User login
What’s Eating You? Caterpillars
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
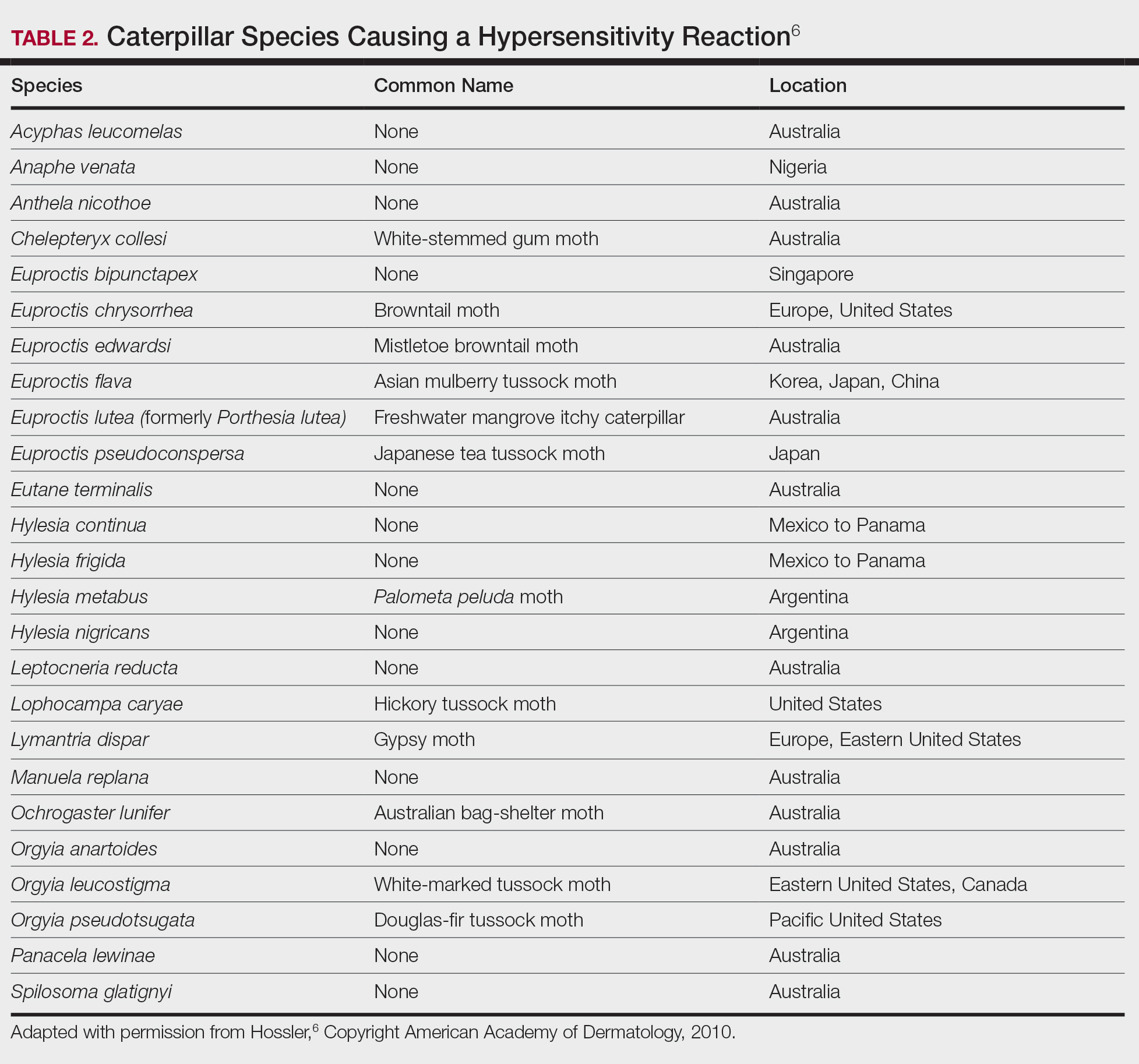
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
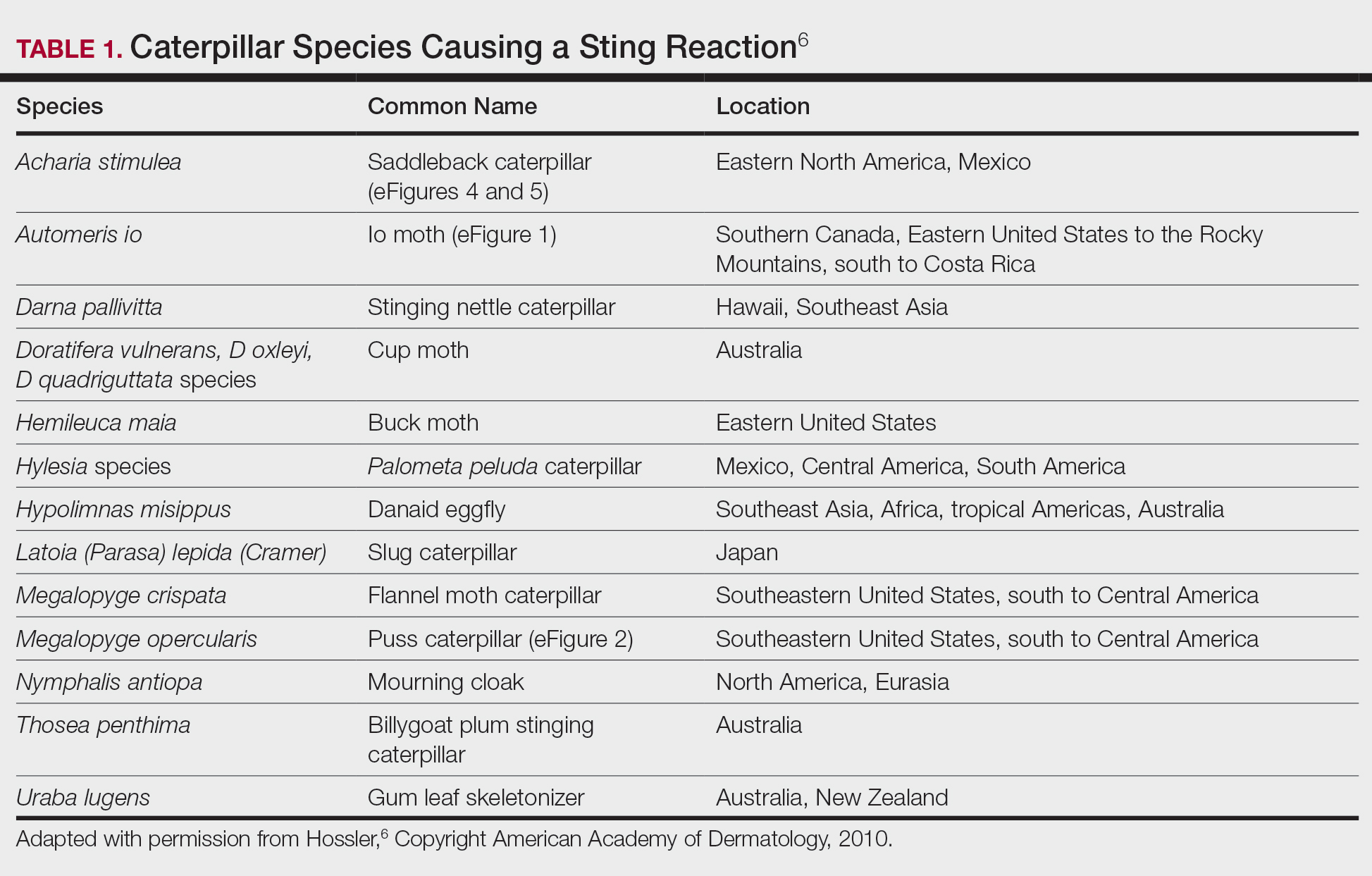
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
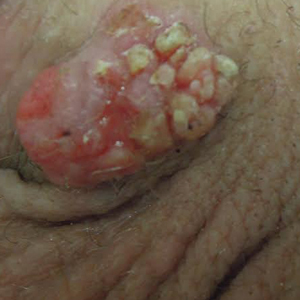
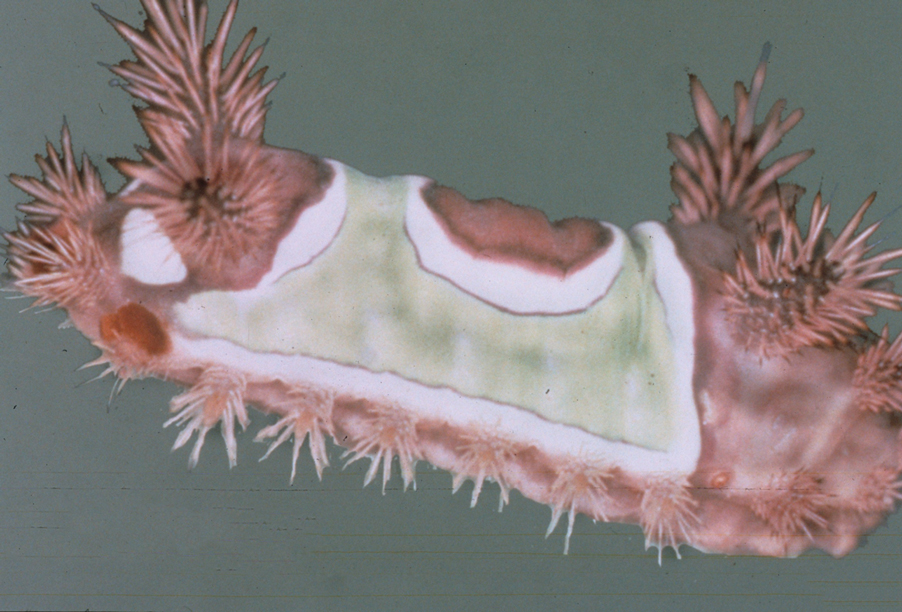
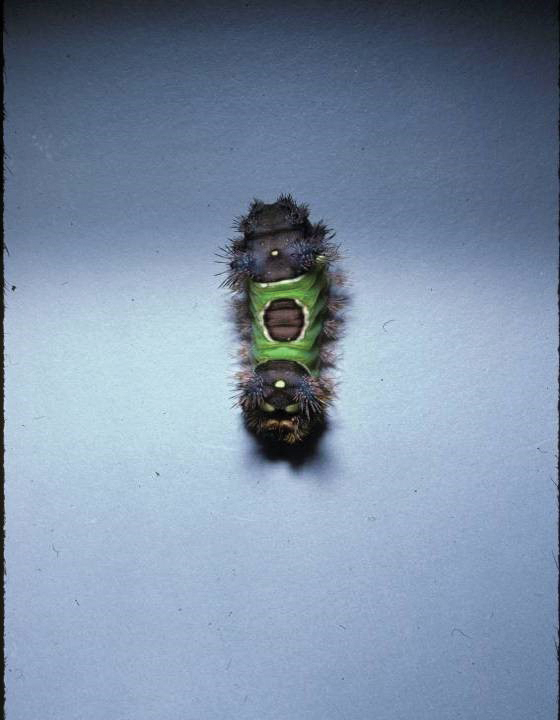
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
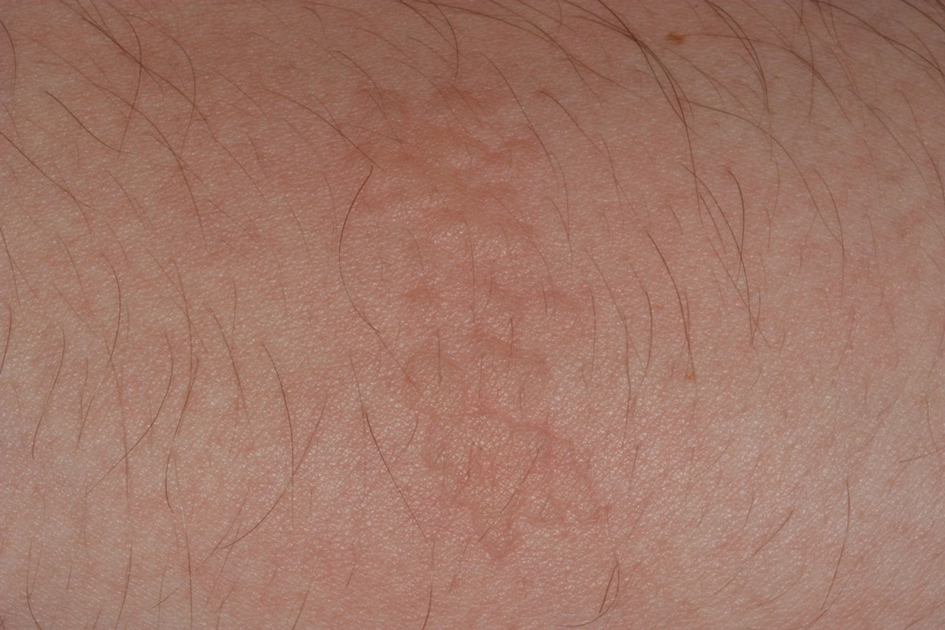



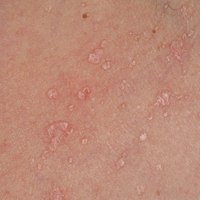
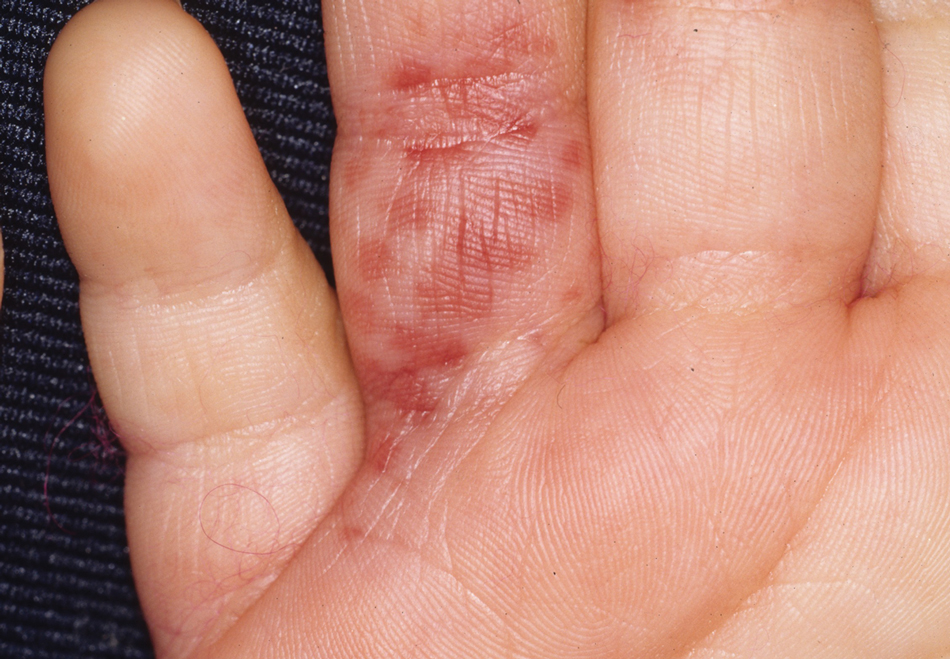
One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.


Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
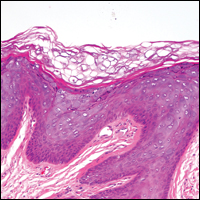
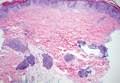
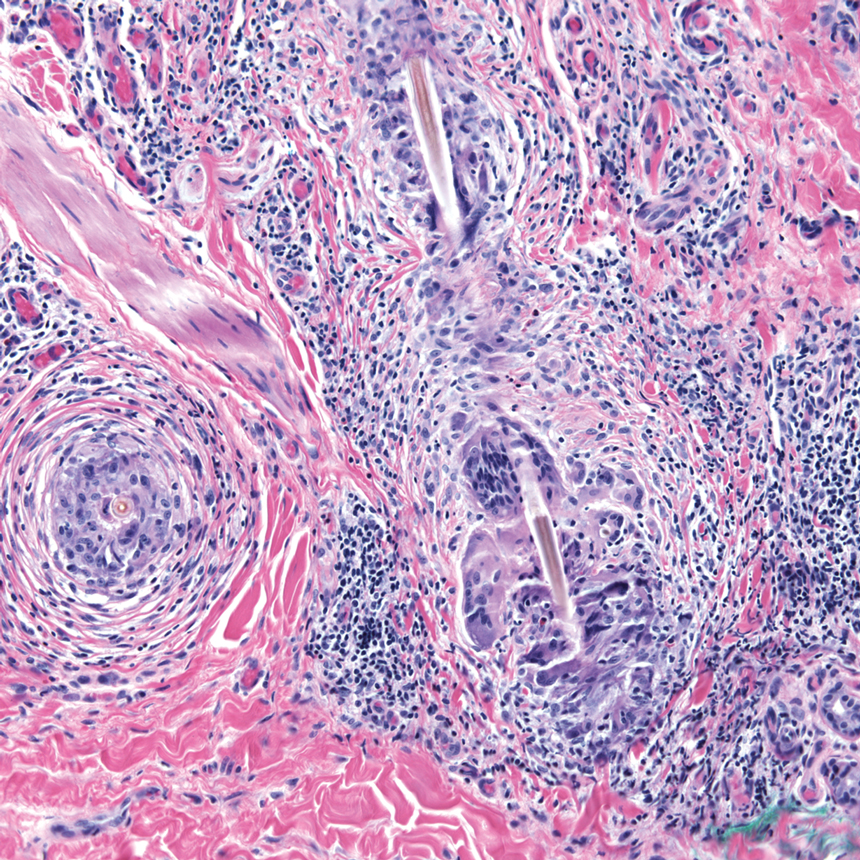
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
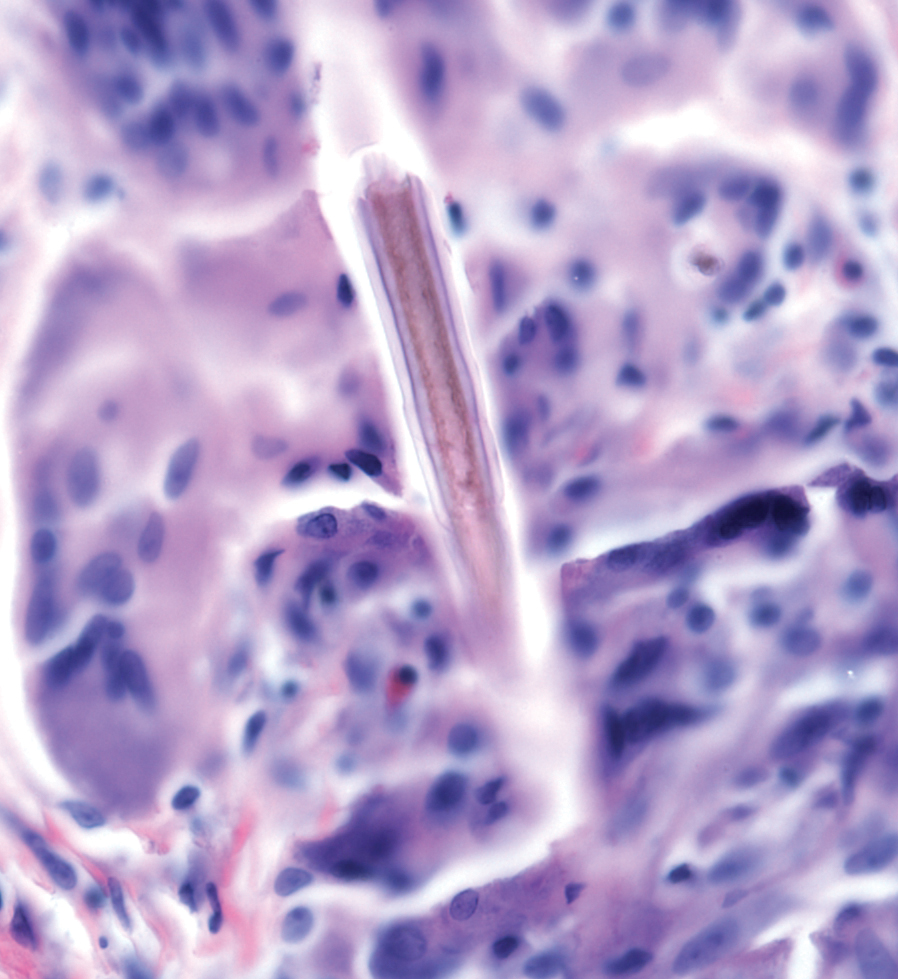
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6



Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties

Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.




One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.



Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.


Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Practice Points
- Lepidopterism describes adverse reactions caused by the stings, hypersensitivity reactions, and lonomism (a hemorrhagic diathesis) of caterpillars, moths, and butterflies.
- Caterpillars can induce an adverse reaction by injecting venom stored in their bristles, inducing a foreign-body reaction to embedded bristles, or a combination of these mechanisms.
- A thorough history, skin scrapings, relevant examination of affected body parts (such as slit-lamp examination, in the case of eyes), and laboratory testing should be conducted to narrow the wide differential diagnosis associated with lepidopterism.
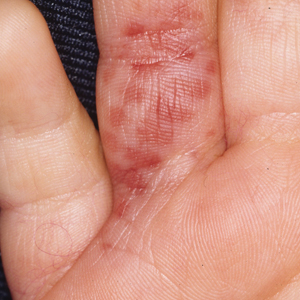
Painless Mobile Nodule on the Shoulder
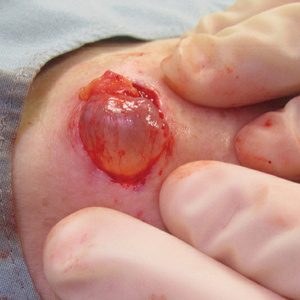
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
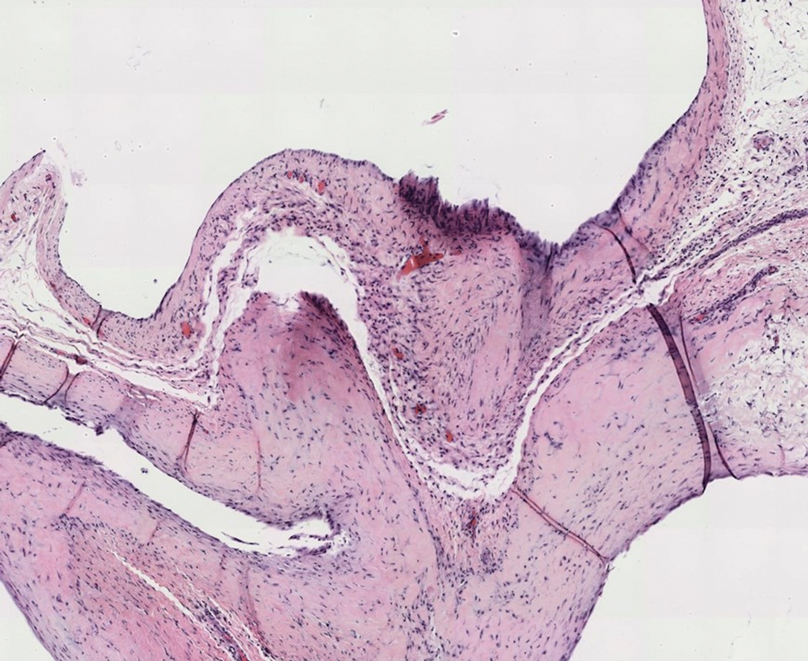
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
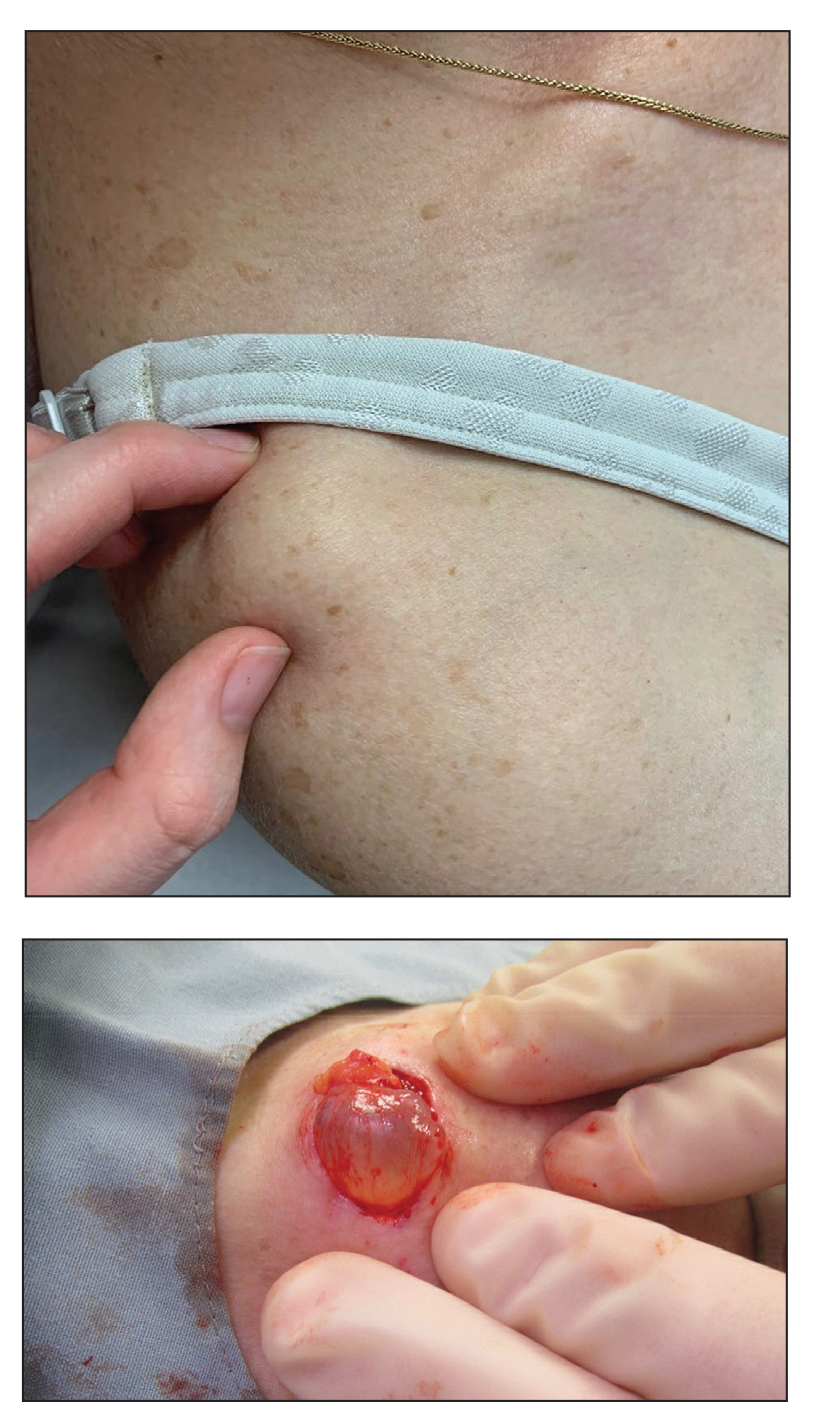
A 70-year-old woman presented to the outpatient dermatology clinic with an acute-onset lesion on the right shoulder. She first noticed a “cyst” developing in the area approximately 3 weeks prior but noted that it may have been present longer. The lesion was bothersome when her undergarments rubbed against it, but she otherwise denied pain, increase in size, or drainage from the site. Her medical history was remarkable for a proliferating trichilemmal tumor on the right parietal scalp treated with Mohs surgery approximately 13 years prior to presentation. She had no personal or family history of skin cancer. Physical examination revealed a 2.5-cm, mobile, nontender, flesh-colored subcutaneous nodule on the right shoulder (top); no ulceration, bleeding, or drainage was present. The surrounding skin demonstrated no clinical changes. The patient was scheduled for outpatient surgical excision of the nodule, which initially was suspected to be a lipoma. During the excision, a translucent cystlike nodule (bottom) was gently dissected and sent for histopathologic examination.
Reliability of Biopsy Margin Status for Basal Cell Carcinoma: A Retrospective Study
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).
We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Practice Points
- Clinicians must recognize the limitations of margin assessment of biopsy specimens and not rely on margin status to dictate treatment.
- Dermatopathologists should consider modifying how margin status is reported, either by omitting it or clarifying its limitations on the pathology report.
Distinct Violaceous Plaques in Conjunction With Blisters
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9
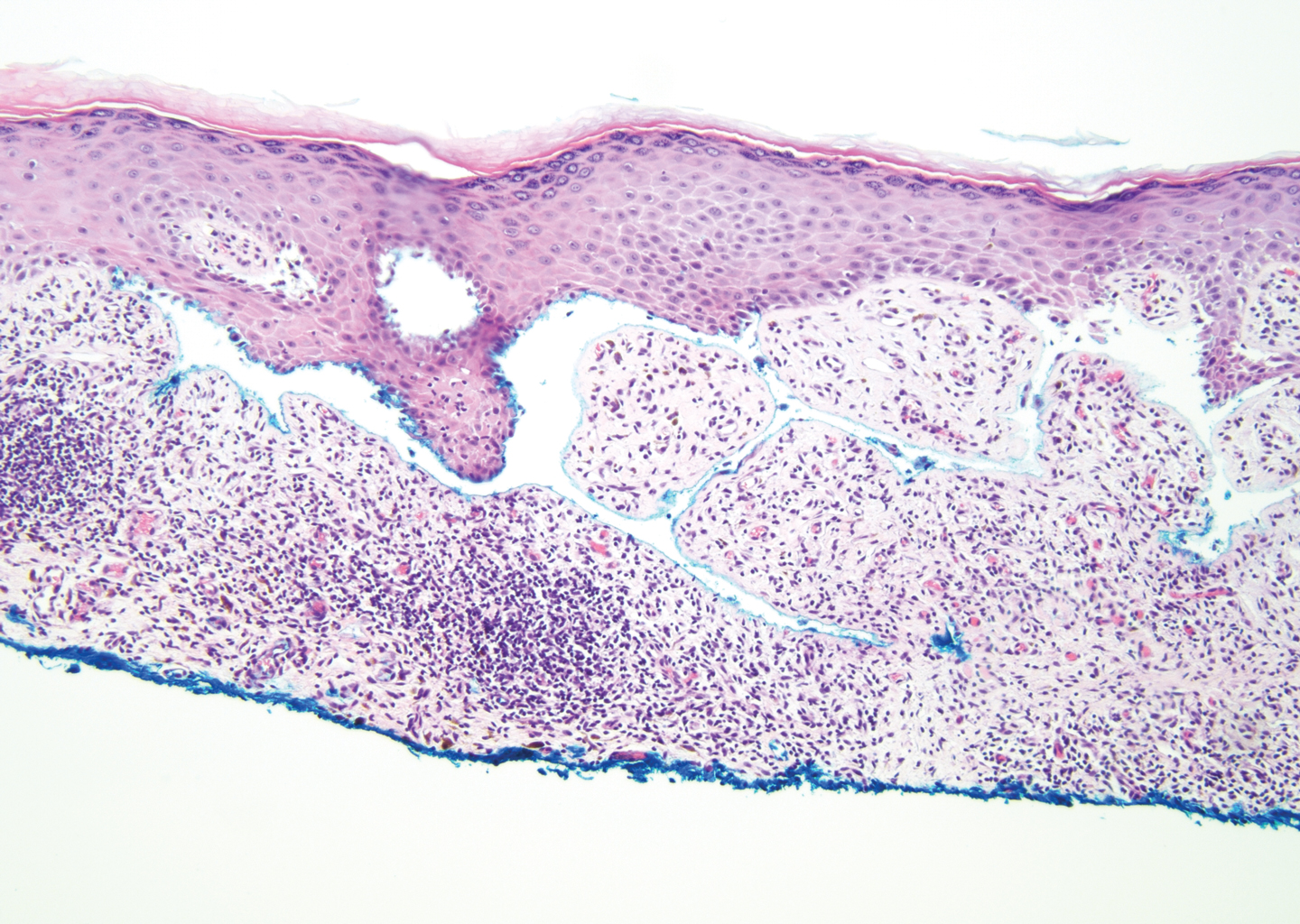
Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10
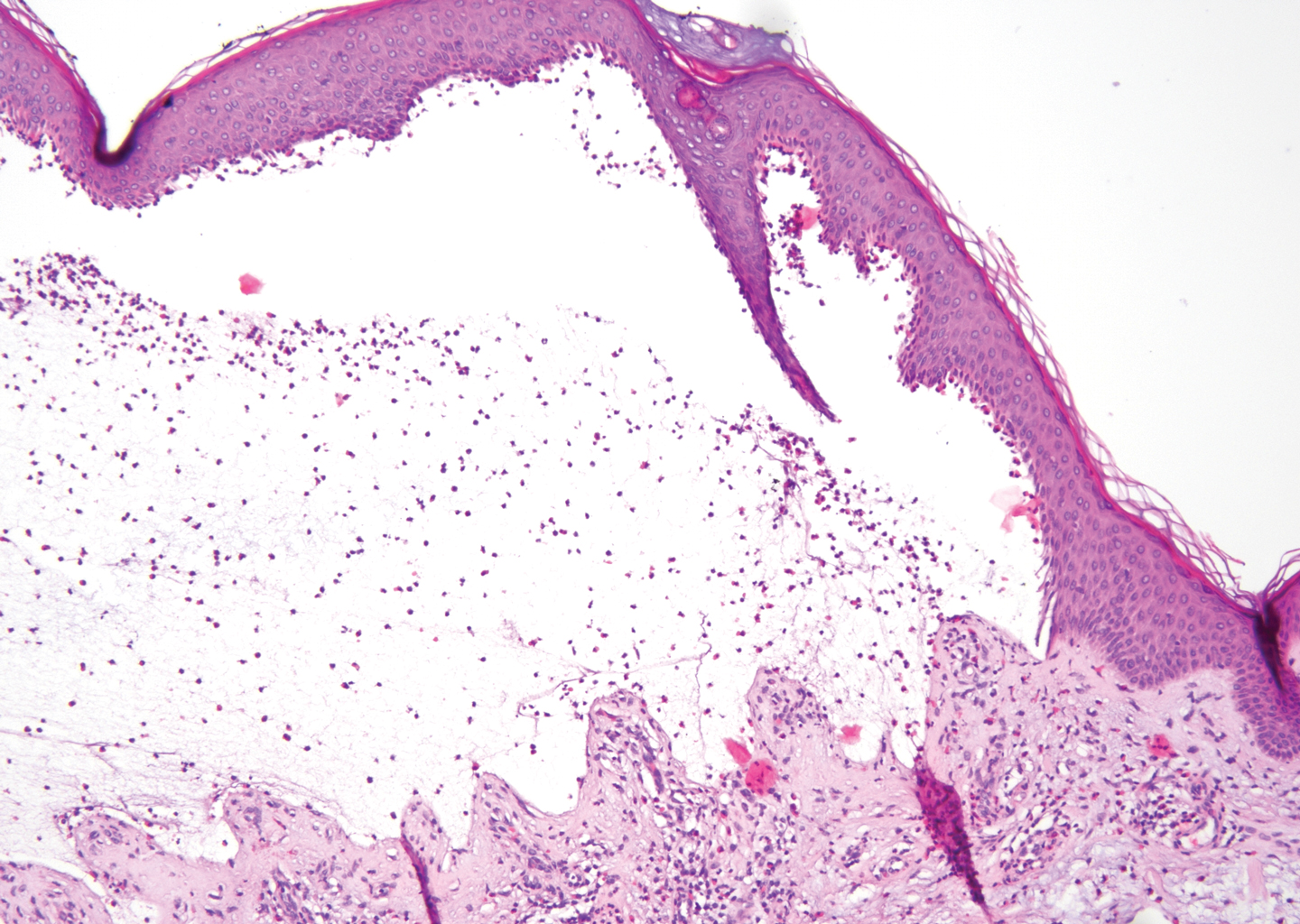
Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9

Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10

Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9

Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10

Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
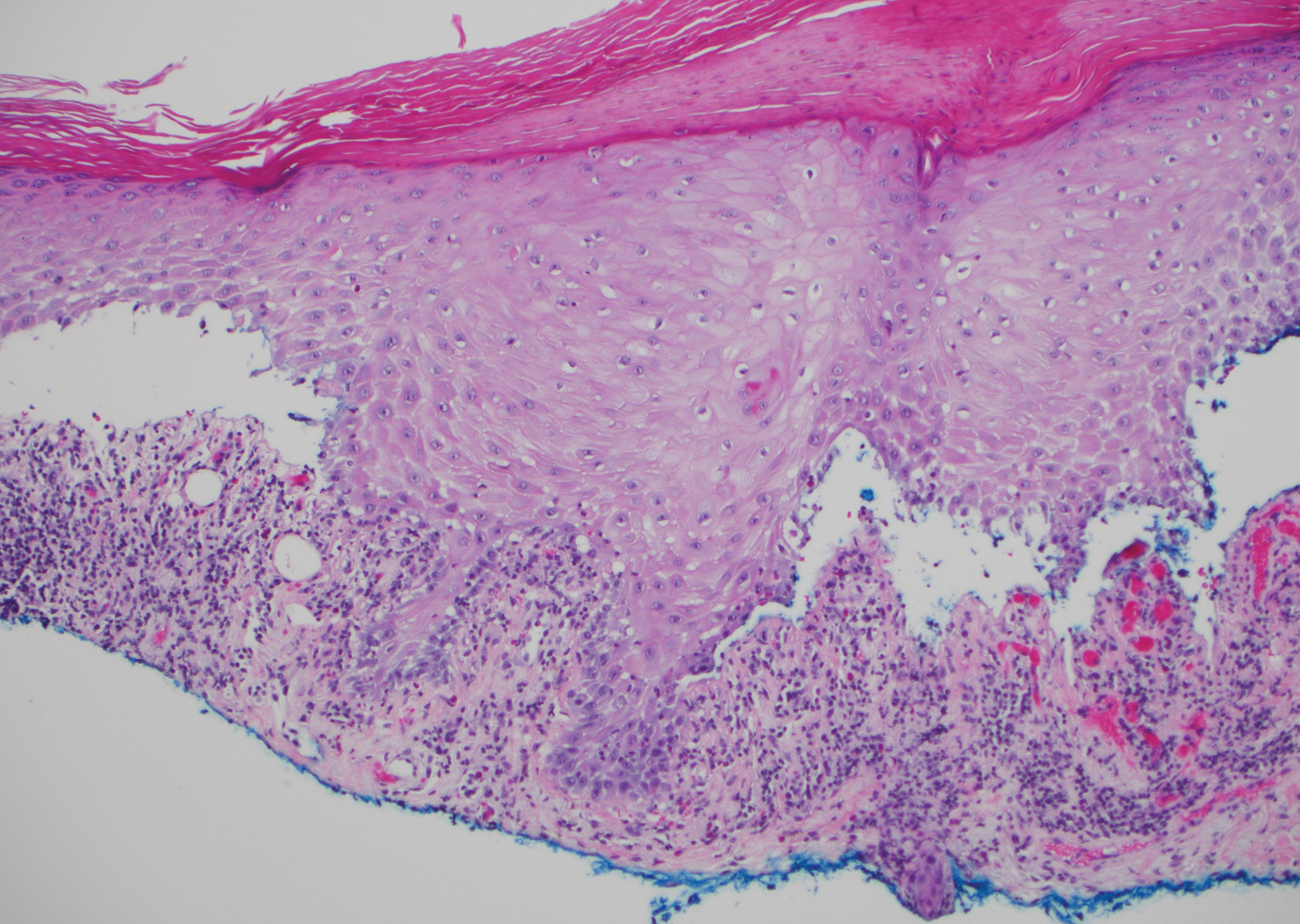
A 72-year-old woman presented to our dermatology clinic with a rash of several months' duration that began as itchy bumps on the wrists and spread to involve the legs. Approximately 2 months prior to presentation, she noted blisters on the feet and legs. She initially went to her primary care physician, who prescribed levofloxacin, cephalexin, and a 5-day course of prednisone. The prednisone initially helped; however the rash worsened on discontinuation. In our clinic, the patient had scattered tense bullae and numerous erosions with crust on the dorsum of the feet and legs, some of which were in conjunction with violaceous papules and plaques. There also was hypertrophic scale on the soles of the feet. A potassium hydroxide preparation of skin scrapings from the feet was negative for fungal elements. Two shave biopsies of a violaceous plaque and bulla as well as a perilesional punch biopsy from the leg were obtained.
How Does Provider Attire Impact Perceived Care and Infection Risk in the Clinical Setting?
Impact of Provider Attire on Patient Satisfaction in an Outpatient Dermatology Clinic
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.
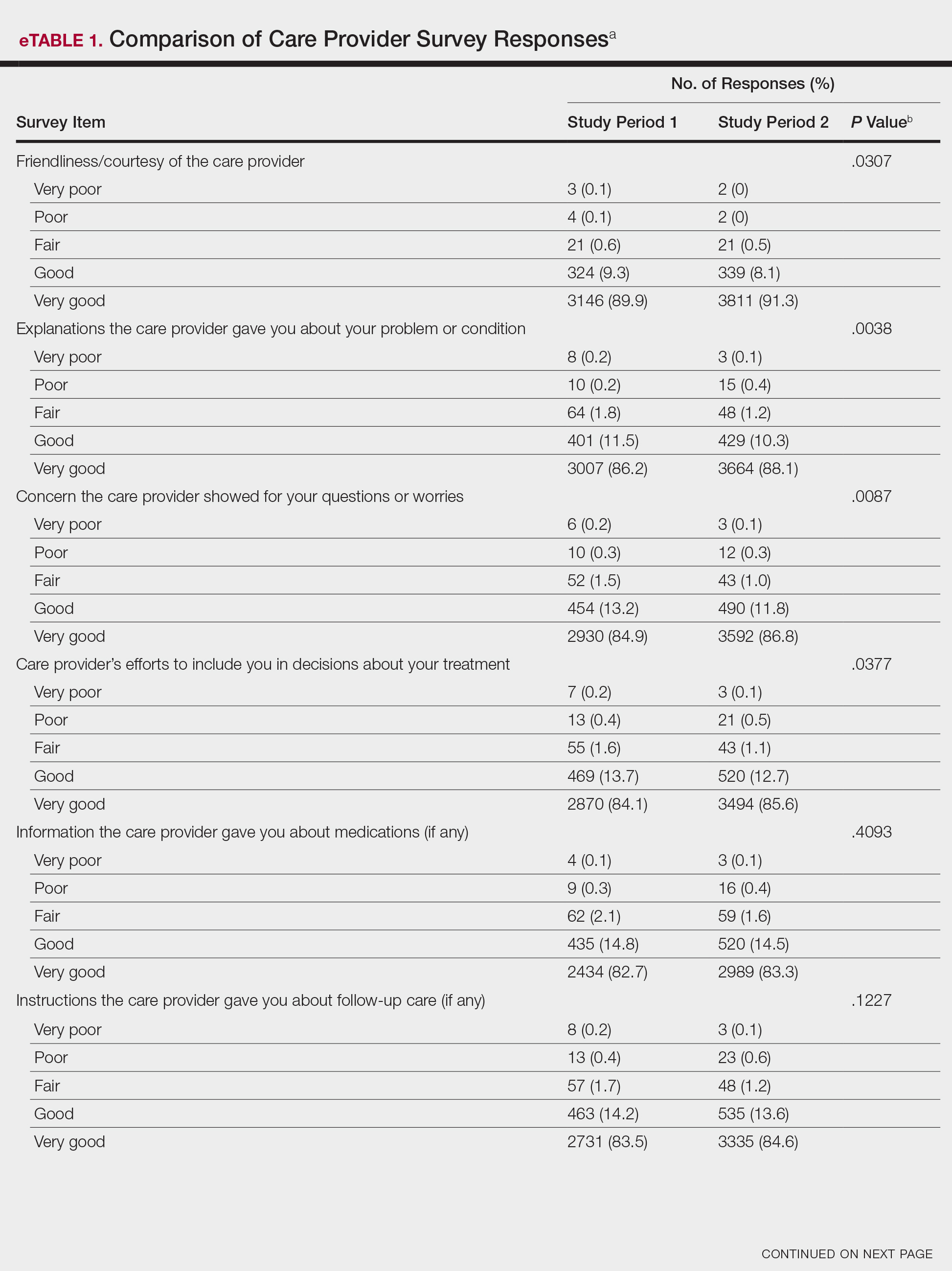
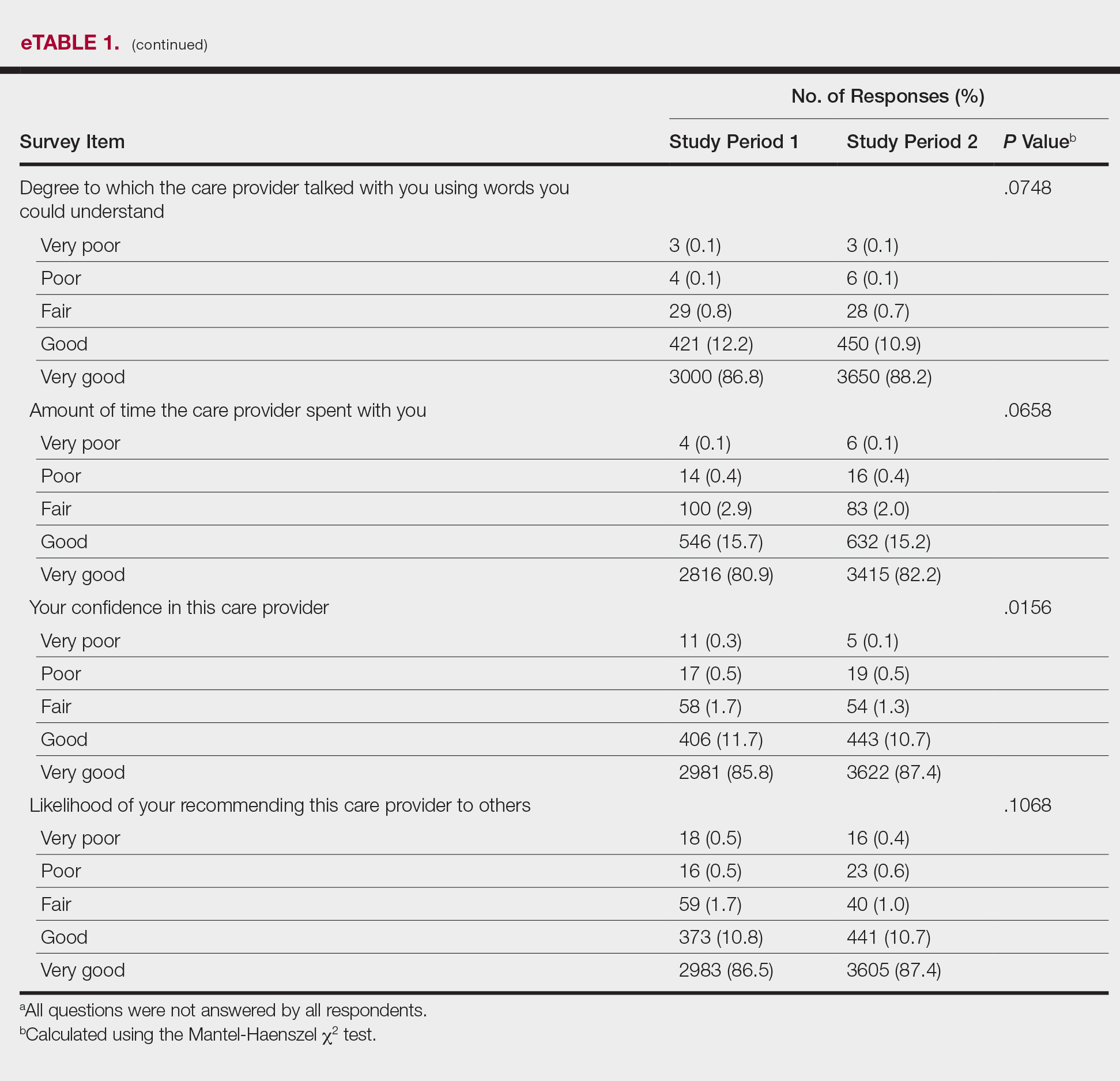
The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.
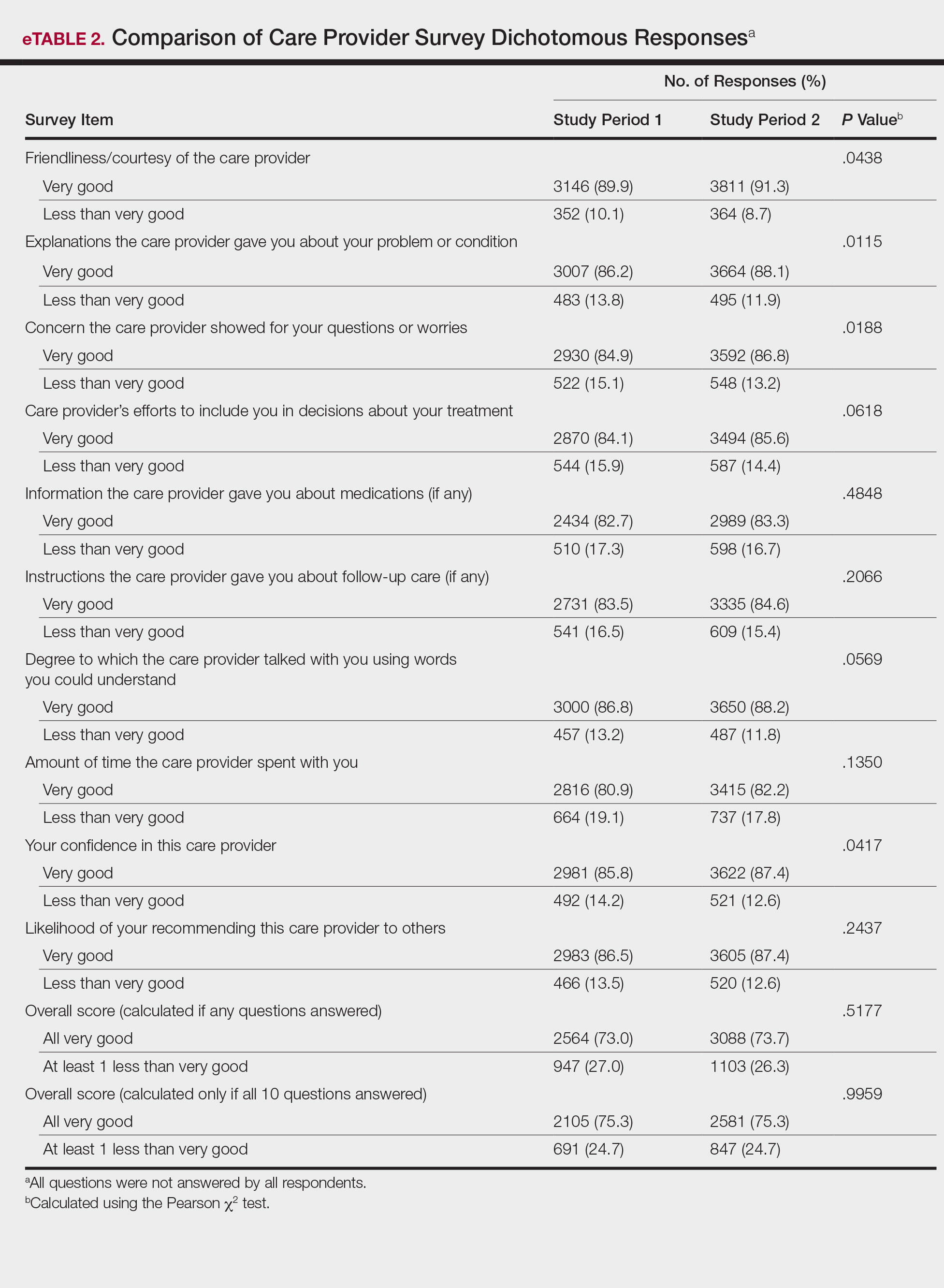
There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.


The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.

There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
Provider attire has come under scrutiny in the more recent medical literature. Epidemiologic data have shown that lab coats, ties, and other articles of clothing are frequently contaminated with disease-causing pathogens including methicillin-resistant Staphylococcus aureus , vancomycin-resistant enterococci, Acinetobacter species, Enterobacteriaceae, Pseudomona s species, and Clostridium difficile.1 Clothing may serve as a vector for spread of these bacteria and may contribute to hospital-acquired infections, increased cost of care, and patient morbidity. Prior to February 2015, the dermatology service line at Geisinger Medical Center in Danville, Pennsylvania, had followed a formal dress code that included white lab coats (white coats) along with long-sleeve shirts and ties/bowties for male providers and blouses, skirts, dress pants, and dresses for female providers. After a review of the recent literature on contamination rates of provider attire,2 we transitioned away from formal attire to adopt fitted, embroidered, black or navy blue scrubs to be worn in the clinic (Figure). Fitted scrubs differ from traditional unisex operating room scrubs, conferring a more professional appearance.

Limited research has shown that dermatology patients may have a slight preference for formal provider attire.2,3 In these studies, patients were shown photographs of providers in various dress (ie, professional attire, business attire, casual attire, scrubs). Patients preferred or had more confidence in the photograph of the provider in professional attire2,3; however, it is unclear if dermatology provider attire has any measurable effect on overall patient satisfaction. Patient satisfaction relies on a myriad of factors, including both spoken and unspoken communication skills. Patient satisfaction has become an integral part of health care, and with an emphasis on value-based care, it will likely be one determining factor in how providers are reimbursed for their services.4,5 In this study, we investigated if a change from formal attire to fitted scrubs influenced patient satisfaction using a common third-party patient satisfaction survey.
Methods
Patient Satisfaction Survey
We conducted a retrospective cohort study analyzing 10 questions from the care provider section of the Press Ganey third-party patient satisfaction survey regarding providers in our dermatology service line. Only providers with at least 12 months of survey data before (study period 1) and after (study period 2) the change in attire were included in the study. Mohs surgeons were excluded, as they already wore fitted scrubs in the clinic. Residents also were excluded, as they are rapidly developing their patient communication skills and may have a notable change in patient satisfaction over a 2-year period.
The survey data were collected, and provider names were removed and replaced with alphanumeric codes to protect anonymity while still allowing individual provider analysis. Aggregate patient comments from surveys before and after the change in attire were digitally searched using the terms scrub, coat, white, attire, and clothing for pertinent positive or negative comments.
Outcomes
We compared individual and aggregate satisfaction scores for our providers during the 12-month periods before and after the adoption of fitted scrubs. The primary outcome was statistically significant change in patient satisfaction scores before and after the institution of fitted scrubs. Secondary outcomes included summation of patient comments, both positive and negative, regarding provider attire, as recorded on satisfaction surveys.
Statistical Analysis
Overall survey scores and scores on individual survey items were summarized using mean (SD), median and interquartile range, or frequency counts and percentage, as appropriate. The overall satisfaction score and responses to individual survey items were compared using Mantel-Haenszel or Pearson χ2 tests, as appropriate.
Assuming an equal number of surveys would be completed during study periods 1 and 2, an average (SD) satisfaction score of 95.4 (15), we calculated that as many as 2136 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −1.9 and 1.9 (a 1% difference). As few as 352 surveys would be needed to conclude satisfaction scores are the same for equivalence limits of −4.7 and 4.7 (a 5% difference). Sample size calculations assume 80% power and a significance level of 0.05. Comparison of responses for study periods 1 and 2 were made using the Mantel-Haenszel χ2 test.
Because more than 80% of respondents selected very good for each question, the responses also were treated as dichotomous variables with a category for very good and a category for responses that were lower than very good (ie, good, fair, poor, very poor). Responses of very good versus less than very good were compared for the study periods 1 and 2 using the Pearson χ2 test.
Two versions of an overall score were analyzed. The first version was for patients who responded to at least 1 of 10 survey items. If responses to all the items were very good, the patient was assigned to the category of all very good. If a patient answered any of the questions with a response less than very good, he/she was categorized as at least 1 less than very good. The second version was for patients who responded to all 10 survey items. If all 10 responses were very good, the patient was assigned to a category of all very good. If any of the 10 responses were less than very good, he/she was categorized as at least 1 less than very good. Differences between study periods for both score versions were tested using the Pearson χ2 test.
Results
Data for 22 providers in the dermatology service line—13 staff dermatologists, 6 physician assistants, 1 nurse practitioner, and 2 podiatrists—were included in the study, with a total of 7702 patient satisfaction surveys completed between February 1, 2014, and January 31, 2016: 3511 were completed between February 1, 2014, and January 31, 2015 (study period 1), and 4191 were completed between February 1, 2015, and January 31, 2016 (study period 2).
Analysis of the overall distribution of possible responses for each survey item showed significant differences between study periods 1 and 2 for friendliness/courtesy of the care provider (P=.0307), explanations the care provider gave about the problem or condition (P=.0038), concern the care provider showed for questions or worries (P=.0087), care provider’s efforts to include the patient in decisions about treatment (P=.0377), and patient confidence in the care provider (P=.0156). These survey items trended toward more positive responses in study period 2. The full results are provided in eTable 1.


The analysis that looked at responses as binary (very good vs less than very good) showed a greater proportion of very good responses for friendliness/courtesy of the care provider (P=.0438), explanations the care provider gave about the problem or condition (P=.0115), concern the care provider showed for questions or worries (P=.0188), and patient confidence in the care provider (P=.0417). The full results are provided in eTable 2.

There were no significant differences in the overall satisfaction scores between the first and second study periods. The differences were statistically significant when the overall score was calculated if any questions were answered (P=.5177) and when the overall score was calculated if all 10 questions were answered (P=.9959). For patients who responded to all survey items, 75.3% selected all very good responses for both the first and second study periods.
Review of the surveys for comments from both study periods revealed only a single patient comment pertaining to attire. The comment, which was submitted during study period 2, was considered positive, referring to the fitted scrubs as neat and professional. No negative comments were found during either period.
Comment
In this study, we did not find that a change from formal attire to fitted scrubs had a measurable negative impact on patient satisfaction scores. Conversely, we found a small but statistically significant improvement on several survey items after the change to fitted scrubs. The data suggest that changing from formal attire to fitted scrubs in an outpatient dermatology clinic had little impact on overall patient satisfaction. Only 1 positive comment and no negative comments were received regarding providers wearing fitted scrubs.
A prior study in an outpatient gynecology/obstetrics clinic showed similar results.6 In that study, providers were randomly assigned to business attire, casual attire, or scrubs. A 10-question patient satisfaction survey was designed that specifically avoided asking about provider attire to reduce any bias. The study found that over a 3-month period, attire had no influence on patient satisfaction.6
Our data suggest that factors beyond provider attire have the greatest influence on patient satisfaction scores. Patient satisfaction is likely driven by other factors such as provider communication skills, concern for patient well-being, ability to empathize, and timeliness. Given the biologic plausibility of increased infection rate from contaminated provider attire, we feel that comfortable, washable, fitted scrubs provide a sanitary and acceptable alternative to more traditional formal provider attire in the office setting. Bearman et al1 suggest consideration of a bare-below-the-elbows policy (with or without scrubs) for inpatient services and lab coats (if worn per facility policy), and other articles of clothing should be laundered frequently or if visibly soiled. We feel these policies also can be applied to outpatient dermatology clinics, as long as the rationale is well communicated to all parties.
Several items on the patient satisfaction survey were statistically improved during the second study period; however, it is impossible to determine if provider attire was an important factor in this change. Improvement in satisfaction scores could be attributed to ongoing departmental and institutional emphasis on patient care and servic
Anecdotally, most providers in our department were enthusiastic and supportive of the change to fitted scrubs. It is possible that provider happiness is reflected in improved patient satisfaction scores. Provider satisfaction has been shown to correlate with patient satisfaction.7
Limitations include possible other unmeasured variables that had a more substantial impact on patient satisfaction survey results. We also recognize that the survey used in this study contained no questions that directly asked patients about their satisfaction with provider attire; however, bias or any preconception patients may have had regarding attire may have been avoided in the process. We also were not able to separate patient surveys based on age or other demographics. Finally, our results may not be generalizable to other settings where patient perceptions may be different from those of central Pennsylvania.
Conclusion
Transitioning from formal provider attire to fitted scrubs did not have a strong impact on overall patient satisfaction scores in an outpatient dermatology clinic. Providers and institutions should consider this information when developing dress code policies.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
- Bearman G, Bryant K, Leekha S, et al. Expert guidance: healthcare personnel attire in non-operating room settings. Infect Control Hosp Epidemiol. 2014;35:107-121.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Maruani A, Léger J, Giraudeau B, et al. Effect of physician dress style on patient confidence. J Eur Acad Dermatol Venereol. 2013;27:E333-E337.
- Guadagnino C. Patient satisfaction critical to hospital value-based purchasing program. The Hospitalist. Published October 2012. http://www.the-hospitalist.org/article/patient-satisfaction-critical-to-hospital-value-based-purchasing-program/. Accessed June 23, 2018.
- Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201-203.
- Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
- Fischer RL, Hansen CE, Hunter RL, et al. Does physician attire influence patient satisfaction in an outpatient obstetrics and gynecology setting? Am J Obstet Gynecol. 2007;196:186.e1-186.e5.
Practice Points
- Provider attire is known to harbor disease-causing microorganisms, potentially serving as a vector and contributing to hospital-acquired infections.
- A change from formal provider attire, including white coats, to fitted scrubs had no measurable impact on patient satisfaction in an outpatient dermatology clinic.
- Patient satisfaction is most strongly linked to other provider characteristics, such as communication skills, concern for patient well-being, ability to empathize, and timeliness.
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
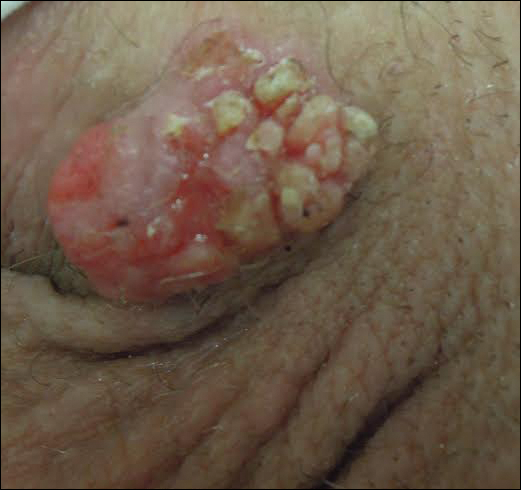
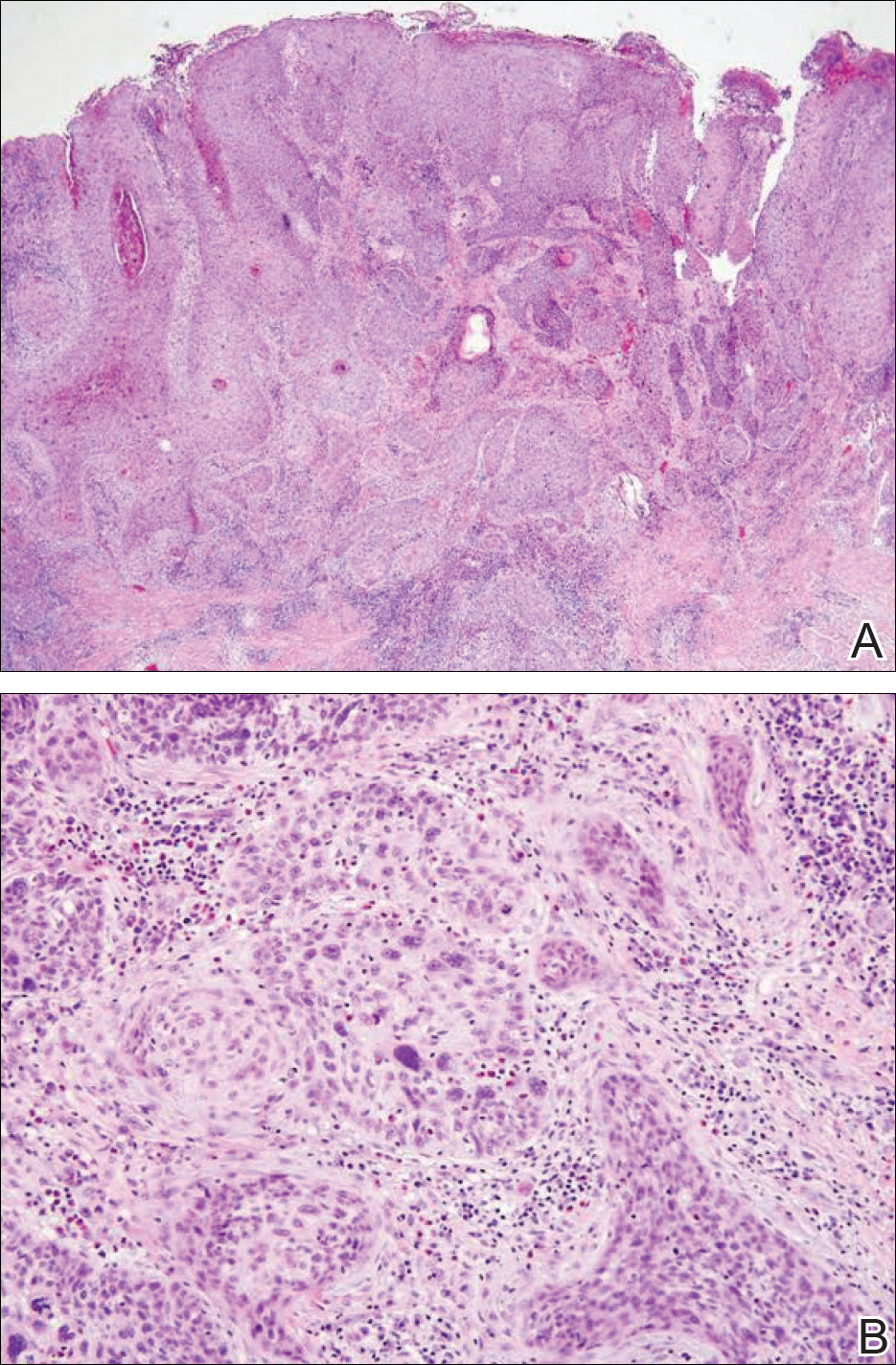
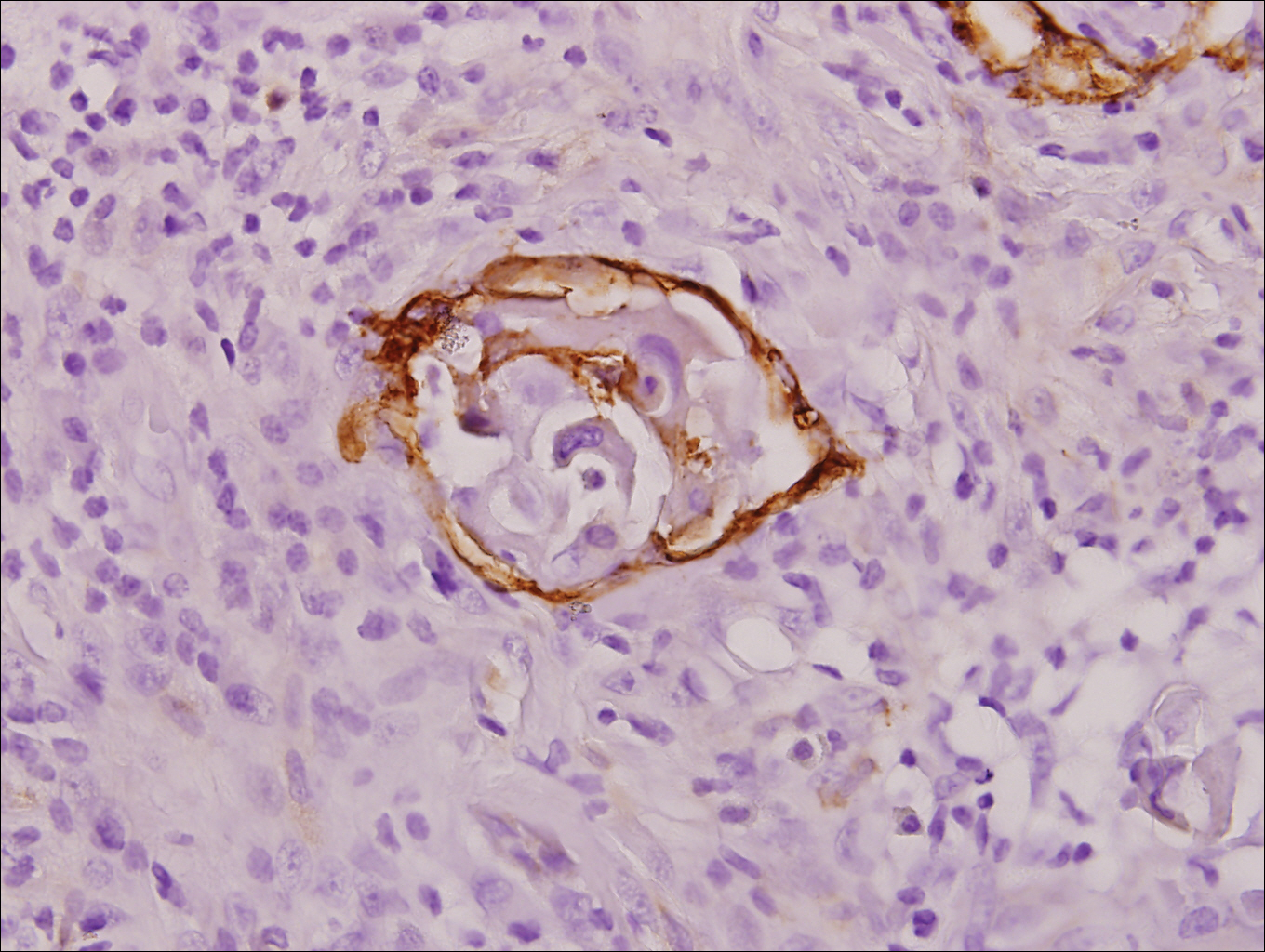
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.
Acquired Epidermodysplasia Verruciformis Occurring in a Renal Transplant Recipient
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.
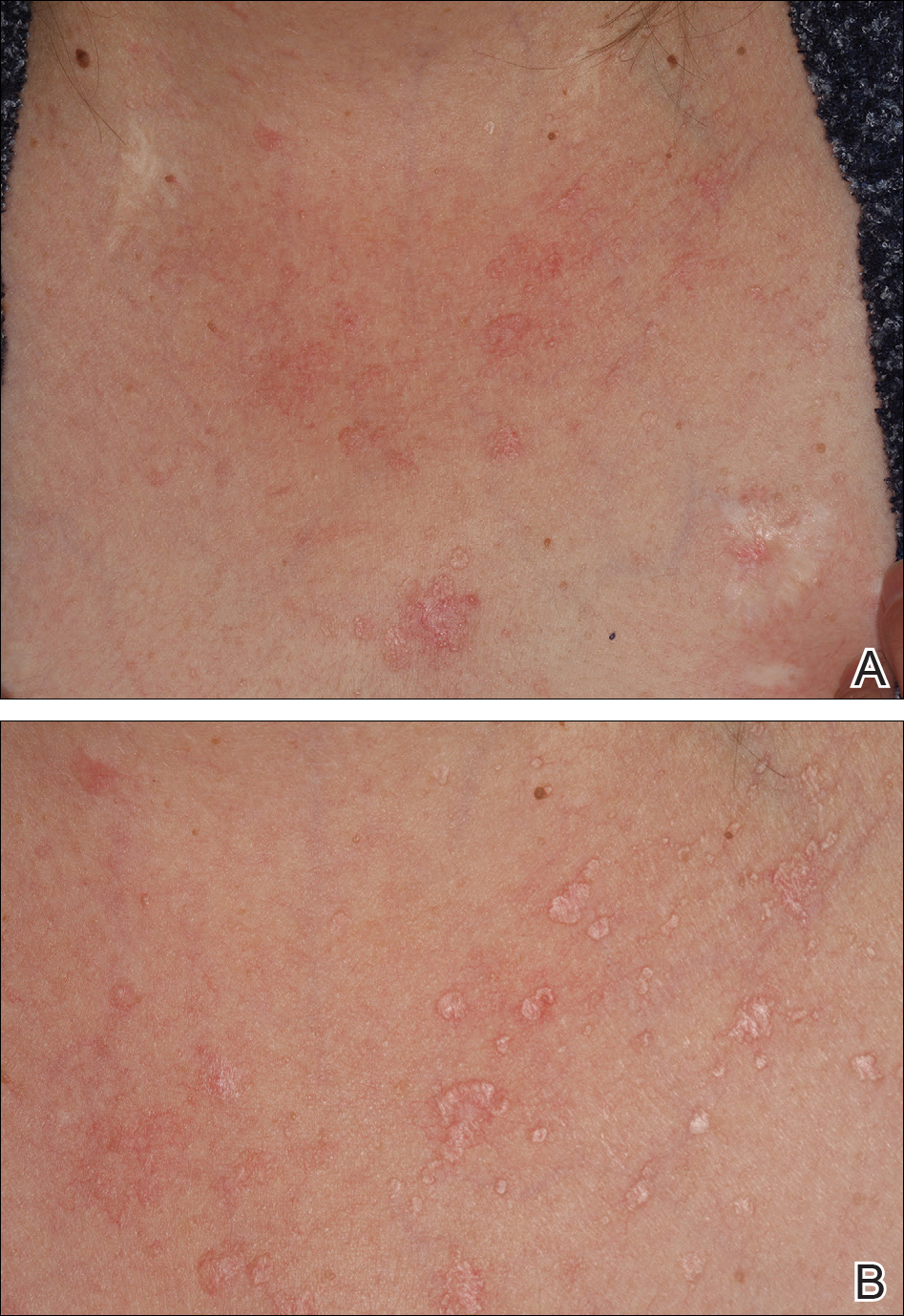
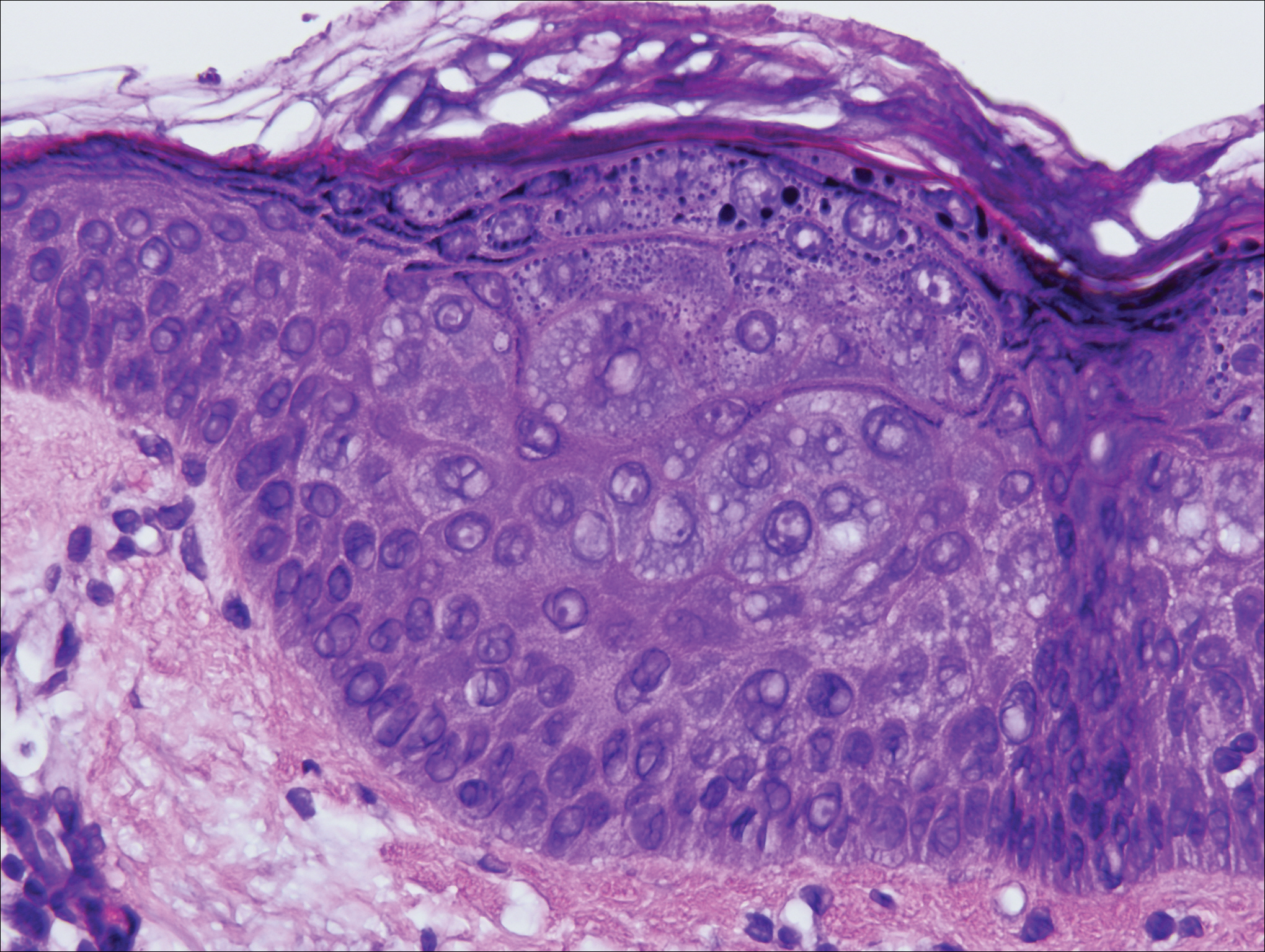
The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is a rare complication of iatrogenic immuno-suppression in the setting of solid organ transplantation.
- Patients with EDV should be counseled to avoid exposure to UV radiation to reduce the risk formalignant transformation.
Progressive Widespread Warty Skin Growths
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1
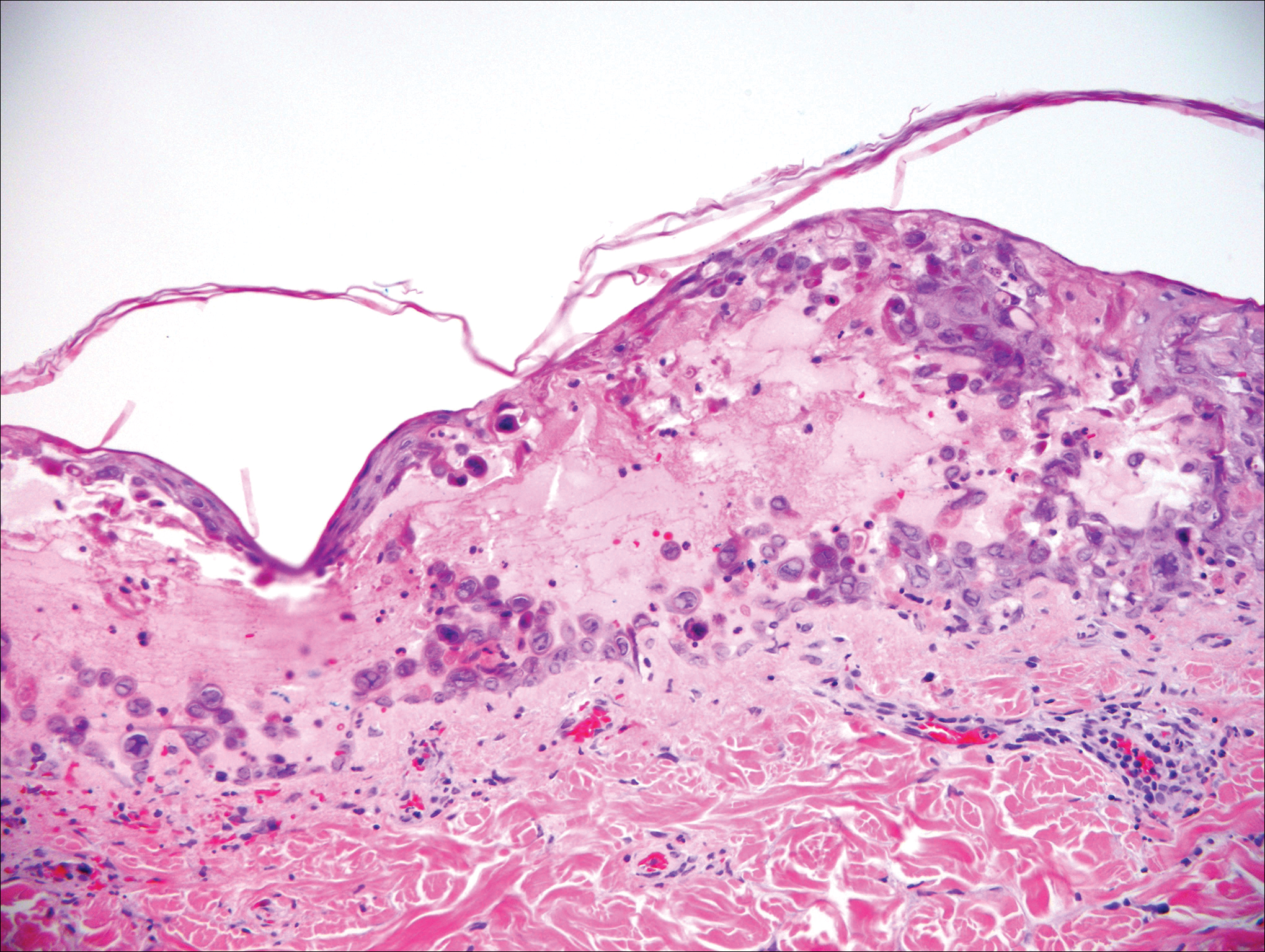
The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4
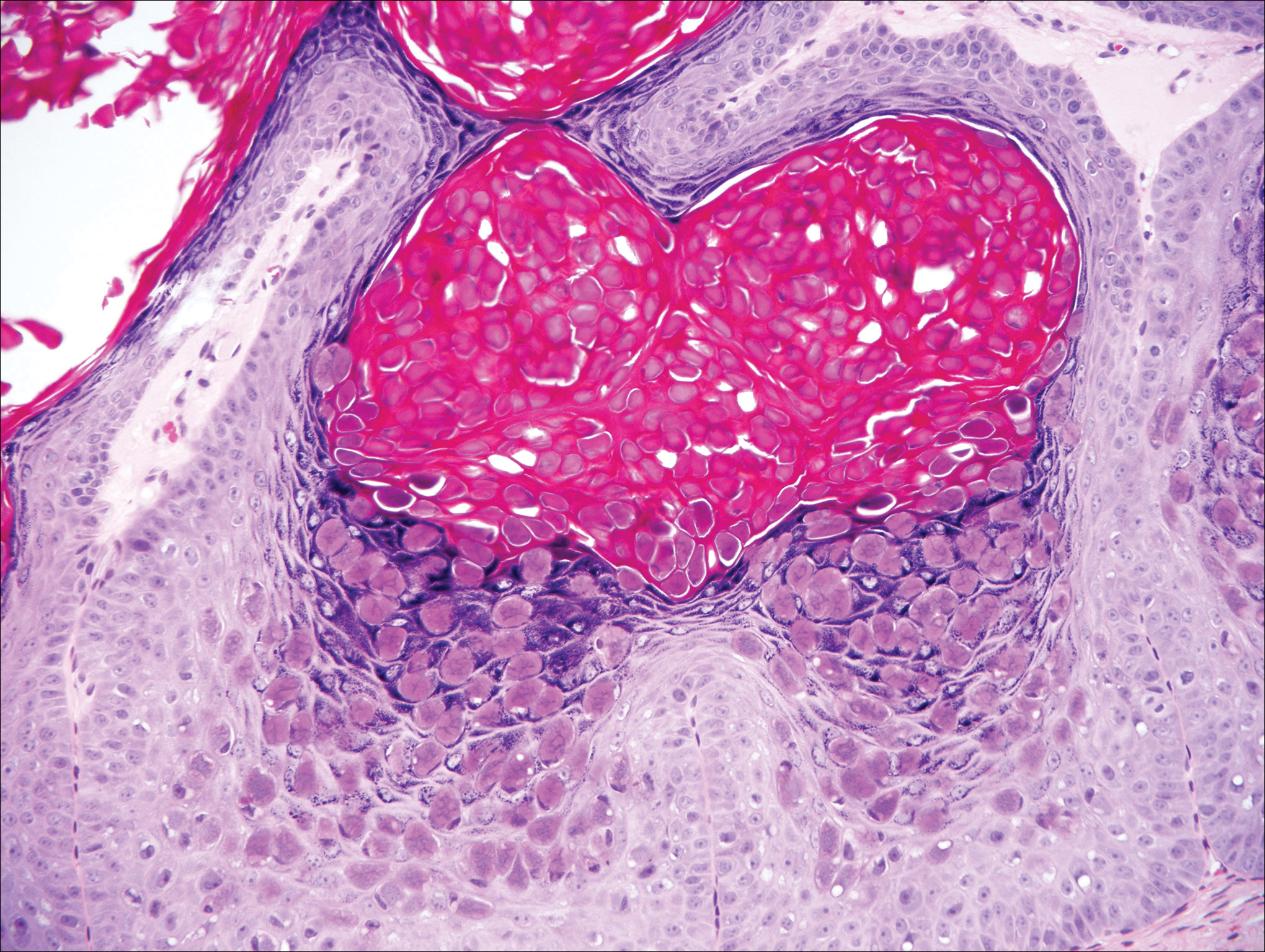
On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4
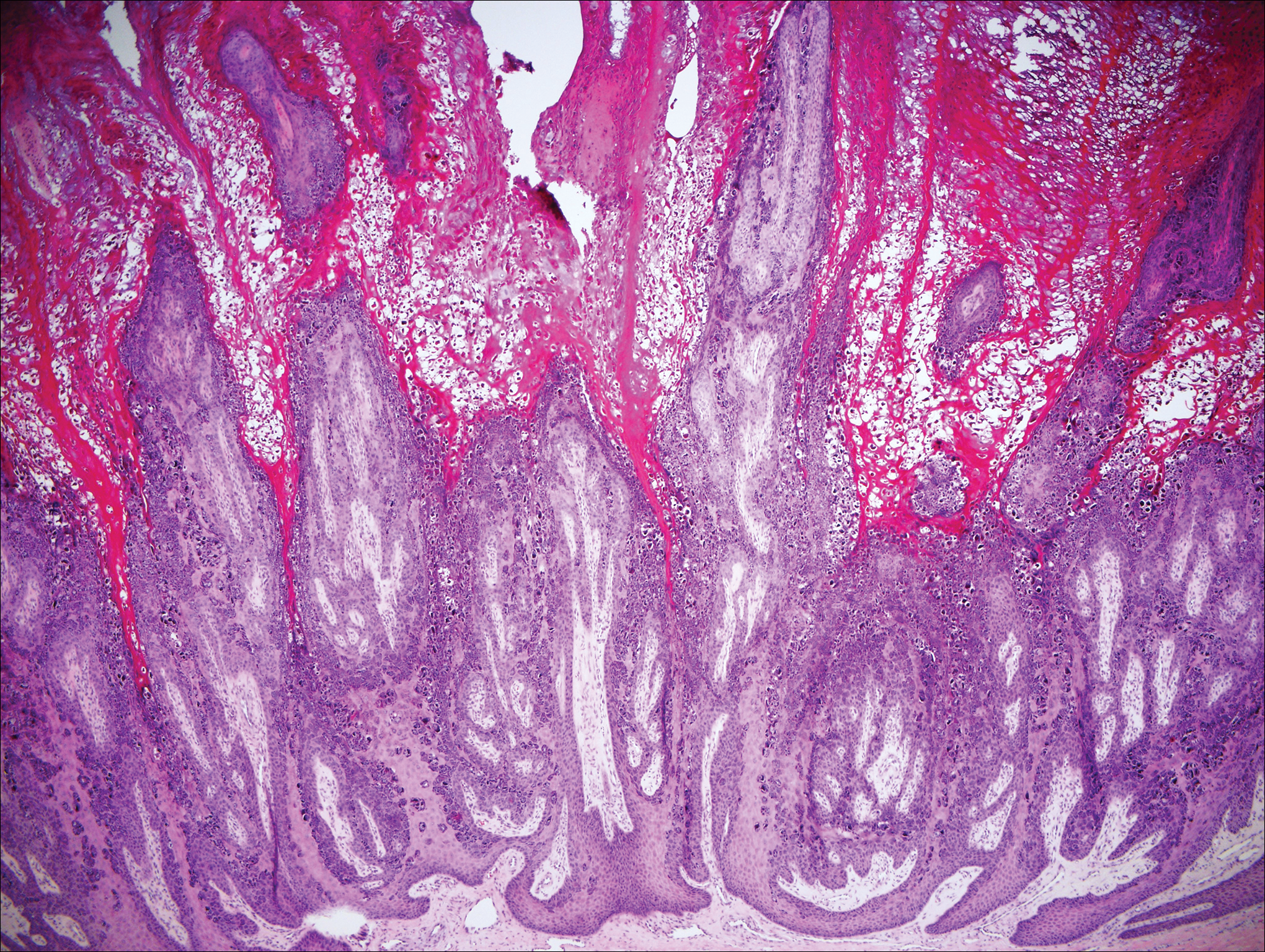
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
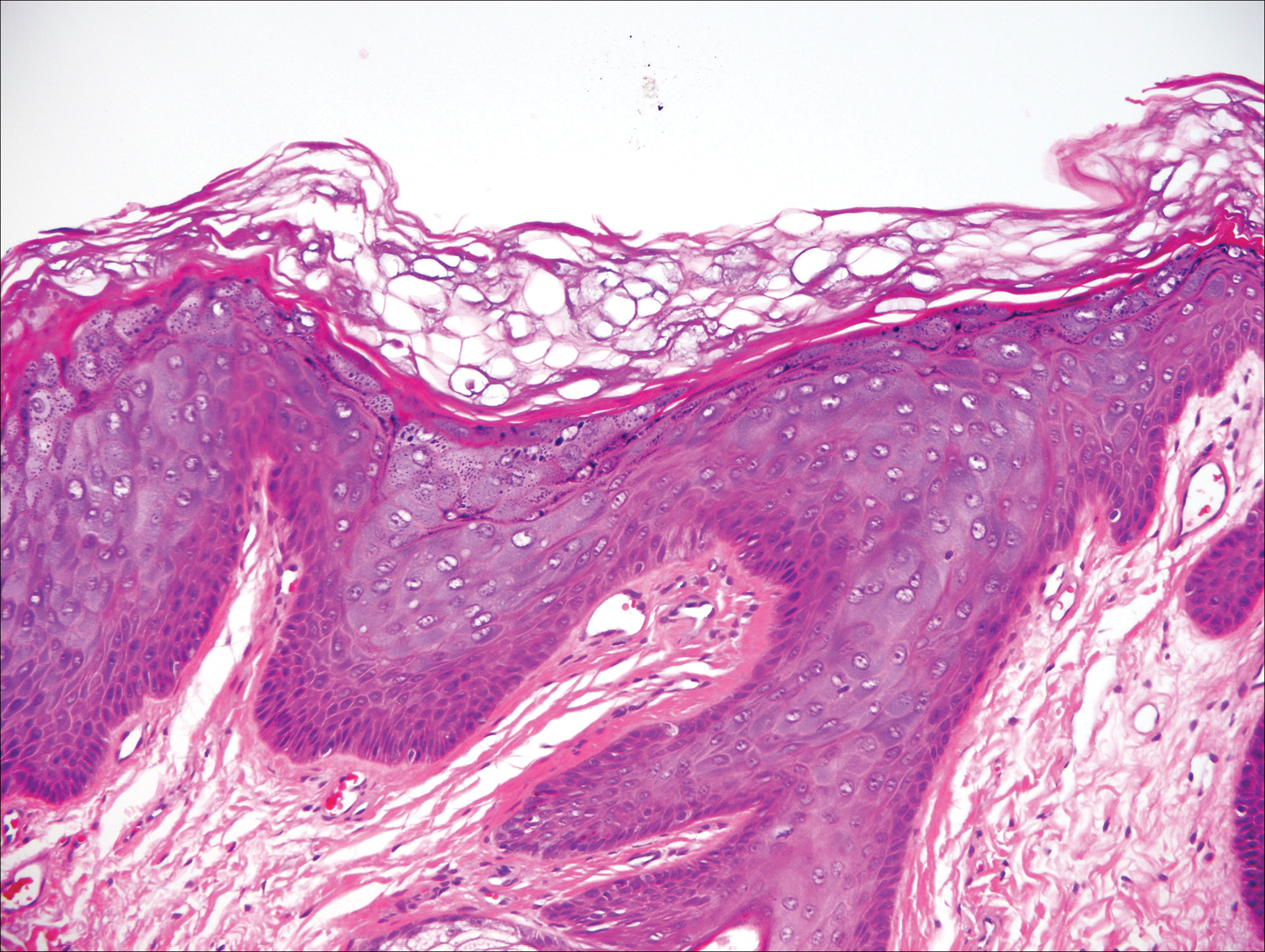
A 33-year-old man presented with progressive widespread warty skin growths that had been present since 6 years of age. Physical examination revealed numerous verrucous papules on the face and neck along with verrucous, tan-pink papules and plaques diffusely scattered on the trunk, arms, and legs. A biopsy of a lesion on the neck was performed.
Lichen Striatus
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.
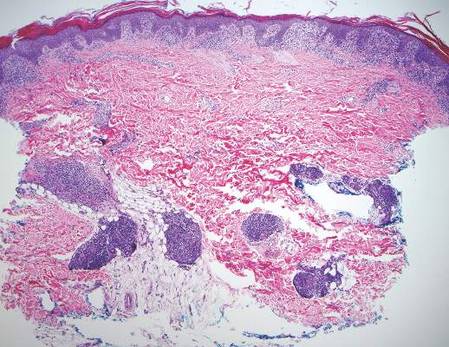
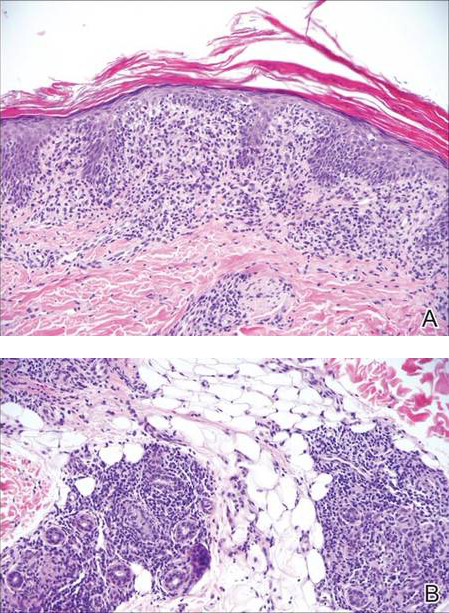
Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.
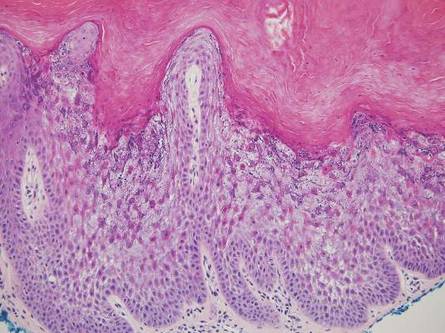
Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1
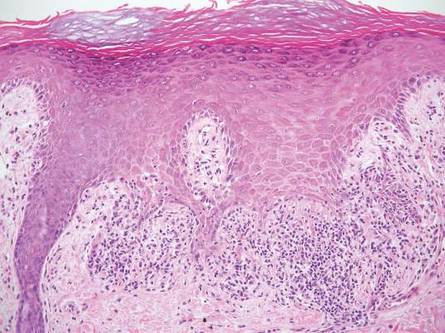
Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.
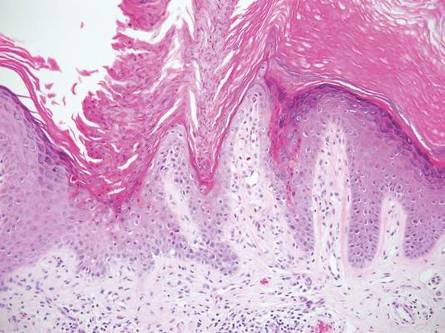
Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4
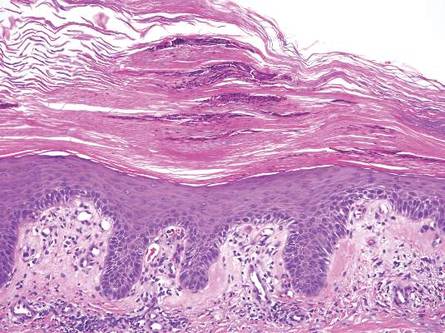
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.