User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
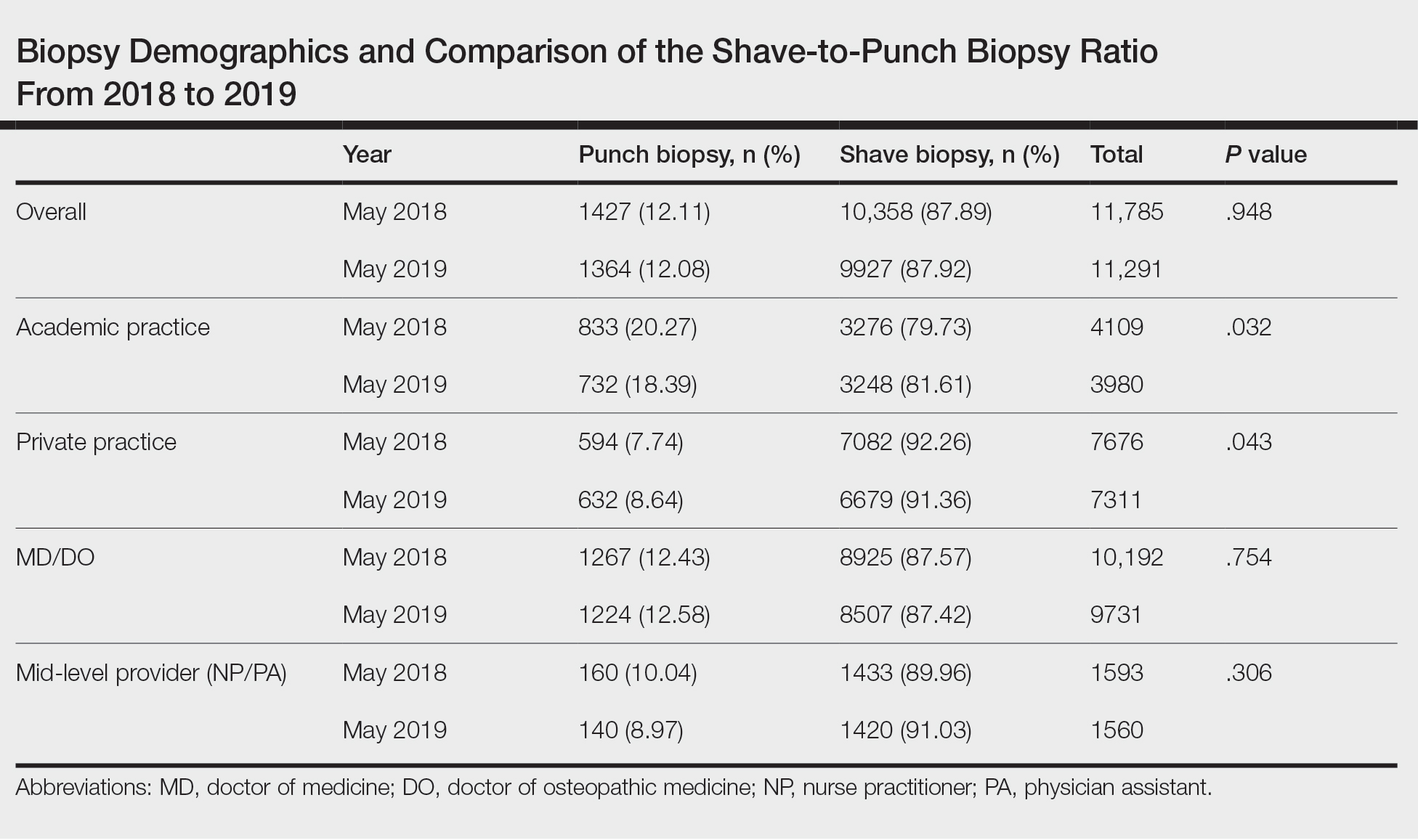
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
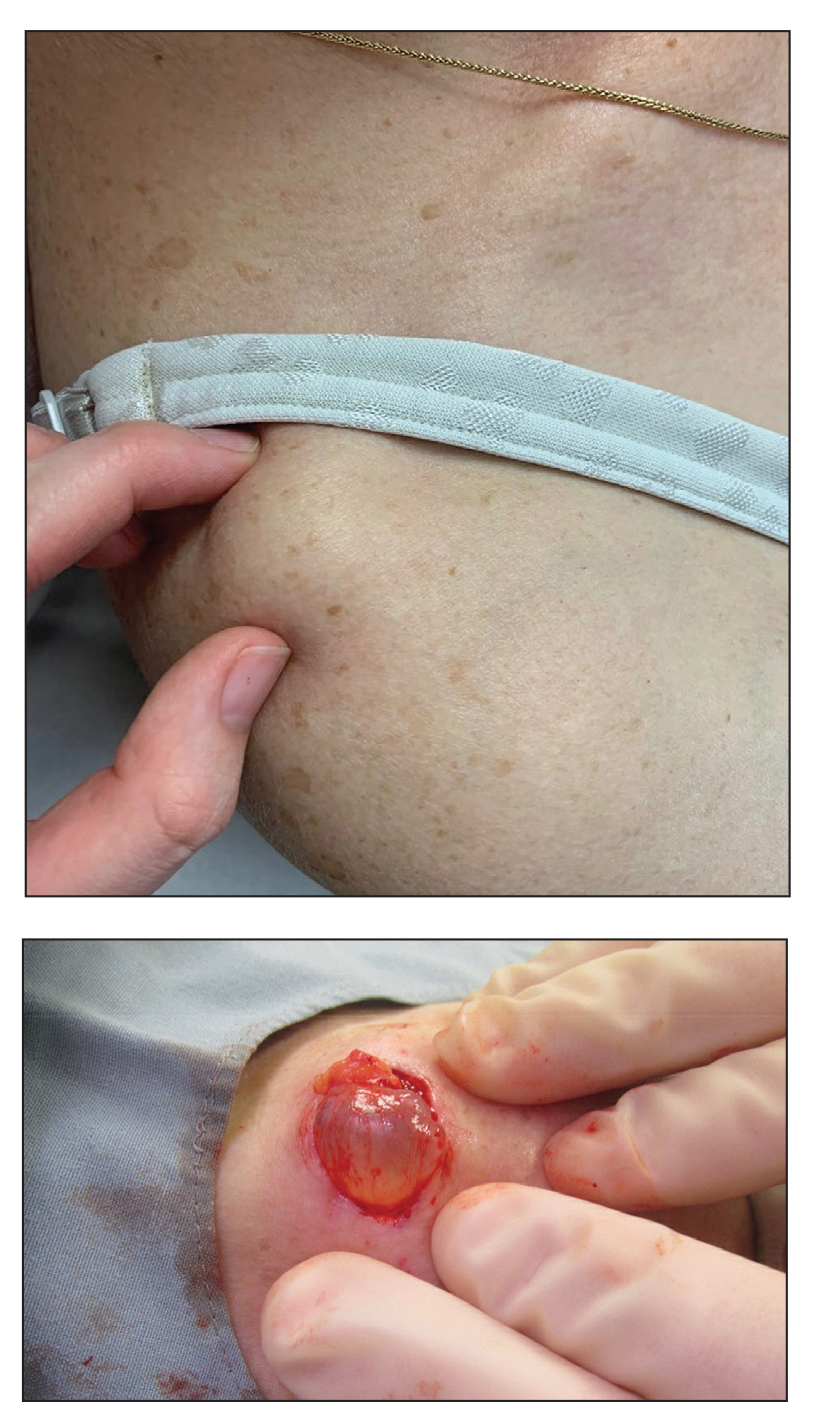
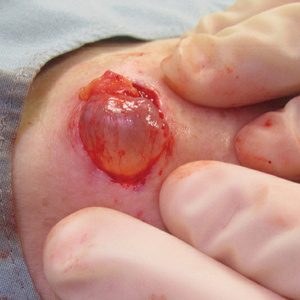
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
Upper Lip Anatomy, Mechanics of Local Flaps, and Considerations for Reconstruction
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
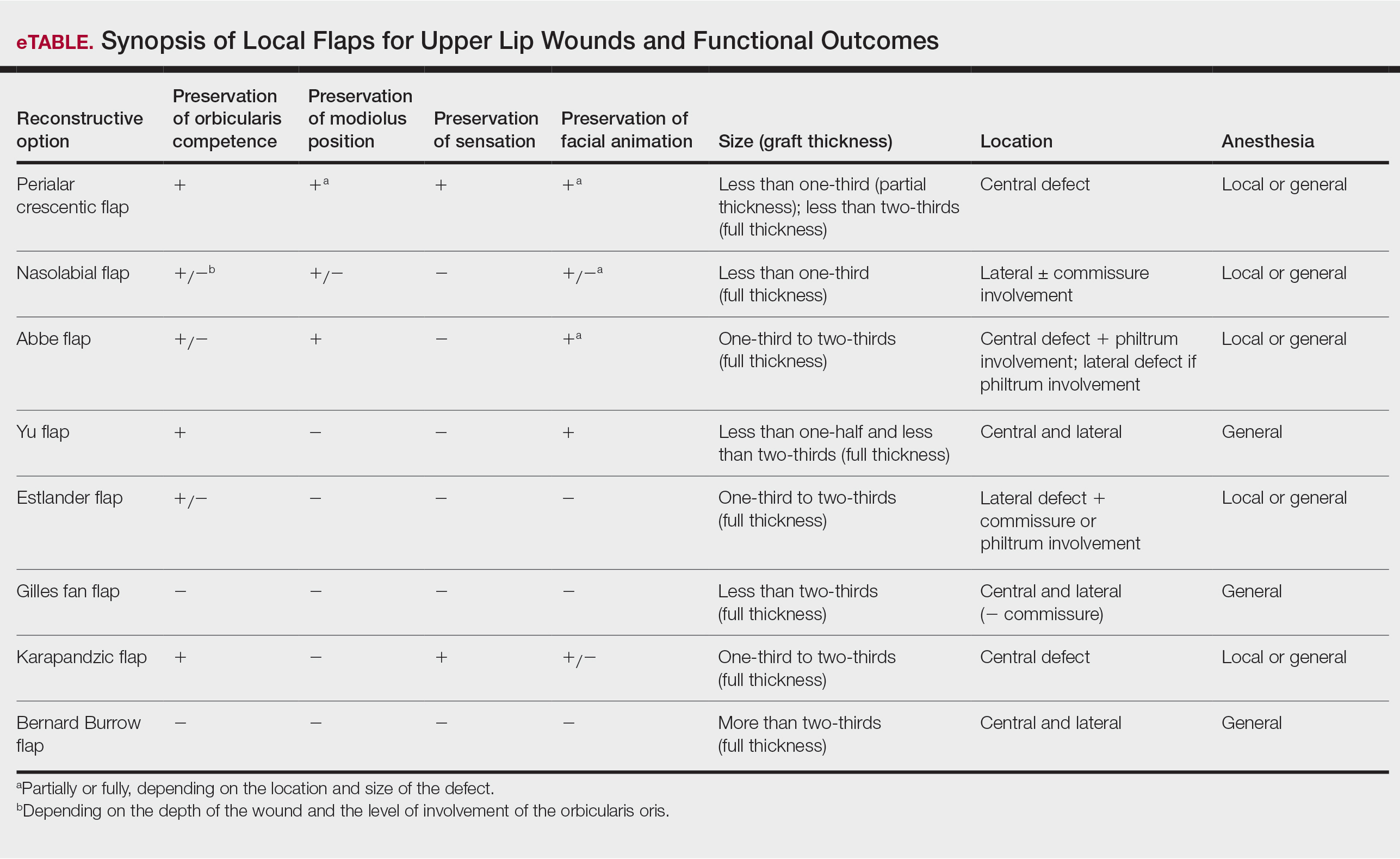
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
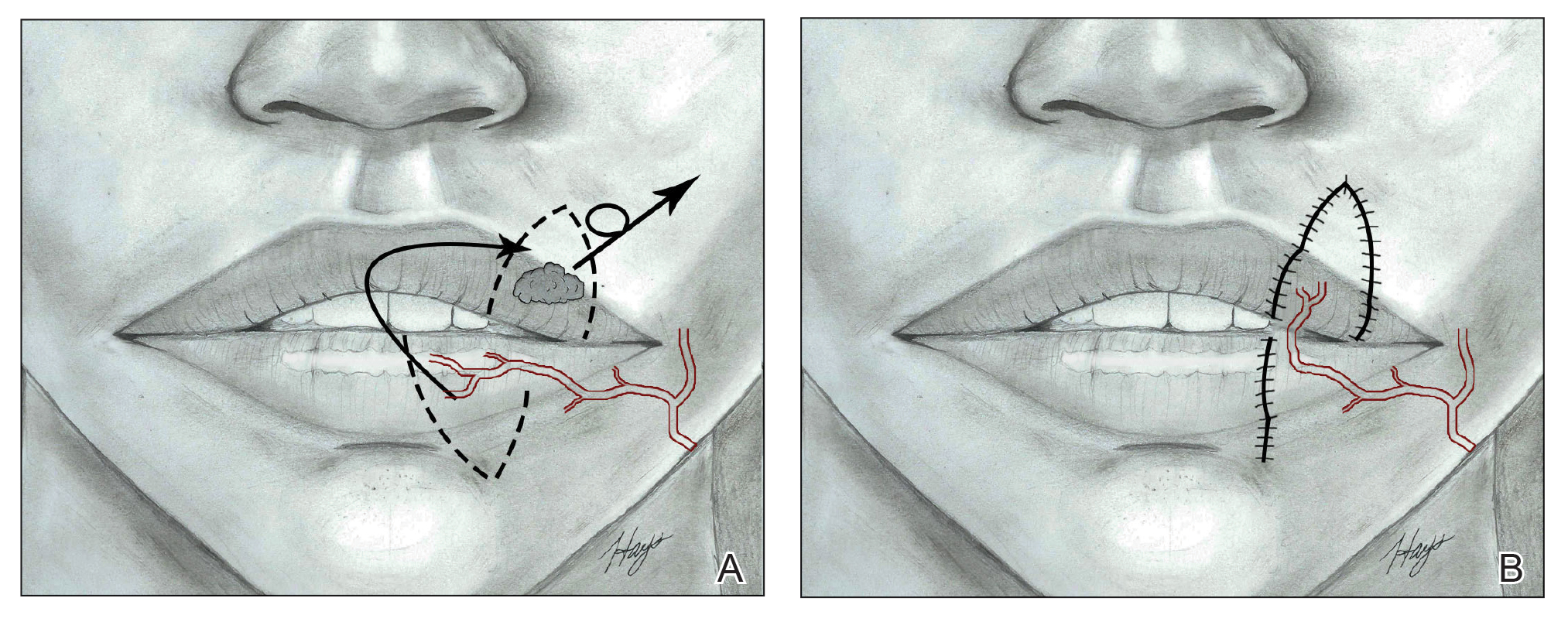
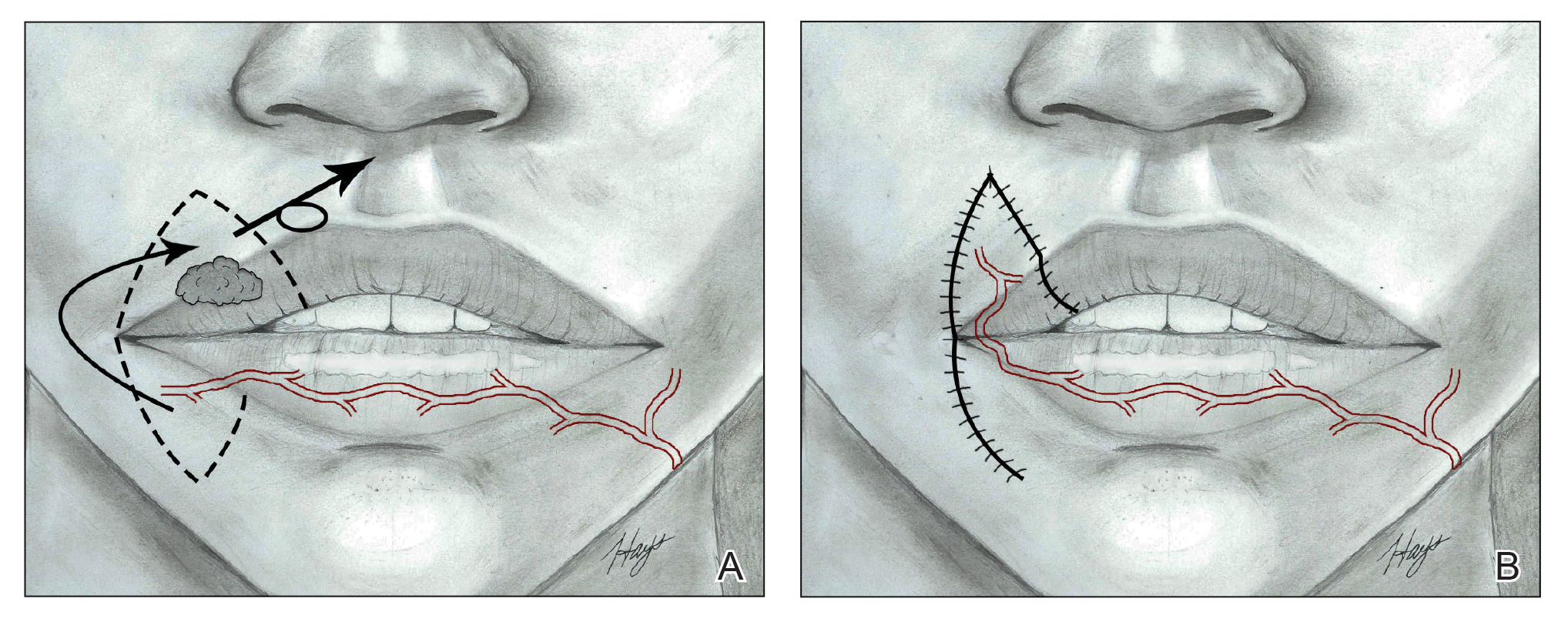
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
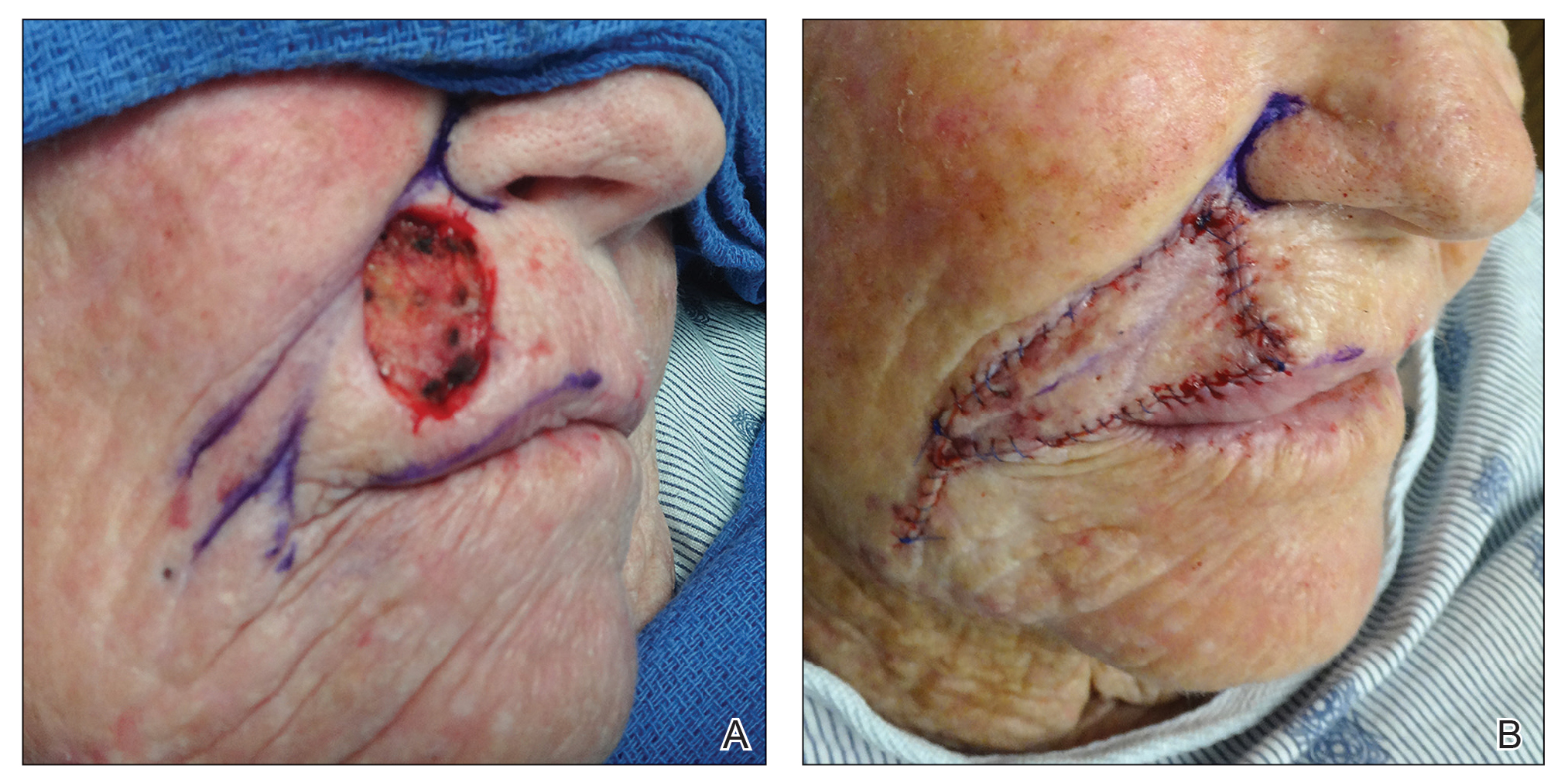
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
Contact Dermatitis of the Hands: Is It Irritant or Allergic?
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Practice Points
- For the hands, irritant contact dermatitis (ICD) is more common than allergic contact dermatitis in both occupational and nonoccupational settings. Because of overlapping clinical features, it can be difficult to differentiate between these conditions.
- Use of hand hygiene products, frequent handwashing, wet work, mechanical trauma, and occlusion can contribute to ICD of the hands.
- Common hand contact allergens include preservatives, metals, fragrances, and rubber accelerators.
- Patch testing often is necessary for diagnosis of hand dermatitis, and both screening and supplemental allergen series may be required.
The Importance of Service Learning in Dermatology Residency: An Actionable Approach to Improve Resident Education and Skin Health Equity
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Practice Points
- In 2019, nearly 30 million Americans did not have health insurance. Dermatologists in the United States should be cognizant of the challenges faced by underserved patients when accessing dermatologic care.
- Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive learning experience, meet societal needs, and fulfill Accreditation Council for Graduate Medical Education requirements.
- Actionable methods to increase service learning in dermatology residency training include volunteering in community service programs offered by national dermatology organizations, implementing service-learning projects, and partnering with free and federally funded community practices.
- Dermatology residents who participate in service learning will help increase access to equitable dermatologic care and experience practicing in settings with limited resources.
Onychomycosis: New Developments in Diagnosis, Treatment, and Antifungal Medication Safety
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
Hyperkeratotic Nummular Plaques on the Upper Trunk
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
A 48-year-old woman with a history of type 2 diabetes mellitus and nonalcoholic steatohepatitis presented with papules and plaques on the upper trunk, proximal extremities, and volar wrists. Clear fluid–filled bullae occasionally developed within the plaques and subsequently ruptured and healed. Aside from intermittent lesion tenderness and irritation with the formation and rupture of the bullae, the patient’s plaques were asymptomatic, and she specifically denied pruritus. A review of systems revealed a history of genital irritation evaluated by a gynecologist; nystatin–triamcinolone cream 0.1% applied as needed provided relief. The patient denied any recent flares or any new or changing oral mucosa findings or symptoms, preceding medications, or family history of similar lesions. Physical examination revealed well-demarcated, round, pink plaques with keratotic scale scattered across the upper trunk and central chest. The bilateral volar wrists were surfaced by well-circumscribed, thin, pink to violaceous, hyperkeratotic papules.
The Genital Examination in Dermatologic Practice
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
Resident Pearls
- Dermatologists should offer a genital examination to all patients who present for a routine total-body skin examination.
- It is critical to educate patients about the importance of examining the genital skin by discussing that skin diseases can arise in all areas of the body including the genital area. Encouraging genital self-examination also is helpful.
- If a patient declines, the dermatologist should strive to ensure that another provider is examining the genital skin.
Acquired Unilateral Nevoid Telangiectasia With Pruritus and Unknown Etiology
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.
Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
Practice Points
- Unilateral nevoid telangiectasia may present in patients without an underlying hyperestrogenic state.
- Unilateral nevoid telangiectasia may present with symptoms including pruritus.
Painless Mobile Nodule on the Shoulder
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
A 70-year-old woman presented to the outpatient dermatology clinic with an acute-onset lesion on the right shoulder. She first noticed a “cyst” developing in the area approximately 3 weeks prior but noted that it may have been present longer. The lesion was bothersome when her undergarments rubbed against it, but she otherwise denied pain, increase in size, or drainage from the site. Her medical history was remarkable for a proliferating trichilemmal tumor on the right parietal scalp treated with Mohs surgery approximately 13 years prior to presentation. She had no personal or family history of skin cancer. Physical examination revealed a 2.5-cm, mobile, nontender, flesh-colored subcutaneous nodule on the right shoulder (top); no ulceration, bleeding, or drainage was present. The surrounding skin demonstrated no clinical changes. The patient was scheduled for outpatient surgical excision of the nodule, which initially was suspected to be a lipoma. During the excision, a translucent cystlike nodule (bottom) was gently dissected and sent for histopathologic examination.