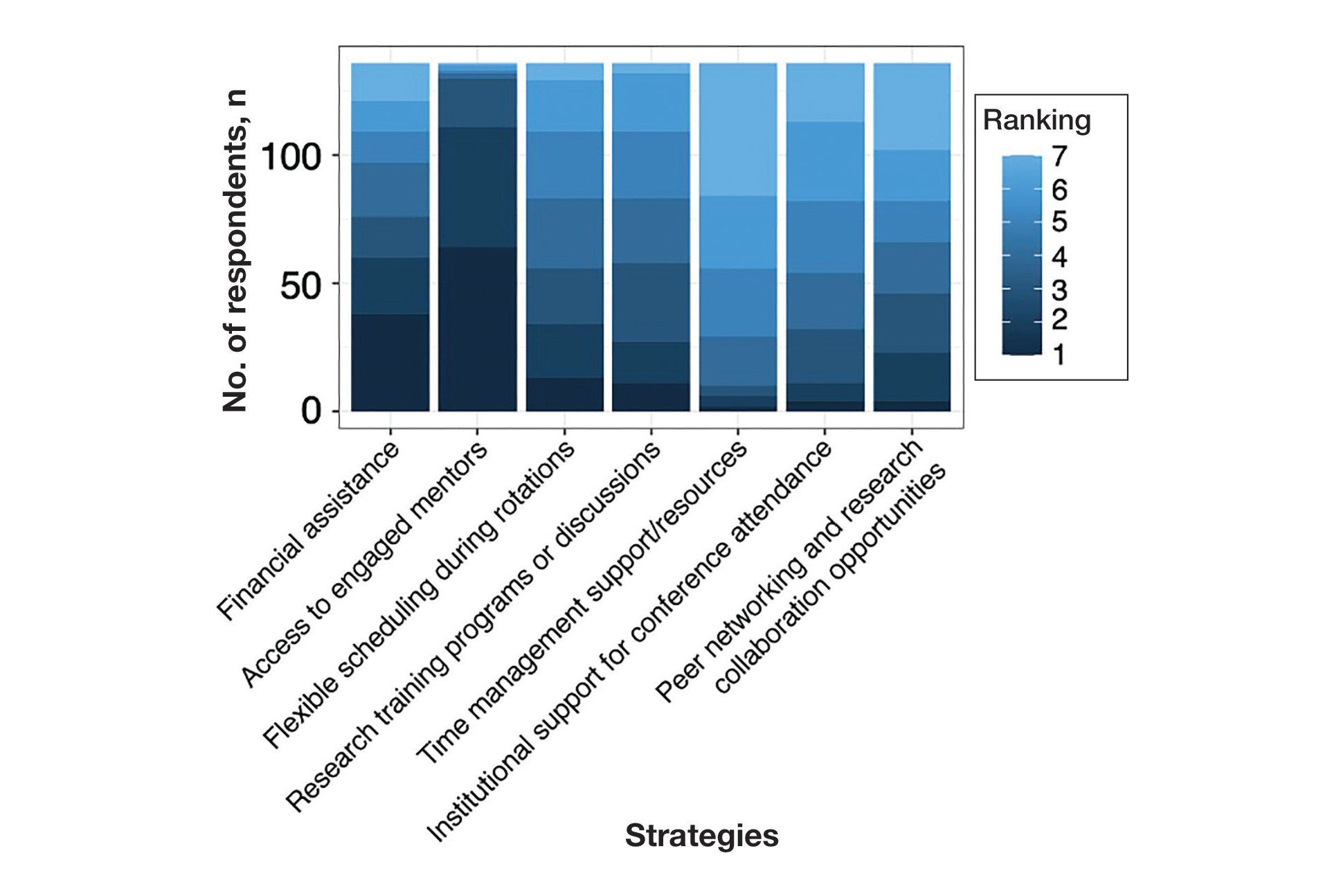
Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.
Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
Practice Points
- Dermatology programs should establish sustainable mentorship networks incorporating faculty, residents, and community dermatologists, as most applicants ranked access to engaged mentors as a top priority for overcoming research barriers.
- Protected research time and funding support for projects are critical, particularly since applicants reporting lack of time and financial barriers were more likely to consider changing their specialty choice.
- Programs should consider implementing caps on reportable research products in residency applications to shift emphasis from quantity to quality while helping address demographic disparities in research access.
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3
Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.
Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
- Mazzoni D, Lin MJ, Dubin DP, et al. Review of non-invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas J Dermatol. 2019;60:278-283. doi:10.1111/ajd.13090
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083. doi:10.1111/j.1524-4725.2009.01186.x&
- Schweiger ES, Kwasniak L, Aires DJ. Treatment of dermatosis papulosa nigra with a 1064 nm Nd:YAG laser: report of two cases. J Cosmet Laser Ther. 2008;10:120-122. doi:10.1080/14764170801950070
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438. doi:10.1002/lsm.20048
- Alajlan AM, Alsuwaidan SN. Acne scars in ethnic skin treated with both non-ablative fractional 1,550 nm and ablative fractional CO2 lasers: comparative retrospective analysis with recommended guidelines. Lasers Surg Med. 2011;43effi:787-791. doi:10.1002/lsm.21092
- Ke R, Cai B, Ni X, et al. Efficacy and safety of non-ablative vs. ablative lasers for acne scarring: a meta-analysis. J Deutschen Dermatologischen Gesellschaft. Published online March 11, 2025. doi: 10.1111/ddg.15651
- Goel A, Krupashankar DS, Aurangabadkar S, et al. Fractional lasers in dermatology—current status and recommendations. Indian J Dermatol Venereol Leprol. 2011;77:369. doi:10.4103/0378-6323.79732
- Lee HS, Lee JH, Ahn GY, et al. Fractional photothermolysis for the treatment of acne scars: a report of 27 Korean patients. J Dermatolog Treat. 2008;19:45-49. doi:10.1080/09546630701691244
- Zhang AD, Clovie J, Lazar M, et al. Treatment of benign pigmented lesions using lasers: a scoping review. J Clin Med. 2025;14li:3985. doi:10.3390/jcm14113985
- Lipper GM, Perez M. Nonablative acne scar reduction after a series of treatments with a short-pulsed 1,064-nm neodymium:YAG laser. Dermatol Surg. 2006;32:998-1006. doi:10.1111/j.1524-4725.2006.32222.x
- Mar K, Khalid B, Maazi M, et al. Treatment of post-inflammatory hyperpigmentation in skin of colour: a systematic review. J Cutan Med Surg. 2024;28:473-480. doi:10.1177/12034754241265716
- Kono T, Chan HH, Groff WF, et al. Prospective direct comparison study of fractional resurfacing using different fluences and densities for skin rejuvenation in Asians. Lasers Surg Med. 2007;39:311-314. doi:10.1002/lsm.20484
- Sharkey JR, Sharf BF, St John JA. “Una persona derechita (staying right in the mind)”: perceptions of Spanish-speaking Mexican American older adults in South Texas colonias. Gerontologist. 2009;49 suppl 1:S79-85. doi:10.1093/geront/gnp086
- Wu X, Cen Q, Jin J, et al. An effective and safe laser treatment strategy of fractional carbon dioxide laser for Chinese populations with periorbital wrinkles: a randomized split-face trial. Dermatol Therapy. 2025;15:1307-1317.
- Milante RR, Doria-Ruiz MJ, Beloso MB, et al. Split-face comparison of grid fractional radiofrequency vs 1064-nm Nd-YAG laser treatment of periorbital rhytides among Filipino patients. Dermatol Ther. 2020;33:e14031. doi:10.1111/dth.14031
- Alexis AF, Andriessen A, Beach RA, et al. Periprocedural skincare for nonenergy and nonablative energy-based aesthetic procedures in patients with skin of color. J Cosmet Dermatol. 2025;24:E16712. doi:10.1111/jocd.16712
- Marmon S, Shek SYN, Yeung CK, et al. Evaluating the safety and efficacy of the 1,440-nm laser in the treatment of photodamage in Asian skin. Lasers Surg Med. 2014;46:375-379. doi:10.1002/lsm.22242
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118. doi:10.1016/j.jaad.2012.08.041
- Jih MH, Goldberg LH, Kimyai-Asadi A. Fractional photothermolysis for photoaging of hands. Dermatol Surg. 2008;34:73-78. doi:10.1111/j.1524-4725.2007.34011.x
- Prohaska J, Hohman MH. Laser complications. StatPearls. Updated August 28, 2023. Accessed July 23, 2025. http://www.ncbi.nlm.nih.gov/books/NBK532248/
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20. doi:10.1016/j.ijwd.2017.01.004
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151:278-284. doi:10.1001/jamadermatol.2014.3045
- Greywal T, Ortiz A. Treating melasma with the 1064 nm Nd:YAG laser with a 650-microsecond pulse duration: a clinical evaluation. J Cosmet Dermatol. 2021;20:3889-3892. doi:10.1111/jocd.14558
- Weaver SM, Sagaral EC. Treatment of pseudofolliculitis barbae using the long-pulse Nd:YAG laser on skin types V and VI. Dermatol Surg. 2003;29:1187-1191. doi:10.1111/j.1524-4725.2003.29387.x
- Negishi K, Tanaka S, Tobita S. Prospective, randomized, evaluator-blinded study of the long pulse 532-nm KTP laser alone or in combination with the long pulse 1064-nm Nd:YAG laser on facial rejuvenation in Asian skin. Lasers Surg Med. 2016;48:844-851. doi:10.1002/lsm.22582
- Kaushik S, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthetic Dermatol. 2017;10:51-67.
- Garg S, Vashisht KR, Garg D, et al. Advancements in laser therapies for dermal hyperpigmentation in skin of color: a comprehensive literature review and experience of sequential laser treatments in a cohort of 122 Indian patients. J Clin Med. 2024;13:2116. doi:10.3390/jcm13072116
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:e36727. doi:10.5812/ijem.36727
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79-93. doi:10.1111/j.1467-2494.2006.00302.x
- El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: Evidence-based effect. J Am Acad Dermatol. 2011;64:524-535. doi:10.1016/j.jaad.2010.06.045
- US Food and Drug Administration. Non-invasive body contouring technologies. Published December 7, 2022. Accessed July 23, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/non-invasive-body-contouring-technologies
- Robinson DM, Kaminer MS, Baumann L, et al. High-intensity focused ultrasound for the reduction of subcutaneous adipose tissue using multiple treatment techniques. Dermatol Surg. 2014;40:641-651. doi:10.1111/dsu.0000000000000022
- Biskanaki F, Tertipi N, Sfyri E, et al. Complications and risks of high-intensity focused ultrasound (HIFU) in esthetic procedures: a review. Applied Sciences. 2025;15:4958. doi:10.3390/app15094958
- Lu PH, Yang CH, Chang YC. Quantitative analysis of face and neck skin tightening by microfocused ultrasound with visualization in Asians. Dermatol Surg. 2017;43:1332-1338. doi:10.1097/DSS.0000000000001181
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708. doi:10.1002/lsm.20864
- Nishikawa A, Aikawa Y. Quantitative assessment of the cryolipolysis method for body contouring in Asian patients. Clin Cosmet Investig Dermatol. 2021;14:1773-1781. doi:10.2147/CCID.S337487
- Bass LS, Doherty ST. Safety and efficacy of a non-invasive 1060 nm diode laser for fat reduction of the abdomen. J Drugs Dermatol. 2018;17:106-112
- Shome D, Khare S, Kapoor R. The use of deoxycholic acid for the clinical reduction of excess submental fat in Indian patients. J Drugs Dermatol. 2019;18:266-272.
- Goodman GJ, Ho WWS, Chang KJ, et al. Efficacy of a novel injection lipolysis to induce targeted adipocyte apoptosis: a randomized, phase IIa study of CBL-514 injection on abdominal subcutaneous fat reduction. Aesthetic Surg J. 2022;42:NP662-NP674. doi:10.1093/asj/sjac162
- McDaniel D, Lozanova P. Human adipocyte apoptosis immediately following high frequency focused field radio frequency: case study.J Drugs Dermatol. 2015;14:622-623.
- Fritz K, Samková P, Salavastru C, et al. A novel selective RF applicator for reducing thigh circumference: a clinical evaluation. Dermatol Ther. 2016;29:92-95. doi:10.1111/dth.12304
- Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51:40-46. doi:10.1002/lsm.23024
- Negosanti F, Cannarozzo G, Zingoni T, et al. Is it possible to reshape the body and tone it at the same time? Schwarzy: the new technology for body sculpting. Bioengineering (Basel). 2022;9:284. doi:10.3390/bioengineering9070284
PRACTICE POINTS
- Nonablative fractional lasers are preferred for acne scars in skin of color (SOC), minimizing hyperpigmentation risk.
- The 1064-nm Nd:YAG and picosecond lasers are safe and effective when used with SOC-appropriate settings.
- Photoprotection and topical lightening agents reduce postprocedure pigmentation risks.
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
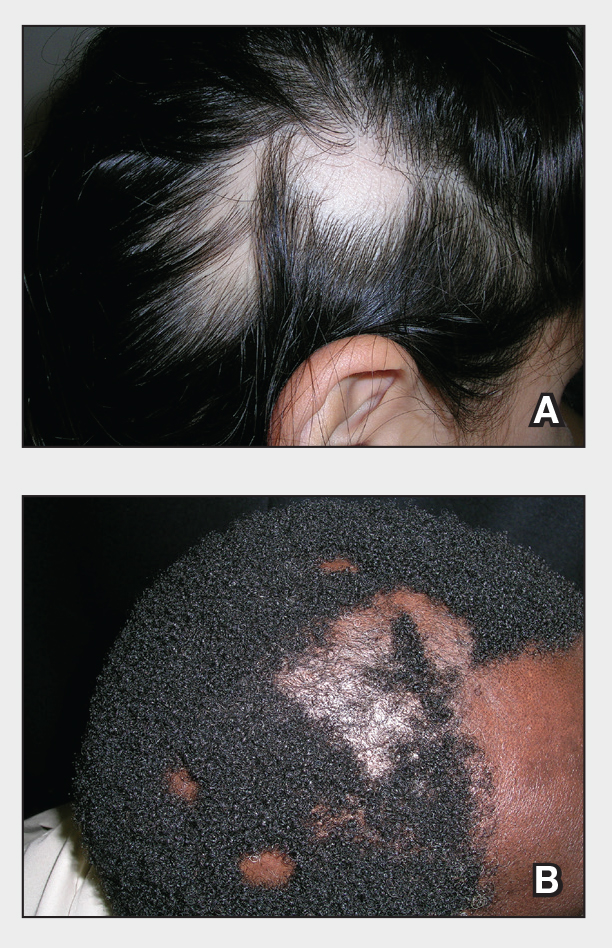
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).
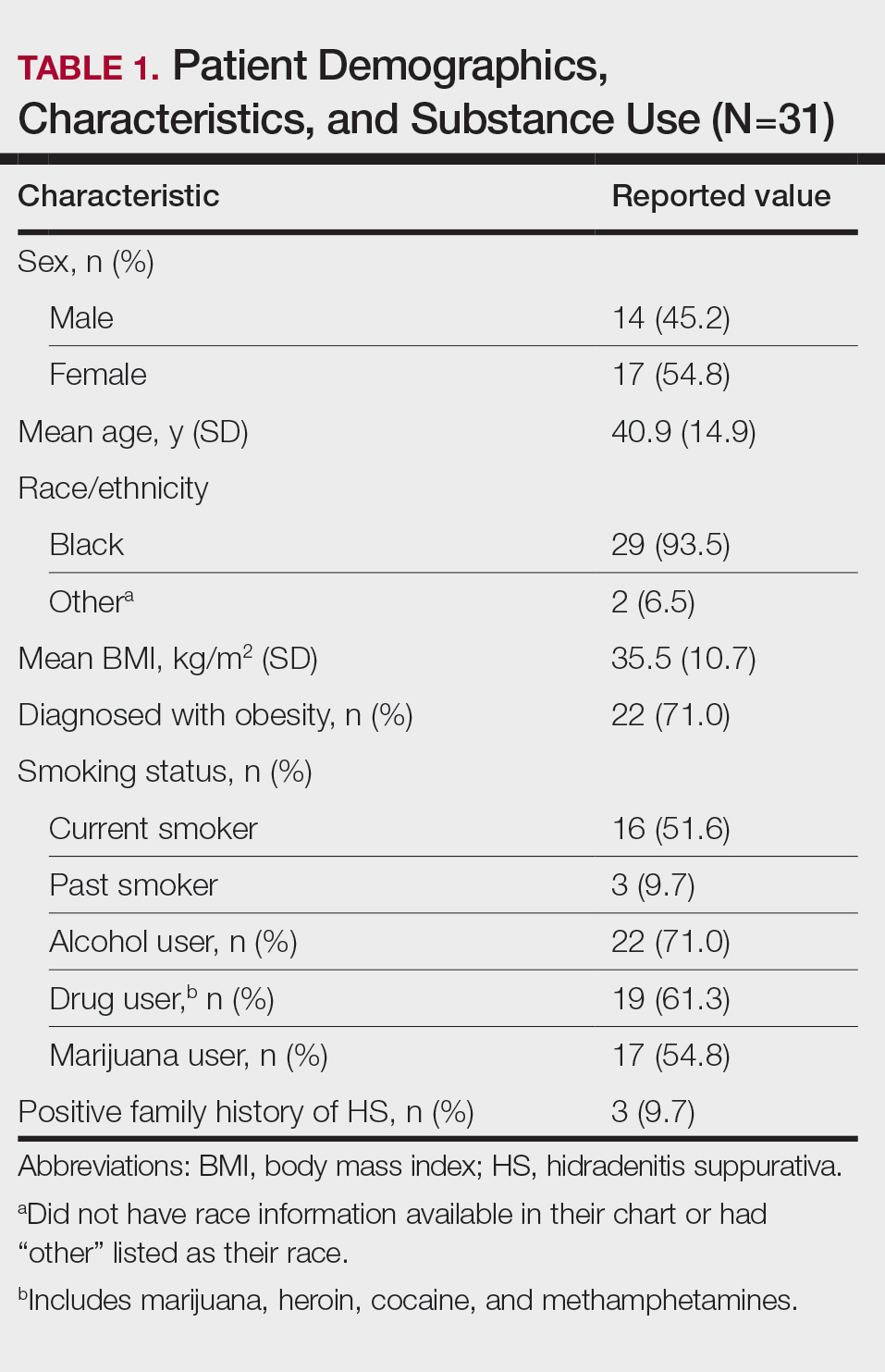
Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.
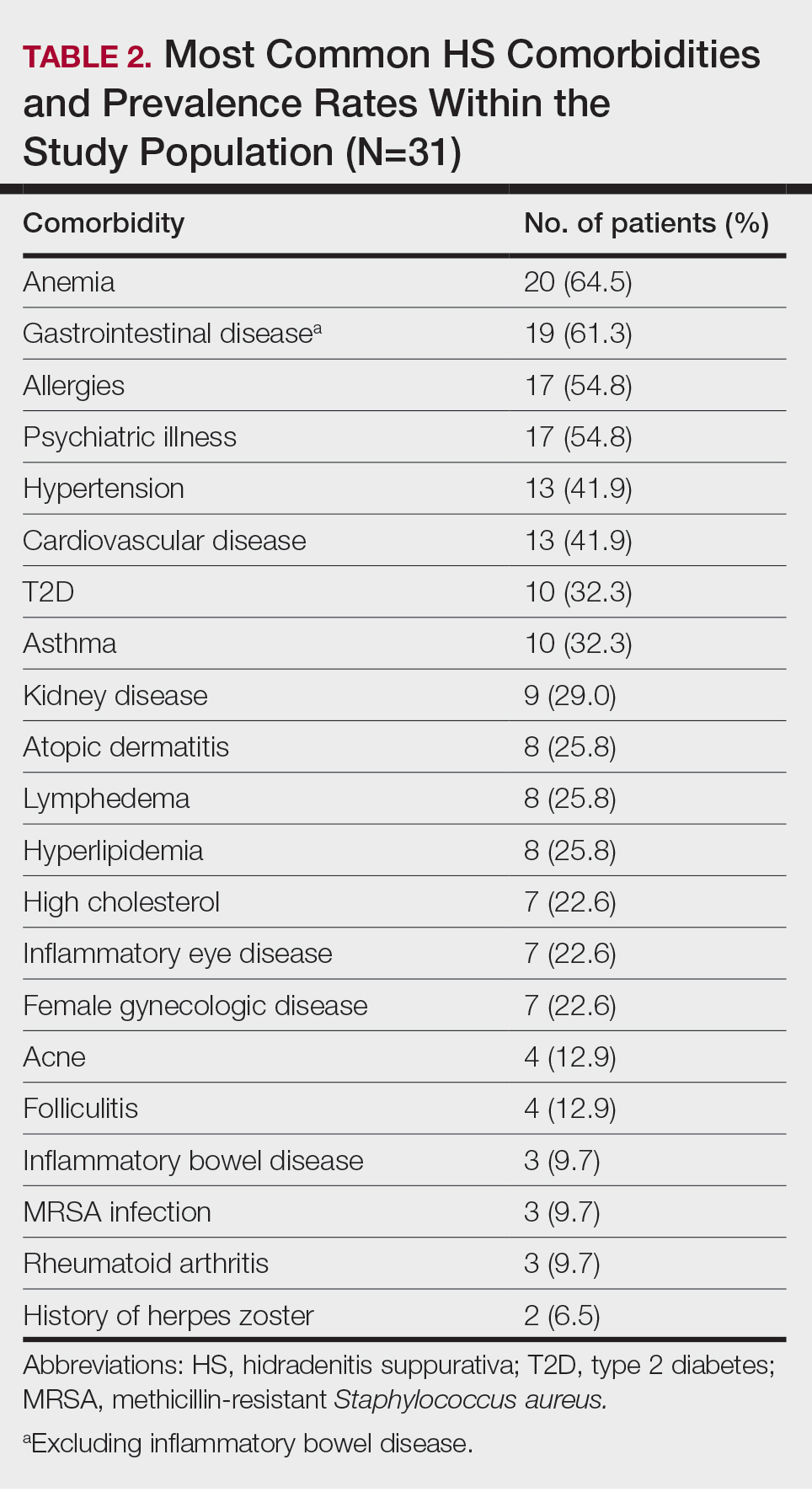
The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.
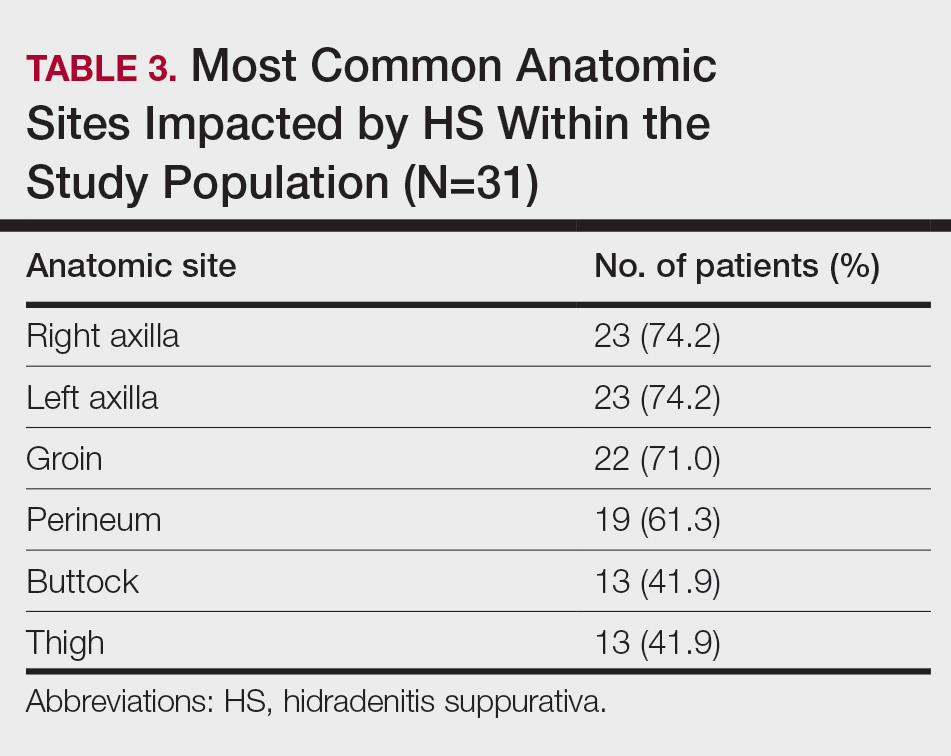
Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).
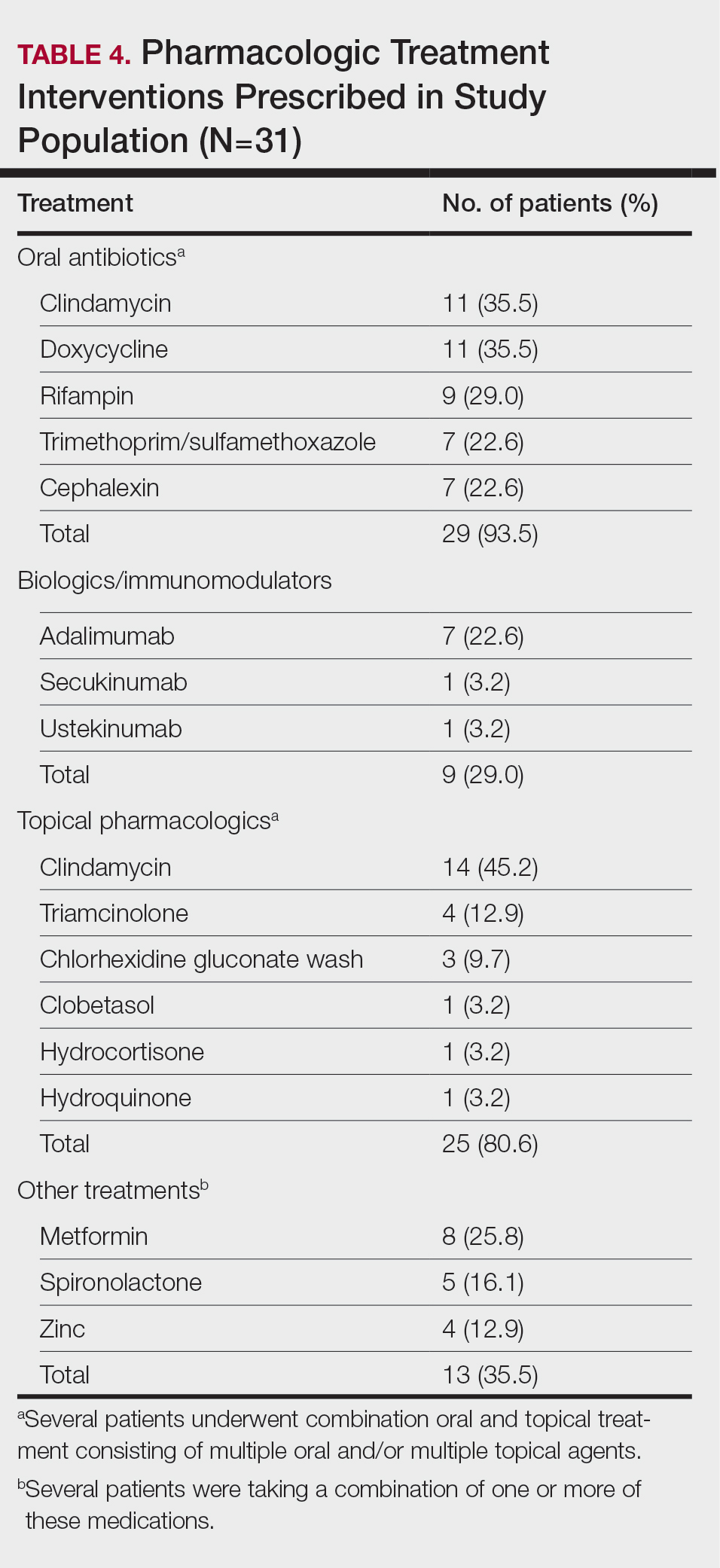
Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
PRACTICE POINTS
- Anxiety and depression are highly prevalent among Black patients with hidradenitis suppurativa (HS). Implementing self-report questionnaires at medical office visits are crucial to identifying patients who could benefit from additional psychiatric resources.
- Hidradenitis suppurativa patients with skin of color may have a higher incidence of comorbid gastrointestinal disease than other HS patients.
- Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
- The patient cohort described here had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology.
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Click to view more from Gastroenterology Data Trends.
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
Click to view more from Gastroenterology Data Trends.
Click to view more from Gastroenterology Data Trends.
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.
Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).
Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
PRACTICE POINTS
- Many funded away rotations are not listed on the most widely used databases for applying to dermatology residency programs.
- Underrepresented in medicine students who are seeking funded dermatology away rotations would benefit from a centralized, comprehensive, and well-organized database to improve equity of opportunity in the dermatology rotation application search process and further diversify the specialty.
- There are limited data assessing outcomes associated with participation in funded rotation and residency match outcomes.