User login
Urinary Metals Linked to Increased Dementia Risk
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Obesity Drug Zepbound Approved for Obstructive Sleep Apnea
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved the obesity treatment tirzepatide (Zepbound, Eli Lilly) for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The new indication is for use in combination with reduced-calorie diet and increased physical activity. The once-weekly injectable is the first-ever drug treatment for OSA.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, MD, director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research. “This is a major step forward for patients with obstructive sleep apnea.”
Excess weight is a major risk factor for OSA, in which the upper airways become blocked multiple times during sleep and obstruct breathing. The condition causes loud snoring, recurrent awakenings, and daytime sleepiness. It is also associated with cardiovascular disease.
Tirzepatide, a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide receptor agonist, was initially approved with brand name Mounjaro in May 2022 for the treatment of type 2 diabetes, and as Zepbound for weight loss in November 2023.
The new OSA approval was based on two phase 3, double-blind randomized controlled trials, SURMOUNT-OSA, in patients with obesity and moderate to severe OSA, conducted at 60 sites in nine countries. Results from both were presented in June 2024 at the annual Scientific Sessions of the American Diabetes Association and were simultaneously published in The New England Journal of Medicine.
The first trial enrolled 469 participants who were unable or unwilling to use PAP therapy, while the second included 234 who had been using PAP for at least 3 months and planned to continue during the trial. In both, the participants randomly received either 10 mg or 15 mg of tirzepatide or placebo once weekly for 52 weeks.
At baseline, 65%-70% of participants had severe OSA, with more than 30 events/h on the apnea-hypopnea index (AHI) and a mean of 51.5 events/h. By 52 weeks, those randomized to tirzepatide had 27-30 fewer events/h, compared with 4-6 fewer events/h for those taking placebo. In addition, significantly more of those on tirzepatide achieved OSA remission or severity reduction to mild.
Those randomized to tirzepatide also averaged up to 20% weight loss, significantly more than with placebo. “The improvement in AHI in participants with OSA is likely related to body weight reduction with Zepbound,” according to an FDA statement.
Side effects of tirzepatide include nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions, fatigue, hypersensitivity reactions (typically fever and rash), burping, hair loss, and gastroesophageal reflux disease.
In an editorial accompanying The New England Journal of Medicine publication of the SURMOUNT-OSA results, Sanjay R. Patel, MD, wrote: “The potential incorporation of tirzepatide into treatment algorithms for obstructive sleep apnea should include consideration of the challenges of adherence to treatment and the imperative to address racial disparities in medical care.”
Patel, who is professor of medicine and epidemiology at the University of Pittsburgh in Pennsylvania, and medical director of the University of Pittsburgh Medical Center’s Comprehensive Sleep Disorders program, pointed out that suboptimal adherence to continuous PAP therapy has been a major limitation, but that adherence to the GLP-1 drug class has also been suboptimal.
“Although adherence to tirzepatide therapy in the SURMOUNT-OSA trial was high, real-world evidence suggests that nearly 50% of patients who begin treatment with a GLP-1 receptor agonist for obesity discontinue therapy within 12 months. Thus, it is likely that any incorporation of tirzepatide into treatment pathways for obstructive sleep apnea will not diminish the importance of long-term strategies to optimize adherence to treatment.”
Moreover, Patel noted, “racial disparities in the use of GLP-1 receptor agonists among patients with diabetes arouse concern that the addition of tirzepatide as a treatment option for obstructive sleep apnea without directly addressing policies relative to coverage of care will only further exacerbate already pervasive disparities in clinical care for obstructive sleep apnea.”
Patel reported consulting for Apnimed, Bayer Pharmaceuticals, Lilly USA, NovaResp Technologies, Philips Respironics, and Powell Mansfield. He is a fiduciary officer of BreathPA.
A version of this article first appeared on Medscape.com.
Reality of Night Shifts: How to Stay Sharp and Healthy
Laura Vater remembers sneaking into her home after 12-hour night shifts during medical training while her husband distracted their toddler. The stealthy tag-teaming effort helped her get enough undisturbed sleep before returning to an Indiana University hospital the following night to repeat the pattern.
“He would pretend to take out the trash when I pulled in,” said Vater, MD, now a gastrointestinal oncologist and assistant professor of medicine at IU Health Simon Cancer Center in Indianapolis. “I would sneak in so she [their daughter] wouldn’t see me, and then he would go back in.”
For Vater, prioritizing sleep during the day to combat sleep deprivation common among doctors-in-training on night shifts required enlisting a supportive spouse. It’s just one of the tips she and a few chief residents shared with this news organization for staying sharp and healthy during overnight rotations.
While the pace of patient rounds may slow from the frenetic daytime rush, training as a doctor after the sun goes down can be quite challenging for residents, they told this news organization. From sleep deprivation working while the rest of us slumbers to the after-effects of late-night caffeine, learning to manage night rotations requires a balance of preparation and attention to personal health while caring for others, the residents and adviser said.
Compromised sleep is one of the biggest hurdles residents have to overcome. Sleep loss comes with risks to cardiovascular disease and type 2 diabetes, among other heath conditions, according to Medscape Medical News reports. And night shift workers who sleep 6 or fewer hours a night have at least one sleep disorder.
Sleep deprivation associated with overnight call schedules also can worsen a resident’s mood and motivation while impairing their judgment, leading to medical errors, according to a new study published in JAMA Open Network. The study proposed shorter consecutive night shifts and naps as ways to offset the results of sleep loss, especially for interns or first-year residents.
Residency programs recently have been experimenting with shorter call schedules.
Catching Zzs
Working the night shift demands a disciplined sleep schedule, said Nat deQuillfeldt, MD, a Denver Health chief resident in the University of Colorado’s internal medicine residency program.
“When I was on night admissions, I was very strict about going to sleep at 8 AM and waking at 3 PM every single day. It can be very tempting to try to stay up and spend time with loved ones, but my husband and I both prioritized my physical well-being for those weeks,” said deQuillfeldt, a PGY-4 resident. “It was especially challenging for me because I had to commute about 50 minutes each way and without such a rigid schedule I would have struggled to be on time.”
deQuillfeldt doesn’t have young children at home, a noisy community, or other distractions to interrupt sleep during the day. But it was still difficult for her to sleep while the sun was out. “I used an eye mask and ear plugs but definitely woke up more often than I would at night.”
Blackout curtains may have helped, she added.
“Without adequate sleep, your clinical thinking is not as sharp. When emergencies happen overnight, you’re often the first person to arrive and need to be able to make rapid, accurate assessments and decisions.”
As a chief resident, she chooses never to sleep during night shifts.
“I personally didn’t want to leave my interns alone or make them feel like they were waking me up or bothering me if they needed help, and I also didn’t want to be groggy in case of a rapid response or code blue.”
But napping on night shift is definitely possible, deQuillfeldt said. Between following up on overnight lab results, answering nurses’ questions, and responding to emergencies, she found downtime on night shift to eat and hydrate. She believes others can catch an hour or 2 of shut eye, even if they work in the intensive care unit, or 3-4 hours on rare quiet nights.
Vater suggests residents transitioning from daytime work to night shift prepare by trying to catch an afternoon nap, staying up later the night before the change, and banking sleep hours in advance.
When he knows he’s starting night shifts, Apurva Popat, MD, said he tries to go to sleep an hour or so later nights before to avoid becoming sleep deprived. The chief resident of internal medicine at Marshfield Clinic Health System in Marshfield, Wisconsin, doesn’t recommend sleeping during the night shift.
“I typically try not to sleep, even if I have time, so I can go home and sleep later in the morning,” said Popat, a PGY-3 resident.
To help him snooze, he uses blackout curtains and a fan to block out noise. His wife, a first-year internal medicine intern, often works a different shift, so she helps set up his sleeping environment and he reciprocates when it’s her turn for night shifts.
Some interns may need to catch a 20- to 30-minute nap on the first night shift, he said.
Popat also seeks out brighter areas of the hospital, such as the emergency department, where there are more people and colleagues to keep him alert.
Bypass Vending Machines
Lack of sleep makes it even more difficult to eat healthy on night shift, said Vater, who advises residents about wellness issues at IU and on social media.
“When you are sleep deprived, when you do not get enough sleep, you eat but you don’t feel full,” she said. “It’s hard to eat well on night shift. It’s harder if you go to the break room and there’s candy and junk food.”
Vater said that, as a resident, she brought a lunch bag to the hospital during night shifts. “I never had time to prep food, so I’d bring a whole apple, a whole orange, a whole avocado or nuts. It allowed me to eat more fruits and vegetables than I normally would.”
She advises caution when considering coffee to stay awake, especially after about 9 PM, which could interfere with sleep residents need later when they finish their shifts. Caffeine may help in the moment, but it prevents deep sleep, Vater said. So when residents finally get sleep after their shifts, they may wake up feeling tired, she said.
To avoid sleepiness, Popat brings protein shakes with him to night shifts. They stave off sugar spikes and keep his energy level high, he said. He might have a protein shake and fruit before he leaves home and carry his vegetarian dinner with him to eat in the early morning hours when the hospital is calm.
Eating small and frequent meals also helps ward off sleepiness, deQuillfeldt said.
Take the Stairs
Trying to stay healthy on night shifts, Vater also checked on patients by taking the stairs. “I’d set the timer on my phone for 30 minutes and if I got paged at 15, I’d pause the timer and reset it if I had a moment later. I’d get at least 30 minutes in, although not always continuous. I think some activity is better than none.”
Vater said her hospital had a gym, but it wasn’t practical for her because it was further away from where she worked. “Sometimes my coresidents would be more creative, and we would do squats.”
Popat tries to lift weights 2 hours before his night shift, but he also takes short walks between patients’ rooms in the early morning hours when it’s quietest. He also promotes deep breathing to stay alert.
Ask for a Ride
Vater urges those coming off night shifts, especially those transitioning for the first time from daytime rotations, not to drive if they’re exhausted. “Get an Uber. ... Make sure you get a ride home.”
The CU residency program covers the cost of a ridesharing service when doctors-in-training are too tired to drive home, deQuillfeldt said. “We really try to encourage people to use this to reduce the risk of car accidents.”
Promoting Mental Health
The residency program also links residents with primary care and mental health services. People who really struggle with shift work sleep disorder may qualify for medications to help them stay awake overnight, in addition to sleep hygiene apps and sleep aides.
“Night shifts can put a strain on mental health, especially when you’re only working, eating, and sleeping and not spending any time with family and friends,” deQuillfeldt said. “My husband works late afternoons, so we often would go weeks seeing each other for 15-20 minutes a day.”
“Sleeping when the sun is out often leads to a lack of light exposure which can compound the problem. Seeking mental health support early is really important to avoiding burnout,” she said.
She also recommended planning a fun weekend activity, trip, or celebration with friends or family after night shifts end “so you have something to look forward to…It’s so important to have a light at the end of the tunnel, which will allow you to enjoy the sense of accomplishment even more.”
For more advice on the subject, consider the American Medical Association guide to managing sleep deprivation in residency or Laura Vater’s tips for night shifts.
A version of this article first appeared on Medscape.com.
Laura Vater remembers sneaking into her home after 12-hour night shifts during medical training while her husband distracted their toddler. The stealthy tag-teaming effort helped her get enough undisturbed sleep before returning to an Indiana University hospital the following night to repeat the pattern.
“He would pretend to take out the trash when I pulled in,” said Vater, MD, now a gastrointestinal oncologist and assistant professor of medicine at IU Health Simon Cancer Center in Indianapolis. “I would sneak in so she [their daughter] wouldn’t see me, and then he would go back in.”
For Vater, prioritizing sleep during the day to combat sleep deprivation common among doctors-in-training on night shifts required enlisting a supportive spouse. It’s just one of the tips she and a few chief residents shared with this news organization for staying sharp and healthy during overnight rotations.
While the pace of patient rounds may slow from the frenetic daytime rush, training as a doctor after the sun goes down can be quite challenging for residents, they told this news organization. From sleep deprivation working while the rest of us slumbers to the after-effects of late-night caffeine, learning to manage night rotations requires a balance of preparation and attention to personal health while caring for others, the residents and adviser said.
Compromised sleep is one of the biggest hurdles residents have to overcome. Sleep loss comes with risks to cardiovascular disease and type 2 diabetes, among other heath conditions, according to Medscape Medical News reports. And night shift workers who sleep 6 or fewer hours a night have at least one sleep disorder.
Sleep deprivation associated with overnight call schedules also can worsen a resident’s mood and motivation while impairing their judgment, leading to medical errors, according to a new study published in JAMA Open Network. The study proposed shorter consecutive night shifts and naps as ways to offset the results of sleep loss, especially for interns or first-year residents.
Residency programs recently have been experimenting with shorter call schedules.
Catching Zzs
Working the night shift demands a disciplined sleep schedule, said Nat deQuillfeldt, MD, a Denver Health chief resident in the University of Colorado’s internal medicine residency program.
“When I was on night admissions, I was very strict about going to sleep at 8 AM and waking at 3 PM every single day. It can be very tempting to try to stay up and spend time with loved ones, but my husband and I both prioritized my physical well-being for those weeks,” said deQuillfeldt, a PGY-4 resident. “It was especially challenging for me because I had to commute about 50 minutes each way and without such a rigid schedule I would have struggled to be on time.”
deQuillfeldt doesn’t have young children at home, a noisy community, or other distractions to interrupt sleep during the day. But it was still difficult for her to sleep while the sun was out. “I used an eye mask and ear plugs but definitely woke up more often than I would at night.”
Blackout curtains may have helped, she added.
“Without adequate sleep, your clinical thinking is not as sharp. When emergencies happen overnight, you’re often the first person to arrive and need to be able to make rapid, accurate assessments and decisions.”
As a chief resident, she chooses never to sleep during night shifts.
“I personally didn’t want to leave my interns alone or make them feel like they were waking me up or bothering me if they needed help, and I also didn’t want to be groggy in case of a rapid response or code blue.”
But napping on night shift is definitely possible, deQuillfeldt said. Between following up on overnight lab results, answering nurses’ questions, and responding to emergencies, she found downtime on night shift to eat and hydrate. She believes others can catch an hour or 2 of shut eye, even if they work in the intensive care unit, or 3-4 hours on rare quiet nights.
Vater suggests residents transitioning from daytime work to night shift prepare by trying to catch an afternoon nap, staying up later the night before the change, and banking sleep hours in advance.
When he knows he’s starting night shifts, Apurva Popat, MD, said he tries to go to sleep an hour or so later nights before to avoid becoming sleep deprived. The chief resident of internal medicine at Marshfield Clinic Health System in Marshfield, Wisconsin, doesn’t recommend sleeping during the night shift.
“I typically try not to sleep, even if I have time, so I can go home and sleep later in the morning,” said Popat, a PGY-3 resident.
To help him snooze, he uses blackout curtains and a fan to block out noise. His wife, a first-year internal medicine intern, often works a different shift, so she helps set up his sleeping environment and he reciprocates when it’s her turn for night shifts.
Some interns may need to catch a 20- to 30-minute nap on the first night shift, he said.
Popat also seeks out brighter areas of the hospital, such as the emergency department, where there are more people and colleagues to keep him alert.
Bypass Vending Machines
Lack of sleep makes it even more difficult to eat healthy on night shift, said Vater, who advises residents about wellness issues at IU and on social media.
“When you are sleep deprived, when you do not get enough sleep, you eat but you don’t feel full,” she said. “It’s hard to eat well on night shift. It’s harder if you go to the break room and there’s candy and junk food.”
Vater said that, as a resident, she brought a lunch bag to the hospital during night shifts. “I never had time to prep food, so I’d bring a whole apple, a whole orange, a whole avocado or nuts. It allowed me to eat more fruits and vegetables than I normally would.”
She advises caution when considering coffee to stay awake, especially after about 9 PM, which could interfere with sleep residents need later when they finish their shifts. Caffeine may help in the moment, but it prevents deep sleep, Vater said. So when residents finally get sleep after their shifts, they may wake up feeling tired, she said.
To avoid sleepiness, Popat brings protein shakes with him to night shifts. They stave off sugar spikes and keep his energy level high, he said. He might have a protein shake and fruit before he leaves home and carry his vegetarian dinner with him to eat in the early morning hours when the hospital is calm.
Eating small and frequent meals also helps ward off sleepiness, deQuillfeldt said.
Take the Stairs
Trying to stay healthy on night shifts, Vater also checked on patients by taking the stairs. “I’d set the timer on my phone for 30 minutes and if I got paged at 15, I’d pause the timer and reset it if I had a moment later. I’d get at least 30 minutes in, although not always continuous. I think some activity is better than none.”
Vater said her hospital had a gym, but it wasn’t practical for her because it was further away from where she worked. “Sometimes my coresidents would be more creative, and we would do squats.”
Popat tries to lift weights 2 hours before his night shift, but he also takes short walks between patients’ rooms in the early morning hours when it’s quietest. He also promotes deep breathing to stay alert.
Ask for a Ride
Vater urges those coming off night shifts, especially those transitioning for the first time from daytime rotations, not to drive if they’re exhausted. “Get an Uber. ... Make sure you get a ride home.”
The CU residency program covers the cost of a ridesharing service when doctors-in-training are too tired to drive home, deQuillfeldt said. “We really try to encourage people to use this to reduce the risk of car accidents.”
Promoting Mental Health
The residency program also links residents with primary care and mental health services. People who really struggle with shift work sleep disorder may qualify for medications to help them stay awake overnight, in addition to sleep hygiene apps and sleep aides.
“Night shifts can put a strain on mental health, especially when you’re only working, eating, and sleeping and not spending any time with family and friends,” deQuillfeldt said. “My husband works late afternoons, so we often would go weeks seeing each other for 15-20 minutes a day.”
“Sleeping when the sun is out often leads to a lack of light exposure which can compound the problem. Seeking mental health support early is really important to avoiding burnout,” she said.
She also recommended planning a fun weekend activity, trip, or celebration with friends or family after night shifts end “so you have something to look forward to…It’s so important to have a light at the end of the tunnel, which will allow you to enjoy the sense of accomplishment even more.”
For more advice on the subject, consider the American Medical Association guide to managing sleep deprivation in residency or Laura Vater’s tips for night shifts.
A version of this article first appeared on Medscape.com.
Laura Vater remembers sneaking into her home after 12-hour night shifts during medical training while her husband distracted their toddler. The stealthy tag-teaming effort helped her get enough undisturbed sleep before returning to an Indiana University hospital the following night to repeat the pattern.
“He would pretend to take out the trash when I pulled in,” said Vater, MD, now a gastrointestinal oncologist and assistant professor of medicine at IU Health Simon Cancer Center in Indianapolis. “I would sneak in so she [their daughter] wouldn’t see me, and then he would go back in.”
For Vater, prioritizing sleep during the day to combat sleep deprivation common among doctors-in-training on night shifts required enlisting a supportive spouse. It’s just one of the tips she and a few chief residents shared with this news organization for staying sharp and healthy during overnight rotations.
While the pace of patient rounds may slow from the frenetic daytime rush, training as a doctor after the sun goes down can be quite challenging for residents, they told this news organization. From sleep deprivation working while the rest of us slumbers to the after-effects of late-night caffeine, learning to manage night rotations requires a balance of preparation and attention to personal health while caring for others, the residents and adviser said.
Compromised sleep is one of the biggest hurdles residents have to overcome. Sleep loss comes with risks to cardiovascular disease and type 2 diabetes, among other heath conditions, according to Medscape Medical News reports. And night shift workers who sleep 6 or fewer hours a night have at least one sleep disorder.
Sleep deprivation associated with overnight call schedules also can worsen a resident’s mood and motivation while impairing their judgment, leading to medical errors, according to a new study published in JAMA Open Network. The study proposed shorter consecutive night shifts and naps as ways to offset the results of sleep loss, especially for interns or first-year residents.
Residency programs recently have been experimenting with shorter call schedules.
Catching Zzs
Working the night shift demands a disciplined sleep schedule, said Nat deQuillfeldt, MD, a Denver Health chief resident in the University of Colorado’s internal medicine residency program.
“When I was on night admissions, I was very strict about going to sleep at 8 AM and waking at 3 PM every single day. It can be very tempting to try to stay up and spend time with loved ones, but my husband and I both prioritized my physical well-being for those weeks,” said deQuillfeldt, a PGY-4 resident. “It was especially challenging for me because I had to commute about 50 minutes each way and without such a rigid schedule I would have struggled to be on time.”
deQuillfeldt doesn’t have young children at home, a noisy community, or other distractions to interrupt sleep during the day. But it was still difficult for her to sleep while the sun was out. “I used an eye mask and ear plugs but definitely woke up more often than I would at night.”
Blackout curtains may have helped, she added.
“Without adequate sleep, your clinical thinking is not as sharp. When emergencies happen overnight, you’re often the first person to arrive and need to be able to make rapid, accurate assessments and decisions.”
As a chief resident, she chooses never to sleep during night shifts.
“I personally didn’t want to leave my interns alone or make them feel like they were waking me up or bothering me if they needed help, and I also didn’t want to be groggy in case of a rapid response or code blue.”
But napping on night shift is definitely possible, deQuillfeldt said. Between following up on overnight lab results, answering nurses’ questions, and responding to emergencies, she found downtime on night shift to eat and hydrate. She believes others can catch an hour or 2 of shut eye, even if they work in the intensive care unit, or 3-4 hours on rare quiet nights.
Vater suggests residents transitioning from daytime work to night shift prepare by trying to catch an afternoon nap, staying up later the night before the change, and banking sleep hours in advance.
When he knows he’s starting night shifts, Apurva Popat, MD, said he tries to go to sleep an hour or so later nights before to avoid becoming sleep deprived. The chief resident of internal medicine at Marshfield Clinic Health System in Marshfield, Wisconsin, doesn’t recommend sleeping during the night shift.
“I typically try not to sleep, even if I have time, so I can go home and sleep later in the morning,” said Popat, a PGY-3 resident.
To help him snooze, he uses blackout curtains and a fan to block out noise. His wife, a first-year internal medicine intern, often works a different shift, so she helps set up his sleeping environment and he reciprocates when it’s her turn for night shifts.
Some interns may need to catch a 20- to 30-minute nap on the first night shift, he said.
Popat also seeks out brighter areas of the hospital, such as the emergency department, where there are more people and colleagues to keep him alert.
Bypass Vending Machines
Lack of sleep makes it even more difficult to eat healthy on night shift, said Vater, who advises residents about wellness issues at IU and on social media.
“When you are sleep deprived, when you do not get enough sleep, you eat but you don’t feel full,” she said. “It’s hard to eat well on night shift. It’s harder if you go to the break room and there’s candy and junk food.”
Vater said that, as a resident, she brought a lunch bag to the hospital during night shifts. “I never had time to prep food, so I’d bring a whole apple, a whole orange, a whole avocado or nuts. It allowed me to eat more fruits and vegetables than I normally would.”
She advises caution when considering coffee to stay awake, especially after about 9 PM, which could interfere with sleep residents need later when they finish their shifts. Caffeine may help in the moment, but it prevents deep sleep, Vater said. So when residents finally get sleep after their shifts, they may wake up feeling tired, she said.
To avoid sleepiness, Popat brings protein shakes with him to night shifts. They stave off sugar spikes and keep his energy level high, he said. He might have a protein shake and fruit before he leaves home and carry his vegetarian dinner with him to eat in the early morning hours when the hospital is calm.
Eating small and frequent meals also helps ward off sleepiness, deQuillfeldt said.
Take the Stairs
Trying to stay healthy on night shifts, Vater also checked on patients by taking the stairs. “I’d set the timer on my phone for 30 minutes and if I got paged at 15, I’d pause the timer and reset it if I had a moment later. I’d get at least 30 minutes in, although not always continuous. I think some activity is better than none.”
Vater said her hospital had a gym, but it wasn’t practical for her because it was further away from where she worked. “Sometimes my coresidents would be more creative, and we would do squats.”
Popat tries to lift weights 2 hours before his night shift, but he also takes short walks between patients’ rooms in the early morning hours when it’s quietest. He also promotes deep breathing to stay alert.
Ask for a Ride
Vater urges those coming off night shifts, especially those transitioning for the first time from daytime rotations, not to drive if they’re exhausted. “Get an Uber. ... Make sure you get a ride home.”
The CU residency program covers the cost of a ridesharing service when doctors-in-training are too tired to drive home, deQuillfeldt said. “We really try to encourage people to use this to reduce the risk of car accidents.”
Promoting Mental Health
The residency program also links residents with primary care and mental health services. People who really struggle with shift work sleep disorder may qualify for medications to help them stay awake overnight, in addition to sleep hygiene apps and sleep aides.
“Night shifts can put a strain on mental health, especially when you’re only working, eating, and sleeping and not spending any time with family and friends,” deQuillfeldt said. “My husband works late afternoons, so we often would go weeks seeing each other for 15-20 minutes a day.”
“Sleeping when the sun is out often leads to a lack of light exposure which can compound the problem. Seeking mental health support early is really important to avoiding burnout,” she said.
She also recommended planning a fun weekend activity, trip, or celebration with friends or family after night shifts end “so you have something to look forward to…It’s so important to have a light at the end of the tunnel, which will allow you to enjoy the sense of accomplishment even more.”
For more advice on the subject, consider the American Medical Association guide to managing sleep deprivation in residency or Laura Vater’s tips for night shifts.
A version of this article first appeared on Medscape.com.
Finally, a New Drug for Posttraumatic Stress Disorder?
A drug that combines the atypical antipsychotic brexpiprazole and the selective serotonin reuptake inhibitor sertraline provides significantly greater relief of posttraumatic stress disorder (PTSD) symptoms than sertraline plus placebo, results of a phase 3 trial showed.
The medication is currently under review by the Food and Drug Administration (FDA) and if approved, will be the first pharmacologic option for PTSD in more than 20 years.
The trial met its primary endpoint of change in the Clinician Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) (CAPS-5) total score at week 10 and secondary patient-reported outcomes of PTSD symptoms, anxiety, and depression.
“And what is really cool, what’s really impactful is the combination worked better than sertraline plus placebo on a brief inventory of psychosocial functioning,” study investigator Lori L. Davis, a senior research psychiatrist, Birmingham Veterans Affairs Health Care System in Alabama, said in an interview.
“We can treat symptoms but that’s where the rubber meets the road, in terms of are they functioning better,” added Davis, who is also an adjunct professor of psychiatry, Heersink School of Medicine, University of Alabama at Birmingham.
The findings were published online on December 18 in JAMA Psychiatry and reported in May 2024 as part of a trio of trials conducted by Otsuka Pharmaceutical and Lundbeck Pharmaceuticals, codevelopers of the drug.
Clinically Meaningful
“This study provides promising results for a medication that may be an important new option for PTSD,” John Krystal, MD, director, Clinical Neuroscience Division, National Center for PTSD, US Department of Veterans Affairs, who was not involved in the research, said in an interview. “New PTSD treatments are a high priority.”
Currently, there are two FDA-approved medication treatments for PTSD — sertraline and paroxetine.
“They are helpful for many people, but patients are often left with residual symptoms or tolerability issues,” noted Krystal, who is also professor and chair of psychiatry, Yale University, New Haven, Connecticut.
“New medications that might address the important ‘effectiveness gap’ in PTSD could help to reduce the remaining distress, disability, and suicide risk associated with PTSD.”
The double-blind, phase 3 trial included 416 adults aged 18-65 years with a DSM-5 diagnosis of PTSD and symptoms for at least 6 months prior to screening. Patients underwent a 1-week placebo-run in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
Participants’ mean age was 37.4 years, 74.5% were women, and mean CAPS-5 total score was 38.4, suggesting moderate to high severity PTSD, Davis said. The average time from the index traumatic event was 4 years and three fourths had no prior exposure to PTSD prescription medications.
At week 10, the mean change in CAPS-5 score from randomization was –19.2 points in the brexpiprazole plus sertraline group and –13.6 points in the sertraline plus placebo group (95% CI, –8.79 to –2.38; P < .001).
Asked whether the 5.59-point treatment difference is clinically meaningful, Davis said there is no widely agreed definition for change in CAPS-5 total score but that a within-group reduction of more than 10-13 points is most-often cited as being clinically meaningful.
The key secondary endpoint of least square mean change in the patient-reported Brief Inventory of Psychosocial Function total score from baseline to week 12 was –33.8 with the combination vs –21.8 with sertraline plus placebo (95% CI, –19.4 to –4.62; P = .002).
“That’s clinically meaningful for me as a provider and a clinician and a researcher when you’re getting the PTSD symptom change differences in parallel with the improvement in functional outcome,” she said. “I see that as the clinically meaningful gauge.”
In terms of safety, 3.9% of the participants in the brexpiprazole/sertraline group and 10.2% of those in the sertraline/placebo group discontinued treatment because of adverse events.
In both the combination and control groups, the only treatment-emergent adverse event with an incidence of more than 10% was nausea (12.2% vs 11.7%, respectively).
At the last visit, the mean change in body weight from baseline was an increase of 1.3 kg for brexpiprazole plus sertraline vs 0 kg for sertraline alone. Rates of fatigue (6.8% vs 4.1%) and somnolence (5.4% vs 2.6%) were also higher with brexpiprazole plus sertraline.
A Trio of Clinical Trials
The findings are part of a larger program reported by the drug makers that includes a flexible-dose brexpiprazole phase 2 trial that met the same CAPS-5 primary endpoint and a second phase 3 trial (072 study) that did not.
“We’ve looked at that data and the sertraline/placebo response was a lot higher, so it was not due to a lack of response with the combination but due to a more robust response with the active control,” Davis said. “But we want to point out for that 072 study, there was still important separation between the combination and sertraline plus placebo on the functional outcome.”
All three trials ran for 12 weeks, so longer-term efficacy and safety data are needed, she said. Other limitations of the published phase 3 study are the patient eligibility criteria, restrictions on concomitant therapy, and lack of non-US sites, which many limit generalizability, the authors noted.
“Specifically, the exclusion of patients with a current major depressive episode is both a strength (to show a specific effect on PTSD) and a limitation (given the high prevalence of comorbid depression in PTSD),” they added.
Kudos, Caveats
Reached for comment, Vincent F. Capaldi, II, MD, ScM, professor and chair, department of psychiatry, Uniformed Services University of the Health Sciences School of Medicine, Bethesda, Maryland, said the exclusion of these patients is a limitation but that the study was well designed and conducted in a large sample across the United States.
“The findings suggest that brexpiprazole plus sertraline is a more effective treatment for PTSD than sertraline alone,” he said. “This finding is significant for our service members, who suffer from PTSD at higher rates than the general population.”
Additionally, the significant improvement in psychosocial functioning at week 12 “is important because PTSD is known to cause significant social and occupational disability, as well as quality-of-life issues,” he said.
Capaldi pointed out, however, that the study was conducted only at US sites and did not specifically target military/veteran persons, which may limit applicability to these unique populations.
“While subgroup analyses were generally consistent with the primary analysis, the study was not powered to detect differences between subgroups,” he added. “These subgroup analyses are quite important when considering military and veteran populations.”
Further research is needed to explore whether certain traumas are more responsive to combination treatment, the efficacy of augmenting existing sertraline therapy, and the specific mechanisms of brexpiprazole driving the improved outcomes, Capaldi said.
This study was funded by Otsuka Pharmaceutical Development & Commercialization, which was involved in the design, conduct, and data analysis. Davis reported receiving advisory board fees from Otsuka and Boehringer Ingelheim; lecture fees from Clinical Care Options; and grants from Alkermes, the Veterans Affairs, Patient-Centered Outcomes Research Institute, Department of Defense, and Social Finance. Several coauthors are employees of Otsuka. Krystal reported serving as a consultant for Otsuka America Pharmaceutical, Aptinyx, Biogen, IDEC, Bionomics, Boehringer Ingelheim International, Clearmind Medicine, Cybin IRL, Enveric Biosciences, Epiodyne, EpiVario, Janssen, Jazz Pharmaceuticals, Perception Neuroscience, Praxis Precision Medicines, Springcare, and Sunovion Pharmaceuticals. Krystal also reported serving as a scientific advisory board member for several companies and holding several patents.
A version of this article appeared on Medscape.com.
A drug that combines the atypical antipsychotic brexpiprazole and the selective serotonin reuptake inhibitor sertraline provides significantly greater relief of posttraumatic stress disorder (PTSD) symptoms than sertraline plus placebo, results of a phase 3 trial showed.
The medication is currently under review by the Food and Drug Administration (FDA) and if approved, will be the first pharmacologic option for PTSD in more than 20 years.
The trial met its primary endpoint of change in the Clinician Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) (CAPS-5) total score at week 10 and secondary patient-reported outcomes of PTSD symptoms, anxiety, and depression.
“And what is really cool, what’s really impactful is the combination worked better than sertraline plus placebo on a brief inventory of psychosocial functioning,” study investigator Lori L. Davis, a senior research psychiatrist, Birmingham Veterans Affairs Health Care System in Alabama, said in an interview.
“We can treat symptoms but that’s where the rubber meets the road, in terms of are they functioning better,” added Davis, who is also an adjunct professor of psychiatry, Heersink School of Medicine, University of Alabama at Birmingham.
The findings were published online on December 18 in JAMA Psychiatry and reported in May 2024 as part of a trio of trials conducted by Otsuka Pharmaceutical and Lundbeck Pharmaceuticals, codevelopers of the drug.
Clinically Meaningful
“This study provides promising results for a medication that may be an important new option for PTSD,” John Krystal, MD, director, Clinical Neuroscience Division, National Center for PTSD, US Department of Veterans Affairs, who was not involved in the research, said in an interview. “New PTSD treatments are a high priority.”
Currently, there are two FDA-approved medication treatments for PTSD — sertraline and paroxetine.
“They are helpful for many people, but patients are often left with residual symptoms or tolerability issues,” noted Krystal, who is also professor and chair of psychiatry, Yale University, New Haven, Connecticut.
“New medications that might address the important ‘effectiveness gap’ in PTSD could help to reduce the remaining distress, disability, and suicide risk associated with PTSD.”
The double-blind, phase 3 trial included 416 adults aged 18-65 years with a DSM-5 diagnosis of PTSD and symptoms for at least 6 months prior to screening. Patients underwent a 1-week placebo-run in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
Participants’ mean age was 37.4 years, 74.5% were women, and mean CAPS-5 total score was 38.4, suggesting moderate to high severity PTSD, Davis said. The average time from the index traumatic event was 4 years and three fourths had no prior exposure to PTSD prescription medications.
At week 10, the mean change in CAPS-5 score from randomization was –19.2 points in the brexpiprazole plus sertraline group and –13.6 points in the sertraline plus placebo group (95% CI, –8.79 to –2.38; P < .001).
Asked whether the 5.59-point treatment difference is clinically meaningful, Davis said there is no widely agreed definition for change in CAPS-5 total score but that a within-group reduction of more than 10-13 points is most-often cited as being clinically meaningful.
The key secondary endpoint of least square mean change in the patient-reported Brief Inventory of Psychosocial Function total score from baseline to week 12 was –33.8 with the combination vs –21.8 with sertraline plus placebo (95% CI, –19.4 to –4.62; P = .002).
“That’s clinically meaningful for me as a provider and a clinician and a researcher when you’re getting the PTSD symptom change differences in parallel with the improvement in functional outcome,” she said. “I see that as the clinically meaningful gauge.”
In terms of safety, 3.9% of the participants in the brexpiprazole/sertraline group and 10.2% of those in the sertraline/placebo group discontinued treatment because of adverse events.
In both the combination and control groups, the only treatment-emergent adverse event with an incidence of more than 10% was nausea (12.2% vs 11.7%, respectively).
At the last visit, the mean change in body weight from baseline was an increase of 1.3 kg for brexpiprazole plus sertraline vs 0 kg for sertraline alone. Rates of fatigue (6.8% vs 4.1%) and somnolence (5.4% vs 2.6%) were also higher with brexpiprazole plus sertraline.
A Trio of Clinical Trials
The findings are part of a larger program reported by the drug makers that includes a flexible-dose brexpiprazole phase 2 trial that met the same CAPS-5 primary endpoint and a second phase 3 trial (072 study) that did not.
“We’ve looked at that data and the sertraline/placebo response was a lot higher, so it was not due to a lack of response with the combination but due to a more robust response with the active control,” Davis said. “But we want to point out for that 072 study, there was still important separation between the combination and sertraline plus placebo on the functional outcome.”
All three trials ran for 12 weeks, so longer-term efficacy and safety data are needed, she said. Other limitations of the published phase 3 study are the patient eligibility criteria, restrictions on concomitant therapy, and lack of non-US sites, which many limit generalizability, the authors noted.
“Specifically, the exclusion of patients with a current major depressive episode is both a strength (to show a specific effect on PTSD) and a limitation (given the high prevalence of comorbid depression in PTSD),” they added.
Kudos, Caveats
Reached for comment, Vincent F. Capaldi, II, MD, ScM, professor and chair, department of psychiatry, Uniformed Services University of the Health Sciences School of Medicine, Bethesda, Maryland, said the exclusion of these patients is a limitation but that the study was well designed and conducted in a large sample across the United States.
“The findings suggest that brexpiprazole plus sertraline is a more effective treatment for PTSD than sertraline alone,” he said. “This finding is significant for our service members, who suffer from PTSD at higher rates than the general population.”
Additionally, the significant improvement in psychosocial functioning at week 12 “is important because PTSD is known to cause significant social and occupational disability, as well as quality-of-life issues,” he said.
Capaldi pointed out, however, that the study was conducted only at US sites and did not specifically target military/veteran persons, which may limit applicability to these unique populations.
“While subgroup analyses were generally consistent with the primary analysis, the study was not powered to detect differences between subgroups,” he added. “These subgroup analyses are quite important when considering military and veteran populations.”
Further research is needed to explore whether certain traumas are more responsive to combination treatment, the efficacy of augmenting existing sertraline therapy, and the specific mechanisms of brexpiprazole driving the improved outcomes, Capaldi said.
This study was funded by Otsuka Pharmaceutical Development & Commercialization, which was involved in the design, conduct, and data analysis. Davis reported receiving advisory board fees from Otsuka and Boehringer Ingelheim; lecture fees from Clinical Care Options; and grants from Alkermes, the Veterans Affairs, Patient-Centered Outcomes Research Institute, Department of Defense, and Social Finance. Several coauthors are employees of Otsuka. Krystal reported serving as a consultant for Otsuka America Pharmaceutical, Aptinyx, Biogen, IDEC, Bionomics, Boehringer Ingelheim International, Clearmind Medicine, Cybin IRL, Enveric Biosciences, Epiodyne, EpiVario, Janssen, Jazz Pharmaceuticals, Perception Neuroscience, Praxis Precision Medicines, Springcare, and Sunovion Pharmaceuticals. Krystal also reported serving as a scientific advisory board member for several companies and holding several patents.
A version of this article appeared on Medscape.com.
A drug that combines the atypical antipsychotic brexpiprazole and the selective serotonin reuptake inhibitor sertraline provides significantly greater relief of posttraumatic stress disorder (PTSD) symptoms than sertraline plus placebo, results of a phase 3 trial showed.
The medication is currently under review by the Food and Drug Administration (FDA) and if approved, will be the first pharmacologic option for PTSD in more than 20 years.
The trial met its primary endpoint of change in the Clinician Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) (CAPS-5) total score at week 10 and secondary patient-reported outcomes of PTSD symptoms, anxiety, and depression.
“And what is really cool, what’s really impactful is the combination worked better than sertraline plus placebo on a brief inventory of psychosocial functioning,” study investigator Lori L. Davis, a senior research psychiatrist, Birmingham Veterans Affairs Health Care System in Alabama, said in an interview.
“We can treat symptoms but that’s where the rubber meets the road, in terms of are they functioning better,” added Davis, who is also an adjunct professor of psychiatry, Heersink School of Medicine, University of Alabama at Birmingham.
The findings were published online on December 18 in JAMA Psychiatry and reported in May 2024 as part of a trio of trials conducted by Otsuka Pharmaceutical and Lundbeck Pharmaceuticals, codevelopers of the drug.
Clinically Meaningful
“This study provides promising results for a medication that may be an important new option for PTSD,” John Krystal, MD, director, Clinical Neuroscience Division, National Center for PTSD, US Department of Veterans Affairs, who was not involved in the research, said in an interview. “New PTSD treatments are a high priority.”
Currently, there are two FDA-approved medication treatments for PTSD — sertraline and paroxetine.
“They are helpful for many people, but patients are often left with residual symptoms or tolerability issues,” noted Krystal, who is also professor and chair of psychiatry, Yale University, New Haven, Connecticut.
“New medications that might address the important ‘effectiveness gap’ in PTSD could help to reduce the remaining distress, disability, and suicide risk associated with PTSD.”
The double-blind, phase 3 trial included 416 adults aged 18-65 years with a DSM-5 diagnosis of PTSD and symptoms for at least 6 months prior to screening. Patients underwent a 1-week placebo-run in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
Participants’ mean age was 37.4 years, 74.5% were women, and mean CAPS-5 total score was 38.4, suggesting moderate to high severity PTSD, Davis said. The average time from the index traumatic event was 4 years and three fourths had no prior exposure to PTSD prescription medications.
At week 10, the mean change in CAPS-5 score from randomization was –19.2 points in the brexpiprazole plus sertraline group and –13.6 points in the sertraline plus placebo group (95% CI, –8.79 to –2.38; P < .001).
Asked whether the 5.59-point treatment difference is clinically meaningful, Davis said there is no widely agreed definition for change in CAPS-5 total score but that a within-group reduction of more than 10-13 points is most-often cited as being clinically meaningful.
The key secondary endpoint of least square mean change in the patient-reported Brief Inventory of Psychosocial Function total score from baseline to week 12 was –33.8 with the combination vs –21.8 with sertraline plus placebo (95% CI, –19.4 to –4.62; P = .002).
“That’s clinically meaningful for me as a provider and a clinician and a researcher when you’re getting the PTSD symptom change differences in parallel with the improvement in functional outcome,” she said. “I see that as the clinically meaningful gauge.”
In terms of safety, 3.9% of the participants in the brexpiprazole/sertraline group and 10.2% of those in the sertraline/placebo group discontinued treatment because of adverse events.
In both the combination and control groups, the only treatment-emergent adverse event with an incidence of more than 10% was nausea (12.2% vs 11.7%, respectively).
At the last visit, the mean change in body weight from baseline was an increase of 1.3 kg for brexpiprazole plus sertraline vs 0 kg for sertraline alone. Rates of fatigue (6.8% vs 4.1%) and somnolence (5.4% vs 2.6%) were also higher with brexpiprazole plus sertraline.
A Trio of Clinical Trials
The findings are part of a larger program reported by the drug makers that includes a flexible-dose brexpiprazole phase 2 trial that met the same CAPS-5 primary endpoint and a second phase 3 trial (072 study) that did not.
“We’ve looked at that data and the sertraline/placebo response was a lot higher, so it was not due to a lack of response with the combination but due to a more robust response with the active control,” Davis said. “But we want to point out for that 072 study, there was still important separation between the combination and sertraline plus placebo on the functional outcome.”
All three trials ran for 12 weeks, so longer-term efficacy and safety data are needed, she said. Other limitations of the published phase 3 study are the patient eligibility criteria, restrictions on concomitant therapy, and lack of non-US sites, which many limit generalizability, the authors noted.
“Specifically, the exclusion of patients with a current major depressive episode is both a strength (to show a specific effect on PTSD) and a limitation (given the high prevalence of comorbid depression in PTSD),” they added.
Kudos, Caveats
Reached for comment, Vincent F. Capaldi, II, MD, ScM, professor and chair, department of psychiatry, Uniformed Services University of the Health Sciences School of Medicine, Bethesda, Maryland, said the exclusion of these patients is a limitation but that the study was well designed and conducted in a large sample across the United States.
“The findings suggest that brexpiprazole plus sertraline is a more effective treatment for PTSD than sertraline alone,” he said. “This finding is significant for our service members, who suffer from PTSD at higher rates than the general population.”
Additionally, the significant improvement in psychosocial functioning at week 12 “is important because PTSD is known to cause significant social and occupational disability, as well as quality-of-life issues,” he said.
Capaldi pointed out, however, that the study was conducted only at US sites and did not specifically target military/veteran persons, which may limit applicability to these unique populations.
“While subgroup analyses were generally consistent with the primary analysis, the study was not powered to detect differences between subgroups,” he added. “These subgroup analyses are quite important when considering military and veteran populations.”
Further research is needed to explore whether certain traumas are more responsive to combination treatment, the efficacy of augmenting existing sertraline therapy, and the specific mechanisms of brexpiprazole driving the improved outcomes, Capaldi said.
This study was funded by Otsuka Pharmaceutical Development & Commercialization, which was involved in the design, conduct, and data analysis. Davis reported receiving advisory board fees from Otsuka and Boehringer Ingelheim; lecture fees from Clinical Care Options; and grants from Alkermes, the Veterans Affairs, Patient-Centered Outcomes Research Institute, Department of Defense, and Social Finance. Several coauthors are employees of Otsuka. Krystal reported serving as a consultant for Otsuka America Pharmaceutical, Aptinyx, Biogen, IDEC, Bionomics, Boehringer Ingelheim International, Clearmind Medicine, Cybin IRL, Enveric Biosciences, Epiodyne, EpiVario, Janssen, Jazz Pharmaceuticals, Perception Neuroscience, Praxis Precision Medicines, Springcare, and Sunovion Pharmaceuticals. Krystal also reported serving as a scientific advisory board member for several companies and holding several patents.
A version of this article appeared on Medscape.com.
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.
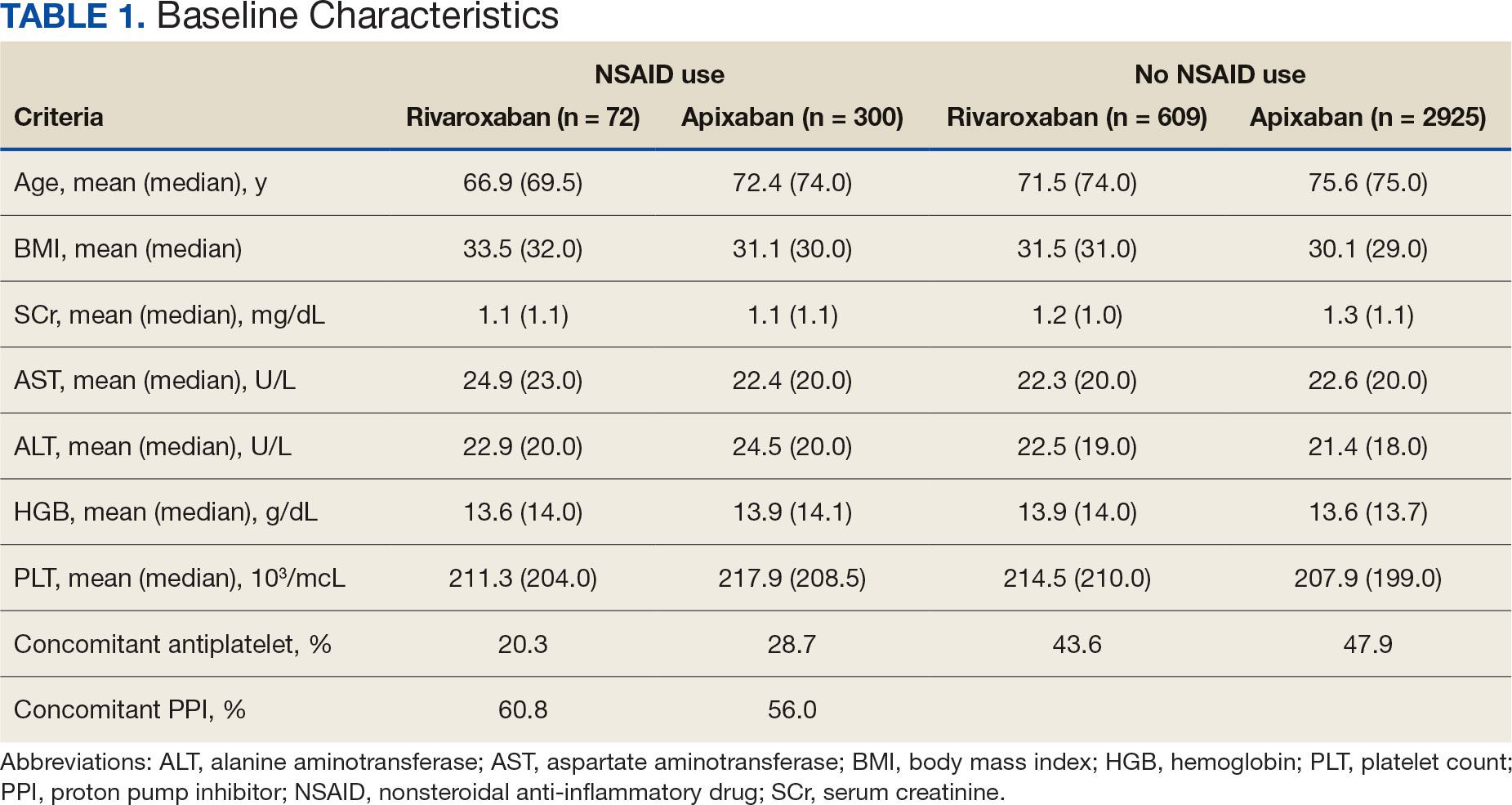
A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).
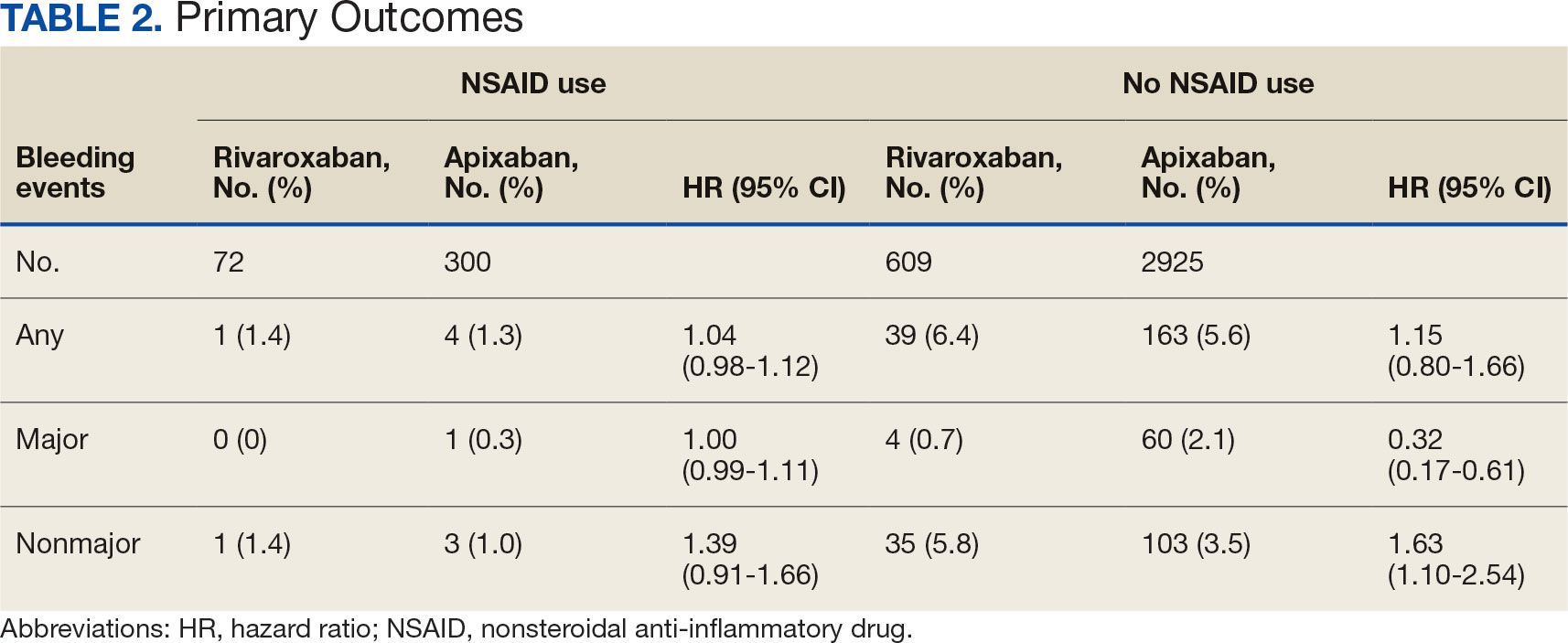
The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Skin Cancer Risk Elevated Among Blood, Marrow Transplant Survivors
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
The Protein Problem: The Unsolved Mystery of AI Drug Dev
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
The question has been lingering for years in medical science circles. Since 2020, when the artificial intelligence (AI) model AlphaFold made it possible to predict protein structures, would the technology open the drug discovery floodgates?
Short answer: No. At least not yet.
The longer answer goes something like this:
A drug target (such as a mutation) is like a lock. The right drug (a protein designed to bind to the mutation, stopping its activity) is the key. But proteins are fidgety and flexible.
“They’re basically molecular springs,” said Gabriel Monteiro da Silva, PhD, a computational chemistry research scientist at Genesis Therapeutics. “Your key can bend and alter the shape of the lock, and if you don’t account for that, your key might fail.”
This is the protein problem in drug development. Another issue making this challenge so vexing is that proteins don’t act in isolation. Their interactions with other proteins, ribonucleic acid, and DNA can affect how they bind to molecules and the shapes they adopt.
Newer versions of AlphaFold, such as AlphaFold Multimer and AlphaFold 3 (the code for which was recently revealed for academic use), can predict many interactions among proteins and between proteins and other molecules. But these tools still have weak points scientists are trying to overcome or work around.
“Those kinds of dynamics and multiple conformations are still quite challenging for the AI models to predict,” said James Zou, PhD, associate professor of biomedical data science at Stanford University in California.
“We’re finding more and more that the only way we can make these structures useful for drug discovery is if we incorporate dynamics, if we incorporate more physics into the model,” said Monteiro da Silva.
Monteiro da Silva spent 3 years during his PhD at Brown University, Providence, Rhode Island, running physics-based simulations in the lab, trying to understand why proteins carrying certain mutations are drug resistant. His results showed how “the changing landscape of shapes that a protein can take” prevented the drug from binding.
It took him 3 years to model just four mutations.
AI can do better — and the struggle is fascinating. By developing models that build on the predictive power of AlphaFold, scientists are uncovering new details about protein activity — insights that can lead to new therapeutics and reveal why existing ones stop working — much faster than they could with traditional methods or AlphaFold alone.
New Windows into Protein Dynamics
A notable step, “but that’s just the starting point,” said Pedro Beltrao, PhD, an associate professor at Institute of Molecular Systems Biology, ETH Zurich in Switzerland. “It’s still very difficult, given a pocket, to actually design the drug or figure out what the pocket binds.”
Going back to the lock-and-key analogy: While he was at Brown, with a team of researchers in the Rubenstein Group, Monteiro da Silva helped create a model to better understand how mutations affect “the shape and dynamics of the lock.” They manipulated the amino acid sequences of proteins, guiding their evolution. This enabled them to use AlphaFold to predict “protein ensembles” and how frequently those ensembles appear. Each ensemble represents the many different shapes a protein can take under given conditions.
“Essentially, it tries to find the most common shapes that a protein will take over an arbitrary amount of time,” Monteiro da Silva said. “If we can predict these ensembles at scale and fast, then we can screen many mutations that cause resistance and develop drugs that will not be affected by that resistance.”
To evaluate their method, the researchers focused on ABL1, a well-studied kinase that causes leukemia. ABL1 can be drugged – unless it carries or develops a mutation that causes drug resistance. Currently there are no drugs that work against proteins carrying those mutations, according to Monteiro da Silva. The researchers used their hybrid AI-meets-physics method to investigate how drugs bind to different ABL1 mutations, screening 100 mutations in just 1 month.
“It’s not going to be perfect for every one of them. But if we have 100 and we get 20 with good accuracy, that’s better than doing four over 3 years,” Monteiro da Silva said.
A forthcoming paper will make their model publicly available in “an easy-to-use graphical interface” that they hope clinicians and medicinal chemists will try out. It can also complement other AI-based tools that dig into protein dynamics, according to Monteiro da Silva.
Complementary Tools to Speed Up Discovery
Another aspect of the protein problem is scale. One protein can interact with hundreds of other proteins, which in turn may interact with hundreds more, all of which comprise the human interactome.
Feixiong Cheng, PhD, helped build PIONEER, a deep learning model that predicts the three-dimensional (3D) structure of interactions between proteins across the interactome.
Most disease mutations disrupt specific interactions between proteins, making their affinity stronger or weaker, explained Cheng. To treat a disease without causing major side effects, scientists need a precise understanding of those interactions.
“From the drug discovery perspective, we cannot just focus on single proteins. We have to understand the protein environment, in particular how the protein interacts with other proteins,” said Cheng, director of Cleveland Clinic Genome Center, Cleveland.
PIONEER helps by blending AlphaFold’s protein structure predictions with next-generation sequencing, a type of genomic research that identifies mutations in the human genome. The model predicts the 3D structure of the places where proteins interact — the binding sites, or interfaces — across the interactome.
“We tell you not only that a binds b, but where on a and where on b the two proteins interact,” said Haiyuan Yu, PhD, director of the Center for Innovative Proteomics, Cornell University, and co-creator of PIONEER.
This can help scientists understand “why a mutation, protein, or even network is a good target for therapeutic discovery,” Cheng said.
The researchers validated PIONEER’s predictions in the lab, testing the impacts of roughly 3000 mutations on 7000 pairs of interacting proteins. Based on their findings, they plan to develop and test treatments for lung and endometrial cancer.
PIONEER can also help scientists home in on how a mutation causes a disease, such as by showing recurrent mutations.
“If you find cancer mutations hitting an interface again and again and again, it means that this is likely to be driving cancer progression,” said Beltrao.
Beltrao’s lab and others have looked for recurrent mutations by using AlphaFold Multimer and AlphaFold 3 to directly model protein interactions. It’s a much slower approach (Pioneer is more than 5000 faster than AlphaFold Multimer, according to Cheng). But it could allow scientists to model interfaces that are not shown by PIONEER.
“You will need many different things to try to come up with a structural modeling of the interactome, and all these will have limitations,” said Beltrao. “Their method is a very good step forward, and there’ll be other approaches that are complementary, to continue to add details.”
And It Wouldn’t be an AI Mission Without ChatGPT
Large language models, such as ChatGPT, are another way that scientists are adding details to protein structure predictions. Zou used GPT-4 to “fine tune” a protein language model, called evolutionary scale modeling (ESM-2), which predicts protein structures directly from a protein sequence.
First, they trained ChatGPT on thousands of papers and studies containing information about the functions, biophysical properties, and disease relevance of different mutations. Next, they used the trained model to “teach” ESM-2, boosting its ability “to predict which mutations are likely to have larger effects or smaller effects,” Zou said. The same could be done for a model like AlphaFold, according to Zou.
“They are quite complementary in that the large language model contains a lot more information about the functions and the biophysics of different mutations and proteins as captured in text,” he said, whereas “you can’t give AlphaFold a piece of paper.”
Exactly how AlphaFold makes its predictions is another mystery. “It will somehow learn protein dynamics phenomenologically,” said Monteiro da Silva. He and others are trying to understand how that happens, in hopes of creating even more accurate predictive models. But for the time being, AI-based methods still need assistance from physics.
“The dream is that we achieve a state where we rely on just the fast methods, and they’re accurate enough,” he said. “But we’re so far from that.”
A version of this article first appeared on Medscape.com.
‘New Hope’ for Alcohol Use Disorder Treatment
Evidence is mounting that new therapies already used to treat gut diseases, type 2 diabetes, and obesity may help people with alcohol use disorder (AUD).
Glucagon-like peptide 1 (GLP-1) receptor agonists, first used to treat diabetes and now widely used for weight loss, and fecal microbiota transplants (FMTs), used to treat diseases such as recurrent Clostridioides difficile infection, are advancing in clinical trials as potential options for treating AUD.
AUD Affects 28.9 Million People in the United States
In 2023, 28.9 million people aged 12 years or older in the United States had AUD (10.2% of the people in this age group). The Food and Drug Administration (FDA) has approved three medical therapies: Acamprosate, naltrexone, and disulfiram to help keep people with the disorder from returning to heavy drinking. Acamprosate’s mechanism of action is not clear, but it is thought to modulate and normalize alcohol-related changes in brain activity, thereby reducing withdrawal symptoms. Naltrexone blocks opioid receptors to reduce alcohol cravings. Disulfiram causes a toxic physical reaction when mixed with alcohol.
Some with AUD also benefit from behavioral therapies and support groups such as Alcoholics Anonymous. But for others, nothing has worked, and that’s part of the reason Lorenzo Leggio, MD, PhD, a scientist in the field of alcohol addiction with the National Institutes of Health (NIH), told this news organization that this is the “most exciting moment” for AUD treatment in his more than 2 decades of research in this area.
GLP-1 Agonists Showing Consistent Results
GLP-1 receptor agonists work by modulating the brain’s reward pathways, including the areas that regulate cravings and motivation.
“By dampening the reward signals associated with alcohol consumption, GLP-1 agonists may reduce cravings and heavy drinking episodes,” Fares Qeadan, PhD, MS, associate professor of biostatistics in the Department of Public Health Sciences at Loyola University Chicago in Illinois, told this news organization.
“The unique aspect of GLP-1 agonists is their ability to target both metabolic and reward systems in the brain,” he said. While naltrexone or acamprosate blocks the effects of alcohol or reduces withdrawal symptoms, “GLP-1 agonists approach addiction through a broader mechanism, potentially addressing underlying factors that contribute to cravings and compulsive behaviors,” he said.
As part of a study published in October in Addiction, Qeadan’s team found that people with AUD who were prescribed GLP-1 agonists had a 50% lower rate of severe intoxication than those who were not prescribed those medications.
“While this is observational and not yet definitive, it highlights the potential of these drugs to complement existing treatments for AUD,” he said.
Another study, a nationwide cohort study published in JAMA Psychiatry, found that using the GLP-1 receptor agonists semaglutide and liraglutide was linked to a lower risk for AUD-related hospitalizations than traditional AUD medications.
A systematic review, published last month in eClinical Medicine, concluded that, though there is little high-quality evidence demonstrating the effect of GLP-1 receptor agonists on alcohol use, “subgroup analysis from two [randomized, controlled trials] and supporting data from four observational studies suggest that GLP-1 [receptor agonists] may reduce alcohol consumption and improve outcomes in some individuals.”
Studying individual differences in response may have implications for personalized medicine, Qeadan said, as treatments could be tailored to those most likely to benefit, such as people with both metabolic dysfunction and AUD.
“These medications may offer hope for patients who struggle with addiction and have not responded to traditional therapies,” Qeadan said.
Exploring FMT as AUD Treatment
FMT is also a new research focus for treating AUD. Jasmohan Bajaj, MD, a gastroenterologist and liver specialist at Virginia Commonwealth University Medical Center, Richmond, is leading the Intestinal Microbiota Transplant in Alcohol-Associated Liver Disease (IMPACT) trial.
AUD has been linked with gut microbial alterations that worsen with cirrhosis. Research has shown that alcohol consumption changes the diversity of bacteria and can lead to bacterial overgrowth and progression of alcohol-associated liver disease.
FMT has been effective in rebalancing gut bacteria by transferring healthy stool from screened donors into patients who have developed an overgrowth of harmful bacteria. In the IMPACT trial, participants, who have not previously received traditional treatment for AUD or for whom treatment has not worked, are randomized either to the oral treatment capsule, which contains freeze-dried stool from a donor with healthy gut bacteria, or placebo.
The trial, sponsored by the NIH, is halfway through its target enrollment of 80.
In a previous smaller, placebo-controlled, phase 1 study, also led by Bajaj and published in Hepatology, 9 of the 10 volunteers who had severe AUD and cirrhosis experienced fewer alcohol cravings and had lower consumption after 15 days. Only three of the placebo participants saw similar improvements. Those who received the microbiota transplant also had fewer AUD-related events over 6 months.
Bajaj said that, if trials show FMT is safe and effective, he envisions the treatment as one tool in a multidisciplinary, integrated clinic that would include a hepatologist and mental health clinicians.
One benefit of the FMT treatment approach is it is given once or twice only, rather than administered regularly.
Current Treatments Work, But More are Needed
Leggio, who is clinical director of the National Institute on Drug Abuse Intramural Research Program, said: “We know that alcohol use disorder, and addiction in general, is a brain disease. We also know that the brain does not work in isolation. The brain is constantly interacting with the rest of the body, including with the gut.”
Leggio said it’s important to note that the three FDA-approved medications do work for alcohol addiction. He said they work as well as selective serotonin reuptake inhibitors for depression and beta-blockers for chronic heart failure.
But there are only three, and they don’t work for everyone, he noted. Those are among the reasons developing new treatments is important. New treatments could be used as an alternative or in combination with already approved treatments.
FMT is in “the very early stages” of trials testing its use for AUD, Leggio noted, adding that the studies by Bajaj’s team are among the very few addressing gut dysbiosis in AUD, and all have involved small numbers of patients. “It’s promising. It’s intriguing. It’s exciting. But we are just at the beginning.”
Results Consistent Across Species, Labs
GLP-1 agonists are further along in trials but still not ready for prescribing for AUD, Leggio said. The positive results have been consistent across species, different labs, and different research teams around the world.
Researchers have also explored through electronic health record emulation trials whether people already taking GLP-1 agonists for diabetes or obesity drink less alcohol compared with matched cohorts not taking GLP-1s. “They consistently show that the people who are on GLP-1s drink less,” he said.
“[Emulation trials] don’t replace the need for randomized controlled trials, Leggio noted. Leggio’s team is currently working on a randomized, placebo-controlled, double-blinded trial studying GLP-1s in relation to AUD.
New Directions 20-Year Highlight
This whole line of research represents “new hope” and has many implications, Leggio said. “I have been in this business for 20-plus years, and I think this is themost exciting moment when we have a very promising target in GLP-1s.”
Regardless of efficacy, he said, the focus on GLP-1 agonists and FMT for AUD has people talking more about addiction and the brain-body connection rather than assuming AUD is a result of poor choices and “bad behavior.”
The momentum of new treatments could also lead to patients and physicians having conversations about existing treatments.
“Hopefully, this momentum will help us destigmatize addiction, and by destigmatizing addiction, there will be an uptick in use of currently approved medications,” Leggio said.
Qeadan, Bajaj, and Leggio reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evidence is mounting that new therapies already used to treat gut diseases, type 2 diabetes, and obesity may help people with alcohol use disorder (AUD).
Glucagon-like peptide 1 (GLP-1) receptor agonists, first used to treat diabetes and now widely used for weight loss, and fecal microbiota transplants (FMTs), used to treat diseases such as recurrent Clostridioides difficile infection, are advancing in clinical trials as potential options for treating AUD.
AUD Affects 28.9 Million People in the United States
In 2023, 28.9 million people aged 12 years or older in the United States had AUD (10.2% of the people in this age group). The Food and Drug Administration (FDA) has approved three medical therapies: Acamprosate, naltrexone, and disulfiram to help keep people with the disorder from returning to heavy drinking. Acamprosate’s mechanism of action is not clear, but it is thought to modulate and normalize alcohol-related changes in brain activity, thereby reducing withdrawal symptoms. Naltrexone blocks opioid receptors to reduce alcohol cravings. Disulfiram causes a toxic physical reaction when mixed with alcohol.
Some with AUD also benefit from behavioral therapies and support groups such as Alcoholics Anonymous. But for others, nothing has worked, and that’s part of the reason Lorenzo Leggio, MD, PhD, a scientist in the field of alcohol addiction with the National Institutes of Health (NIH), told this news organization that this is the “most exciting moment” for AUD treatment in his more than 2 decades of research in this area.
GLP-1 Agonists Showing Consistent Results
GLP-1 receptor agonists work by modulating the brain’s reward pathways, including the areas that regulate cravings and motivation.
“By dampening the reward signals associated with alcohol consumption, GLP-1 agonists may reduce cravings and heavy drinking episodes,” Fares Qeadan, PhD, MS, associate professor of biostatistics in the Department of Public Health Sciences at Loyola University Chicago in Illinois, told this news organization.
“The unique aspect of GLP-1 agonists is their ability to target both metabolic and reward systems in the brain,” he said. While naltrexone or acamprosate blocks the effects of alcohol or reduces withdrawal symptoms, “GLP-1 agonists approach addiction through a broader mechanism, potentially addressing underlying factors that contribute to cravings and compulsive behaviors,” he said.
As part of a study published in October in Addiction, Qeadan’s team found that people with AUD who were prescribed GLP-1 agonists had a 50% lower rate of severe intoxication than those who were not prescribed those medications.
“While this is observational and not yet definitive, it highlights the potential of these drugs to complement existing treatments for AUD,” he said.
Another study, a nationwide cohort study published in JAMA Psychiatry, found that using the GLP-1 receptor agonists semaglutide and liraglutide was linked to a lower risk for AUD-related hospitalizations than traditional AUD medications.
A systematic review, published last month in eClinical Medicine, concluded that, though there is little high-quality evidence demonstrating the effect of GLP-1 receptor agonists on alcohol use, “subgroup analysis from two [randomized, controlled trials] and supporting data from four observational studies suggest that GLP-1 [receptor agonists] may reduce alcohol consumption and improve outcomes in some individuals.”
Studying individual differences in response may have implications for personalized medicine, Qeadan said, as treatments could be tailored to those most likely to benefit, such as people with both metabolic dysfunction and AUD.
“These medications may offer hope for patients who struggle with addiction and have not responded to traditional therapies,” Qeadan said.
Exploring FMT as AUD Treatment
FMT is also a new research focus for treating AUD. Jasmohan Bajaj, MD, a gastroenterologist and liver specialist at Virginia Commonwealth University Medical Center, Richmond, is leading the Intestinal Microbiota Transplant in Alcohol-Associated Liver Disease (IMPACT) trial.
AUD has been linked with gut microbial alterations that worsen with cirrhosis. Research has shown that alcohol consumption changes the diversity of bacteria and can lead to bacterial overgrowth and progression of alcohol-associated liver disease.
FMT has been effective in rebalancing gut bacteria by transferring healthy stool from screened donors into patients who have developed an overgrowth of harmful bacteria. In the IMPACT trial, participants, who have not previously received traditional treatment for AUD or for whom treatment has not worked, are randomized either to the oral treatment capsule, which contains freeze-dried stool from a donor with healthy gut bacteria, or placebo.
The trial, sponsored by the NIH, is halfway through its target enrollment of 80.
In a previous smaller, placebo-controlled, phase 1 study, also led by Bajaj and published in Hepatology, 9 of the 10 volunteers who had severe AUD and cirrhosis experienced fewer alcohol cravings and had lower consumption after 15 days. Only three of the placebo participants saw similar improvements. Those who received the microbiota transplant also had fewer AUD-related events over 6 months.
Bajaj said that, if trials show FMT is safe and effective, he envisions the treatment as one tool in a multidisciplinary, integrated clinic that would include a hepatologist and mental health clinicians.
One benefit of the FMT treatment approach is it is given once or twice only, rather than administered regularly.
Current Treatments Work, But More are Needed
Leggio, who is clinical director of the National Institute on Drug Abuse Intramural Research Program, said: “We know that alcohol use disorder, and addiction in general, is a brain disease. We also know that the brain does not work in isolation. The brain is constantly interacting with the rest of the body, including with the gut.”
Leggio said it’s important to note that the three FDA-approved medications do work for alcohol addiction. He said they work as well as selective serotonin reuptake inhibitors for depression and beta-blockers for chronic heart failure.
But there are only three, and they don’t work for everyone, he noted. Those are among the reasons developing new treatments is important. New treatments could be used as an alternative or in combination with already approved treatments.
FMT is in “the very early stages” of trials testing its use for AUD, Leggio noted, adding that the studies by Bajaj’s team are among the very few addressing gut dysbiosis in AUD, and all have involved small numbers of patients. “It’s promising. It’s intriguing. It’s exciting. But we are just at the beginning.”
Results Consistent Across Species, Labs
GLP-1 agonists are further along in trials but still not ready for prescribing for AUD, Leggio said. The positive results have been consistent across species, different labs, and different research teams around the world.
Researchers have also explored through electronic health record emulation trials whether people already taking GLP-1 agonists for diabetes or obesity drink less alcohol compared with matched cohorts not taking GLP-1s. “They consistently show that the people who are on GLP-1s drink less,” he said.
“[Emulation trials] don’t replace the need for randomized controlled trials, Leggio noted. Leggio’s team is currently working on a randomized, placebo-controlled, double-blinded trial studying GLP-1s in relation to AUD.
New Directions 20-Year Highlight
This whole line of research represents “new hope” and has many implications, Leggio said. “I have been in this business for 20-plus years, and I think this is themost exciting moment when we have a very promising target in GLP-1s.”
Regardless of efficacy, he said, the focus on GLP-1 agonists and FMT for AUD has people talking more about addiction and the brain-body connection rather than assuming AUD is a result of poor choices and “bad behavior.”
The momentum of new treatments could also lead to patients and physicians having conversations about existing treatments.
“Hopefully, this momentum will help us destigmatize addiction, and by destigmatizing addiction, there will be an uptick in use of currently approved medications,” Leggio said.
Qeadan, Bajaj, and Leggio reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evidence is mounting that new therapies already used to treat gut diseases, type 2 diabetes, and obesity may help people with alcohol use disorder (AUD).
Glucagon-like peptide 1 (GLP-1) receptor agonists, first used to treat diabetes and now widely used for weight loss, and fecal microbiota transplants (FMTs), used to treat diseases such as recurrent Clostridioides difficile infection, are advancing in clinical trials as potential options for treating AUD.
AUD Affects 28.9 Million People in the United States
In 2023, 28.9 million people aged 12 years or older in the United States had AUD (10.2% of the people in this age group). The Food and Drug Administration (FDA) has approved three medical therapies: Acamprosate, naltrexone, and disulfiram to help keep people with the disorder from returning to heavy drinking. Acamprosate’s mechanism of action is not clear, but it is thought to modulate and normalize alcohol-related changes in brain activity, thereby reducing withdrawal symptoms. Naltrexone blocks opioid receptors to reduce alcohol cravings. Disulfiram causes a toxic physical reaction when mixed with alcohol.
Some with AUD also benefit from behavioral therapies and support groups such as Alcoholics Anonymous. But for others, nothing has worked, and that’s part of the reason Lorenzo Leggio, MD, PhD, a scientist in the field of alcohol addiction with the National Institutes of Health (NIH), told this news organization that this is the “most exciting moment” for AUD treatment in his more than 2 decades of research in this area.
GLP-1 Agonists Showing Consistent Results
GLP-1 receptor agonists work by modulating the brain’s reward pathways, including the areas that regulate cravings and motivation.
“By dampening the reward signals associated with alcohol consumption, GLP-1 agonists may reduce cravings and heavy drinking episodes,” Fares Qeadan, PhD, MS, associate professor of biostatistics in the Department of Public Health Sciences at Loyola University Chicago in Illinois, told this news organization.
“The unique aspect of GLP-1 agonists is their ability to target both metabolic and reward systems in the brain,” he said. While naltrexone or acamprosate blocks the effects of alcohol or reduces withdrawal symptoms, “GLP-1 agonists approach addiction through a broader mechanism, potentially addressing underlying factors that contribute to cravings and compulsive behaviors,” he said.
As part of a study published in October in Addiction, Qeadan’s team found that people with AUD who were prescribed GLP-1 agonists had a 50% lower rate of severe intoxication than those who were not prescribed those medications.
“While this is observational and not yet definitive, it highlights the potential of these drugs to complement existing treatments for AUD,” he said.
Another study, a nationwide cohort study published in JAMA Psychiatry, found that using the GLP-1 receptor agonists semaglutide and liraglutide was linked to a lower risk for AUD-related hospitalizations than traditional AUD medications.
A systematic review, published last month in eClinical Medicine, concluded that, though there is little high-quality evidence demonstrating the effect of GLP-1 receptor agonists on alcohol use, “subgroup analysis from two [randomized, controlled trials] and supporting data from four observational studies suggest that GLP-1 [receptor agonists] may reduce alcohol consumption and improve outcomes in some individuals.”
Studying individual differences in response may have implications for personalized medicine, Qeadan said, as treatments could be tailored to those most likely to benefit, such as people with both metabolic dysfunction and AUD.
“These medications may offer hope for patients who struggle with addiction and have not responded to traditional therapies,” Qeadan said.
Exploring FMT as AUD Treatment
FMT is also a new research focus for treating AUD. Jasmohan Bajaj, MD, a gastroenterologist and liver specialist at Virginia Commonwealth University Medical Center, Richmond, is leading the Intestinal Microbiota Transplant in Alcohol-Associated Liver Disease (IMPACT) trial.
AUD has been linked with gut microbial alterations that worsen with cirrhosis. Research has shown that alcohol consumption changes the diversity of bacteria and can lead to bacterial overgrowth and progression of alcohol-associated liver disease.
FMT has been effective in rebalancing gut bacteria by transferring healthy stool from screened donors into patients who have developed an overgrowth of harmful bacteria. In the IMPACT trial, participants, who have not previously received traditional treatment for AUD or for whom treatment has not worked, are randomized either to the oral treatment capsule, which contains freeze-dried stool from a donor with healthy gut bacteria, or placebo.
The trial, sponsored by the NIH, is halfway through its target enrollment of 80.
In a previous smaller, placebo-controlled, phase 1 study, also led by Bajaj and published in Hepatology, 9 of the 10 volunteers who had severe AUD and cirrhosis experienced fewer alcohol cravings and had lower consumption after 15 days. Only three of the placebo participants saw similar improvements. Those who received the microbiota transplant also had fewer AUD-related events over 6 months.
Bajaj said that, if trials show FMT is safe and effective, he envisions the treatment as one tool in a multidisciplinary, integrated clinic that would include a hepatologist and mental health clinicians.
One benefit of the FMT treatment approach is it is given once or twice only, rather than administered regularly.
Current Treatments Work, But More are Needed
Leggio, who is clinical director of the National Institute on Drug Abuse Intramural Research Program, said: “We know that alcohol use disorder, and addiction in general, is a brain disease. We also know that the brain does not work in isolation. The brain is constantly interacting with the rest of the body, including with the gut.”
Leggio said it’s important to note that the three FDA-approved medications do work for alcohol addiction. He said they work as well as selective serotonin reuptake inhibitors for depression and beta-blockers for chronic heart failure.
But there are only three, and they don’t work for everyone, he noted. Those are among the reasons developing new treatments is important. New treatments could be used as an alternative or in combination with already approved treatments.
FMT is in “the very early stages” of trials testing its use for AUD, Leggio noted, adding that the studies by Bajaj’s team are among the very few addressing gut dysbiosis in AUD, and all have involved small numbers of patients. “It’s promising. It’s intriguing. It’s exciting. But we are just at the beginning.”
Results Consistent Across Species, Labs
GLP-1 agonists are further along in trials but still not ready for prescribing for AUD, Leggio said. The positive results have been consistent across species, different labs, and different research teams around the world.
Researchers have also explored through electronic health record emulation trials whether people already taking GLP-1 agonists for diabetes or obesity drink less alcohol compared with matched cohorts not taking GLP-1s. “They consistently show that the people who are on GLP-1s drink less,” he said.
“[Emulation trials] don’t replace the need for randomized controlled trials, Leggio noted. Leggio’s team is currently working on a randomized, placebo-controlled, double-blinded trial studying GLP-1s in relation to AUD.
New Directions 20-Year Highlight
This whole line of research represents “new hope” and has many implications, Leggio said. “I have been in this business for 20-plus years, and I think this is themost exciting moment when we have a very promising target in GLP-1s.”
Regardless of efficacy, he said, the focus on GLP-1 agonists and FMT for AUD has people talking more about addiction and the brain-body connection rather than assuming AUD is a result of poor choices and “bad behavior.”
The momentum of new treatments could also lead to patients and physicians having conversations about existing treatments.
“Hopefully, this momentum will help us destigmatize addiction, and by destigmatizing addiction, there will be an uptick in use of currently approved medications,” Leggio said.
Qeadan, Bajaj, and Leggio reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A New Weight Loss Drug With No Side Effects? Yes... So Far
For people with obesity or type 2 diabetes, glucagon-like peptide 1 (GLP-1) agonists (including Mounjaro, Wegovy, and Ozempic) have been labeled miracle drugs. But they aren’t miraculous for everyone. Research indicates a significant portion of people discontinue using them within a year.
The main problems with GLP-1 agonists are that they are expensive and have a fairly high rate of side effects — such as nausea, vomiting, diarrhea, or constipation. Another big one is muscle loss.
This lack of side effects, particularly in how the potential drug causes no muscle loss — and in fact engages muscle for some of its effect — sets it apart and makes it a potential alternative to GLP-1s. The key is not just reducing appetite but also increasing energy expenditure.
How It Works
The new approach targets a protein called NK2R — a member of the neurokinin receptor family, which has a role in a variety of physiological processes, including pain sensation, anxiety, and inflammation.
“We were looking to see genetic linkages to metabolic health, and there NK2R was,” said Zach Gerhart-Hines, PhD, a professor studying molecular metabolism at the University of Copenhagen in Denmark and principal investigator of the study. The group then created a few long-acting agonists that are selective for NK2R. So far, they’ve tested them in mice and nonhuman primates.
“The data on new medicines targeting NK2R is very promising and highlights the potential of both reducing food intake and increasing energy expenditure,” said Daniel Drucker, MD, an endocrinologist and researcher at Lunenfeld-Tanenbaum Research Institute in Toronto who was not involved in the study.
“The drug activates a certain region in the hindbrain of the animal, which is controlling food intake, and it does so by reducing appetite without increasing nausea or vomiting,” explained Frederike Sass, a research assistant at the University of Copenhagen in Copenhagen, Denmark, who led the study.
Gerhart-Hines said that even at the highest dose, there were no incidents of vomiting among the nonhuman primates. Mice can’t vomit, but there are ways to tell if they feel unwell from a drug. One way researchers test that is to start feeding the mice sweetened water at the same time they’re given a drug. Then later, when the mice are no longer on the drug, they’re given a choice between sweetened and unsweetened water. If they weren’t feeling well on the drug, they’ll choose plain water because they associate the sweet water with feeling bad, otherwise mice prefer sweet water. Sass said that with the NK2R agonist, they continued to drink sweet water after the treatment, whereas when they gave the mice semaglutide, the mice preferred plain water posttreatment.
The researchers also monitored the animals’ psychological health, as NK2R has been associated with anxiety, but they observed no behavioral changes.
The Key Mechanism at Work
One big question is how the NK2R agonists work. The amphetamines people used for weight loss during the 1950s and 1960s worked by making people more active. GLP-1 agonists reduce appetite and lower blood sugar. This is not that. In their studies with animals, the researchers didn’t observe that the animals were more active nor were there changes in other biomarkers like insulin. So far, the main difference they found with the NK2R agonists is an increase in thermogenesis in certain muscles.
Another benefit of the NK2R treatments is that they don’t seem to have a big impact on lean mass — the nonfat component of body weight, namely muscle, bones, and organs. Studies indicate that 25%-39% of weight loss on GLP-1 agonists is lost muscle. According to DEXA scans of the mice, Gerhart-Hines said they observed no lean mass loss. (In mice, he noted, GLP-1 agonists can cause up to 50% lean mass loss).
And for people with both diabetes and obesity, “what we found with NK2R is that obese and diabetic models, whether mice or monkeys, respond much better to that treatment in terms of glucose control and body weight loss,” Gerhart-Hines said. He explained that GLP-1 agonists don’t work quite as well for weight loss in people with diabetes because the drug stimulates insulin production in a system that already has insulin issues and can cause more sugar to be stored as fat.
Further, GLP-1 agonists are peptide drugs, which are expensive to make. The NK2R agonists are small molecules that would be cheaper to produce, Gerhart-Hines believes. One candidate they’re testing would likely be given once daily, another once weekly.
The current surge in obesity and diabetes may be a direct consequence of our bodies’ decreased energy expenditure. “Compared to 80s and 90s, the average person is more physically active, but the overarching basal resting energy expenditure has gone down,” said Gerhart-Hines, according to research by John Speakman at the University of Aberdeen, Scotland. We don’t know why, though, he said, but guesses it could be our diets or climate controlled environments.
But the NK2R agonists are among the many currently being studied for weight loss, and it may be hard to compete with the GLP-1 agonists. “As GLP-1 medicines will soon achieve 25% weight loss and have an extensively studied safety profile, the task of producing better drugs that work well in most people, are well tolerated and also reduce the complications of cardiometabolic disease, is challenging but not impossible,” said Drucker.
Gerhart-Hines said they plan to start trials in humans in the next year, but he suspects it will be another 6 or 7 years before it comes to market, if the trials are successful.
“There’s people who want [a GLP-1 agonist] and can’t even get it,” Gerhart-Hines said. As far as weight loss drugs, he noted, “we are not even saturating the market right now.”
A version of this article first appeared on Medscape.com.
For people with obesity or type 2 diabetes, glucagon-like peptide 1 (GLP-1) agonists (including Mounjaro, Wegovy, and Ozempic) have been labeled miracle drugs. But they aren’t miraculous for everyone. Research indicates a significant portion of people discontinue using them within a year.
The main problems with GLP-1 agonists are that they are expensive and have a fairly high rate of side effects — such as nausea, vomiting, diarrhea, or constipation. Another big one is muscle loss.
This lack of side effects, particularly in how the potential drug causes no muscle loss — and in fact engages muscle for some of its effect — sets it apart and makes it a potential alternative to GLP-1s. The key is not just reducing appetite but also increasing energy expenditure.
How It Works
The new approach targets a protein called NK2R — a member of the neurokinin receptor family, which has a role in a variety of physiological processes, including pain sensation, anxiety, and inflammation.
“We were looking to see genetic linkages to metabolic health, and there NK2R was,” said Zach Gerhart-Hines, PhD, a professor studying molecular metabolism at the University of Copenhagen in Denmark and principal investigator of the study. The group then created a few long-acting agonists that are selective for NK2R. So far, they’ve tested them in mice and nonhuman primates.
“The data on new medicines targeting NK2R is very promising and highlights the potential of both reducing food intake and increasing energy expenditure,” said Daniel Drucker, MD, an endocrinologist and researcher at Lunenfeld-Tanenbaum Research Institute in Toronto who was not involved in the study.
“The drug activates a certain region in the hindbrain of the animal, which is controlling food intake, and it does so by reducing appetite without increasing nausea or vomiting,” explained Frederike Sass, a research assistant at the University of Copenhagen in Copenhagen, Denmark, who led the study.
Gerhart-Hines said that even at the highest dose, there were no incidents of vomiting among the nonhuman primates. Mice can’t vomit, but there are ways to tell if they feel unwell from a drug. One way researchers test that is to start feeding the mice sweetened water at the same time they’re given a drug. Then later, when the mice are no longer on the drug, they’re given a choice between sweetened and unsweetened water. If they weren’t feeling well on the drug, they’ll choose plain water because they associate the sweet water with feeling bad, otherwise mice prefer sweet water. Sass said that with the NK2R agonist, they continued to drink sweet water after the treatment, whereas when they gave the mice semaglutide, the mice preferred plain water posttreatment.
The researchers also monitored the animals’ psychological health, as NK2R has been associated with anxiety, but they observed no behavioral changes.
The Key Mechanism at Work
One big question is how the NK2R agonists work. The amphetamines people used for weight loss during the 1950s and 1960s worked by making people more active. GLP-1 agonists reduce appetite and lower blood sugar. This is not that. In their studies with animals, the researchers didn’t observe that the animals were more active nor were there changes in other biomarkers like insulin. So far, the main difference they found with the NK2R agonists is an increase in thermogenesis in certain muscles.
Another benefit of the NK2R treatments is that they don’t seem to have a big impact on lean mass — the nonfat component of body weight, namely muscle, bones, and organs. Studies indicate that 25%-39% of weight loss on GLP-1 agonists is lost muscle. According to DEXA scans of the mice, Gerhart-Hines said they observed no lean mass loss. (In mice, he noted, GLP-1 agonists can cause up to 50% lean mass loss).
And for people with both diabetes and obesity, “what we found with NK2R is that obese and diabetic models, whether mice or monkeys, respond much better to that treatment in terms of glucose control and body weight loss,” Gerhart-Hines said. He explained that GLP-1 agonists don’t work quite as well for weight loss in people with diabetes because the drug stimulates insulin production in a system that already has insulin issues and can cause more sugar to be stored as fat.
Further, GLP-1 agonists are peptide drugs, which are expensive to make. The NK2R agonists are small molecules that would be cheaper to produce, Gerhart-Hines believes. One candidate they’re testing would likely be given once daily, another once weekly.
The current surge in obesity and diabetes may be a direct consequence of our bodies’ decreased energy expenditure. “Compared to 80s and 90s, the average person is more physically active, but the overarching basal resting energy expenditure has gone down,” said Gerhart-Hines, according to research by John Speakman at the University of Aberdeen, Scotland. We don’t know why, though, he said, but guesses it could be our diets or climate controlled environments.
But the NK2R agonists are among the many currently being studied for weight loss, and it may be hard to compete with the GLP-1 agonists. “As GLP-1 medicines will soon achieve 25% weight loss and have an extensively studied safety profile, the task of producing better drugs that work well in most people, are well tolerated and also reduce the complications of cardiometabolic disease, is challenging but not impossible,” said Drucker.
Gerhart-Hines said they plan to start trials in humans in the next year, but he suspects it will be another 6 or 7 years before it comes to market, if the trials are successful.
“There’s people who want [a GLP-1 agonist] and can’t even get it,” Gerhart-Hines said. As far as weight loss drugs, he noted, “we are not even saturating the market right now.”
A version of this article first appeared on Medscape.com.
For people with obesity or type 2 diabetes, glucagon-like peptide 1 (GLP-1) agonists (including Mounjaro, Wegovy, and Ozempic) have been labeled miracle drugs. But they aren’t miraculous for everyone. Research indicates a significant portion of people discontinue using them within a year.
The main problems with GLP-1 agonists are that they are expensive and have a fairly high rate of side effects — such as nausea, vomiting, diarrhea, or constipation. Another big one is muscle loss.
This lack of side effects, particularly in how the potential drug causes no muscle loss — and in fact engages muscle for some of its effect — sets it apart and makes it a potential alternative to GLP-1s. The key is not just reducing appetite but also increasing energy expenditure.
How It Works
The new approach targets a protein called NK2R — a member of the neurokinin receptor family, which has a role in a variety of physiological processes, including pain sensation, anxiety, and inflammation.
“We were looking to see genetic linkages to metabolic health, and there NK2R was,” said Zach Gerhart-Hines, PhD, a professor studying molecular metabolism at the University of Copenhagen in Denmark and principal investigator of the study. The group then created a few long-acting agonists that are selective for NK2R. So far, they’ve tested them in mice and nonhuman primates.
“The data on new medicines targeting NK2R is very promising and highlights the potential of both reducing food intake and increasing energy expenditure,” said Daniel Drucker, MD, an endocrinologist and researcher at Lunenfeld-Tanenbaum Research Institute in Toronto who was not involved in the study.
“The drug activates a certain region in the hindbrain of the animal, which is controlling food intake, and it does so by reducing appetite without increasing nausea or vomiting,” explained Frederike Sass, a research assistant at the University of Copenhagen in Copenhagen, Denmark, who led the study.
Gerhart-Hines said that even at the highest dose, there were no incidents of vomiting among the nonhuman primates. Mice can’t vomit, but there are ways to tell if they feel unwell from a drug. One way researchers test that is to start feeding the mice sweetened water at the same time they’re given a drug. Then later, when the mice are no longer on the drug, they’re given a choice between sweetened and unsweetened water. If they weren’t feeling well on the drug, they’ll choose plain water because they associate the sweet water with feeling bad, otherwise mice prefer sweet water. Sass said that with the NK2R agonist, they continued to drink sweet water after the treatment, whereas when they gave the mice semaglutide, the mice preferred plain water posttreatment.
The researchers also monitored the animals’ psychological health, as NK2R has been associated with anxiety, but they observed no behavioral changes.
The Key Mechanism at Work
One big question is how the NK2R agonists work. The amphetamines people used for weight loss during the 1950s and 1960s worked by making people more active. GLP-1 agonists reduce appetite and lower blood sugar. This is not that. In their studies with animals, the researchers didn’t observe that the animals were more active nor were there changes in other biomarkers like insulin. So far, the main difference they found with the NK2R agonists is an increase in thermogenesis in certain muscles.
Another benefit of the NK2R treatments is that they don’t seem to have a big impact on lean mass — the nonfat component of body weight, namely muscle, bones, and organs. Studies indicate that 25%-39% of weight loss on GLP-1 agonists is lost muscle. According to DEXA scans of the mice, Gerhart-Hines said they observed no lean mass loss. (In mice, he noted, GLP-1 agonists can cause up to 50% lean mass loss).
And for people with both diabetes and obesity, “what we found with NK2R is that obese and diabetic models, whether mice or monkeys, respond much better to that treatment in terms of glucose control and body weight loss,” Gerhart-Hines said. He explained that GLP-1 agonists don’t work quite as well for weight loss in people with diabetes because the drug stimulates insulin production in a system that already has insulin issues and can cause more sugar to be stored as fat.
Further, GLP-1 agonists are peptide drugs, which are expensive to make. The NK2R agonists are small molecules that would be cheaper to produce, Gerhart-Hines believes. One candidate they’re testing would likely be given once daily, another once weekly.
The current surge in obesity and diabetes may be a direct consequence of our bodies’ decreased energy expenditure. “Compared to 80s and 90s, the average person is more physically active, but the overarching basal resting energy expenditure has gone down,” said Gerhart-Hines, according to research by John Speakman at the University of Aberdeen, Scotland. We don’t know why, though, he said, but guesses it could be our diets or climate controlled environments.
But the NK2R agonists are among the many currently being studied for weight loss, and it may be hard to compete with the GLP-1 agonists. “As GLP-1 medicines will soon achieve 25% weight loss and have an extensively studied safety profile, the task of producing better drugs that work well in most people, are well tolerated and also reduce the complications of cardiometabolic disease, is challenging but not impossible,” said Drucker.
Gerhart-Hines said they plan to start trials in humans in the next year, but he suspects it will be another 6 or 7 years before it comes to market, if the trials are successful.
“There’s people who want [a GLP-1 agonist] and can’t even get it,” Gerhart-Hines said. As far as weight loss drugs, he noted, “we are not even saturating the market right now.”
A version of this article first appeared on Medscape.com.
FROM NATURE
Most Effective Treatments for Adult ADHD Identified
, results of a large comprehensive meta-analysis showed.
The study of 113 randomized controlled trials with nearly 15,000 adults with a formal diagnosis of ADHD also revealed that atomoxetine is less acceptable to patients and that results of efficacy of nonpharmacological strategies are inconsistent.
Data on long-term efficacy of ADHD therapies are lacking, investigators noted, so these results only apply to short-term efficacy.
“There is a lot of controversy about medication, so these are quite reassuring data and certainly reinforce the role of medication as a treatment for ADHD,” study investigator Samuele Cortese, MD, PhD, with University of Southampton, England, said during a press briefing hosted by the UK Science Media Center where the findings were released.
The results also point to the “possible role of nonpharmacological interventions, which are currently not well established in current guidelines. However, there is a need for better evidence to fully understand the exact effect of these nonpharmacological interventions,” Cortese noted.
The study was published online in The Lancet Psychiatry.
Bridging the Knowledge Gap
Once thought to be a childhood disorder only, ADHD is now well-known to persist into adulthood, affecting roughly 2.5% of the general adult population worldwide. The comparative benefits and harms of available interventions for ADHD in adults remain unclear.
To address this knowledge gap, researchers did a comprehensive systematic review and component network meta-analysis comparing a broad range of drug and nondrug treatments for adults with ADHD across several outcomes.
For reducing core ADHD symptoms at 12 weeks, only stimulants and atomoxetine were better than placebo in self-reported and clinician-reported rating scales, the study team found.
For stimulants, the standardized mean differences (SMDs) on the self-reported and clinician-reported scales were 0.39 and 0.61, respectively. The corresponding SMDs for atomoxetine were 0.38 and 0.51.
There was no evidence that ADHD medications were better than placebo in improving additional relevant outcomes such as quality of life.
In terms of nondrug interventions, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation were better than placebo only on clinician-reported measures, with SMDs of −1.35, −0.79, −0.77, and −0.78, respectively.
However, the evidence for nondrug strategies is less conclusive overall, with “discordant results across types of raters and based on a small body of evidence,” the authors wrote in their article.
And evidence for long-term efficacy (beyond 12 weeks) for ADHD interventions is “limited and under-investigated,” they said.
Regarding acceptability, all strategies were similar to placebo except for atomoxetine and guanfacine which had lower acceptability than placebo.
“It’s very important to emphasize that we focused on the average effect, not at an individual level,” first author Edoardo Ostinelli, MD, with University of Oxford, England, said at the briefing. “Therefore, we cannot make any recommendation at an individual level. We need studies with individual participant data so that we can personalize treatment.”
Cortese said the information from this analysis may be particularly important for “psychoeducation” of the patient before actually starting with a treatment plan. Patients often ask about nonpharmacological interventions and this study provides the “best synthesis of available data to inform these discussions,” he said.
Experts Weigh In
Several experts weighed in on the results in a statement from the UK Science Media Center.
Celso Arango, MD, PhD, psychiatrist with Gregorio Marañón General University Hospital, Madrid, Spain, noted that there is a “clear shortage of research on ADHD in adulthood, particularly regarding medium-term (beyond 12 weeks) and long-term treatment outcomes. Consequently, the findings are applicable only to short-term treatment.”
Another strength of the study is that it was developed with input from people with ADHD, Arango added, making it “highly relevant.”
The majority of studies available for the analysis involved pharmacological treatments, which is important to consider when interpreting the findings, noted Katya Rubia, PhD, professor of cognitive neuroscience, King’s College London, England.
“For example, for neurostimulation, only 10 studies were included and on very heterogeneous stimulation methods,” Rubia said. “The evidence on the efficacy of neurostimulation is therefore hardly conclusive and more studies are needed to establish their efficacy.”
Roi Cohen Kadosh, PhD, professor of cognitive neuroscience, University of Surrey, Guildford, England, agreed. While the study is a “valuable contribution to the literature,” it sheds light on “both the scarcity of neurostimulation research and the limited exploration of combined treatment approaches for ADHD,” he said.
“While novel neurostimulation methods linked to neuroplasticity — such as those we have demonstrated to be superior in children with ADHD — were not covered here, they have shown promising and lasting benefits. In contrast, research in adults remains relatively underdeveloped. Moving forward, greater emphasis on innovative, tolerable, personalized, and sustainable neurostimulation approaches is essential to meet the unmet clinical needs of adults with ADHD,” Kadosh added.
In a commentary in The Lancet Psychiatry, David Coghill, MD, with The University of Melbourne, Australia, cautioned that the findings do not mean that potential benefits of nonpharmacological interventions should be dismissed.
“While some of the nonpharmacological treatments (eg, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation) showed effects on clinician-rated outcomes similar to, and in some cases greater than, the pharmacological treatments, they did not show the same effects on self-reported outcomes. These interventions were therefore considered less robust than the pharmacological treatments that showed changes on both measurement types,” he wrote.
This study had no commercial funding. Ostinelli had received research and consultancy fees from Angelini Pharma. Cortese received reimbursement for travel and accommodation expenses in relation to lectures delivered for the Association for Child and Adolescent Central Health, the Canadian ADHD Alliance Resource, and the British Association of Psychopharmacology; and had received honoraria from MEDICE; and is chair of the European ADHD Guidelines Group. Arango, Rubia, and Kadosh had no relevant disclosures. Coghill had received honoraria from CCM Conecta, Takeda, Novartis, Servier, and MEDICE.
A version of this article first appeared on Medscape.com.
, results of a large comprehensive meta-analysis showed.
The study of 113 randomized controlled trials with nearly 15,000 adults with a formal diagnosis of ADHD also revealed that atomoxetine is less acceptable to patients and that results of efficacy of nonpharmacological strategies are inconsistent.
Data on long-term efficacy of ADHD therapies are lacking, investigators noted, so these results only apply to short-term efficacy.
“There is a lot of controversy about medication, so these are quite reassuring data and certainly reinforce the role of medication as a treatment for ADHD,” study investigator Samuele Cortese, MD, PhD, with University of Southampton, England, said during a press briefing hosted by the UK Science Media Center where the findings were released.
The results also point to the “possible role of nonpharmacological interventions, which are currently not well established in current guidelines. However, there is a need for better evidence to fully understand the exact effect of these nonpharmacological interventions,” Cortese noted.
The study was published online in The Lancet Psychiatry.
Bridging the Knowledge Gap
Once thought to be a childhood disorder only, ADHD is now well-known to persist into adulthood, affecting roughly 2.5% of the general adult population worldwide. The comparative benefits and harms of available interventions for ADHD in adults remain unclear.
To address this knowledge gap, researchers did a comprehensive systematic review and component network meta-analysis comparing a broad range of drug and nondrug treatments for adults with ADHD across several outcomes.
For reducing core ADHD symptoms at 12 weeks, only stimulants and atomoxetine were better than placebo in self-reported and clinician-reported rating scales, the study team found.
For stimulants, the standardized mean differences (SMDs) on the self-reported and clinician-reported scales were 0.39 and 0.61, respectively. The corresponding SMDs for atomoxetine were 0.38 and 0.51.
There was no evidence that ADHD medications were better than placebo in improving additional relevant outcomes such as quality of life.
In terms of nondrug interventions, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation were better than placebo only on clinician-reported measures, with SMDs of −1.35, −0.79, −0.77, and −0.78, respectively.
However, the evidence for nondrug strategies is less conclusive overall, with “discordant results across types of raters and based on a small body of evidence,” the authors wrote in their article.
And evidence for long-term efficacy (beyond 12 weeks) for ADHD interventions is “limited and under-investigated,” they said.
Regarding acceptability, all strategies were similar to placebo except for atomoxetine and guanfacine which had lower acceptability than placebo.
“It’s very important to emphasize that we focused on the average effect, not at an individual level,” first author Edoardo Ostinelli, MD, with University of Oxford, England, said at the briefing. “Therefore, we cannot make any recommendation at an individual level. We need studies with individual participant data so that we can personalize treatment.”
Cortese said the information from this analysis may be particularly important for “psychoeducation” of the patient before actually starting with a treatment plan. Patients often ask about nonpharmacological interventions and this study provides the “best synthesis of available data to inform these discussions,” he said.
Experts Weigh In
Several experts weighed in on the results in a statement from the UK Science Media Center.
Celso Arango, MD, PhD, psychiatrist with Gregorio Marañón General University Hospital, Madrid, Spain, noted that there is a “clear shortage of research on ADHD in adulthood, particularly regarding medium-term (beyond 12 weeks) and long-term treatment outcomes. Consequently, the findings are applicable only to short-term treatment.”
Another strength of the study is that it was developed with input from people with ADHD, Arango added, making it “highly relevant.”
The majority of studies available for the analysis involved pharmacological treatments, which is important to consider when interpreting the findings, noted Katya Rubia, PhD, professor of cognitive neuroscience, King’s College London, England.
“For example, for neurostimulation, only 10 studies were included and on very heterogeneous stimulation methods,” Rubia said. “The evidence on the efficacy of neurostimulation is therefore hardly conclusive and more studies are needed to establish their efficacy.”
Roi Cohen Kadosh, PhD, professor of cognitive neuroscience, University of Surrey, Guildford, England, agreed. While the study is a “valuable contribution to the literature,” it sheds light on “both the scarcity of neurostimulation research and the limited exploration of combined treatment approaches for ADHD,” he said.
“While novel neurostimulation methods linked to neuroplasticity — such as those we have demonstrated to be superior in children with ADHD — were not covered here, they have shown promising and lasting benefits. In contrast, research in adults remains relatively underdeveloped. Moving forward, greater emphasis on innovative, tolerable, personalized, and sustainable neurostimulation approaches is essential to meet the unmet clinical needs of adults with ADHD,” Kadosh added.
In a commentary in The Lancet Psychiatry, David Coghill, MD, with The University of Melbourne, Australia, cautioned that the findings do not mean that potential benefits of nonpharmacological interventions should be dismissed.
“While some of the nonpharmacological treatments (eg, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation) showed effects on clinician-rated outcomes similar to, and in some cases greater than, the pharmacological treatments, they did not show the same effects on self-reported outcomes. These interventions were therefore considered less robust than the pharmacological treatments that showed changes on both measurement types,” he wrote.
This study had no commercial funding. Ostinelli had received research and consultancy fees from Angelini Pharma. Cortese received reimbursement for travel and accommodation expenses in relation to lectures delivered for the Association for Child and Adolescent Central Health, the Canadian ADHD Alliance Resource, and the British Association of Psychopharmacology; and had received honoraria from MEDICE; and is chair of the European ADHD Guidelines Group. Arango, Rubia, and Kadosh had no relevant disclosures. Coghill had received honoraria from CCM Conecta, Takeda, Novartis, Servier, and MEDICE.
A version of this article first appeared on Medscape.com.
, results of a large comprehensive meta-analysis showed.
The study of 113 randomized controlled trials with nearly 15,000 adults with a formal diagnosis of ADHD also revealed that atomoxetine is less acceptable to patients and that results of efficacy of nonpharmacological strategies are inconsistent.
Data on long-term efficacy of ADHD therapies are lacking, investigators noted, so these results only apply to short-term efficacy.
“There is a lot of controversy about medication, so these are quite reassuring data and certainly reinforce the role of medication as a treatment for ADHD,” study investigator Samuele Cortese, MD, PhD, with University of Southampton, England, said during a press briefing hosted by the UK Science Media Center where the findings were released.
The results also point to the “possible role of nonpharmacological interventions, which are currently not well established in current guidelines. However, there is a need for better evidence to fully understand the exact effect of these nonpharmacological interventions,” Cortese noted.
The study was published online in The Lancet Psychiatry.
Bridging the Knowledge Gap
Once thought to be a childhood disorder only, ADHD is now well-known to persist into adulthood, affecting roughly 2.5% of the general adult population worldwide. The comparative benefits and harms of available interventions for ADHD in adults remain unclear.
To address this knowledge gap, researchers did a comprehensive systematic review and component network meta-analysis comparing a broad range of drug and nondrug treatments for adults with ADHD across several outcomes.
For reducing core ADHD symptoms at 12 weeks, only stimulants and atomoxetine were better than placebo in self-reported and clinician-reported rating scales, the study team found.
For stimulants, the standardized mean differences (SMDs) on the self-reported and clinician-reported scales were 0.39 and 0.61, respectively. The corresponding SMDs for atomoxetine were 0.38 and 0.51.
There was no evidence that ADHD medications were better than placebo in improving additional relevant outcomes such as quality of life.
In terms of nondrug interventions, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation were better than placebo only on clinician-reported measures, with SMDs of −1.35, −0.79, −0.77, and −0.78, respectively.
However, the evidence for nondrug strategies is less conclusive overall, with “discordant results across types of raters and based on a small body of evidence,” the authors wrote in their article.
And evidence for long-term efficacy (beyond 12 weeks) for ADHD interventions is “limited and under-investigated,” they said.
Regarding acceptability, all strategies were similar to placebo except for atomoxetine and guanfacine which had lower acceptability than placebo.
“It’s very important to emphasize that we focused on the average effect, not at an individual level,” first author Edoardo Ostinelli, MD, with University of Oxford, England, said at the briefing. “Therefore, we cannot make any recommendation at an individual level. We need studies with individual participant data so that we can personalize treatment.”
Cortese said the information from this analysis may be particularly important for “psychoeducation” of the patient before actually starting with a treatment plan. Patients often ask about nonpharmacological interventions and this study provides the “best synthesis of available data to inform these discussions,” he said.
Experts Weigh In
Several experts weighed in on the results in a statement from the UK Science Media Center.
Celso Arango, MD, PhD, psychiatrist with Gregorio Marañón General University Hospital, Madrid, Spain, noted that there is a “clear shortage of research on ADHD in adulthood, particularly regarding medium-term (beyond 12 weeks) and long-term treatment outcomes. Consequently, the findings are applicable only to short-term treatment.”
Another strength of the study is that it was developed with input from people with ADHD, Arango added, making it “highly relevant.”
The majority of studies available for the analysis involved pharmacological treatments, which is important to consider when interpreting the findings, noted Katya Rubia, PhD, professor of cognitive neuroscience, King’s College London, England.
“For example, for neurostimulation, only 10 studies were included and on very heterogeneous stimulation methods,” Rubia said. “The evidence on the efficacy of neurostimulation is therefore hardly conclusive and more studies are needed to establish their efficacy.”
Roi Cohen Kadosh, PhD, professor of cognitive neuroscience, University of Surrey, Guildford, England, agreed. While the study is a “valuable contribution to the literature,” it sheds light on “both the scarcity of neurostimulation research and the limited exploration of combined treatment approaches for ADHD,” he said.
“While novel neurostimulation methods linked to neuroplasticity — such as those we have demonstrated to be superior in children with ADHD — were not covered here, they have shown promising and lasting benefits. In contrast, research in adults remains relatively underdeveloped. Moving forward, greater emphasis on innovative, tolerable, personalized, and sustainable neurostimulation approaches is essential to meet the unmet clinical needs of adults with ADHD,” Kadosh added.
In a commentary in The Lancet Psychiatry, David Coghill, MD, with The University of Melbourne, Australia, cautioned that the findings do not mean that potential benefits of nonpharmacological interventions should be dismissed.
“While some of the nonpharmacological treatments (eg, cognitive behavioral therapy, cognitive remediation, mindfulness, psychoeducation, and transcranial direct current stimulation) showed effects on clinician-rated outcomes similar to, and in some cases greater than, the pharmacological treatments, they did not show the same effects on self-reported outcomes. These interventions were therefore considered less robust than the pharmacological treatments that showed changes on both measurement types,” he wrote.
This study had no commercial funding. Ostinelli had received research and consultancy fees from Angelini Pharma. Cortese received reimbursement for travel and accommodation expenses in relation to lectures delivered for the Association for Child and Adolescent Central Health, the Canadian ADHD Alliance Resource, and the British Association of Psychopharmacology; and had received honoraria from MEDICE; and is chair of the European ADHD Guidelines Group. Arango, Rubia, and Kadosh had no relevant disclosures. Coghill had received honoraria from CCM Conecta, Takeda, Novartis, Servier, and MEDICE.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY