User login
High Breast Cancer Risk With Menopausal Hormone Therapy & Strong Family History
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Breast Cancer Hormone Therapy May Protect Against Dementia
TOPLINE:
with the greatest benefit seen in younger Black women.
METHODOLOGY:
- Hormone-modulating therapy is widely used to treat hormone receptor–positive breast cancer, but the cognitive effects of the treatment, including a potential link to dementia, remain unclear.
- To investigate, researchers used the SEER-Medicare linked database to identify women aged 65 years or older with breast cancer who did and did not receive hormone-modulating therapy within 3 years following their diagnosis.
- The researchers excluded women with preexisting Alzheimer’s disease/dementia diagnoses or those who had received hormone-modulating therapy before their breast cancer diagnosis.
- Analyses were adjusted for demographic, sociocultural, and clinical variables, and subgroup analyses evaluated the impact of age, race, and type of hormone-modulating therapy on Alzheimer’s disease/dementia risk.
TAKEAWAY:
- Among the 18,808 women included in the analysis, 66% received hormone-modulating therapy and 34% did not. During the mean follow-up of 12 years, 24% of hormone-modulating therapy users and 28% of nonusers developed Alzheimer’s disease/dementia.
- Overall, hormone-modulating therapy use (vs nonuse) was associated with a significant 7% lower risk for Alzheimer’s disease/dementia (hazard ratio [HR], 0.93; P = .005), with notable age and racial differences.
- Hormone-modulating therapy use was associated with a 24% lower risk for Alzheimer’s disease/dementia in Black women aged 65-74 years (HR, 0.76), but that protective effect decreased to 19% in Black women aged 75 years or older (HR, 0.81). White women aged 65-74 years who received hormone-modulating therapy (vs those who did not) had an 11% lower risk for Alzheimer’s disease/dementia (HR, 0.89), but the association disappeared among those aged 75 years or older (HR, 0.96; 95% CI, 0.90-1.02). Other races demonstrated no significant association between hormone-modulating therapy use and Alzheimer’s disease/dementia.
- Overall, the use of an aromatase inhibitor or a selective estrogen receptor modulator was associated with a significantly lower risk for Alzheimer’s disease/dementia (HR, 0.93 and HR, 0.89, respectively).
IN PRACTICE:
Overall, the retrospective study found that “hormone therapy was associated with protection against [Alzheimer’s/dementia] in women aged 65 years or older with newly diagnosed breast cancer,” with the decrease in risk relatively greater for Black women and women younger than 75 years, the authors concluded.
“The results highlight the critical need for personalized breast cancer treatment plans that are tailored to the individual characteristics of each patient, particularly given the significantly higher likelihood (two to three times more) of Black women developing [Alzheimer’s/dementia], compared with their White counterparts,” the researchers added.
SOURCE:
The study, with first author Chao Cai, PhD, Department of Clinical Pharmacy and Outcomes Sciences, University of South Carolina, Columbia, was published online on July 16 in JAMA Network Open.
LIMITATIONS:
The study included only women aged 65 years or older, limiting generalizability to younger women. The dataset lacked genetic information and laboratory data related to dementia. The duration of hormone-modulating therapy use beyond 3 years and specific formulations were not assessed. Potential confounders such as variations in chemotherapy, radiation, and surgery were not fully addressed.
DISCLOSURES:
Support for the study was provided by the National Institutes of Health; Carolina Center on Alzheimer’s Disease and Minority Research pilot project; and the Dean’s Faculty Advancement Fund, University of Pittsburgh, Pennsylvania. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
with the greatest benefit seen in younger Black women.
METHODOLOGY:
- Hormone-modulating therapy is widely used to treat hormone receptor–positive breast cancer, but the cognitive effects of the treatment, including a potential link to dementia, remain unclear.
- To investigate, researchers used the SEER-Medicare linked database to identify women aged 65 years or older with breast cancer who did and did not receive hormone-modulating therapy within 3 years following their diagnosis.
- The researchers excluded women with preexisting Alzheimer’s disease/dementia diagnoses or those who had received hormone-modulating therapy before their breast cancer diagnosis.
- Analyses were adjusted for demographic, sociocultural, and clinical variables, and subgroup analyses evaluated the impact of age, race, and type of hormone-modulating therapy on Alzheimer’s disease/dementia risk.
TAKEAWAY:
- Among the 18,808 women included in the analysis, 66% received hormone-modulating therapy and 34% did not. During the mean follow-up of 12 years, 24% of hormone-modulating therapy users and 28% of nonusers developed Alzheimer’s disease/dementia.
- Overall, hormone-modulating therapy use (vs nonuse) was associated with a significant 7% lower risk for Alzheimer’s disease/dementia (hazard ratio [HR], 0.93; P = .005), with notable age and racial differences.
- Hormone-modulating therapy use was associated with a 24% lower risk for Alzheimer’s disease/dementia in Black women aged 65-74 years (HR, 0.76), but that protective effect decreased to 19% in Black women aged 75 years or older (HR, 0.81). White women aged 65-74 years who received hormone-modulating therapy (vs those who did not) had an 11% lower risk for Alzheimer’s disease/dementia (HR, 0.89), but the association disappeared among those aged 75 years or older (HR, 0.96; 95% CI, 0.90-1.02). Other races demonstrated no significant association between hormone-modulating therapy use and Alzheimer’s disease/dementia.
- Overall, the use of an aromatase inhibitor or a selective estrogen receptor modulator was associated with a significantly lower risk for Alzheimer’s disease/dementia (HR, 0.93 and HR, 0.89, respectively).
IN PRACTICE:
Overall, the retrospective study found that “hormone therapy was associated with protection against [Alzheimer’s/dementia] in women aged 65 years or older with newly diagnosed breast cancer,” with the decrease in risk relatively greater for Black women and women younger than 75 years, the authors concluded.
“The results highlight the critical need for personalized breast cancer treatment plans that are tailored to the individual characteristics of each patient, particularly given the significantly higher likelihood (two to three times more) of Black women developing [Alzheimer’s/dementia], compared with their White counterparts,” the researchers added.
SOURCE:
The study, with first author Chao Cai, PhD, Department of Clinical Pharmacy and Outcomes Sciences, University of South Carolina, Columbia, was published online on July 16 in JAMA Network Open.
LIMITATIONS:
The study included only women aged 65 years or older, limiting generalizability to younger women. The dataset lacked genetic information and laboratory data related to dementia. The duration of hormone-modulating therapy use beyond 3 years and specific formulations were not assessed. Potential confounders such as variations in chemotherapy, radiation, and surgery were not fully addressed.
DISCLOSURES:
Support for the study was provided by the National Institutes of Health; Carolina Center on Alzheimer’s Disease and Minority Research pilot project; and the Dean’s Faculty Advancement Fund, University of Pittsburgh, Pennsylvania. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
with the greatest benefit seen in younger Black women.
METHODOLOGY:
- Hormone-modulating therapy is widely used to treat hormone receptor–positive breast cancer, but the cognitive effects of the treatment, including a potential link to dementia, remain unclear.
- To investigate, researchers used the SEER-Medicare linked database to identify women aged 65 years or older with breast cancer who did and did not receive hormone-modulating therapy within 3 years following their diagnosis.
- The researchers excluded women with preexisting Alzheimer’s disease/dementia diagnoses or those who had received hormone-modulating therapy before their breast cancer diagnosis.
- Analyses were adjusted for demographic, sociocultural, and clinical variables, and subgroup analyses evaluated the impact of age, race, and type of hormone-modulating therapy on Alzheimer’s disease/dementia risk.
TAKEAWAY:
- Among the 18,808 women included in the analysis, 66% received hormone-modulating therapy and 34% did not. During the mean follow-up of 12 years, 24% of hormone-modulating therapy users and 28% of nonusers developed Alzheimer’s disease/dementia.
- Overall, hormone-modulating therapy use (vs nonuse) was associated with a significant 7% lower risk for Alzheimer’s disease/dementia (hazard ratio [HR], 0.93; P = .005), with notable age and racial differences.
- Hormone-modulating therapy use was associated with a 24% lower risk for Alzheimer’s disease/dementia in Black women aged 65-74 years (HR, 0.76), but that protective effect decreased to 19% in Black women aged 75 years or older (HR, 0.81). White women aged 65-74 years who received hormone-modulating therapy (vs those who did not) had an 11% lower risk for Alzheimer’s disease/dementia (HR, 0.89), but the association disappeared among those aged 75 years or older (HR, 0.96; 95% CI, 0.90-1.02). Other races demonstrated no significant association between hormone-modulating therapy use and Alzheimer’s disease/dementia.
- Overall, the use of an aromatase inhibitor or a selective estrogen receptor modulator was associated with a significantly lower risk for Alzheimer’s disease/dementia (HR, 0.93 and HR, 0.89, respectively).
IN PRACTICE:
Overall, the retrospective study found that “hormone therapy was associated with protection against [Alzheimer’s/dementia] in women aged 65 years or older with newly diagnosed breast cancer,” with the decrease in risk relatively greater for Black women and women younger than 75 years, the authors concluded.
“The results highlight the critical need for personalized breast cancer treatment plans that are tailored to the individual characteristics of each patient, particularly given the significantly higher likelihood (two to three times more) of Black women developing [Alzheimer’s/dementia], compared with their White counterparts,” the researchers added.
SOURCE:
The study, with first author Chao Cai, PhD, Department of Clinical Pharmacy and Outcomes Sciences, University of South Carolina, Columbia, was published online on July 16 in JAMA Network Open.
LIMITATIONS:
The study included only women aged 65 years or older, limiting generalizability to younger women. The dataset lacked genetic information and laboratory data related to dementia. The duration of hormone-modulating therapy use beyond 3 years and specific formulations were not assessed. Potential confounders such as variations in chemotherapy, radiation, and surgery were not fully addressed.
DISCLOSURES:
Support for the study was provided by the National Institutes of Health; Carolina Center on Alzheimer’s Disease and Minority Research pilot project; and the Dean’s Faculty Advancement Fund, University of Pittsburgh, Pennsylvania. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
How Do Plant-Based Foods Reduce Type 2 Diabetes Risk?
TOPLINE:
especially in individuals with obesity or premenopausal women.
METHODOLOGY:
- Lignans, polyphenolic compounds abundant in plant-based foods, are the primary dietary source of phytoestrogens in Western diets and are associated with a reduced risk for cardiometabolic conditions, but the relative associations of individual lignans with T2D are unknown.
- Researchers assessed the associations between the risk for T2D and the intake of total and four primary lignans using the data of 201,111 participants (mean age, 44.7 years; 80.2% women; 96.7% White individuals) from three large prospective US cohorts with over 30 years of follow-up, as well as the association between lignan intake and hemoglobin A1c in 496 participants from the Men’s Lifestyle Validation Study (MLVS).
- For the three large cohorts, lignan intake (total, secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol) was assessed using a validated food frequency questionnaire updated every 2-4 years and categorized into quintiles. For MLVS, diet was assessed by two sets of 7-day diet records and presented as percentage changes in A1c for linear increases in lignan intake.
- Incident T2D was confirmed using diagnostic tests, symptoms, hypoglycemic medication, elevated glucose by several measures.
TAKEAWAY:
- Across the three cohorts, 20,291 cases of T2D were recorded in the full follow-up.
- Higher intakes of total and individual ligands, except for lariciresinol, were associated with about 8%-27% lower T2D incidents (approximate hazard ratio [HR], 0.72-0.93)
- Of the individual lignans, secoisolariciresinol (but not others) showed a significant inverse association with the risk for T2D among those with a body mass index ≥ 30 (HR, 0.75; 95% CI, 0.71-0.79) and premenopausal women (HR, 0.67; 95% CI, 0.65-0.69).
- The dietary intake of lignans assessed using the 7-day diet records in MLVS was associated with lower levels of A1c (percentage changes ranging from −0.92% to −1.50%.
IN PRACTICE:
“Our findings underscore the importance of a healthy plant-based diet rich in lignan-containing foods, including flaxseed products, whole grains, and coffee for the primary prevention of T2D,” the authors wrote.
SOURCE:
The study, led by Siyue Wang, PhD, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts, and the School of Public Health, Peking University, Beijing, China, was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the potential for measurement errors in dietary assessments. Flax seed, the most concentrated source of lignans, was not assessed until midway through the three large cohort follow-ups, and this may have resulted in misclassification of the intake levels of secoisolariciresinol. The lack of diversity in the socioeconomic status and race within the population may restrict the generalizability of the findings. Despite making multivariable adjustments, residual confounding cannot be fully ruled out.
DISCLOSURES:
The three cohort studies were supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
especially in individuals with obesity or premenopausal women.
METHODOLOGY:
- Lignans, polyphenolic compounds abundant in plant-based foods, are the primary dietary source of phytoestrogens in Western diets and are associated with a reduced risk for cardiometabolic conditions, but the relative associations of individual lignans with T2D are unknown.
- Researchers assessed the associations between the risk for T2D and the intake of total and four primary lignans using the data of 201,111 participants (mean age, 44.7 years; 80.2% women; 96.7% White individuals) from three large prospective US cohorts with over 30 years of follow-up, as well as the association between lignan intake and hemoglobin A1c in 496 participants from the Men’s Lifestyle Validation Study (MLVS).
- For the three large cohorts, lignan intake (total, secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol) was assessed using a validated food frequency questionnaire updated every 2-4 years and categorized into quintiles. For MLVS, diet was assessed by two sets of 7-day diet records and presented as percentage changes in A1c for linear increases in lignan intake.
- Incident T2D was confirmed using diagnostic tests, symptoms, hypoglycemic medication, elevated glucose by several measures.
TAKEAWAY:
- Across the three cohorts, 20,291 cases of T2D were recorded in the full follow-up.
- Higher intakes of total and individual ligands, except for lariciresinol, were associated with about 8%-27% lower T2D incidents (approximate hazard ratio [HR], 0.72-0.93)
- Of the individual lignans, secoisolariciresinol (but not others) showed a significant inverse association with the risk for T2D among those with a body mass index ≥ 30 (HR, 0.75; 95% CI, 0.71-0.79) and premenopausal women (HR, 0.67; 95% CI, 0.65-0.69).
- The dietary intake of lignans assessed using the 7-day diet records in MLVS was associated with lower levels of A1c (percentage changes ranging from −0.92% to −1.50%.
IN PRACTICE:
“Our findings underscore the importance of a healthy plant-based diet rich in lignan-containing foods, including flaxseed products, whole grains, and coffee for the primary prevention of T2D,” the authors wrote.
SOURCE:
The study, led by Siyue Wang, PhD, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts, and the School of Public Health, Peking University, Beijing, China, was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the potential for measurement errors in dietary assessments. Flax seed, the most concentrated source of lignans, was not assessed until midway through the three large cohort follow-ups, and this may have resulted in misclassification of the intake levels of secoisolariciresinol. The lack of diversity in the socioeconomic status and race within the population may restrict the generalizability of the findings. Despite making multivariable adjustments, residual confounding cannot be fully ruled out.
DISCLOSURES:
The three cohort studies were supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
especially in individuals with obesity or premenopausal women.
METHODOLOGY:
- Lignans, polyphenolic compounds abundant in plant-based foods, are the primary dietary source of phytoestrogens in Western diets and are associated with a reduced risk for cardiometabolic conditions, but the relative associations of individual lignans with T2D are unknown.
- Researchers assessed the associations between the risk for T2D and the intake of total and four primary lignans using the data of 201,111 participants (mean age, 44.7 years; 80.2% women; 96.7% White individuals) from three large prospective US cohorts with over 30 years of follow-up, as well as the association between lignan intake and hemoglobin A1c in 496 participants from the Men’s Lifestyle Validation Study (MLVS).
- For the three large cohorts, lignan intake (total, secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol) was assessed using a validated food frequency questionnaire updated every 2-4 years and categorized into quintiles. For MLVS, diet was assessed by two sets of 7-day diet records and presented as percentage changes in A1c for linear increases in lignan intake.
- Incident T2D was confirmed using diagnostic tests, symptoms, hypoglycemic medication, elevated glucose by several measures.
TAKEAWAY:
- Across the three cohorts, 20,291 cases of T2D were recorded in the full follow-up.
- Higher intakes of total and individual ligands, except for lariciresinol, were associated with about 8%-27% lower T2D incidents (approximate hazard ratio [HR], 0.72-0.93)
- Of the individual lignans, secoisolariciresinol (but not others) showed a significant inverse association with the risk for T2D among those with a body mass index ≥ 30 (HR, 0.75; 95% CI, 0.71-0.79) and premenopausal women (HR, 0.67; 95% CI, 0.65-0.69).
- The dietary intake of lignans assessed using the 7-day diet records in MLVS was associated with lower levels of A1c (percentage changes ranging from −0.92% to −1.50%.
IN PRACTICE:
“Our findings underscore the importance of a healthy plant-based diet rich in lignan-containing foods, including flaxseed products, whole grains, and coffee for the primary prevention of T2D,” the authors wrote.
SOURCE:
The study, led by Siyue Wang, PhD, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts, and the School of Public Health, Peking University, Beijing, China, was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the potential for measurement errors in dietary assessments. Flax seed, the most concentrated source of lignans, was not assessed until midway through the three large cohort follow-ups, and this may have resulted in misclassification of the intake levels of secoisolariciresinol. The lack of diversity in the socioeconomic status and race within the population may restrict the generalizability of the findings. Despite making multivariable adjustments, residual confounding cannot be fully ruled out.
DISCLOSURES:
The three cohort studies were supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Men, Women, & Exercise: How Metabolism Differs
TOPLINE:
METHODOLOGY:
- By stimulating skeletal muscle, exercise can help prevent muscle loss associated with weight loss and improve insulin sensitivity and glucose control in type 2 diabetes, but biological sex-based differences have been reported for many measures.
- This study of sedentary men and women evaluated the molecular differences in skeletal muscle in response to a training program.
- Researchers collected muscle biopsies from 16 women and nine men with overweight or obesity (average age, 30 years) at three time points — baseline, after the first exercise session, and after the last session at the end of training.
- Training involved 1 hour of moderate to intense endurance exercise under supervision (30 minutes cycling on an ergometer and 30 minutes walking on a treadmill) thrice a week for 8 weeks.
- The biopsies were profiled for patterns of three sets of omics data — DNA methylation for insight into genes switched on and off (epigenomics), RNA molecules transcribed from genes (transcriptomics), and proteins (proteomics).
TAKEAWAY:
- At baseline, sex-specific differences were observed most tellingly in 120 proteins and also in DNA methylation sites of 16,012 genes and in 1366 RNA transcripts.
- Men displayed a higher abundance of glycolysis-related proteins and other fast-twitch fiber–type proteins, which are involved in the processing of glucose, while women showed more proteins responsible for regulating fatty acid metabolism.
- The response to the first exercise session differed between men and women, with the cellular stress response upregulated predominantly in men.
- The 8-week exercise training mitigated these sex-specific differences in the skeletal muscle, leading to an upregulation of mitochondrial proteins responsible for substrate oxidation and ATP generation in both men and women.
IN PRACTICE:
“This is important because the increased capacity after exercise to use glucose and lipids for energy production is generally regarded as key to prevent type 2 diabetes,” study leader Professor Cora Weigert from the University of Tübingen, Germany, said in a news release from the meeting organizers. “While initial response of skeletal muscles to exercise differs between females and males, repeated exercise appears to cancel out these differences and trigger beneficial metabolic changes in both sexes,” she added.
SOURCE:
The study was led by Simon I. Dreher, PhD, Institute for Clinical Chemistry and Pathobiochemistry, Department for Diagnostic Laboratory Medicine, Tübingen, Germany. It was published on August 15, 2024, as an early release from the annual meeting of the European Association for the Study of Diabetes 2024, Madrid, September 9-13.
LIMITATIONS:
This abstract did not discuss any limitations.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- By stimulating skeletal muscle, exercise can help prevent muscle loss associated with weight loss and improve insulin sensitivity and glucose control in type 2 diabetes, but biological sex-based differences have been reported for many measures.
- This study of sedentary men and women evaluated the molecular differences in skeletal muscle in response to a training program.
- Researchers collected muscle biopsies from 16 women and nine men with overweight or obesity (average age, 30 years) at three time points — baseline, after the first exercise session, and after the last session at the end of training.
- Training involved 1 hour of moderate to intense endurance exercise under supervision (30 minutes cycling on an ergometer and 30 minutes walking on a treadmill) thrice a week for 8 weeks.
- The biopsies were profiled for patterns of three sets of omics data — DNA methylation for insight into genes switched on and off (epigenomics), RNA molecules transcribed from genes (transcriptomics), and proteins (proteomics).
TAKEAWAY:
- At baseline, sex-specific differences were observed most tellingly in 120 proteins and also in DNA methylation sites of 16,012 genes and in 1366 RNA transcripts.
- Men displayed a higher abundance of glycolysis-related proteins and other fast-twitch fiber–type proteins, which are involved in the processing of glucose, while women showed more proteins responsible for regulating fatty acid metabolism.
- The response to the first exercise session differed between men and women, with the cellular stress response upregulated predominantly in men.
- The 8-week exercise training mitigated these sex-specific differences in the skeletal muscle, leading to an upregulation of mitochondrial proteins responsible for substrate oxidation and ATP generation in both men and women.
IN PRACTICE:
“This is important because the increased capacity after exercise to use glucose and lipids for energy production is generally regarded as key to prevent type 2 diabetes,” study leader Professor Cora Weigert from the University of Tübingen, Germany, said in a news release from the meeting organizers. “While initial response of skeletal muscles to exercise differs between females and males, repeated exercise appears to cancel out these differences and trigger beneficial metabolic changes in both sexes,” she added.
SOURCE:
The study was led by Simon I. Dreher, PhD, Institute for Clinical Chemistry and Pathobiochemistry, Department for Diagnostic Laboratory Medicine, Tübingen, Germany. It was published on August 15, 2024, as an early release from the annual meeting of the European Association for the Study of Diabetes 2024, Madrid, September 9-13.
LIMITATIONS:
This abstract did not discuss any limitations.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- By stimulating skeletal muscle, exercise can help prevent muscle loss associated with weight loss and improve insulin sensitivity and glucose control in type 2 diabetes, but biological sex-based differences have been reported for many measures.
- This study of sedentary men and women evaluated the molecular differences in skeletal muscle in response to a training program.
- Researchers collected muscle biopsies from 16 women and nine men with overweight or obesity (average age, 30 years) at three time points — baseline, after the first exercise session, and after the last session at the end of training.
- Training involved 1 hour of moderate to intense endurance exercise under supervision (30 minutes cycling on an ergometer and 30 minutes walking on a treadmill) thrice a week for 8 weeks.
- The biopsies were profiled for patterns of three sets of omics data — DNA methylation for insight into genes switched on and off (epigenomics), RNA molecules transcribed from genes (transcriptomics), and proteins (proteomics).
TAKEAWAY:
- At baseline, sex-specific differences were observed most tellingly in 120 proteins and also in DNA methylation sites of 16,012 genes and in 1366 RNA transcripts.
- Men displayed a higher abundance of glycolysis-related proteins and other fast-twitch fiber–type proteins, which are involved in the processing of glucose, while women showed more proteins responsible for regulating fatty acid metabolism.
- The response to the first exercise session differed between men and women, with the cellular stress response upregulated predominantly in men.
- The 8-week exercise training mitigated these sex-specific differences in the skeletal muscle, leading to an upregulation of mitochondrial proteins responsible for substrate oxidation and ATP generation in both men and women.
IN PRACTICE:
“This is important because the increased capacity after exercise to use glucose and lipids for energy production is generally regarded as key to prevent type 2 diabetes,” study leader Professor Cora Weigert from the University of Tübingen, Germany, said in a news release from the meeting organizers. “While initial response of skeletal muscles to exercise differs between females and males, repeated exercise appears to cancel out these differences and trigger beneficial metabolic changes in both sexes,” she added.
SOURCE:
The study was led by Simon I. Dreher, PhD, Institute for Clinical Chemistry and Pathobiochemistry, Department for Diagnostic Laboratory Medicine, Tübingen, Germany. It was published on August 15, 2024, as an early release from the annual meeting of the European Association for the Study of Diabetes 2024, Madrid, September 9-13.
LIMITATIONS:
This abstract did not discuss any limitations.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A Step-by-Step Guide for Diagnosing Cushing Syndrome
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
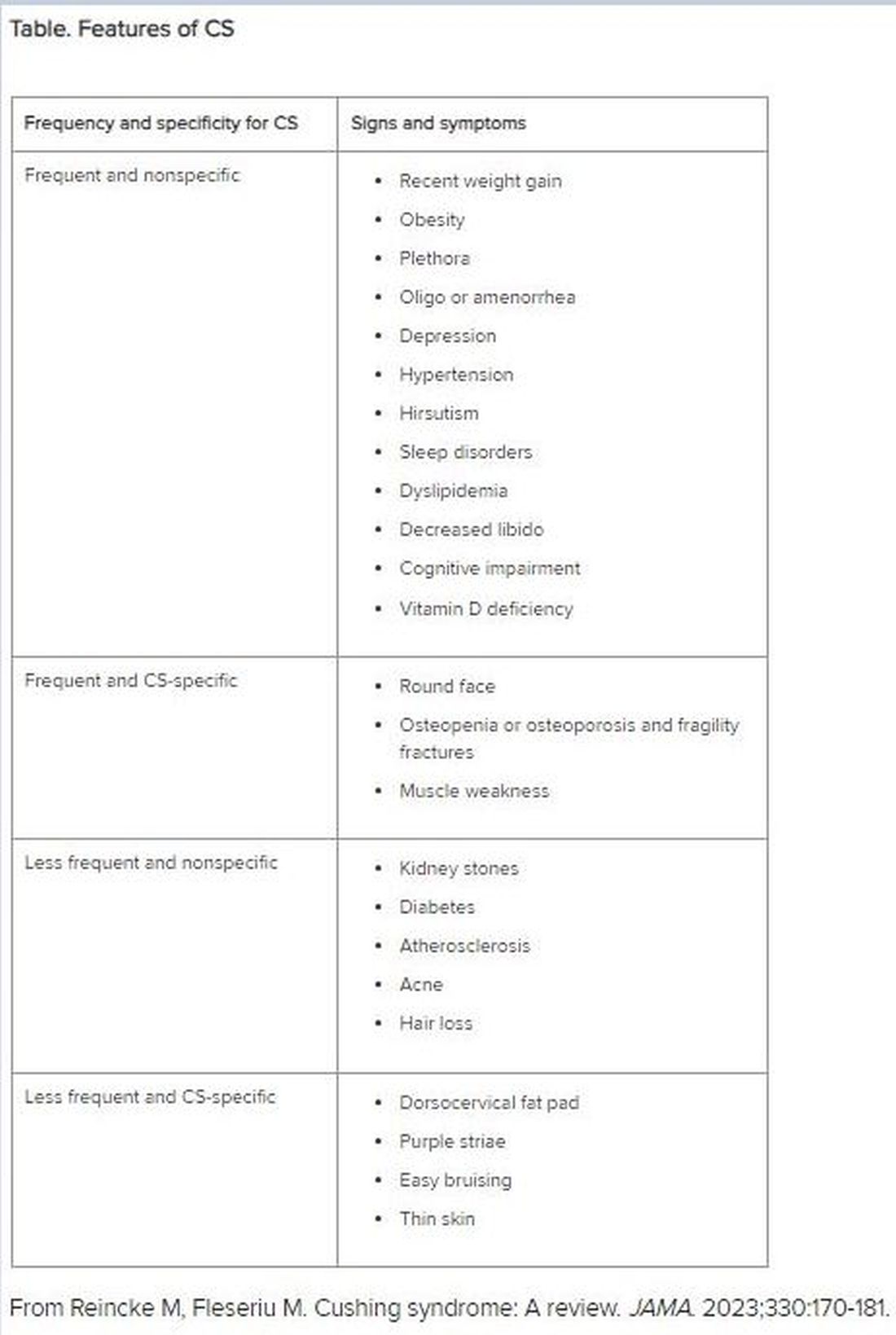
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
Patients With Immune-Mediated Inflammatory Diseases, Type 2 Diabetes Reap GLP-1 Receptor Agonist Benefits, Too
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Gender and Sports: Can Science Enable Fair Competition?
The allegations against Algerian boxer Imane Khelif at the Paris Olympics raised the questions of intersexuality and its implications in competitive sports. This news organization has decided to delve into the topic to assist doctors who suspect a similar condition in their patients. No certain clinical data about Ms. Khelif have been made public, so this article does not concern the boxer but rather takes inspiration from the media controversy.
What Is Intersexuality?
Intersexuality encompasses a spectrum of variations in sexual development that lead to the simultaneous presence of typical male and female characteristics. As reiterated by the United Nations Office of the High Commissioner for Human Rights, the medical definition does not affect the patient’s self-identification of gender or sexual orientation.
“The percentage of people who fall within the intersexuality spectrum is less than 0.5 per thousand of the general population, but there are no precise statistics, given the difficulty of definition,” said Roberto Lala, MD, pediatric endocrinologist and president of the Federation of Rare Childhood Diseases.
Indeed, there is not only a strict definition of intersexuality that involves a significant presence of these mixed physical characteristics in a way that conditions the self-image of the subject but also a broad definition, said Dr. Lala. “For example, clitoral hypertrophy in a female otherwise conforming to the female gender, which does not raise doubts about identity,” he said.
Chromosomes, Genes, and Hormones
“The human body is, so to speak, programmed to take on female appearances in development and shifts toward male ones only if exposed to testosterone and other factors. For this to happen, testosterone must be produced during embryonic development, and it must function properly,” said Paolo Moghetti, full professor of endocrinology at the University of Verona, Italy.
The protein encoded by SRY, which is located on the Y chromosome, determines the development of the testicles from undifferentiated tissue of the embryonic gonads. The testicles of the embryo then produce testosterone. The absence of the Y chromosome is a common characteristic of most female individuals. However, there are individuals with a female phenotype who have X and Y chromosomes but lack SRY or have a variant of it that is not entirely functional.
Numerous other chromosomal or genetic variations can lead to alterations in sexual differentiation. “In phenotypically male adult subjects (with a chromosomal makeup of 46XY) with complete androgen insensitivity (so-called Morris syndrome), testosterone levels in the blood are elevated, above normal even for a male, but the hormone is totally ineffective, and the phenotype is totally female at birth, with completely female development of secondary sexual characteristics at puberty,” said Dr. Moghetti.
This means that affected individuals have well-developed breasts and a complete lack or extremely reduced presence of hair, including underarm and pubic hair. Menstruation is also completely absent because there is no uterus, and there are testes, not visible because they are considered in the abdomen.
“There are syndromes that are currently considered congenital but not genetic, of which a genetic origin will probably be identified in the future,” said Dr. Lala.
Some variations in sexual development can be diagnosed prenatally, such as an alteration of the number of sex chromosomes or a discordance between the morphologic characteristics highlighted by ultrasound and the genotype detected by amniocentesis. Some variations are evident at birth because of atypical anatomical characteristics. Others are diagnosed during puberty or later in adulthood, in the presence of infertility. The Italian National Institute of Health details these variations on its website, describing the characteristics that determine diagnosis and treatment.
Pathologies or Variations?
Some anomalies in sexual development negatively affect the patient’s physical health. One example is congenital adrenal hyperplasia. “It results from an inherited defect of the adrenal glands, which reduces cortisol production while increasing testosterone production,” said Dr. Lala. “In addition to the appearance of male characteristics in females, in more severe forms, it carries the risk of collapse and shock and requires pharmacological treatment.” It is undoubtedly a pathology.
Other variations in sexual characteristics do not affect the patient’s physical health negatively. They may, however, have a psychologic effect, sometimes a significant one, because of the lack of social acceptance of a person who cannot be classified within the binary classification of sexes.
“Conditions in which mixed male and female aspects are clearly evident have been and are still pathologized by the family, the treating physician, and society,” said Dr. Lala. “In the late 1970s, when a child was born with intersexual anatomical characteristics, it was common practice to surgically intervene, making them female, because it was technically easier.”
Over the years, patients who, as they grew up, were dissatisfied with the solution adopted at birth began to make their voices heard, Dr. Lala added. Scientific societies and international organizations have spoken out against subjecting intersexual newborns to surgical interventions that are not medically necessary. “Nowadays, decisions are made on a case-by-case basis, taking into account the families’ wishes. Interventions are justified with medical reasons, which are often very nuanced,” Dr. Lala concluded.
Implications for Sports
Traditionally, athletes participating in competitions in certain sports have been divided into male and female categories to ensure a certain equity and uniformity in performance. Over the years, the emergence of new information about sexual development has made it necessary to update the criteria used in this division.
The main factor responsible for the performance diversity between males and females is the action of testosterone on the male and female organism. “Testosterone has important effects on muscle mass and enhances training results,” said Dr. Moghetti. “As a demonstration of this fact, before puberty, the best performances in athletics or swimming by males and females are similar, then males gain a significant advantage of around 10%-20%.”
A few years ago, the World Athletics Federation conducted widespread screening of athletes participating in its world championships. “It identified a small group of individuals with potentially abnormal testosterone levels for the female sex,” said Dr. Moghetti. “Some were found to be doping, others had genetic defects, and for some, an interpretation was not even possible.”
Some of the individuals had a male genotype but a defect in 5-alpha-reductase, an enzyme essential for the formation of male genitals and hair growth. An athlete with these characteristics, assigned female sex at birth, has a male level of testosterone that stimulates the accumulation of muscle mass, Dr. Moghetti explained. Therefore, the individual has a considerable advantage in performances influenced by this hormone.
“In the end, the Federation decided to set limits on the testosterone levels of athletes participating in certain types of races, especially those in middle distance, that appeared to be more sensitive to differences in hormone levels,” said Dr. Moghetti. “The limitation does not apply to athletes with Morris syndrome, ie, with a male genotype and complete resistance to testosterone, for whom the high level of this hormone does not provide any advantage.” Given the complexity of the problem, he hopes for a case-by-case policy that considers the needs of patients with genetic alterations and those of athletes who have to compete with them.
Not the First Time
A recent incident underscored the difficulty of regulating such complex issues. The World Athletics Federation excluded South African middle-distance runner Caster Semenya from competitions years ago because of excessively high testosterone levels.
“The Federation’s regulations recommend that athletes in these cases reduce hormone levels to values below the threshold of 5 nmol/L of blood for a period of at least 6 months before the race by using hormonal contraceptives. The use of such drugs does not pose a health risk, as they are substances normally taken by women for contraception purposes,” said Amelia Filippelli, a pharmacologist at the University of Salerno in Italy. The South African middle-distance runner refused the drug and appealed to the Court of Arbitration for Sport and later to the Swiss Federal Court. Both rejected her appeal. Finally, Ms. Semenya appealed to the European Court of Human Rights, which in 2023 recognized a violation of her rights but does not have the authority to order a change in the Federation’s regulations.
Beyond the ideologic positions of nonexperts, therefore, the issue is still the subject of debate in the scientific community, which is evaluating not only its medical aspects but also its ethical implications.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The allegations against Algerian boxer Imane Khelif at the Paris Olympics raised the questions of intersexuality and its implications in competitive sports. This news organization has decided to delve into the topic to assist doctors who suspect a similar condition in their patients. No certain clinical data about Ms. Khelif have been made public, so this article does not concern the boxer but rather takes inspiration from the media controversy.
What Is Intersexuality?
Intersexuality encompasses a spectrum of variations in sexual development that lead to the simultaneous presence of typical male and female characteristics. As reiterated by the United Nations Office of the High Commissioner for Human Rights, the medical definition does not affect the patient’s self-identification of gender or sexual orientation.
“The percentage of people who fall within the intersexuality spectrum is less than 0.5 per thousand of the general population, but there are no precise statistics, given the difficulty of definition,” said Roberto Lala, MD, pediatric endocrinologist and president of the Federation of Rare Childhood Diseases.
Indeed, there is not only a strict definition of intersexuality that involves a significant presence of these mixed physical characteristics in a way that conditions the self-image of the subject but also a broad definition, said Dr. Lala. “For example, clitoral hypertrophy in a female otherwise conforming to the female gender, which does not raise doubts about identity,” he said.
Chromosomes, Genes, and Hormones
“The human body is, so to speak, programmed to take on female appearances in development and shifts toward male ones only if exposed to testosterone and other factors. For this to happen, testosterone must be produced during embryonic development, and it must function properly,” said Paolo Moghetti, full professor of endocrinology at the University of Verona, Italy.
The protein encoded by SRY, which is located on the Y chromosome, determines the development of the testicles from undifferentiated tissue of the embryonic gonads. The testicles of the embryo then produce testosterone. The absence of the Y chromosome is a common characteristic of most female individuals. However, there are individuals with a female phenotype who have X and Y chromosomes but lack SRY or have a variant of it that is not entirely functional.
Numerous other chromosomal or genetic variations can lead to alterations in sexual differentiation. “In phenotypically male adult subjects (with a chromosomal makeup of 46XY) with complete androgen insensitivity (so-called Morris syndrome), testosterone levels in the blood are elevated, above normal even for a male, but the hormone is totally ineffective, and the phenotype is totally female at birth, with completely female development of secondary sexual characteristics at puberty,” said Dr. Moghetti.
This means that affected individuals have well-developed breasts and a complete lack or extremely reduced presence of hair, including underarm and pubic hair. Menstruation is also completely absent because there is no uterus, and there are testes, not visible because they are considered in the abdomen.
“There are syndromes that are currently considered congenital but not genetic, of which a genetic origin will probably be identified in the future,” said Dr. Lala.
Some variations in sexual development can be diagnosed prenatally, such as an alteration of the number of sex chromosomes or a discordance between the morphologic characteristics highlighted by ultrasound and the genotype detected by amniocentesis. Some variations are evident at birth because of atypical anatomical characteristics. Others are diagnosed during puberty or later in adulthood, in the presence of infertility. The Italian National Institute of Health details these variations on its website, describing the characteristics that determine diagnosis and treatment.
Pathologies or Variations?
Some anomalies in sexual development negatively affect the patient’s physical health. One example is congenital adrenal hyperplasia. “It results from an inherited defect of the adrenal glands, which reduces cortisol production while increasing testosterone production,” said Dr. Lala. “In addition to the appearance of male characteristics in females, in more severe forms, it carries the risk of collapse and shock and requires pharmacological treatment.” It is undoubtedly a pathology.
Other variations in sexual characteristics do not affect the patient’s physical health negatively. They may, however, have a psychologic effect, sometimes a significant one, because of the lack of social acceptance of a person who cannot be classified within the binary classification of sexes.
“Conditions in which mixed male and female aspects are clearly evident have been and are still pathologized by the family, the treating physician, and society,” said Dr. Lala. “In the late 1970s, when a child was born with intersexual anatomical characteristics, it was common practice to surgically intervene, making them female, because it was technically easier.”
Over the years, patients who, as they grew up, were dissatisfied with the solution adopted at birth began to make their voices heard, Dr. Lala added. Scientific societies and international organizations have spoken out against subjecting intersexual newborns to surgical interventions that are not medically necessary. “Nowadays, decisions are made on a case-by-case basis, taking into account the families’ wishes. Interventions are justified with medical reasons, which are often very nuanced,” Dr. Lala concluded.
Implications for Sports
Traditionally, athletes participating in competitions in certain sports have been divided into male and female categories to ensure a certain equity and uniformity in performance. Over the years, the emergence of new information about sexual development has made it necessary to update the criteria used in this division.
The main factor responsible for the performance diversity between males and females is the action of testosterone on the male and female organism. “Testosterone has important effects on muscle mass and enhances training results,” said Dr. Moghetti. “As a demonstration of this fact, before puberty, the best performances in athletics or swimming by males and females are similar, then males gain a significant advantage of around 10%-20%.”
A few years ago, the World Athletics Federation conducted widespread screening of athletes participating in its world championships. “It identified a small group of individuals with potentially abnormal testosterone levels for the female sex,” said Dr. Moghetti. “Some were found to be doping, others had genetic defects, and for some, an interpretation was not even possible.”
Some of the individuals had a male genotype but a defect in 5-alpha-reductase, an enzyme essential for the formation of male genitals and hair growth. An athlete with these characteristics, assigned female sex at birth, has a male level of testosterone that stimulates the accumulation of muscle mass, Dr. Moghetti explained. Therefore, the individual has a considerable advantage in performances influenced by this hormone.
“In the end, the Federation decided to set limits on the testosterone levels of athletes participating in certain types of races, especially those in middle distance, that appeared to be more sensitive to differences in hormone levels,” said Dr. Moghetti. “The limitation does not apply to athletes with Morris syndrome, ie, with a male genotype and complete resistance to testosterone, for whom the high level of this hormone does not provide any advantage.” Given the complexity of the problem, he hopes for a case-by-case policy that considers the needs of patients with genetic alterations and those of athletes who have to compete with them.
Not the First Time
A recent incident underscored the difficulty of regulating such complex issues. The World Athletics Federation excluded South African middle-distance runner Caster Semenya from competitions years ago because of excessively high testosterone levels.
“The Federation’s regulations recommend that athletes in these cases reduce hormone levels to values below the threshold of 5 nmol/L of blood for a period of at least 6 months before the race by using hormonal contraceptives. The use of such drugs does not pose a health risk, as they are substances normally taken by women for contraception purposes,” said Amelia Filippelli, a pharmacologist at the University of Salerno in Italy. The South African middle-distance runner refused the drug and appealed to the Court of Arbitration for Sport and later to the Swiss Federal Court. Both rejected her appeal. Finally, Ms. Semenya appealed to the European Court of Human Rights, which in 2023 recognized a violation of her rights but does not have the authority to order a change in the Federation’s regulations.
Beyond the ideologic positions of nonexperts, therefore, the issue is still the subject of debate in the scientific community, which is evaluating not only its medical aspects but also its ethical implications.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The allegations against Algerian boxer Imane Khelif at the Paris Olympics raised the questions of intersexuality and its implications in competitive sports. This news organization has decided to delve into the topic to assist doctors who suspect a similar condition in their patients. No certain clinical data about Ms. Khelif have been made public, so this article does not concern the boxer but rather takes inspiration from the media controversy.
What Is Intersexuality?
Intersexuality encompasses a spectrum of variations in sexual development that lead to the simultaneous presence of typical male and female characteristics. As reiterated by the United Nations Office of the High Commissioner for Human Rights, the medical definition does not affect the patient’s self-identification of gender or sexual orientation.
“The percentage of people who fall within the intersexuality spectrum is less than 0.5 per thousand of the general population, but there are no precise statistics, given the difficulty of definition,” said Roberto Lala, MD, pediatric endocrinologist and president of the Federation of Rare Childhood Diseases.
Indeed, there is not only a strict definition of intersexuality that involves a significant presence of these mixed physical characteristics in a way that conditions the self-image of the subject but also a broad definition, said Dr. Lala. “For example, clitoral hypertrophy in a female otherwise conforming to the female gender, which does not raise doubts about identity,” he said.
Chromosomes, Genes, and Hormones
“The human body is, so to speak, programmed to take on female appearances in development and shifts toward male ones only if exposed to testosterone and other factors. For this to happen, testosterone must be produced during embryonic development, and it must function properly,” said Paolo Moghetti, full professor of endocrinology at the University of Verona, Italy.
The protein encoded by SRY, which is located on the Y chromosome, determines the development of the testicles from undifferentiated tissue of the embryonic gonads. The testicles of the embryo then produce testosterone. The absence of the Y chromosome is a common characteristic of most female individuals. However, there are individuals with a female phenotype who have X and Y chromosomes but lack SRY or have a variant of it that is not entirely functional.
Numerous other chromosomal or genetic variations can lead to alterations in sexual differentiation. “In phenotypically male adult subjects (with a chromosomal makeup of 46XY) with complete androgen insensitivity (so-called Morris syndrome), testosterone levels in the blood are elevated, above normal even for a male, but the hormone is totally ineffective, and the phenotype is totally female at birth, with completely female development of secondary sexual characteristics at puberty,” said Dr. Moghetti.
This means that affected individuals have well-developed breasts and a complete lack or extremely reduced presence of hair, including underarm and pubic hair. Menstruation is also completely absent because there is no uterus, and there are testes, not visible because they are considered in the abdomen.
“There are syndromes that are currently considered congenital but not genetic, of which a genetic origin will probably be identified in the future,” said Dr. Lala.
Some variations in sexual development can be diagnosed prenatally, such as an alteration of the number of sex chromosomes or a discordance between the morphologic characteristics highlighted by ultrasound and the genotype detected by amniocentesis. Some variations are evident at birth because of atypical anatomical characteristics. Others are diagnosed during puberty or later in adulthood, in the presence of infertility. The Italian National Institute of Health details these variations on its website, describing the characteristics that determine diagnosis and treatment.
Pathologies or Variations?
Some anomalies in sexual development negatively affect the patient’s physical health. One example is congenital adrenal hyperplasia. “It results from an inherited defect of the adrenal glands, which reduces cortisol production while increasing testosterone production,” said Dr. Lala. “In addition to the appearance of male characteristics in females, in more severe forms, it carries the risk of collapse and shock and requires pharmacological treatment.” It is undoubtedly a pathology.
Other variations in sexual characteristics do not affect the patient’s physical health negatively. They may, however, have a psychologic effect, sometimes a significant one, because of the lack of social acceptance of a person who cannot be classified within the binary classification of sexes.
“Conditions in which mixed male and female aspects are clearly evident have been and are still pathologized by the family, the treating physician, and society,” said Dr. Lala. “In the late 1970s, when a child was born with intersexual anatomical characteristics, it was common practice to surgically intervene, making them female, because it was technically easier.”
Over the years, patients who, as they grew up, were dissatisfied with the solution adopted at birth began to make their voices heard, Dr. Lala added. Scientific societies and international organizations have spoken out against subjecting intersexual newborns to surgical interventions that are not medically necessary. “Nowadays, decisions are made on a case-by-case basis, taking into account the families’ wishes. Interventions are justified with medical reasons, which are often very nuanced,” Dr. Lala concluded.
Implications for Sports
Traditionally, athletes participating in competitions in certain sports have been divided into male and female categories to ensure a certain equity and uniformity in performance. Over the years, the emergence of new information about sexual development has made it necessary to update the criteria used in this division.
The main factor responsible for the performance diversity between males and females is the action of testosterone on the male and female organism. “Testosterone has important effects on muscle mass and enhances training results,” said Dr. Moghetti. “As a demonstration of this fact, before puberty, the best performances in athletics or swimming by males and females are similar, then males gain a significant advantage of around 10%-20%.”
A few years ago, the World Athletics Federation conducted widespread screening of athletes participating in its world championships. “It identified a small group of individuals with potentially abnormal testosterone levels for the female sex,” said Dr. Moghetti. “Some were found to be doping, others had genetic defects, and for some, an interpretation was not even possible.”
Some of the individuals had a male genotype but a defect in 5-alpha-reductase, an enzyme essential for the formation of male genitals and hair growth. An athlete with these characteristics, assigned female sex at birth, has a male level of testosterone that stimulates the accumulation of muscle mass, Dr. Moghetti explained. Therefore, the individual has a considerable advantage in performances influenced by this hormone.
“In the end, the Federation decided to set limits on the testosterone levels of athletes participating in certain types of races, especially those in middle distance, that appeared to be more sensitive to differences in hormone levels,” said Dr. Moghetti. “The limitation does not apply to athletes with Morris syndrome, ie, with a male genotype and complete resistance to testosterone, for whom the high level of this hormone does not provide any advantage.” Given the complexity of the problem, he hopes for a case-by-case policy that considers the needs of patients with genetic alterations and those of athletes who have to compete with them.
Not the First Time
A recent incident underscored the difficulty of regulating such complex issues. The World Athletics Federation excluded South African middle-distance runner Caster Semenya from competitions years ago because of excessively high testosterone levels.
“The Federation’s regulations recommend that athletes in these cases reduce hormone levels to values below the threshold of 5 nmol/L of blood for a period of at least 6 months before the race by using hormonal contraceptives. The use of such drugs does not pose a health risk, as they are substances normally taken by women for contraception purposes,” said Amelia Filippelli, a pharmacologist at the University of Salerno in Italy. The South African middle-distance runner refused the drug and appealed to the Court of Arbitration for Sport and later to the Swiss Federal Court. Both rejected her appeal. Finally, Ms. Semenya appealed to the European Court of Human Rights, which in 2023 recognized a violation of her rights but does not have the authority to order a change in the Federation’s regulations.
Beyond the ideologic positions of nonexperts, therefore, the issue is still the subject of debate in the scientific community, which is evaluating not only its medical aspects but also its ethical implications.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Recurrent Pancreatitis Triples Risk for Chronic Disease
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The overall progression to chronic pancreatitis among adults was three times higher following recurrent episodes of acute pancreatitis than occurring after just the first acute pancreatitis episode.
METHODOLOGY:
- The progression of acute pancreatitis is time-dependent, with the recurrence and progression rates to recurrent acute pancreatitis and chronic pancreatitis varying based on the follow-up duration and may be affected by the cause and severity of the first acute episode.
- To better understand the progression of acute pancreatitis to recurrent acute pancreatitis and chronic pancreatitis, researchers conducted a systematic review and meta-analysis of 119 studies, all of which were used for qualitative and quantitative synthesis and 29 of which also were used for calculating incidence rates.
- The primary outcomes were the incidence rates of recurrent acute and chronic pancreatitis following the initial episode of acute pancreatitis and the incidence rate of chronic pancreatitis after recurrent episodes of acute pancreatitis.
- The secondary outcomes were the cumulative incidences and proportions of recurrent acute and chronic pancreatitis following the initial acute pancreatitis episode and the proportion of chronic pancreatitis occurring after recurrent acute pancreatitis episodes.
TAKEAWAY:
- The incidence rate of recurrent acute pancreatitis after the first acute episode was 5.26 per 100 person-years in adults and 4.64 per 100 person-years in children, a difference that did not reach statistical significance.
- The progression rate to chronic pancreatitis in adults was threefold higher after recurrent acute pancreatitis episodes than after the first acute pancreatitis episode (4.31 vs 1.38 per 100 person-years).
- Hypertriglyceridemia-induced acute pancreatitis had the highest recurrence rates, followed by alcohol-induced, idiopathic, and biliary pancreatitis.
- The overall progression rate into chronic pancreatitis was 8% after the first acute pancreatitis episode and 24% after recurrent episodes of acute pancreatitis. Progression to chronic pancreatitis among adults was highest among those with alcohol-induced disease, followed by idiopathic and biliary pancreatitis.
- A moderately severe first episode of acute pancreatitis was associated with the highest recurrence rate, followed by mild and severe first episodes.
IN PRACTICE:
The authors emphasized the need to develop new interventions to address the factors associated with acute pancreatitis and its progression and to better utilize existing approaches, such as brief and repeated psychological interventions and alcohol and smoking cessation programs. Deeper investigation into the underlying causes of the disease’s etiology is warranted to reduce recurrence and progression rates, they noted.
SOURCE:
The study, led by Endre-Botond Gagyi, MD, of the Center for Translational Medicine, Semmelweis University, Budapest, Hungary, was published online in Therapeutic Advances in Gastroenterology.
LIMITATIONS:
Most of the studies included in the analysis were retrospective, and there was high heterogeneity between them. The researchers could only analyze the presence of recurrent acute pancreatitis but could not explore the number of episodes or their impact on progression due to the lack of reported data.
DISCLOSURES:
The study was funded by the New National Excellence Program of the Ministry for Innovation and Technology from the National Research, Development and Innovation Fund. The authors declared no conflict of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
What Every Provider Should Know About Type 1 Diabetes
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.