User login
Updated Guideline Reflects New Drugs for Type 2 Diabetes
Type 2 diabetes (T2D) is the most common form of diabetes, representing more than 90% of all cases worldwide. The prevalence of T2D is increasing globally, mainly because of behavioral and social factors related to obesity, diet, and physical activity. The International Diabetes Federation estimated in its 2021 report that 537 million adults aged between 20 and 79 years have been diagnosed with diabetes worldwide. The organization predicts an increase to 643 million by 2030 and 743 million by 2045.
The main therapeutic goals for patients with T2D include adequate glycemic control and primary and secondary prevention of atherosclerotic cardiovascular and renal diseases, which represent nearly half of all deaths among adults with T2D. Despite the multiple treatment options available, 16% of adults with T2D have inadequate glycemic control, including hemoglobin A1c levels greater than 9%, even though glycemic control was the focus of the 2017 guidelines of the American College of Physicians.
Therefore, the ACP deemed it necessary to update the previous guidelines, considering new evidence on the efficacy and harms of new pharmacologic treatments in adults with T2D with the goal of reducing the risk for all-cause mortality, cardiovascular morbidity, and progression of chronic kidney disease (CKD) in these patients.
New Drugs
The pharmacologic treatments that the ACP considered while updating its guidelines include glucagon-like peptide 1 (GLP-1) receptor agonists (that is, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide), a GLP-1 receptor agonist and a glucose-dependent insulinotropic polypeptide receptor agonist (that is, tirzepatide), sodium-glucose cotransporter 2 (SGLT-2) inhibitors (that is, canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin), dipeptidyl peptidase 4 (DPP-4) inhibitors (that is, alogliptin, linagliptin, saxagliptin, and sitagliptin), and long-acting insulins (that is, insulin glargine and insulin degludec).
Recommendations
The ACP recommends adding an SGLT-2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with inadequately controlled T2D (strong recommendation, high certainty of evidence). Use an SGLT-2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events (MACE), CKD progression, and hospitalization resulting from heart failure, according to the document. Use a GLP-1 agonist to reduce the risk for all-cause mortality, MACE, and strokes.
SGLT-2 inhibitors and GLP-1 agonists are the only newer pharmacological treatments for T2D that have reduced all-cause mortality than placebo or usual care. In indirect comparison, SGLT-2 inhibitors probably reduce the risk for hospitalization resulting from heart failure, while GLP-1 agonists probably reduce the risk for strokes.
Neither class of drugs causes severe hypoglycemia, but both are associated with various harms, as reported in specific warnings. Both classes of drugs lead to weight loss.
Compared with long-acting insulins, SGLT-2 inhibitors can reduce, and GLP-1 agonists probably reduce, all-cause mortality. Compared with DPP-4 inhibitors, GLP-1 agonists probably reduce all-cause mortality.
Compared with DPP-4 inhibitors, SGLT-2 inhibitors probably reduce MACE, as well as compared with sulfonylureas.
The ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with inadequately controlled T2D to reduce morbidity and all-cause mortality (strong recommendation, high certainty of evidence).
Compared with usual therapy, DPP-4 inhibitors do not result in differences in all-cause mortality, MACE, myocardial infarction, stroke, hospitalization for chronic heart failure (CHF), CKD progression, or severe hypoglycemia. Compared with SGLT-2 inhibitors, DPP-4 inhibitors may increase hospitalization caused by CHF and probably increase the risk for MACE and CKD progression. Compared with GLP-1 agonists, they probably increase all-cause mortality and hospitalization caused by CHF and the risk for MACE. Metformin is the most common usual therapy in the studies considered.
Considerations for Practice
Metformin (unless contraindicated) and lifestyle modifications represent the first step in managing T2D in most patients, according to the ACP.
The choice of additional therapy requires a risk/benefit assessment and should be personalized on the basis of patient preferences, glycemic control goals, comorbidities, and the risk for hypoglycemia. SGLT-2 inhibitors can be added in patients with T2D and CHF or CKD, according to the ACP. GLP-1 agonists can be added in patients with T2D at increased risk for stroke or for whom total body weight loss is a significant therapeutic goal.
The A1c target should be considered between 7% and 8% in most adults with T2D, and de-escalation of pharmacologic treatments should be considered for A1c levels less than 6.5%. Self-monitoring of blood glucose may not be necessary in patients treated with metformin in combination with an SGLT-2 inhibitor or a GLP-1 agonist, according to the ACP.
The document also holds that, in cases of adequate glycemic control with the addition of an SGLT-2 inhibitor or a GLP-1 agonist, existing treatment with sulfonylureas or long-acting insulin should be reduced or stopped due to the increased risk for severe hypoglycemia.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Type 2 diabetes (T2D) is the most common form of diabetes, representing more than 90% of all cases worldwide. The prevalence of T2D is increasing globally, mainly because of behavioral and social factors related to obesity, diet, and physical activity. The International Diabetes Federation estimated in its 2021 report that 537 million adults aged between 20 and 79 years have been diagnosed with diabetes worldwide. The organization predicts an increase to 643 million by 2030 and 743 million by 2045.
The main therapeutic goals for patients with T2D include adequate glycemic control and primary and secondary prevention of atherosclerotic cardiovascular and renal diseases, which represent nearly half of all deaths among adults with T2D. Despite the multiple treatment options available, 16% of adults with T2D have inadequate glycemic control, including hemoglobin A1c levels greater than 9%, even though glycemic control was the focus of the 2017 guidelines of the American College of Physicians.
Therefore, the ACP deemed it necessary to update the previous guidelines, considering new evidence on the efficacy and harms of new pharmacologic treatments in adults with T2D with the goal of reducing the risk for all-cause mortality, cardiovascular morbidity, and progression of chronic kidney disease (CKD) in these patients.
New Drugs
The pharmacologic treatments that the ACP considered while updating its guidelines include glucagon-like peptide 1 (GLP-1) receptor agonists (that is, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide), a GLP-1 receptor agonist and a glucose-dependent insulinotropic polypeptide receptor agonist (that is, tirzepatide), sodium-glucose cotransporter 2 (SGLT-2) inhibitors (that is, canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin), dipeptidyl peptidase 4 (DPP-4) inhibitors (that is, alogliptin, linagliptin, saxagliptin, and sitagliptin), and long-acting insulins (that is, insulin glargine and insulin degludec).
Recommendations
The ACP recommends adding an SGLT-2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with inadequately controlled T2D (strong recommendation, high certainty of evidence). Use an SGLT-2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events (MACE), CKD progression, and hospitalization resulting from heart failure, according to the document. Use a GLP-1 agonist to reduce the risk for all-cause mortality, MACE, and strokes.
SGLT-2 inhibitors and GLP-1 agonists are the only newer pharmacological treatments for T2D that have reduced all-cause mortality than placebo or usual care. In indirect comparison, SGLT-2 inhibitors probably reduce the risk for hospitalization resulting from heart failure, while GLP-1 agonists probably reduce the risk for strokes.
Neither class of drugs causes severe hypoglycemia, but both are associated with various harms, as reported in specific warnings. Both classes of drugs lead to weight loss.
Compared with long-acting insulins, SGLT-2 inhibitors can reduce, and GLP-1 agonists probably reduce, all-cause mortality. Compared with DPP-4 inhibitors, GLP-1 agonists probably reduce all-cause mortality.
Compared with DPP-4 inhibitors, SGLT-2 inhibitors probably reduce MACE, as well as compared with sulfonylureas.
The ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with inadequately controlled T2D to reduce morbidity and all-cause mortality (strong recommendation, high certainty of evidence).
Compared with usual therapy, DPP-4 inhibitors do not result in differences in all-cause mortality, MACE, myocardial infarction, stroke, hospitalization for chronic heart failure (CHF), CKD progression, or severe hypoglycemia. Compared with SGLT-2 inhibitors, DPP-4 inhibitors may increase hospitalization caused by CHF and probably increase the risk for MACE and CKD progression. Compared with GLP-1 agonists, they probably increase all-cause mortality and hospitalization caused by CHF and the risk for MACE. Metformin is the most common usual therapy in the studies considered.
Considerations for Practice
Metformin (unless contraindicated) and lifestyle modifications represent the first step in managing T2D in most patients, according to the ACP.
The choice of additional therapy requires a risk/benefit assessment and should be personalized on the basis of patient preferences, glycemic control goals, comorbidities, and the risk for hypoglycemia. SGLT-2 inhibitors can be added in patients with T2D and CHF or CKD, according to the ACP. GLP-1 agonists can be added in patients with T2D at increased risk for stroke or for whom total body weight loss is a significant therapeutic goal.
The A1c target should be considered between 7% and 8% in most adults with T2D, and de-escalation of pharmacologic treatments should be considered for A1c levels less than 6.5%. Self-monitoring of blood glucose may not be necessary in patients treated with metformin in combination with an SGLT-2 inhibitor or a GLP-1 agonist, according to the ACP.
The document also holds that, in cases of adequate glycemic control with the addition of an SGLT-2 inhibitor or a GLP-1 agonist, existing treatment with sulfonylureas or long-acting insulin should be reduced or stopped due to the increased risk for severe hypoglycemia.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Type 2 diabetes (T2D) is the most common form of diabetes, representing more than 90% of all cases worldwide. The prevalence of T2D is increasing globally, mainly because of behavioral and social factors related to obesity, diet, and physical activity. The International Diabetes Federation estimated in its 2021 report that 537 million adults aged between 20 and 79 years have been diagnosed with diabetes worldwide. The organization predicts an increase to 643 million by 2030 and 743 million by 2045.
The main therapeutic goals for patients with T2D include adequate glycemic control and primary and secondary prevention of atherosclerotic cardiovascular and renal diseases, which represent nearly half of all deaths among adults with T2D. Despite the multiple treatment options available, 16% of adults with T2D have inadequate glycemic control, including hemoglobin A1c levels greater than 9%, even though glycemic control was the focus of the 2017 guidelines of the American College of Physicians.
Therefore, the ACP deemed it necessary to update the previous guidelines, considering new evidence on the efficacy and harms of new pharmacologic treatments in adults with T2D with the goal of reducing the risk for all-cause mortality, cardiovascular morbidity, and progression of chronic kidney disease (CKD) in these patients.
New Drugs
The pharmacologic treatments that the ACP considered while updating its guidelines include glucagon-like peptide 1 (GLP-1) receptor agonists (that is, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide), a GLP-1 receptor agonist and a glucose-dependent insulinotropic polypeptide receptor agonist (that is, tirzepatide), sodium-glucose cotransporter 2 (SGLT-2) inhibitors (that is, canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin), dipeptidyl peptidase 4 (DPP-4) inhibitors (that is, alogliptin, linagliptin, saxagliptin, and sitagliptin), and long-acting insulins (that is, insulin glargine and insulin degludec).
Recommendations
The ACP recommends adding an SGLT-2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with inadequately controlled T2D (strong recommendation, high certainty of evidence). Use an SGLT-2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events (MACE), CKD progression, and hospitalization resulting from heart failure, according to the document. Use a GLP-1 agonist to reduce the risk for all-cause mortality, MACE, and strokes.
SGLT-2 inhibitors and GLP-1 agonists are the only newer pharmacological treatments for T2D that have reduced all-cause mortality than placebo or usual care. In indirect comparison, SGLT-2 inhibitors probably reduce the risk for hospitalization resulting from heart failure, while GLP-1 agonists probably reduce the risk for strokes.
Neither class of drugs causes severe hypoglycemia, but both are associated with various harms, as reported in specific warnings. Both classes of drugs lead to weight loss.
Compared with long-acting insulins, SGLT-2 inhibitors can reduce, and GLP-1 agonists probably reduce, all-cause mortality. Compared with DPP-4 inhibitors, GLP-1 agonists probably reduce all-cause mortality.
Compared with DPP-4 inhibitors, SGLT-2 inhibitors probably reduce MACE, as well as compared with sulfonylureas.
The ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with inadequately controlled T2D to reduce morbidity and all-cause mortality (strong recommendation, high certainty of evidence).
Compared with usual therapy, DPP-4 inhibitors do not result in differences in all-cause mortality, MACE, myocardial infarction, stroke, hospitalization for chronic heart failure (CHF), CKD progression, or severe hypoglycemia. Compared with SGLT-2 inhibitors, DPP-4 inhibitors may increase hospitalization caused by CHF and probably increase the risk for MACE and CKD progression. Compared with GLP-1 agonists, they probably increase all-cause mortality and hospitalization caused by CHF and the risk for MACE. Metformin is the most common usual therapy in the studies considered.
Considerations for Practice
Metformin (unless contraindicated) and lifestyle modifications represent the first step in managing T2D in most patients, according to the ACP.
The choice of additional therapy requires a risk/benefit assessment and should be personalized on the basis of patient preferences, glycemic control goals, comorbidities, and the risk for hypoglycemia. SGLT-2 inhibitors can be added in patients with T2D and CHF or CKD, according to the ACP. GLP-1 agonists can be added in patients with T2D at increased risk for stroke or for whom total body weight loss is a significant therapeutic goal.
The A1c target should be considered between 7% and 8% in most adults with T2D, and de-escalation of pharmacologic treatments should be considered for A1c levels less than 6.5%. Self-monitoring of blood glucose may not be necessary in patients treated with metformin in combination with an SGLT-2 inhibitor or a GLP-1 agonist, according to the ACP.
The document also holds that, in cases of adequate glycemic control with the addition of an SGLT-2 inhibitor or a GLP-1 agonist, existing treatment with sulfonylureas or long-acting insulin should be reduced or stopped due to the increased risk for severe hypoglycemia.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Acute Sore Throat in Primary Care: When to Reach for the Antibiotics
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
There is a helpful consensus from experts on the best management of patients with acute sore throat. This is a common problem in primary care, and one for which there is a lot of evidence, opinion, and ultimately overprescribing of antibiotics. This consensus presents a pragmatic clinical approach aimed at decreasing overprescribing, yet detecting which patients are likely to benefit from treatment with antibiotics.
Let’s first go over the evidence that forms the basis for the recommendations, then the recommended approach. First, a sore throat can be caused by many different viruses, as well as group A streptococcus (GAS), the group C streptococcus S dysgalactiae, and fusobacterium. We sometimes think of throat cultures as telling us the definitive etiology of a sore throat. In fact, children commonly are colonized with GAS even when not infected — 35% of the time, when GAS is detected on throat swab in a child, GAS is not the cause of the sore throat. Very few adults are colonized with GAS.
Sore throats are usually self-limited, whether they are treated with antibiotics or not, but occasionally complications can occur. Suppurative complications include peritonsillar abscess, sinusitis and sepsis. Nonsuppurative complications are primarily glomerulonephritis and rheumatic fever, which can lead to rheumatic heart disease.
Antibiotics. Antibiotics have three potential benefits in acute sore throat: to reduce the risk of developing rheumatic heart disease, reduce the duration and severity of symptoms, and treat suppurative complications. The risk for rheumatic heart disease has almost vanished in high-income countries, but not in low-income countries. Thus, antibiotic treatment of acute sore throat due to GAS may benefit those in living in, and those who recently emigrated from, low-income countries.
Patients with suppurative complications should be identified because antibiotics are important for this group. Although antibiotics are prescribed primarily to prevent rheumatic fever in this population, they may be mildly helpful in reducing a patient’s symptoms.
Testing. The sensitivity and specificity of high-quality point-of-care tests (POCTs) are on par with those of cultures, with the advantage that the results are available within minutes. Negative tests reduce unneeded antibiotic prescriptions.
Given this evidence, the authors recommend an approach that puts a lot of emphasis on two major things: the risk for rheumatic fever, and clinical assessment. On the basis of these factors, a decision is made about the utility of POCTs and treatment with antibiotics for GAS. The risk for rheumatic fever is based on epidemiology: If the patient is in a low-income country or has recently immigrated from one, then the risk is high, and if not, the risk is low.
Complicated vs uncomplicated? This is determined by clinical assessment of the severity of the patient’s illness, including general appearance. Uncomplicated sore throat means that the patient:
- Is not getting worse after 3 days of illness
- Has a duration of illness ≤ 5 days or is getting better after day 5
- Has mild to moderate symptom severity (bilateral throat pain, the ability to open the mouth fully, and absence of a sandpaper or scarlatiniform rash or strawberry tongue)
For patients with uncomplicated sore throat and low risk for rheumatic fever, the main goals are to reduce antibiotic use and provide symptomatic relief. For these patients, an assessment such as the Centor score can be done. Those with a low Centor score (0-2) can be treated with analgesics and there is no need for a POCT.
In patients with a higher Centor score, the consensus gives two choices: They can either be tested (and treated if the testing is positive), or it is reasonable to forgo testing and use a wait-and-see strategy, with reevaluation if they are getting worse after day 3 or not improving after day 5 days of their illness. Illnesses that last longer than 5 days with sore throat and fatigue should prompt consideration of alternative diagnoses, such as infectious mononucleosis.
For patients with potentially complicated sore throat — including indicators such as worsening symptoms after 3 days or worsening after initiation of antibiotics, inability to open the mouth fully, unilateral neck pain or swelling, or rigors — should undergo a careful evaluation. The need for further testing in these patients, including labs and imaging, should be decided on a case-by-case basis. If the patient appears seriously ill, don’t rely solely on POCT for GAS, but think about other diagnoses.
Rheumatic fever. The approach is very different in patients at high risk for rheumatic fever. POCT for GAS is recommended irrespective of their clinical score, and antibiotics should be prescribed if it’s positive for GAS. If a POCT is unavailable, then the consensus recommends prescribing antibiotics for all high-risk patients who have acute sore throat.
This approach is sensible and puts a lot of emphasis on clinical evaluation, though it should be noted that this approach is considerably different from that in the 2012 Infectious Diseases Society of America guidelines.
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
Flesh-Colored Pinpoint Papules With Fine White Spicules on the Upper Body
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
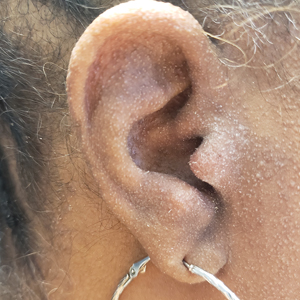
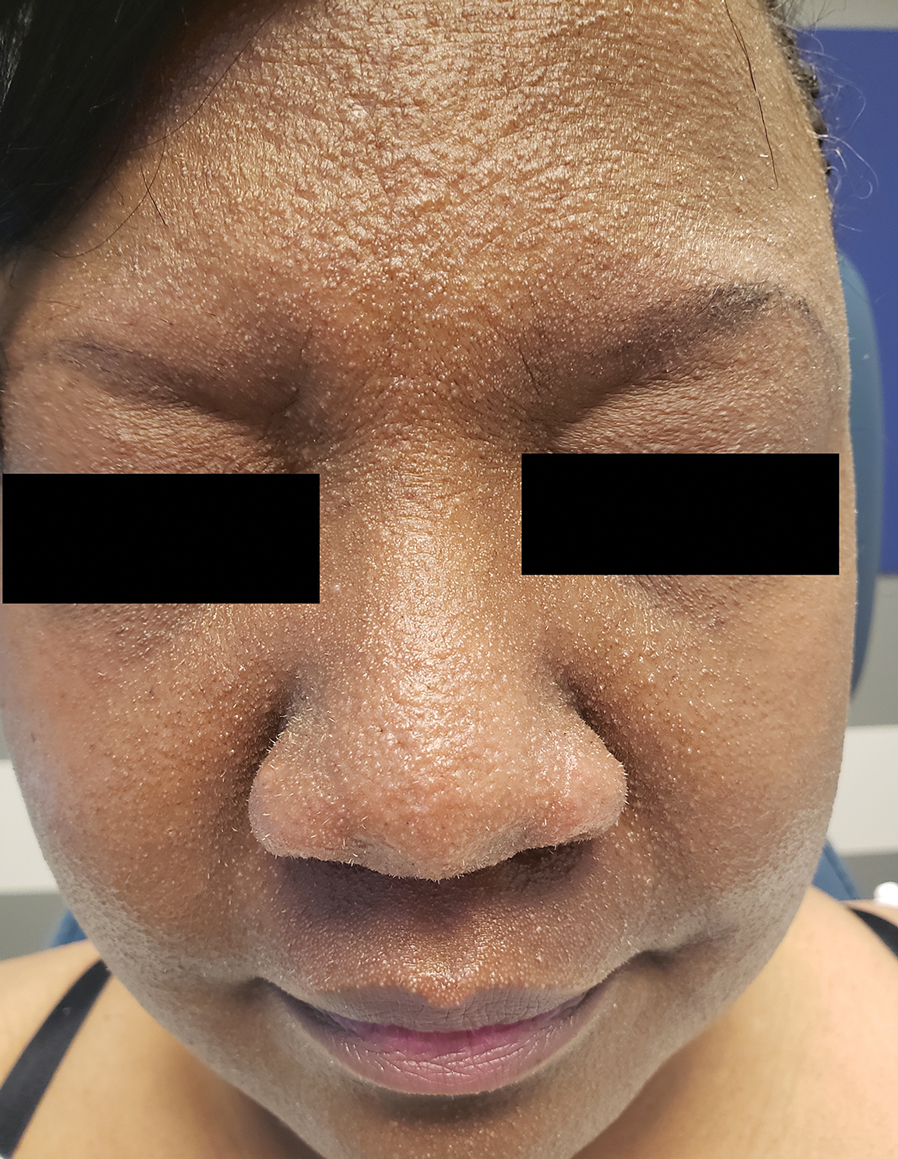
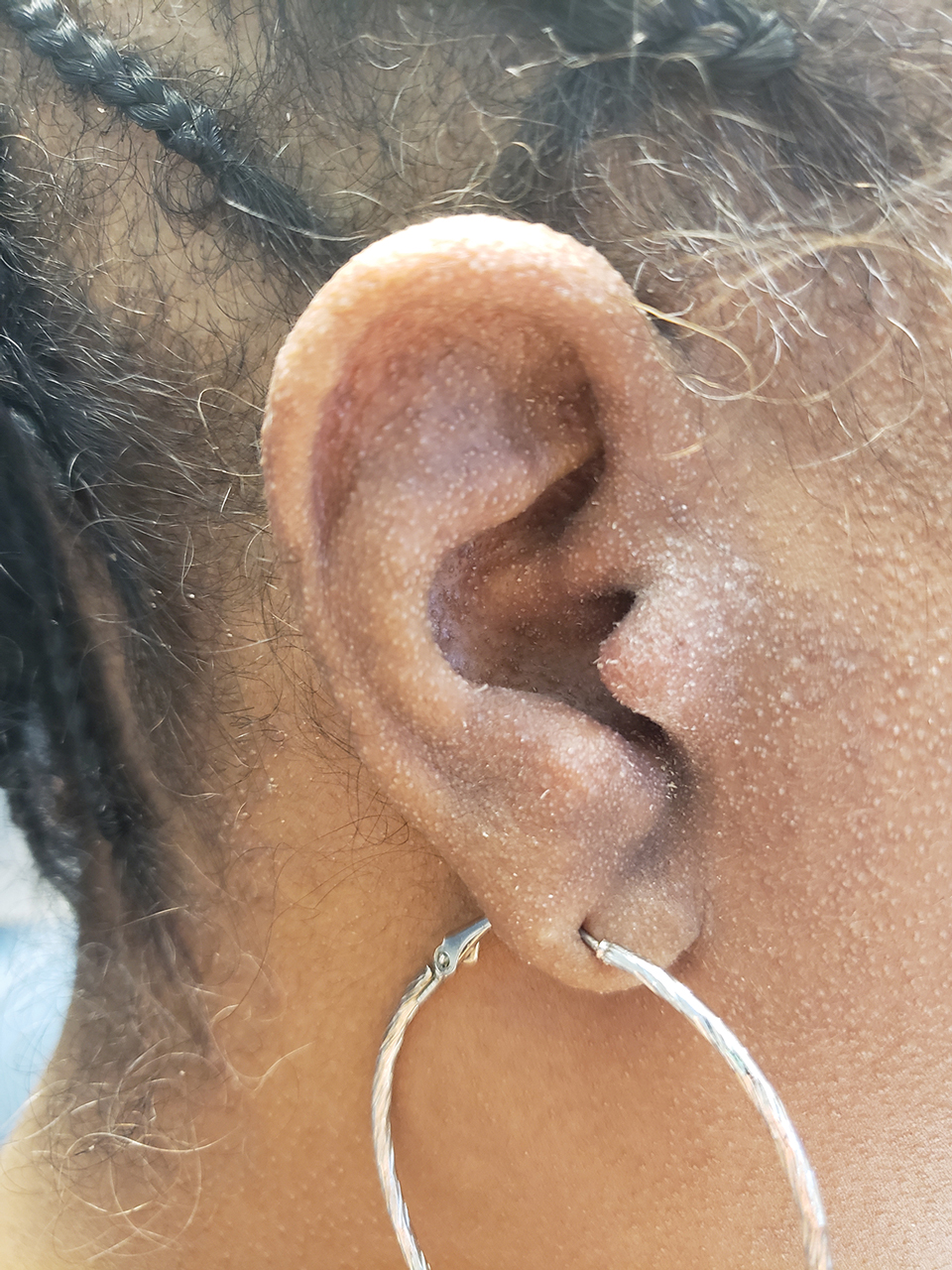
A 54-year-old Black woman presented with a rash that developed 6 months after a renal transplant due to a history of systemic lupus erythematosus with lupus nephritis. She was started on mycophenolate mofetil and tacrolimus after the transplant but was switched to cyclosporine because of BK viremia. The rash developed 1 week after cyclosporine was initiated and consisted of pruritic papules that started on the face and spread to the trunk and arms. Physical examination revealed innumerable follicular-based, keratotic, flesh-colored, pinpoint papules with fine white spicules on the face (top), neck, chest, arms, and back. Leonine facies was seen along the glabella with madarosis of the lateral eyebrows (top) and ears (bottom).
Doctors Endorsing Products on X May Not Disclose Company Ties
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
One Patient Changed This Oncologist’s View of Hope. Here’s How.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
FROM ASCO 2024
Lidocaine Effective Against Pediatric Migraine
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
FROM AHS 2024
ChatGPT Enhances Readability of Cancer Information for Patients
TOPLINE:
The artificial intelligence (AI) chatbot ChatGPT can significantly improve the readability of online cancer-related patient information while maintaining the content’s quality, a recent study found.
METHODOLOGY:
- Patients with cancer often search for cancer information online after their diagnosis, with most seeking information from their oncologists’ websites. However, the online materials often exceed the average reading level of the US population, limiting accessibility and comprehension.
- Researchers asked ChatGPT 4.0 to rewrite content about breast, colon, lung, prostate, and pancreas cancer, aiming for a sixth-grade readability level. The content came from a random sample of documents from 34 patient-facing websites associated with National Comprehensive Cancer Network (NCCN) member institutions.
- Readability, accuracy, similarity, and quality of the rewritten content were assessed using several established metrics and tools, including an F1 score, which assesses the precision and recall of a machine-learning model; a cosine similarity score, which measures similarities and is often used to detect plagiarism; and the DISCERN instrument, which helps assess the quality of the AI-rewritten information.
- The primary outcome was the mean readability score for the original and AI-generated content.
TAKEAWAY:
- The original content had an average readability level equivalent to a university freshman (grade 13). Following the AI revision, the readability level improved to a high school freshman level (grade 9).
- The rewritten content had high accuracy, with an overall F1 score of 0.87 (a good score is 0.8-0.9).
- The rewritten content had a high cosine similarity score of 0.915 (scores range from 0 to 1, with 0 indicating no similarity and 1 indicating complete similarity). Researchers attributed the improved readability to the use of simpler words and shorter sentences.
- Quality assessment using the DISCERN instrument showed that the AI-rewritten content maintained a “good” quality rating, similar to that of the original content.
IN PRACTICE:
Society has become increasingly dependent on online educational materials, and considering that more than half of Americans may not be literate beyond an eighth-grade level, our AI intervention offers a potential low-cost solution to narrow the gap between patient health literacy and content received from the nation’s leading cancer centers, the authors wrote.
SOURCE:
The study, with first author Andres A. Abreu, MD, with UT Southwestern Medical Center, Dallas, Texas, was published online in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The study was limited to English-language content from NCCN member websites, so the findings may not be generalizable to other sources or languages. Readability alone cannot guarantee comprehension. Factors such as material design and audiovisual aids were not evaluated.
DISCLOSURES:
The study did not report a funding source. The authors reported several disclosures but none related to the study. Herbert J. Zeh disclosed serving as a scientific advisor for Surgical Safety Technologies; Dr. Polanco disclosed serving as a consultant for Iota Biosciences and Palisade Bio and as a proctor for Intuitive Surgical.
A version of this article first appeared on Medscape.com.
TOPLINE:
The artificial intelligence (AI) chatbot ChatGPT can significantly improve the readability of online cancer-related patient information while maintaining the content’s quality, a recent study found.
METHODOLOGY:
- Patients with cancer often search for cancer information online after their diagnosis, with most seeking information from their oncologists’ websites. However, the online materials often exceed the average reading level of the US population, limiting accessibility and comprehension.
- Researchers asked ChatGPT 4.0 to rewrite content about breast, colon, lung, prostate, and pancreas cancer, aiming for a sixth-grade readability level. The content came from a random sample of documents from 34 patient-facing websites associated with National Comprehensive Cancer Network (NCCN) member institutions.
- Readability, accuracy, similarity, and quality of the rewritten content were assessed using several established metrics and tools, including an F1 score, which assesses the precision and recall of a machine-learning model; a cosine similarity score, which measures similarities and is often used to detect plagiarism; and the DISCERN instrument, which helps assess the quality of the AI-rewritten information.
- The primary outcome was the mean readability score for the original and AI-generated content.
TAKEAWAY:
- The original content had an average readability level equivalent to a university freshman (grade 13). Following the AI revision, the readability level improved to a high school freshman level (grade 9).
- The rewritten content had high accuracy, with an overall F1 score of 0.87 (a good score is 0.8-0.9).
- The rewritten content had a high cosine similarity score of 0.915 (scores range from 0 to 1, with 0 indicating no similarity and 1 indicating complete similarity). Researchers attributed the improved readability to the use of simpler words and shorter sentences.
- Quality assessment using the DISCERN instrument showed that the AI-rewritten content maintained a “good” quality rating, similar to that of the original content.
IN PRACTICE:
Society has become increasingly dependent on online educational materials, and considering that more than half of Americans may not be literate beyond an eighth-grade level, our AI intervention offers a potential low-cost solution to narrow the gap between patient health literacy and content received from the nation’s leading cancer centers, the authors wrote.
SOURCE:
The study, with first author Andres A. Abreu, MD, with UT Southwestern Medical Center, Dallas, Texas, was published online in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The study was limited to English-language content from NCCN member websites, so the findings may not be generalizable to other sources or languages. Readability alone cannot guarantee comprehension. Factors such as material design and audiovisual aids were not evaluated.
DISCLOSURES:
The study did not report a funding source. The authors reported several disclosures but none related to the study. Herbert J. Zeh disclosed serving as a scientific advisor for Surgical Safety Technologies; Dr. Polanco disclosed serving as a consultant for Iota Biosciences and Palisade Bio and as a proctor for Intuitive Surgical.
A version of this article first appeared on Medscape.com.
TOPLINE:
The artificial intelligence (AI) chatbot ChatGPT can significantly improve the readability of online cancer-related patient information while maintaining the content’s quality, a recent study found.
METHODOLOGY:
- Patients with cancer often search for cancer information online after their diagnosis, with most seeking information from their oncologists’ websites. However, the online materials often exceed the average reading level of the US population, limiting accessibility and comprehension.
- Researchers asked ChatGPT 4.0 to rewrite content about breast, colon, lung, prostate, and pancreas cancer, aiming for a sixth-grade readability level. The content came from a random sample of documents from 34 patient-facing websites associated with National Comprehensive Cancer Network (NCCN) member institutions.
- Readability, accuracy, similarity, and quality of the rewritten content were assessed using several established metrics and tools, including an F1 score, which assesses the precision and recall of a machine-learning model; a cosine similarity score, which measures similarities and is often used to detect plagiarism; and the DISCERN instrument, which helps assess the quality of the AI-rewritten information.
- The primary outcome was the mean readability score for the original and AI-generated content.
TAKEAWAY:
- The original content had an average readability level equivalent to a university freshman (grade 13). Following the AI revision, the readability level improved to a high school freshman level (grade 9).
- The rewritten content had high accuracy, with an overall F1 score of 0.87 (a good score is 0.8-0.9).
- The rewritten content had a high cosine similarity score of 0.915 (scores range from 0 to 1, with 0 indicating no similarity and 1 indicating complete similarity). Researchers attributed the improved readability to the use of simpler words and shorter sentences.
- Quality assessment using the DISCERN instrument showed that the AI-rewritten content maintained a “good” quality rating, similar to that of the original content.
IN PRACTICE:
Society has become increasingly dependent on online educational materials, and considering that more than half of Americans may not be literate beyond an eighth-grade level, our AI intervention offers a potential low-cost solution to narrow the gap between patient health literacy and content received from the nation’s leading cancer centers, the authors wrote.
SOURCE:
The study, with first author Andres A. Abreu, MD, with UT Southwestern Medical Center, Dallas, Texas, was published online in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The study was limited to English-language content from NCCN member websites, so the findings may not be generalizable to other sources or languages. Readability alone cannot guarantee comprehension. Factors such as material design and audiovisual aids were not evaluated.
DISCLOSURES:
The study did not report a funding source. The authors reported several disclosures but none related to the study. Herbert J. Zeh disclosed serving as a scientific advisor for Surgical Safety Technologies; Dr. Polanco disclosed serving as a consultant for Iota Biosciences and Palisade Bio and as a proctor for Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Is Location a Risk Factor for Early-Onset Cancer?
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Long-Term OA, RA Symptom Improvement Seen with Plant-Based Diet, Lifestyle Changes
VIENNA — An intervention consisting of a plant-based diet, exercise, and sleep and stress advice improved pain, stiffness, and physical function in people with knee and/or hip osteoarthritis (OA) and metabolic syndrome, while in patients with rheumatoid arthritis (RA), disease activity improved significantly, and medication use was reduced.
At the annual European Congress of Rheumatology, Carlijn Wagenaar, MD, a PhD candidate in Clinical Immunology and Rheumatology at Amsterdam University Medical Center, presented 2-year extension study results for OA and RA and an overview of the possible biological mechanisms underpinning the plant-based intervention in RA.
“At 2 years, RA patients on the PFJ [Plants for Joints] intervention resulted in a significant improvement in disease activity of RA, and these outcomes were maintained 2 years after program end,” Dr. Wagenaar reported.
“Some initial improvements in body composition and metabolic outcomes were also maintained at the end of the 2-year extension phase, and there was a net decrease in antirheumatic medication use,” she continued.
In the patients with OA, Dr. Wagenaar said the PFJ intervention improved pain, stiffness, and physical function in people with knee and/or hip OA and metabolic syndrome. “In the 2-year extension study, these effects were maintained, and we saw lasting body composition changes and a decrease in cholesterol-lowering medications. There was also high acceptability of the program; the study shows long-term maintenance of clinically relevant effects.”
Significant Improvement in OA Pain, Stiffness, Physical Function
In the OA randomized controlled trial, 64 people with hip and/or knee OA and metabolic syndrome were randomized to the PFJ intervention or usual care (waitlist control group). A total of 62 participants (including those in the control group previously) entered the long-term effectiveness study, and 44 had 2 years of follow-up data for analysis. Twenty participants dropped out, with most being unreachable or too busy.
“The PFJ program is a theoretical and practical program where people learn about and follow a whole food, plant-based diet, and receive advice on sleep and stress management and exercise,” said Dr. Wagenaar.
The program lasted 16 weeks with group sessions of 6-12 participants. The diet was a plant-based version of the Dutch dietary guidelines with a focus on unprocessed food. It was rich in whole grains, legumes, nuts, seeds, fruit, and vegetables, but without calorie restrictions and participants had one-to-one contact with a dietitian. The exercise advice followed the Dutch exercise guidelines, which advise 150 minutes of moderate to intense exercise per week, as well as twice-weekly muscle strength exercises, noted Dr. Wagenaar.
The 2-year follow-up study involved twice-yearly visits and six adherence-promoting webinars per year, as well as monthly newsletters. Researchers also monitored changes in medication intensity (classified as “increased,” “stable,” or “decreased/stopped”) between the start of the PFJ intervention and end of the 2-year extension study, and they were grouped into medications for pain, blood pressure, glucose, and cholesterol.
Participants were encouraged to try to avoid making changes to medication during the intervention phase, but they could do so during the 2-year extension study, said Dr. Wagenaar. In fact, the researchers actively monitored and quantified medication changes between the start of the PFJ intervention and end of the 2-year follow-up period.
Patients in the 16-week trial had an average age of 64 years, 84% were women, and their mean body mass index (BMI) was 33 kg/m2. A total of 73% had knee OA and 78% hip OA, and their mean WOMAC score was 38.2, indicative of moderate to severe OA.
In participants who completed the 2-year extension study, the primary outcome (WOMAC score for mean stiffness and physical function) showed a significant improvement of −9.1 (95% CI, −12.8 to −5.3; P < .0001) compared with the start of the PFJ intervention.
“Looking at individual components of the WOMAC score — pain, stiffness, and physical function — we found these also all significantly improved at the end of the 2-year extension phase,” reported Dr. Wagenaar.
She added that after 2 years, there were significant improvements in weight loss (from 94.9 to 92.1 kg), BMI (from 33.3 to 32 kg/m2), and waist circumference (from 110 to 106.7 cm).
By the end of the trial and at 1 year of the extension study, there were significant improvements in A1c, fasting blood glucose, and low-density lipoprotein cholesterol, but at 2 years, these were no longer significant.
Regarding medications use, Dr. Wagenaar reported that, overall, there was no net change in use of pain, glucose-lowering, or hypertension medications, but 44% of patients using cholesterol-lowering medications were able to lower their dose or stop them.
Disease Activity Improvement and Medication Reduction in RA
Turning to the study of the intervention in patients with RA, 77 people (DAS28 ≥ 2.6 and ≤ 5.1, mild to moderate disease) were randomized to receive either the PFJ intervention in addition to usual care or only usual care (control group). Of these, 48 (62%) from both the intervention and control groups also completed the 2-year follow-up. The details of the PFJ intervention and the extension study for RA were the same as for the OA patient group.
Dr. Wagenaar commented on how they tried to individualize the exercise part of the program. “We noticed many of the RA patients asked too much of their body, while in contrast, those with OA were too hesitant,” she said. “We decided to focus on people’s own physical barriers, and we wanted to protect these. Sometimes, people needed to move more, and at other times, we had to tell people to slow down. Often, we advised people to move more by integrating exercise into their daily life.”
Similar to the OA study, patients were asked to try to avoid changing their medications in the 16-week study. “In the extension study, they were encouraged to reduce their medication in collaboration with their rheumatologist,” explained Wagenaar, who monitored any changes.
Differences were quantified according to medication groups comprising rheumatic medications, as well as pain, blood pressure, glucose-lowering, and cholesterol medications, and changes were categorized as increased, stable, or decreased/stopped.
Again, participants were mostly women (92%) with an average age of 55 years, BMI of 26 kg/m2, and DAS28 of 3.85 at baseline. Dropout reasons were similar to those for OA, and over 85% of participants were on medications.
During the 16-week trial period, the DAS28 changed more in the intervention participants than in the controls, and after 2 years of follow-up, DAS28 was significantly lower than baseline with a mean difference of −0.9 (95% CI, −1.2 to −0.6; P < .0001).
“Comparing with the literature, the drop in DAS28 was similar to that seen with medication, so it’s a very significant reduction,” remarked Dr. Wagenaar.
Mean tender joint count dropped from 3 to 0, and general health components of the DAS28 improved significantly over the intervention and over the 2-year follow-up, whereas there was no significant difference in the already low erythrocyte sedimentation rate and swollen joint count compared with baseline. C-reactive protein (CRP) changed from 3.2 to 1.3 mg/L over the 2-year follow-up. High-density lipoprotein increased from 1.6 to 1.8 mmol/L.
A total of 44% of people using antirheumatic medication decreased or stopped them after the 2-year extension.
Dr. Wagenaar went on to say that focus group findings suggested that “participants were very enthusiastic about the program despite it largely involving lifestyle change, and this is reflected in our low dropout rates after the trial and 1-year extension [20% for OA and RA].” There were more dropouts in year 2 of the extension.
In an interview, Dr. Wagenaar explained why she felt the program had been so well received. “People in the program felt like they had more control over their disease, and they felt listened to.”
Mechanisms Underpinning PFJ
Dr. Wagenaar and colleagues also sought to determine the possible mechanisms underlying the clinical effects of the plant-based diet on RA. “With RA, we have the mucosal origins hypothesis, which suggests RA is triggered at the mucosal site [of the gut] in genetically predisposed individuals, and this consequently transfers to the synovial [fluid in] joints,” she said.
“On top of this, we know that fiber protects our gut barrier and therefore reduces inflammation. The PFJ intervention is a very high-fiber program, so our hypothesis is that it might help [strengthen] the barrier,” she explained.
Dr. Wagenaar and colleagues collected fecal samples from patients and measured the albumin and calprotectin in them, which are both indicators of the gut barrier function. The researchers analyzed metabolomic data and found that fecal albumin — considered a gut barrier integrity marker — decreased significantly in the intervention group. In patients with RA, this improvement corresponded with an improvement in DAS28, the researchers reported in a poster at the meeting.
“Patients who had the greatest improvement in their gut barrier function also showed the greatest improvement in the DAS28 score, suggestive of a possible link between gut barrier improvement and clinical effects.”
They did not identify any change in calprotectin, an inflammation marker, but Dr. Wagenaar said this might change later. “We found that in those on the intervention, at 4 months, the CRP wasn’t reduced, but 1 year later it was.”
The metabolite lenticin, a lentil intake biomarker considered protective against inflammation and osteoclastic differentiation, also increased. Tryptophan was also reduced in people on the PFJ intervention.
Fernando Estevez-Lopez, PhD, a sports scientist at Harvard T.H. Chan School of Public Health, Boston, who specializes in physical activity and behavioral change in rheumatology patients, co-moderated the session and remarked that, “In this study, they did a brilliant job with encouraging participants to follow the program. The design and methods were really good — the sample size was good, and they followed people up. Also, these researchers come from Reade [a medical research center in Amsterdam University Medical Center] where they are well known for applying their research findings to the clinic,” he said.
“In terms of physical activity, we really mean increasing the time spent moving, for example, gentle activity such as walking, or changing behaviors in people with OA and RA. We don’t want them to have more pain the next day.”
Dr. Wagenaar reported receiving a grant from ZonMw (The Netherlands Organization for Health Research and Development). She and colleagues hold shares in Plants for Health, a limited liability company. Dr. Estevez-Lopez reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
VIENNA — An intervention consisting of a plant-based diet, exercise, and sleep and stress advice improved pain, stiffness, and physical function in people with knee and/or hip osteoarthritis (OA) and metabolic syndrome, while in patients with rheumatoid arthritis (RA), disease activity improved significantly, and medication use was reduced.
At the annual European Congress of Rheumatology, Carlijn Wagenaar, MD, a PhD candidate in Clinical Immunology and Rheumatology at Amsterdam University Medical Center, presented 2-year extension study results for OA and RA and an overview of the possible biological mechanisms underpinning the plant-based intervention in RA.
“At 2 years, RA patients on the PFJ [Plants for Joints] intervention resulted in a significant improvement in disease activity of RA, and these outcomes were maintained 2 years after program end,” Dr. Wagenaar reported.
“Some initial improvements in body composition and metabolic outcomes were also maintained at the end of the 2-year extension phase, and there was a net decrease in antirheumatic medication use,” she continued.
In the patients with OA, Dr. Wagenaar said the PFJ intervention improved pain, stiffness, and physical function in people with knee and/or hip OA and metabolic syndrome. “In the 2-year extension study, these effects were maintained, and we saw lasting body composition changes and a decrease in cholesterol-lowering medications. There was also high acceptability of the program; the study shows long-term maintenance of clinically relevant effects.”
Significant Improvement in OA Pain, Stiffness, Physical Function
In the OA randomized controlled trial, 64 people with hip and/or knee OA and metabolic syndrome were randomized to the PFJ intervention or usual care (waitlist control group). A total of 62 participants (including those in the control group previously) entered the long-term effectiveness study, and 44 had 2 years of follow-up data for analysis. Twenty participants dropped out, with most being unreachable or too busy.
“The PFJ program is a theoretical and practical program where people learn about and follow a whole food, plant-based diet, and receive advice on sleep and stress management and exercise,” said Dr. Wagenaar.
The program lasted 16 weeks with group sessions of 6-12 participants. The diet was a plant-based version of the Dutch dietary guidelines with a focus on unprocessed food. It was rich in whole grains, legumes, nuts, seeds, fruit, and vegetables, but without calorie restrictions and participants had one-to-one contact with a dietitian. The exercise advice followed the Dutch exercise guidelines, which advise 150 minutes of moderate to intense exercise per week, as well as twice-weekly muscle strength exercises, noted Dr. Wagenaar.
The 2-year follow-up study involved twice-yearly visits and six adherence-promoting webinars per year, as well as monthly newsletters. Researchers also monitored changes in medication intensity (classified as “increased,” “stable,” or “decreased/stopped”) between the start of the PFJ intervention and end of the 2-year extension study, and they were grouped into medications for pain, blood pressure, glucose, and cholesterol.
Participants were encouraged to try to avoid making changes to medication during the intervention phase, but they could do so during the 2-year extension study, said Dr. Wagenaar. In fact, the researchers actively monitored and quantified medication changes between the start of the PFJ intervention and end of the 2-year follow-up period.
Patients in the 16-week trial had an average age of 64 years, 84% were women, and their mean body mass index (BMI) was 33 kg/m2. A total of 73% had knee OA and 78% hip OA, and their mean WOMAC score was 38.2, indicative of moderate to severe OA.
In participants who completed the 2-year extension study, the primary outcome (WOMAC score for mean stiffness and physical function) showed a significant improvement of −9.1 (95% CI, −12.8 to −5.3; P < .0001) compared with the start of the PFJ intervention.
“Looking at individual components of the WOMAC score — pain, stiffness, and physical function — we found these also all significantly improved at the end of the 2-year extension phase,” reported Dr. Wagenaar.
She added that after 2 years, there were significant improvements in weight loss (from 94.9 to 92.1 kg), BMI (from 33.3 to 32 kg/m2), and waist circumference (from 110 to 106.7 cm).
By the end of the trial and at 1 year of the extension study, there were significant improvements in A1c, fasting blood glucose, and low-density lipoprotein cholesterol, but at 2 years, these were no longer significant.
Regarding medications use, Dr. Wagenaar reported that, overall, there was no net change in use of pain, glucose-lowering, or hypertension medications, but 44% of patients using cholesterol-lowering medications were able to lower their dose or stop them.
Disease Activity Improvement and Medication Reduction in RA
Turning to the study of the intervention in patients with RA, 77 people (DAS28 ≥ 2.6 and ≤ 5.1, mild to moderate disease) were randomized to receive either the PFJ intervention in addition to usual care or only usual care (control group). Of these, 48 (62%) from both the intervention and control groups also completed the 2-year follow-up. The details of the PFJ intervention and the extension study for RA were the same as for the OA patient group.
Dr. Wagenaar commented on how they tried to individualize the exercise part of the program. “We noticed many of the RA patients asked too much of their body, while in contrast, those with OA were too hesitant,” she said. “We decided to focus on people’s own physical barriers, and we wanted to protect these. Sometimes, people needed to move more, and at other times, we had to tell people to slow down. Often, we advised people to move more by integrating exercise into their daily life.”
Similar to the OA study, patients were asked to try to avoid changing their medications in the 16-week study. “In the extension study, they were encouraged to reduce their medication in collaboration with their rheumatologist,” explained Wagenaar, who monitored any changes.
Differences were quantified according to medication groups comprising rheumatic medications, as well as pain, blood pressure, glucose-lowering, and cholesterol medications, and changes were categorized as increased, stable, or decreased/stopped.
Again, participants were mostly women (92%) with an average age of 55 years, BMI of 26 kg/m2, and DAS28 of 3.85 at baseline. Dropout reasons were similar to those for OA, and over 85% of participants were on medications.
During the 16-week trial period, the DAS28 changed more in the intervention participants than in the controls, and after 2 years of follow-up, DAS28 was significantly lower than baseline with a mean difference of −0.9 (95% CI, −1.2 to −0.6; P < .0001).
“Comparing with the literature, the drop in DAS28 was similar to that seen with medication, so it’s a very significant reduction,” remarked Dr. Wagenaar.
Mean tender joint count dropped from 3 to 0, and general health components of the DAS28 improved significantly over the intervention and over the 2-year follow-up, whereas there was no significant difference in the already low erythrocyte sedimentation rate and swollen joint count compared with baseline. C-reactive protein (CRP) changed from 3.2 to 1.3 mg/L over the 2-year follow-up. High-density lipoprotein increased from 1.6 to 1.8 mmol/L.
A total of 44% of people using antirheumatic medication decreased or stopped them after the 2-year extension.
Dr. Wagenaar went on to say that focus group findings suggested that “participants were very enthusiastic about the program despite it largely involving lifestyle change, and this is reflected in our low dropout rates after the trial and 1-year extension [20% for OA and RA].” There were more dropouts in year 2 of the extension.
In an interview, Dr. Wagenaar explained why she felt the program had been so well received. “People in the program felt like they had more control over their disease, and they felt listened to.”
Mechanisms Underpinning PFJ
Dr. Wagenaar and colleagues also sought to determine the possible mechanisms underlying the clinical effects of the plant-based diet on RA. “With RA, we have the mucosal origins hypothesis, which suggests RA is triggered at the mucosal site [of the gut] in genetically predisposed individuals, and this consequently transfers to the synovial [fluid in] joints,” she said.
“On top of this, we know that fiber protects our gut barrier and therefore reduces inflammation. The PFJ intervention is a very high-fiber program, so our hypothesis is that it might help [strengthen] the barrier,” she explained.
Dr. Wagenaar and colleagues collected fecal samples from patients and measured the albumin and calprotectin in them, which are both indicators of the gut barrier function. The researchers analyzed metabolomic data and found that fecal albumin — considered a gut barrier integrity marker — decreased significantly in the intervention group. In patients with RA, this improvement corresponded with an improvement in DAS28, the researchers reported in a poster at the meeting.
“Patients who had the greatest improvement in their gut barrier function also showed the greatest improvement in the DAS28 score, suggestive of a possible link between gut barrier improvement and clinical effects.”
They did not identify any change in calprotectin, an inflammation marker, but Dr. Wagenaar said this might change later. “We found that in those on the intervention, at 4 months, the CRP wasn’t reduced, but 1 year later it was.”
The metabolite lenticin, a lentil intake biomarker considered protective against inflammation and osteoclastic differentiation, also increased. Tryptophan was also reduced in people on the PFJ intervention.
Fernando Estevez-Lopez, PhD, a sports scientist at Harvard T.H. Chan School of Public Health, Boston, who specializes in physical activity and behavioral change in rheumatology patients, co-moderated the session and remarked that, “In this study, they did a brilliant job with encouraging participants to follow the program. The design and methods were really good — the sample size was good, and they followed people up. Also, these researchers come from Reade [a medical research center in Amsterdam University Medical Center] where they are well known for applying their research findings to the clinic,” he said.
“In terms of physical activity, we really mean increasing the time spent moving, for example, gentle activity such as walking, or changing behaviors in people with OA and RA. We don’t want them to have more pain the next day.”
Dr. Wagenaar reported receiving a grant from ZonMw (The Netherlands Organization for Health Research and Development). She and colleagues hold shares in Plants for Health, a limited liability company. Dr. Estevez-Lopez reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
VIENNA — An intervention consisting of a plant-based diet, exercise, and sleep and stress advice improved pain, stiffness, and physical function in people with knee and/or hip osteoarthritis (OA) and metabolic syndrome, while in patients with rheumatoid arthritis (RA), disease activity improved significantly, and medication use was reduced.
At the annual European Congress of Rheumatology, Carlijn Wagenaar, MD, a PhD candidate in Clinical Immunology and Rheumatology at Amsterdam University Medical Center, presented 2-year extension study results for OA and RA and an overview of the possible biological mechanisms underpinning the plant-based intervention in RA.
“At 2 years, RA patients on the PFJ [Plants for Joints] intervention resulted in a significant improvement in disease activity of RA, and these outcomes were maintained 2 years after program end,” Dr. Wagenaar reported.
“Some initial improvements in body composition and metabolic outcomes were also maintained at the end of the 2-year extension phase, and there was a net decrease in antirheumatic medication use,” she continued.
In the patients with OA, Dr. Wagenaar said the PFJ intervention improved pain, stiffness, and physical function in people with knee and/or hip OA and metabolic syndrome. “In the 2-year extension study, these effects were maintained, and we saw lasting body composition changes and a decrease in cholesterol-lowering medications. There was also high acceptability of the program; the study shows long-term maintenance of clinically relevant effects.”
Significant Improvement in OA Pain, Stiffness, Physical Function
In the OA randomized controlled trial, 64 people with hip and/or knee OA and metabolic syndrome were randomized to the PFJ intervention or usual care (waitlist control group). A total of 62 participants (including those in the control group previously) entered the long-term effectiveness study, and 44 had 2 years of follow-up data for analysis. Twenty participants dropped out, with most being unreachable or too busy.
“The PFJ program is a theoretical and practical program where people learn about and follow a whole food, plant-based diet, and receive advice on sleep and stress management and exercise,” said Dr. Wagenaar.
The program lasted 16 weeks with group sessions of 6-12 participants. The diet was a plant-based version of the Dutch dietary guidelines with a focus on unprocessed food. It was rich in whole grains, legumes, nuts, seeds, fruit, and vegetables, but without calorie restrictions and participants had one-to-one contact with a dietitian. The exercise advice followed the Dutch exercise guidelines, which advise 150 minutes of moderate to intense exercise per week, as well as twice-weekly muscle strength exercises, noted Dr. Wagenaar.
The 2-year follow-up study involved twice-yearly visits and six adherence-promoting webinars per year, as well as monthly newsletters. Researchers also monitored changes in medication intensity (classified as “increased,” “stable,” or “decreased/stopped”) between the start of the PFJ intervention and end of the 2-year extension study, and they were grouped into medications for pain, blood pressure, glucose, and cholesterol.
Participants were encouraged to try to avoid making changes to medication during the intervention phase, but they could do so during the 2-year extension study, said Dr. Wagenaar. In fact, the researchers actively monitored and quantified medication changes between the start of the PFJ intervention and end of the 2-year follow-up period.
Patients in the 16-week trial had an average age of 64 years, 84% were women, and their mean body mass index (BMI) was 33 kg/m2. A total of 73% had knee OA and 78% hip OA, and their mean WOMAC score was 38.2, indicative of moderate to severe OA.
In participants who completed the 2-year extension study, the primary outcome (WOMAC score for mean stiffness and physical function) showed a significant improvement of −9.1 (95% CI, −12.8 to −5.3; P < .0001) compared with the start of the PFJ intervention.
“Looking at individual components of the WOMAC score — pain, stiffness, and physical function — we found these also all significantly improved at the end of the 2-year extension phase,” reported Dr. Wagenaar.
She added that after 2 years, there were significant improvements in weight loss (from 94.9 to 92.1 kg), BMI (from 33.3 to 32 kg/m2), and waist circumference (from 110 to 106.7 cm).
By the end of the trial and at 1 year of the extension study, there were significant improvements in A1c, fasting blood glucose, and low-density lipoprotein cholesterol, but at 2 years, these were no longer significant.
Regarding medications use, Dr. Wagenaar reported that, overall, there was no net change in use of pain, glucose-lowering, or hypertension medications, but 44% of patients using cholesterol-lowering medications were able to lower their dose or stop them.
Disease Activity Improvement and Medication Reduction in RA
Turning to the study of the intervention in patients with RA, 77 people (DAS28 ≥ 2.6 and ≤ 5.1, mild to moderate disease) were randomized to receive either the PFJ intervention in addition to usual care or only usual care (control group). Of these, 48 (62%) from both the intervention and control groups also completed the 2-year follow-up. The details of the PFJ intervention and the extension study for RA were the same as for the OA patient group.
Dr. Wagenaar commented on how they tried to individualize the exercise part of the program. “We noticed many of the RA patients asked too much of their body, while in contrast, those with OA were too hesitant,” she said. “We decided to focus on people’s own physical barriers, and we wanted to protect these. Sometimes, people needed to move more, and at other times, we had to tell people to slow down. Often, we advised people to move more by integrating exercise into their daily life.”
Similar to the OA study, patients were asked to try to avoid changing their medications in the 16-week study. “In the extension study, they were encouraged to reduce their medication in collaboration with their rheumatologist,” explained Wagenaar, who monitored any changes.
Differences were quantified according to medication groups comprising rheumatic medications, as well as pain, blood pressure, glucose-lowering, and cholesterol medications, and changes were categorized as increased, stable, or decreased/stopped.
Again, participants were mostly women (92%) with an average age of 55 years, BMI of 26 kg/m2, and DAS28 of 3.85 at baseline. Dropout reasons were similar to those for OA, and over 85% of participants were on medications.
During the 16-week trial period, the DAS28 changed more in the intervention participants than in the controls, and after 2 years of follow-up, DAS28 was significantly lower than baseline with a mean difference of −0.9 (95% CI, −1.2 to −0.6; P < .0001).
“Comparing with the literature, the drop in DAS28 was similar to that seen with medication, so it’s a very significant reduction,” remarked Dr. Wagenaar.
Mean tender joint count dropped from 3 to 0, and general health components of the DAS28 improved significantly over the intervention and over the 2-year follow-up, whereas there was no significant difference in the already low erythrocyte sedimentation rate and swollen joint count compared with baseline. C-reactive protein (CRP) changed from 3.2 to 1.3 mg/L over the 2-year follow-up. High-density lipoprotein increased from 1.6 to 1.8 mmol/L.
A total of 44% of people using antirheumatic medication decreased or stopped them after the 2-year extension.
Dr. Wagenaar went on to say that focus group findings suggested that “participants were very enthusiastic about the program despite it largely involving lifestyle change, and this is reflected in our low dropout rates after the trial and 1-year extension [20% for OA and RA].” There were more dropouts in year 2 of the extension.
In an interview, Dr. Wagenaar explained why she felt the program had been so well received. “People in the program felt like they had more control over their disease, and they felt listened to.”
Mechanisms Underpinning PFJ
Dr. Wagenaar and colleagues also sought to determine the possible mechanisms underlying the clinical effects of the plant-based diet on RA. “With RA, we have the mucosal origins hypothesis, which suggests RA is triggered at the mucosal site [of the gut] in genetically predisposed individuals, and this consequently transfers to the synovial [fluid in] joints,” she said.
“On top of this, we know that fiber protects our gut barrier and therefore reduces inflammation. The PFJ intervention is a very high-fiber program, so our hypothesis is that it might help [strengthen] the barrier,” she explained.
Dr. Wagenaar and colleagues collected fecal samples from patients and measured the albumin and calprotectin in them, which are both indicators of the gut barrier function. The researchers analyzed metabolomic data and found that fecal albumin — considered a gut barrier integrity marker — decreased significantly in the intervention group. In patients with RA, this improvement corresponded with an improvement in DAS28, the researchers reported in a poster at the meeting.
“Patients who had the greatest improvement in their gut barrier function also showed the greatest improvement in the DAS28 score, suggestive of a possible link between gut barrier improvement and clinical effects.”
They did not identify any change in calprotectin, an inflammation marker, but Dr. Wagenaar said this might change later. “We found that in those on the intervention, at 4 months, the CRP wasn’t reduced, but 1 year later it was.”
The metabolite lenticin, a lentil intake biomarker considered protective against inflammation and osteoclastic differentiation, also increased. Tryptophan was also reduced in people on the PFJ intervention.
Fernando Estevez-Lopez, PhD, a sports scientist at Harvard T.H. Chan School of Public Health, Boston, who specializes in physical activity and behavioral change in rheumatology patients, co-moderated the session and remarked that, “In this study, they did a brilliant job with encouraging participants to follow the program. The design and methods were really good — the sample size was good, and they followed people up. Also, these researchers come from Reade [a medical research center in Amsterdam University Medical Center] where they are well known for applying their research findings to the clinic,” he said.
“In terms of physical activity, we really mean increasing the time spent moving, for example, gentle activity such as walking, or changing behaviors in people with OA and RA. We don’t want them to have more pain the next day.”
Dr. Wagenaar reported receiving a grant from ZonMw (The Netherlands Organization for Health Research and Development). She and colleagues hold shares in Plants for Health, a limited liability company. Dr. Estevez-Lopez reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
Trial Confirms Treating Gout Based on Uric Acid Level, Not Symptoms
UPDATED July 8, 2024 // Editor's note: This article has been revised to give a more nuanced view from Yael Klionsky, MD, about the need for more accurate and consistent gout management guidelines for busy primary care clinicians who often rely on them in clinical practice.
VIENNA — The first multicenter randomized trial in gout to compare treat-to-target (T2T) and treat for symptom avoidance (T2S) strategies has finally generated data to make the guideline-recommended practice of T2T evidence-based.
The T2T strategy may be guideline-endorsed, but it has never been validated, contended Anusha Moses, MSc, a researcher and PhD candidate at the University of Twente, Enschede, the Netherlands. She argued that this controlled trial fills an evidence gap.
T2T is defined as maintaining a serum uric acid (sUA) level below the physiologic threshold level of 36 mmol/L (< 6 mg/dL). T2S, in contrast, is a strategy of symptom control, typically basing therapy on suppression of symptoms independent of sUA, Dr. Moses explained.
Both the American College of Rheumatology and European Alliance of Associations for Rheumatology (EULAR) have already endorsed T2T, but other organizations, such as the American College of Physicians (ACP), still accept symptom-based treatment in its gout clinical practice guideline, according to Dr. Moses.
The results of the trial were not surprising based on the pathophysiology of gout. Elevated sUA is considered the driver of both flares and the complications of gout. This well-established association led to endorsement of T2T in guidelines from organizations such as EULAR, but Dr. Moses said a controlled trial allows this to be declared as evidence based.
To provide proof that T2T is superior, 308 gout patients at eight centers were randomized to one of the two strategies in a trial called GO TEST OVERTURE. In the T2T arm, commonly used therapies, such as allopurinol, benzbromarone, and febuxostat were employed to achieve and maintain a target sUA of < 0.36 mmol/L. In the T2S comparator arm, the same drugs were offered to control symptoms and prevent recurrences, but sUA levels were not used to guide treatment.
The 1-year results of a planned 2-year study were presented in an oral abstract session at the annual European Congress of Rheumatology. For this analysis, outcomes were compared in the last 6 months prior to the 1-year data analysis. When assessed at 2 years, the comparison will again be made in the prior 6 months of the study.
For the primary endpoint of flares defined by the validated Gallo criteria, the mean rates were 1.3 for T2T and 1.85 for T2S (P < .001), Dr. Moses reported.
The reduced risk for flares correlated with the greater proportion of patients with sUA < 0.36 mmol/L. These proportions were 72% and 26% (P < .001) for the T2T and T2S groups, respectively. The mean sUA levels were 0.31 mmol/L and 0.42 mmol/L (P < .001), respectively.
At the 1-year mark, none of the secondary endpoints reached statistical significance. These included mean numeric rating pain scale (2.46 vs 2.41), the proportion of patients in remission (8% vs 5.7%), and the mean Health Assessment Questionnaire-Disability Index score (0.65 vs 0.62), according to Dr. Moses, who said all of these endpoints will continue to be followed in the planned second year of the study.
At baseline, there were no differences in any of the variables evaluated, including age (about 62.5 years in both groups), proportion of patients with a body mass index > 30 kg/m2 (about 62%), sUA (about 0.5 mmol/L), or estimated glomerular filtration rate (about 70 mL/min/1.73 m2).
Nonspecialists Should Heed the Results
According to Yael Klionsky, MD, a clinical assistant professor of rheumatology and immunology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, these data are not a surprise.
Even before this trial was completed, the message for clinicians is that they “should be focused on maintaining serum uric acid levels below physiological levels to improve outcomes” in patients with recurrent flares, said Klionsky, citing the validated EULAR and ACR guidelines.
While the ACP does still consider T2S acceptable as a strategy for chronic gout management, Klionsky pointed out that those guidelines have not been updated recently. Specialists in the treatment of gout do not need any more evidence that the T2T approach leads to better outcomes.
However, she agreed with the principle that non-rheumatologists need to be reached with better guidance in regard to gout management. While she expects the ACP to endorse T2T the next time their guidelines are updated, she hopes that primary care physicians recognize that T2T should now be a standard.
“In a 10- to 20-minute visit, managing multiple chronic conditions can be a challenge in primary care,” Klionsky said. “Many clinicians rely on guidelines so it is important to have consistent and accurate information.”
There is currently some distance between specialists and primary care physicians regarding the goals of gout management, according to a study that Klionsky presented at EULAR 2024. In a survey, nonspecialists and specialists did not perceive treatment priorities in the same way.
In this survey, which elicited responses from 151 rheumatologists, 150 nephrologists, and 102 primary care physicians, there was general agreement that preventing flares is a priority, but only 30% of primary care physicians and 35% of nephrologists vs 64% of rheumatologists identified the T2T target of < 0.36 mmol/L as a key step in reaching this goal.
Conversely, 58% of primary care physicians and 42% of nephrologists vs only 34% of rheumatologists considered absence of gout pain to be in the top three criteria.
In addition to the fact that primary care physicians differ from specialists in their goals for gout treatment, these data “highlight the need for the importance of a standardized definition of gout remission that includes serum uric acid control,” Dr. Klionsky said. She further thinks that this type of guidance should be disseminated to nonspecialists.
Dr. Moses reported no potential conflicts of interest. Dr. Klionsky reported financial relationships with Amgen, AstraZeneca, Eli Lilly, and MedIQ.
A version of this article appeared on Medscape.com.
UPDATED July 8, 2024 // Editor's note: This article has been revised to give a more nuanced view from Yael Klionsky, MD, about the need for more accurate and consistent gout management guidelines for busy primary care clinicians who often rely on them in clinical practice.
VIENNA — The first multicenter randomized trial in gout to compare treat-to-target (T2T) and treat for symptom avoidance (T2S) strategies has finally generated data to make the guideline-recommended practice of T2T evidence-based.
The T2T strategy may be guideline-endorsed, but it has never been validated, contended Anusha Moses, MSc, a researcher and PhD candidate at the University of Twente, Enschede, the Netherlands. She argued that this controlled trial fills an evidence gap.
T2T is defined as maintaining a serum uric acid (sUA) level below the physiologic threshold level of 36 mmol/L (< 6 mg/dL). T2S, in contrast, is a strategy of symptom control, typically basing therapy on suppression of symptoms independent of sUA, Dr. Moses explained.
Both the American College of Rheumatology and European Alliance of Associations for Rheumatology (EULAR) have already endorsed T2T, but other organizations, such as the American College of Physicians (ACP), still accept symptom-based treatment in its gout clinical practice guideline, according to Dr. Moses.
The results of the trial were not surprising based on the pathophysiology of gout. Elevated sUA is considered the driver of both flares and the complications of gout. This well-established association led to endorsement of T2T in guidelines from organizations such as EULAR, but Dr. Moses said a controlled trial allows this to be declared as evidence based.
To provide proof that T2T is superior, 308 gout patients at eight centers were randomized to one of the two strategies in a trial called GO TEST OVERTURE. In the T2T arm, commonly used therapies, such as allopurinol, benzbromarone, and febuxostat were employed to achieve and maintain a target sUA of < 0.36 mmol/L. In the T2S comparator arm, the same drugs were offered to control symptoms and prevent recurrences, but sUA levels were not used to guide treatment.
The 1-year results of a planned 2-year study were presented in an oral abstract session at the annual European Congress of Rheumatology. For this analysis, outcomes were compared in the last 6 months prior to the 1-year data analysis. When assessed at 2 years, the comparison will again be made in the prior 6 months of the study.
For the primary endpoint of flares defined by the validated Gallo criteria, the mean rates were 1.3 for T2T and 1.85 for T2S (P < .001), Dr. Moses reported.
The reduced risk for flares correlated with the greater proportion of patients with sUA < 0.36 mmol/L. These proportions were 72% and 26% (P < .001) for the T2T and T2S groups, respectively. The mean sUA levels were 0.31 mmol/L and 0.42 mmol/L (P < .001), respectively.
At the 1-year mark, none of the secondary endpoints reached statistical significance. These included mean numeric rating pain scale (2.46 vs 2.41), the proportion of patients in remission (8% vs 5.7%), and the mean Health Assessment Questionnaire-Disability Index score (0.65 vs 0.62), according to Dr. Moses, who said all of these endpoints will continue to be followed in the planned second year of the study.
At baseline, there were no differences in any of the variables evaluated, including age (about 62.5 years in both groups), proportion of patients with a body mass index > 30 kg/m2 (about 62%), sUA (about 0.5 mmol/L), or estimated glomerular filtration rate (about 70 mL/min/1.73 m2).
Nonspecialists Should Heed the Results
According to Yael Klionsky, MD, a clinical assistant professor of rheumatology and immunology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, these data are not a surprise.
Even before this trial was completed, the message for clinicians is that they “should be focused on maintaining serum uric acid levels below physiological levels to improve outcomes” in patients with recurrent flares, said Klionsky, citing the validated EULAR and ACR guidelines.
While the ACP does still consider T2S acceptable as a strategy for chronic gout management, Klionsky pointed out that those guidelines have not been updated recently. Specialists in the treatment of gout do not need any more evidence that the T2T approach leads to better outcomes.
However, she agreed with the principle that non-rheumatologists need to be reached with better guidance in regard to gout management. While she expects the ACP to endorse T2T the next time their guidelines are updated, she hopes that primary care physicians recognize that T2T should now be a standard.
“In a 10- to 20-minute visit, managing multiple chronic conditions can be a challenge in primary care,” Klionsky said. “Many clinicians rely on guidelines so it is important to have consistent and accurate information.”
There is currently some distance between specialists and primary care physicians regarding the goals of gout management, according to a study that Klionsky presented at EULAR 2024. In a survey, nonspecialists and specialists did not perceive treatment priorities in the same way.
In this survey, which elicited responses from 151 rheumatologists, 150 nephrologists, and 102 primary care physicians, there was general agreement that preventing flares is a priority, but only 30% of primary care physicians and 35% of nephrologists vs 64% of rheumatologists identified the T2T target of < 0.36 mmol/L as a key step in reaching this goal.
Conversely, 58% of primary care physicians and 42% of nephrologists vs only 34% of rheumatologists considered absence of gout pain to be in the top three criteria.
In addition to the fact that primary care physicians differ from specialists in their goals for gout treatment, these data “highlight the need for the importance of a standardized definition of gout remission that includes serum uric acid control,” Dr. Klionsky said. She further thinks that this type of guidance should be disseminated to nonspecialists.
Dr. Moses reported no potential conflicts of interest. Dr. Klionsky reported financial relationships with Amgen, AstraZeneca, Eli Lilly, and MedIQ.
A version of this article appeared on Medscape.com.
UPDATED July 8, 2024 // Editor's note: This article has been revised to give a more nuanced view from Yael Klionsky, MD, about the need for more accurate and consistent gout management guidelines for busy primary care clinicians who often rely on them in clinical practice.
VIENNA — The first multicenter randomized trial in gout to compare treat-to-target (T2T) and treat for symptom avoidance (T2S) strategies has finally generated data to make the guideline-recommended practice of T2T evidence-based.
The T2T strategy may be guideline-endorsed, but it has never been validated, contended Anusha Moses, MSc, a researcher and PhD candidate at the University of Twente, Enschede, the Netherlands. She argued that this controlled trial fills an evidence gap.
T2T is defined as maintaining a serum uric acid (sUA) level below the physiologic threshold level of 36 mmol/L (< 6 mg/dL). T2S, in contrast, is a strategy of symptom control, typically basing therapy on suppression of symptoms independent of sUA, Dr. Moses explained.
Both the American College of Rheumatology and European Alliance of Associations for Rheumatology (EULAR) have already endorsed T2T, but other organizations, such as the American College of Physicians (ACP), still accept symptom-based treatment in its gout clinical practice guideline, according to Dr. Moses.
The results of the trial were not surprising based on the pathophysiology of gout. Elevated sUA is considered the driver of both flares and the complications of gout. This well-established association led to endorsement of T2T in guidelines from organizations such as EULAR, but Dr. Moses said a controlled trial allows this to be declared as evidence based.
To provide proof that T2T is superior, 308 gout patients at eight centers were randomized to one of the two strategies in a trial called GO TEST OVERTURE. In the T2T arm, commonly used therapies, such as allopurinol, benzbromarone, and febuxostat were employed to achieve and maintain a target sUA of < 0.36 mmol/L. In the T2S comparator arm, the same drugs were offered to control symptoms and prevent recurrences, but sUA levels were not used to guide treatment.
The 1-year results of a planned 2-year study were presented in an oral abstract session at the annual European Congress of Rheumatology. For this analysis, outcomes were compared in the last 6 months prior to the 1-year data analysis. When assessed at 2 years, the comparison will again be made in the prior 6 months of the study.
For the primary endpoint of flares defined by the validated Gallo criteria, the mean rates were 1.3 for T2T and 1.85 for T2S (P < .001), Dr. Moses reported.
The reduced risk for flares correlated with the greater proportion of patients with sUA < 0.36 mmol/L. These proportions were 72% and 26% (P < .001) for the T2T and T2S groups, respectively. The mean sUA levels were 0.31 mmol/L and 0.42 mmol/L (P < .001), respectively.
At the 1-year mark, none of the secondary endpoints reached statistical significance. These included mean numeric rating pain scale (2.46 vs 2.41), the proportion of patients in remission (8% vs 5.7%), and the mean Health Assessment Questionnaire-Disability Index score (0.65 vs 0.62), according to Dr. Moses, who said all of these endpoints will continue to be followed in the planned second year of the study.
At baseline, there were no differences in any of the variables evaluated, including age (about 62.5 years in both groups), proportion of patients with a body mass index > 30 kg/m2 (about 62%), sUA (about 0.5 mmol/L), or estimated glomerular filtration rate (about 70 mL/min/1.73 m2).
Nonspecialists Should Heed the Results
According to Yael Klionsky, MD, a clinical assistant professor of rheumatology and immunology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, these data are not a surprise.
Even before this trial was completed, the message for clinicians is that they “should be focused on maintaining serum uric acid levels below physiological levels to improve outcomes” in patients with recurrent flares, said Klionsky, citing the validated EULAR and ACR guidelines.
While the ACP does still consider T2S acceptable as a strategy for chronic gout management, Klionsky pointed out that those guidelines have not been updated recently. Specialists in the treatment of gout do not need any more evidence that the T2T approach leads to better outcomes.
However, she agreed with the principle that non-rheumatologists need to be reached with better guidance in regard to gout management. While she expects the ACP to endorse T2T the next time their guidelines are updated, she hopes that primary care physicians recognize that T2T should now be a standard.
“In a 10- to 20-minute visit, managing multiple chronic conditions can be a challenge in primary care,” Klionsky said. “Many clinicians rely on guidelines so it is important to have consistent and accurate information.”
There is currently some distance between specialists and primary care physicians regarding the goals of gout management, according to a study that Klionsky presented at EULAR 2024. In a survey, nonspecialists and specialists did not perceive treatment priorities in the same way.
In this survey, which elicited responses from 151 rheumatologists, 150 nephrologists, and 102 primary care physicians, there was general agreement that preventing flares is a priority, but only 30% of primary care physicians and 35% of nephrologists vs 64% of rheumatologists identified the T2T target of < 0.36 mmol/L as a key step in reaching this goal.
Conversely, 58% of primary care physicians and 42% of nephrologists vs only 34% of rheumatologists considered absence of gout pain to be in the top three criteria.
In addition to the fact that primary care physicians differ from specialists in their goals for gout treatment, these data “highlight the need for the importance of a standardized definition of gout remission that includes serum uric acid control,” Dr. Klionsky said. She further thinks that this type of guidance should be disseminated to nonspecialists.
Dr. Moses reported no potential conflicts of interest. Dr. Klionsky reported financial relationships with Amgen, AstraZeneca, Eli Lilly, and MedIQ.
A version of this article appeared on Medscape.com.
FROM EULAR 2024