User login
Painless Mobile Nodule on the Shoulder
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
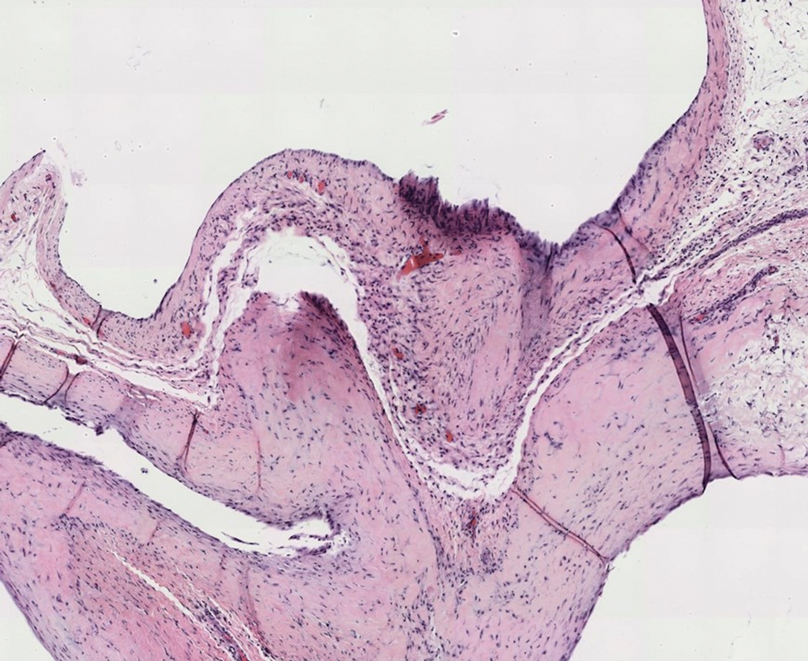
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
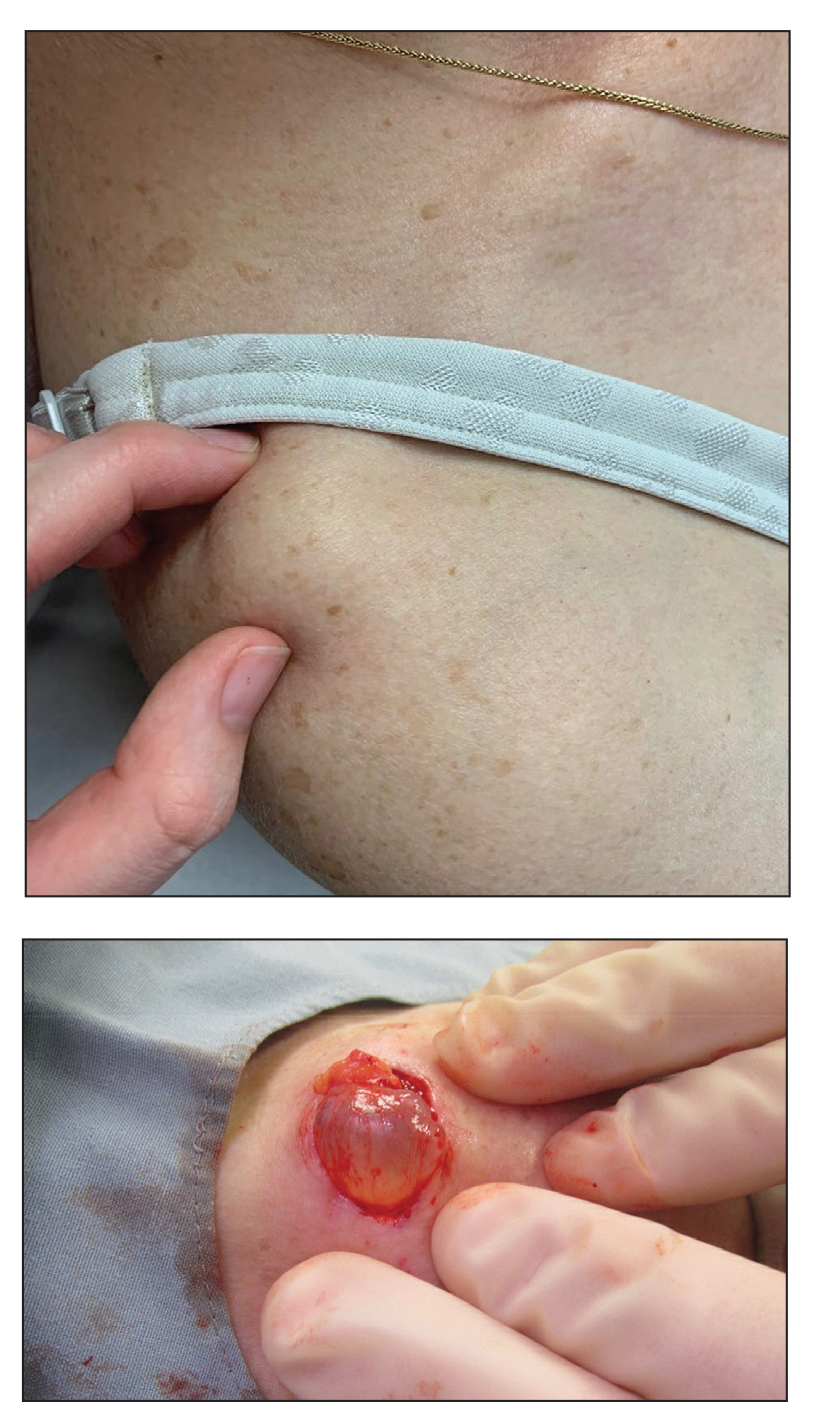
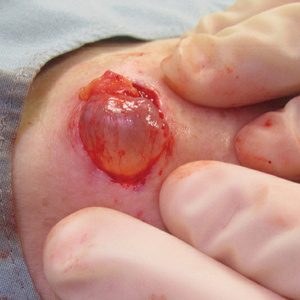
A 70-year-old woman presented to the outpatient dermatology clinic with an acute-onset lesion on the right shoulder. She first noticed a “cyst” developing in the area approximately 3 weeks prior but noted that it may have been present longer. The lesion was bothersome when her undergarments rubbed against it, but she otherwise denied pain, increase in size, or drainage from the site. Her medical history was remarkable for a proliferating trichilemmal tumor on the right parietal scalp treated with Mohs surgery approximately 13 years prior to presentation. She had no personal or family history of skin cancer. Physical examination revealed a 2.5-cm, mobile, nontender, flesh-colored subcutaneous nodule on the right shoulder (top); no ulceration, bleeding, or drainage was present. The surrounding skin demonstrated no clinical changes. The patient was scheduled for outpatient surgical excision of the nodule, which initially was suspected to be a lipoma. During the excision, a translucent cystlike nodule (bottom) was gently dissected and sent for histopathologic examination.
Black Adherence Nodules on the Scalp Hair Shaft
The Diagnosis: Piedra
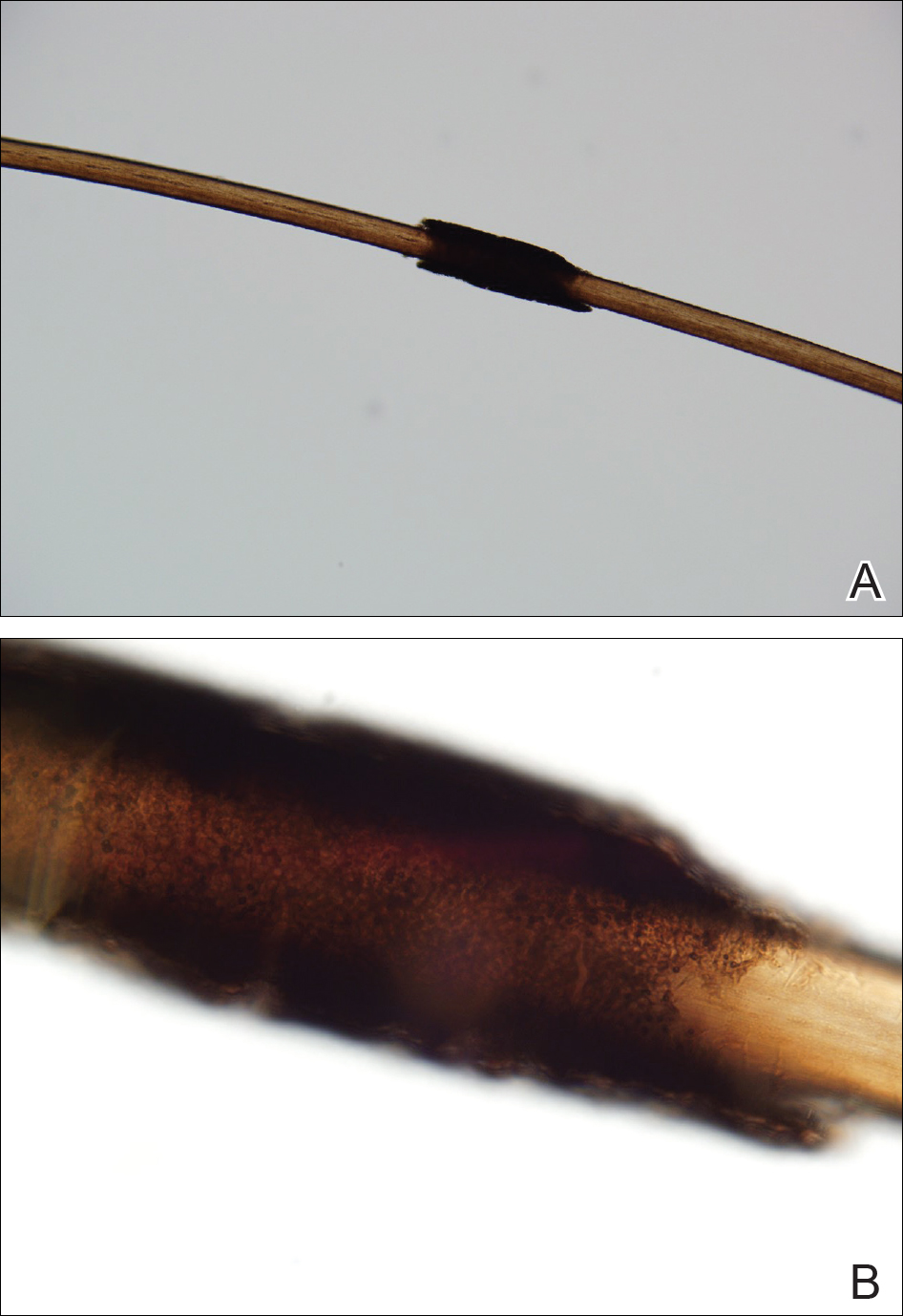
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).
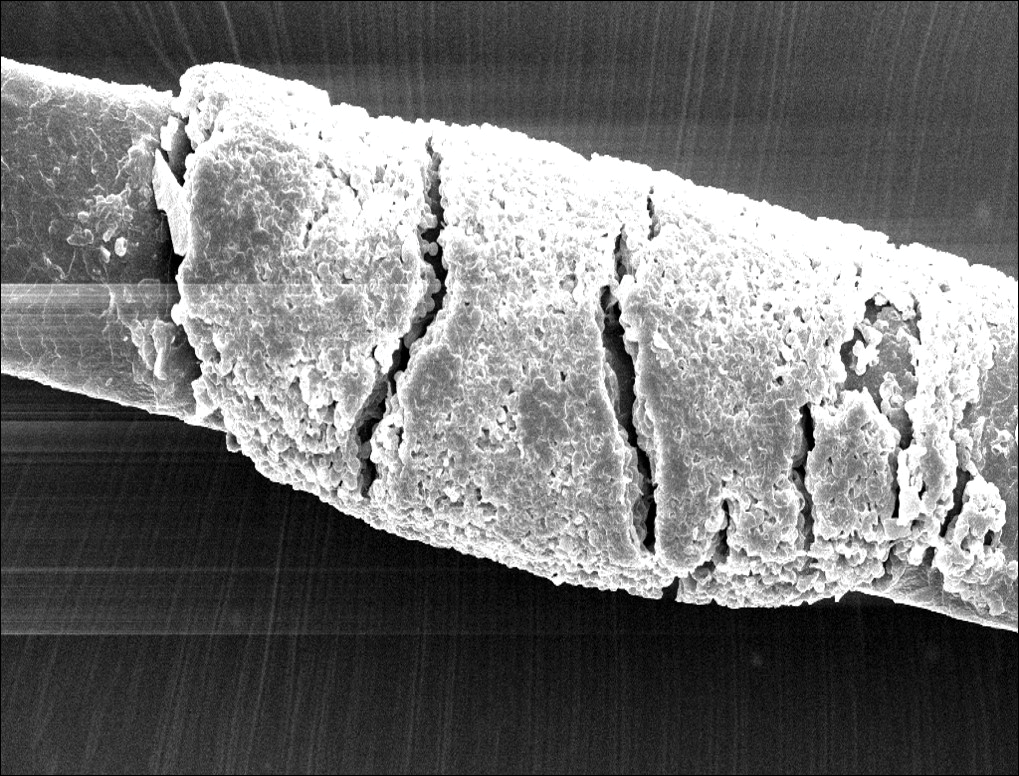
Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.

A 21-year-old woman presented to the dermatology clinic with what she described as small black dots in her hair that she first noted 3 months prior to presentation. The black nodules were asymptomatic, but the patient noted that they seemed to be moving up the hair shaft. They were firmly attached and great effort was required to remove them. The patient's sister recently developed similar nodules. The patient and her sister work as missionaries and had spent time in India, Southeast Asia, and Central America within the last few years. Physical examination revealed firmly adherent black nodules involving the mid to distal portions of the hair shafts on the scalp. There were no nail or skin findings. Cultures were obtained, and microscopic examination was performed.