User login
Lichen Striatus
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
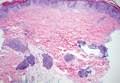
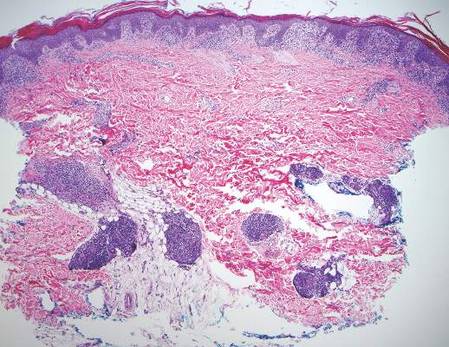
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.
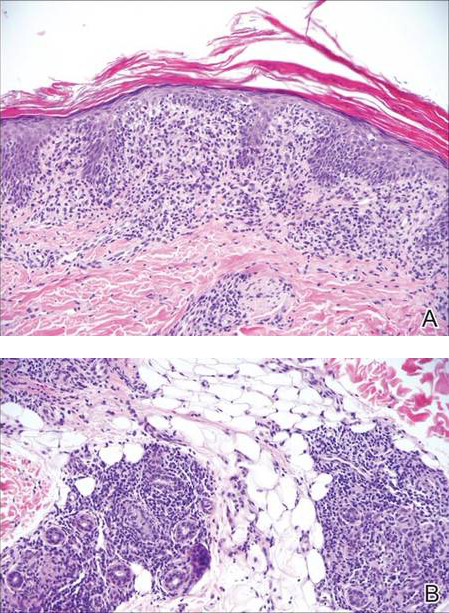
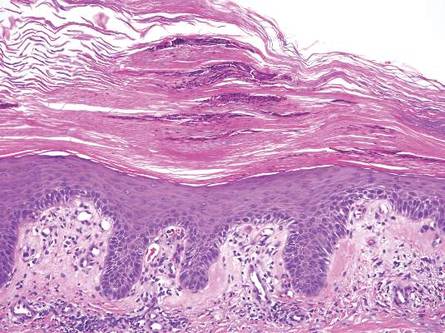
Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.
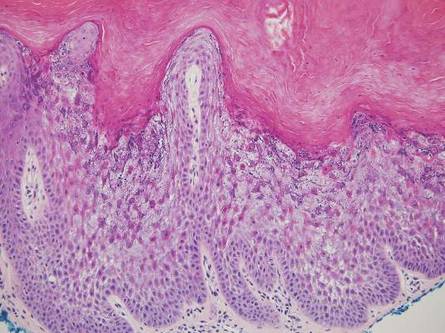
Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1
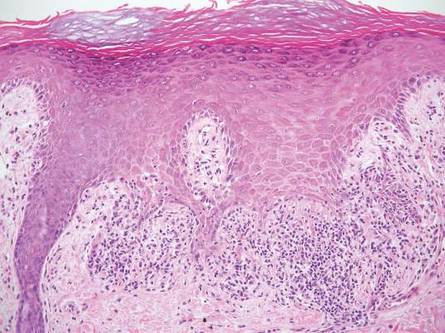
Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.
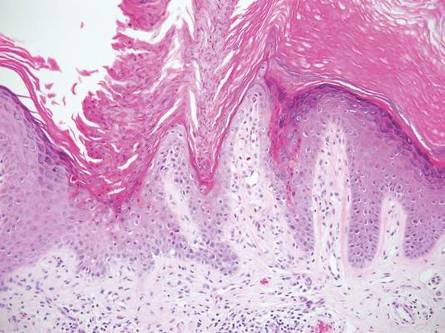
Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.