User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cold water immersion can have benefits
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF CIRCUMPOLAR HEALTH
No, you can’t see a different doctor: We need zero tolerance of patient bias
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Don’t lift weights – lower them instead
as you would do with a typical rep.
That means, for example, that you could use two hands to lift a dumbbell, then one hand to slowly lower it, while sacrificing little in the way of results. Focusing on the lowering – or the “eccentric” contraction – can lead to a more efficient gym session, Japanese researchers say.
In the study, published in the European Journal of Applied Physiology, researchers divided people into three groups of 14 for a 5-week, twice-weekly comparison.
One group performed dumbbell curls from full extension to about one-quarter of the way up, for 2 seconds up and 2 seconds down, in three sets of 10 reps. Another 14 people performed only the lift portion of the movement (a researcher helped them reset the weight after each rep), and another 14 did only the lowering part of the move.
The group that both lifted and lowered the weights increased the maximum force they could produce on a lift by 18% and increased the thickness of the biceps muscle by 11%.
The people who only lowered the weights nearly matched that, increasing their maximum force by 14% and muscle size by 10%. The lifting-only group increased their max force by 11%, while muscle size increase was insignificant.
Your muscle fibers work two ways. When you lift a dumbbell from a straight arm up to your shoulder, your biceps muscle is using a “concentric” contraction. As you lower that dumbbell back down, the biceps muscle is working to put the brakes on the descent – that’s called an “eccentric” contraction.
The lifting-plus-lowering group saw the biggest gains because they were pretty much doing twice the number of reps. The lowering-only group made similar improvements in strength and muscle with only half the work.
Study author Masatoshi Nakamura, PhD, a professor at Nishikyushu University, Japan, believes that eccentric muscle contractions produce greater neurological adaptations in the spine and brain than concentric contractions. In other words, your nerves learn to send more of the “pull harder” signal to your muscles.
At the same time, the spring action of a large protein called “titin” in the muscle fibers produces greater force during eccentric contractions while using less energy, and more titin could account for the increase in muscle size, which is called hypertrophy.
“Titin in the muscle fibers could be the best explanation for muscle hypertrophy,” Dr. Nakamura says. “However, we believe that other factors, such as neurological adaptations, also play a large role in increasing muscle strength.”
The short range of motion used in the dumbbell curls was an important factor. A study, published in the Journal of Strength and Conditioning Research, found that a partial range-of-motion triceps exercise produced greater muscle growth than full range-of-motion movements.
Although the people in this newest study only performed dumbbell curls, “we think the effect is similar in other muscles,” Dr. Nakamura says.
Your muscles are much stronger when lowering than they are lifting, so Dr. Nakamura suggests choosing a heavy weight to perform single-arm dumbbell curls. Use both arms to raise the dumbbell into the 50-degree position, then lower it over a 2-second count. For two-handed bent- or straight-bar curls, you can ask a spotter to help you lift the weights into position between slow lowering moves.
You can also try the same trick with leg curl or leg extension exercise machines, using two legs to lift the weight and allowing one leg to lower it.
In the near future, your gym might contain more equipment that was designed specifically around lowering movements.
“Other machines that can emphasize eccentric contraction are gradually being developed,” Dr. Nakamura says.
A version of this article first appeared on WebMD.com.
as you would do with a typical rep.
That means, for example, that you could use two hands to lift a dumbbell, then one hand to slowly lower it, while sacrificing little in the way of results. Focusing on the lowering – or the “eccentric” contraction – can lead to a more efficient gym session, Japanese researchers say.
In the study, published in the European Journal of Applied Physiology, researchers divided people into three groups of 14 for a 5-week, twice-weekly comparison.
One group performed dumbbell curls from full extension to about one-quarter of the way up, for 2 seconds up and 2 seconds down, in three sets of 10 reps. Another 14 people performed only the lift portion of the movement (a researcher helped them reset the weight after each rep), and another 14 did only the lowering part of the move.
The group that both lifted and lowered the weights increased the maximum force they could produce on a lift by 18% and increased the thickness of the biceps muscle by 11%.
The people who only lowered the weights nearly matched that, increasing their maximum force by 14% and muscle size by 10%. The lifting-only group increased their max force by 11%, while muscle size increase was insignificant.
Your muscle fibers work two ways. When you lift a dumbbell from a straight arm up to your shoulder, your biceps muscle is using a “concentric” contraction. As you lower that dumbbell back down, the biceps muscle is working to put the brakes on the descent – that’s called an “eccentric” contraction.
The lifting-plus-lowering group saw the biggest gains because they were pretty much doing twice the number of reps. The lowering-only group made similar improvements in strength and muscle with only half the work.
Study author Masatoshi Nakamura, PhD, a professor at Nishikyushu University, Japan, believes that eccentric muscle contractions produce greater neurological adaptations in the spine and brain than concentric contractions. In other words, your nerves learn to send more of the “pull harder” signal to your muscles.
At the same time, the spring action of a large protein called “titin” in the muscle fibers produces greater force during eccentric contractions while using less energy, and more titin could account for the increase in muscle size, which is called hypertrophy.
“Titin in the muscle fibers could be the best explanation for muscle hypertrophy,” Dr. Nakamura says. “However, we believe that other factors, such as neurological adaptations, also play a large role in increasing muscle strength.”
The short range of motion used in the dumbbell curls was an important factor. A study, published in the Journal of Strength and Conditioning Research, found that a partial range-of-motion triceps exercise produced greater muscle growth than full range-of-motion movements.
Although the people in this newest study only performed dumbbell curls, “we think the effect is similar in other muscles,” Dr. Nakamura says.
Your muscles are much stronger when lowering than they are lifting, so Dr. Nakamura suggests choosing a heavy weight to perform single-arm dumbbell curls. Use both arms to raise the dumbbell into the 50-degree position, then lower it over a 2-second count. For two-handed bent- or straight-bar curls, you can ask a spotter to help you lift the weights into position between slow lowering moves.
You can also try the same trick with leg curl or leg extension exercise machines, using two legs to lift the weight and allowing one leg to lower it.
In the near future, your gym might contain more equipment that was designed specifically around lowering movements.
“Other machines that can emphasize eccentric contraction are gradually being developed,” Dr. Nakamura says.
A version of this article first appeared on WebMD.com.
as you would do with a typical rep.
That means, for example, that you could use two hands to lift a dumbbell, then one hand to slowly lower it, while sacrificing little in the way of results. Focusing on the lowering – or the “eccentric” contraction – can lead to a more efficient gym session, Japanese researchers say.
In the study, published in the European Journal of Applied Physiology, researchers divided people into three groups of 14 for a 5-week, twice-weekly comparison.
One group performed dumbbell curls from full extension to about one-quarter of the way up, for 2 seconds up and 2 seconds down, in three sets of 10 reps. Another 14 people performed only the lift portion of the movement (a researcher helped them reset the weight after each rep), and another 14 did only the lowering part of the move.
The group that both lifted and lowered the weights increased the maximum force they could produce on a lift by 18% and increased the thickness of the biceps muscle by 11%.
The people who only lowered the weights nearly matched that, increasing their maximum force by 14% and muscle size by 10%. The lifting-only group increased their max force by 11%, while muscle size increase was insignificant.
Your muscle fibers work two ways. When you lift a dumbbell from a straight arm up to your shoulder, your biceps muscle is using a “concentric” contraction. As you lower that dumbbell back down, the biceps muscle is working to put the brakes on the descent – that’s called an “eccentric” contraction.
The lifting-plus-lowering group saw the biggest gains because they were pretty much doing twice the number of reps. The lowering-only group made similar improvements in strength and muscle with only half the work.
Study author Masatoshi Nakamura, PhD, a professor at Nishikyushu University, Japan, believes that eccentric muscle contractions produce greater neurological adaptations in the spine and brain than concentric contractions. In other words, your nerves learn to send more of the “pull harder” signal to your muscles.
At the same time, the spring action of a large protein called “titin” in the muscle fibers produces greater force during eccentric contractions while using less energy, and more titin could account for the increase in muscle size, which is called hypertrophy.
“Titin in the muscle fibers could be the best explanation for muscle hypertrophy,” Dr. Nakamura says. “However, we believe that other factors, such as neurological adaptations, also play a large role in increasing muscle strength.”
The short range of motion used in the dumbbell curls was an important factor. A study, published in the Journal of Strength and Conditioning Research, found that a partial range-of-motion triceps exercise produced greater muscle growth than full range-of-motion movements.
Although the people in this newest study only performed dumbbell curls, “we think the effect is similar in other muscles,” Dr. Nakamura says.
Your muscles are much stronger when lowering than they are lifting, so Dr. Nakamura suggests choosing a heavy weight to perform single-arm dumbbell curls. Use both arms to raise the dumbbell into the 50-degree position, then lower it over a 2-second count. For two-handed bent- or straight-bar curls, you can ask a spotter to help you lift the weights into position between slow lowering moves.
You can also try the same trick with leg curl or leg extension exercise machines, using two legs to lift the weight and allowing one leg to lower it.
In the near future, your gym might contain more equipment that was designed specifically around lowering movements.
“Other machines that can emphasize eccentric contraction are gradually being developed,” Dr. Nakamura says.
A version of this article first appeared on WebMD.com.
FROM THE EUROPEAN JOURNAL OF APPLIED PHYSIOLOGY
Multidrug-resistant gram-negative infections treatable with newer antibiotics, but guidance is needed
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CURRENT OPINION IN INFECTIOUS DISEASES
Poison centers fielding more calls about teen cannabis use
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Study implicates myelin plasticity in absence seizures
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
AT AES 2022
Newer brand-name drugs fuel spending on antiseizure medications
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
AT AES 2022
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.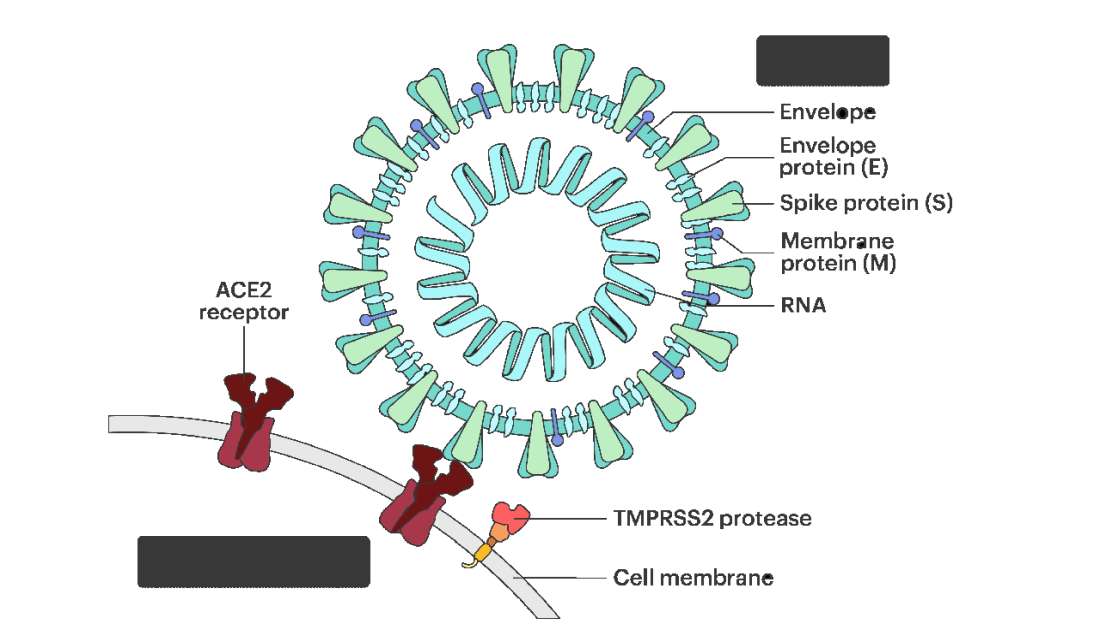
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.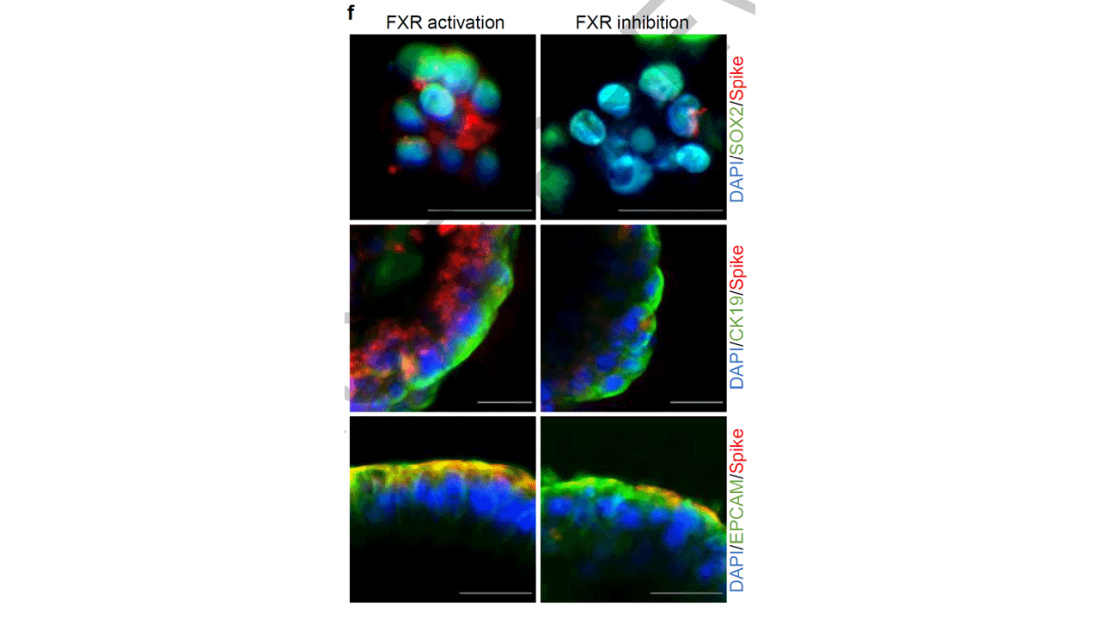
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies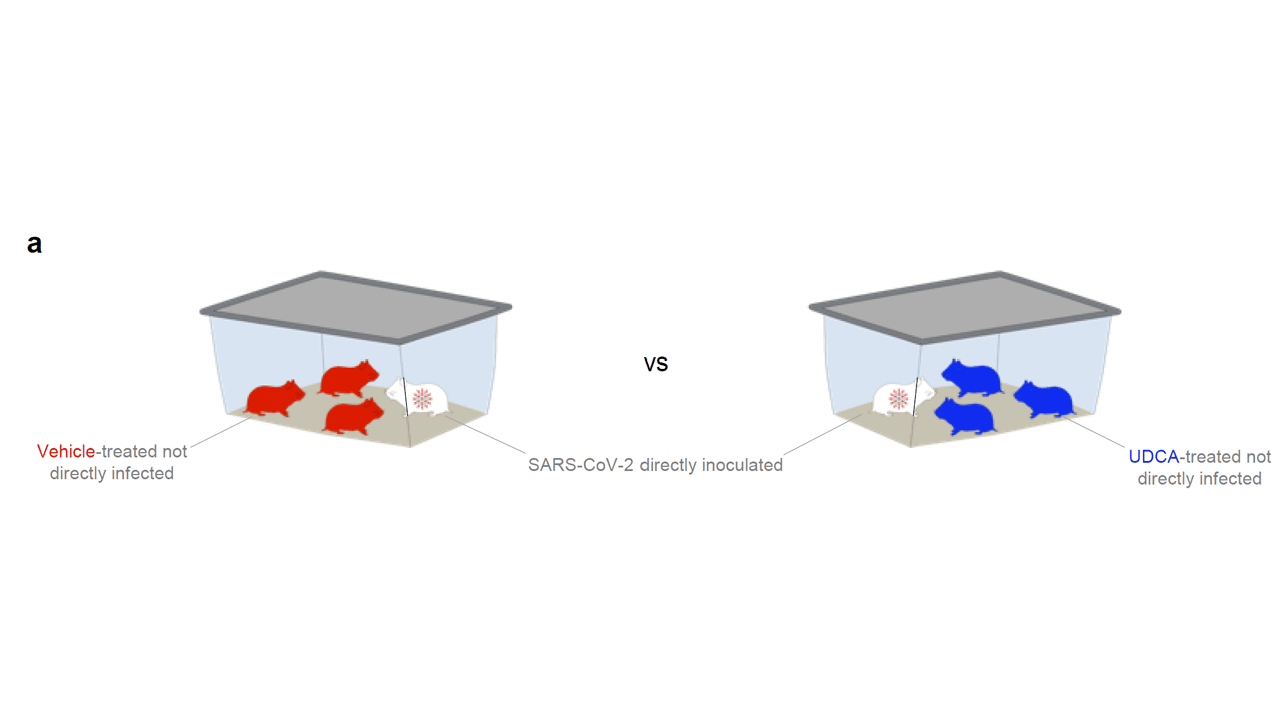
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.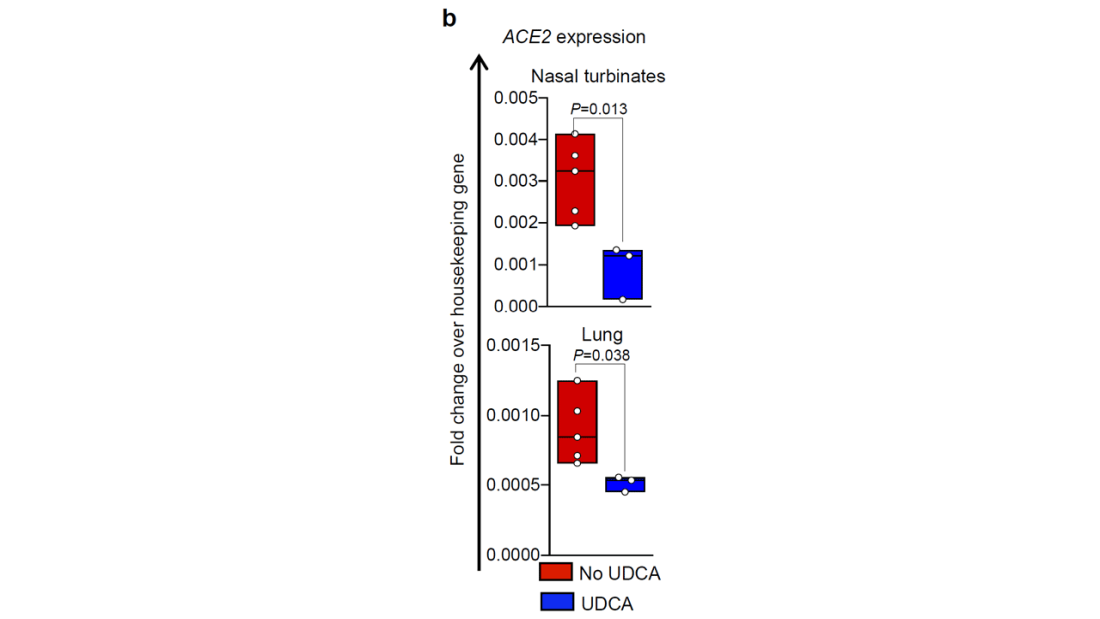
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.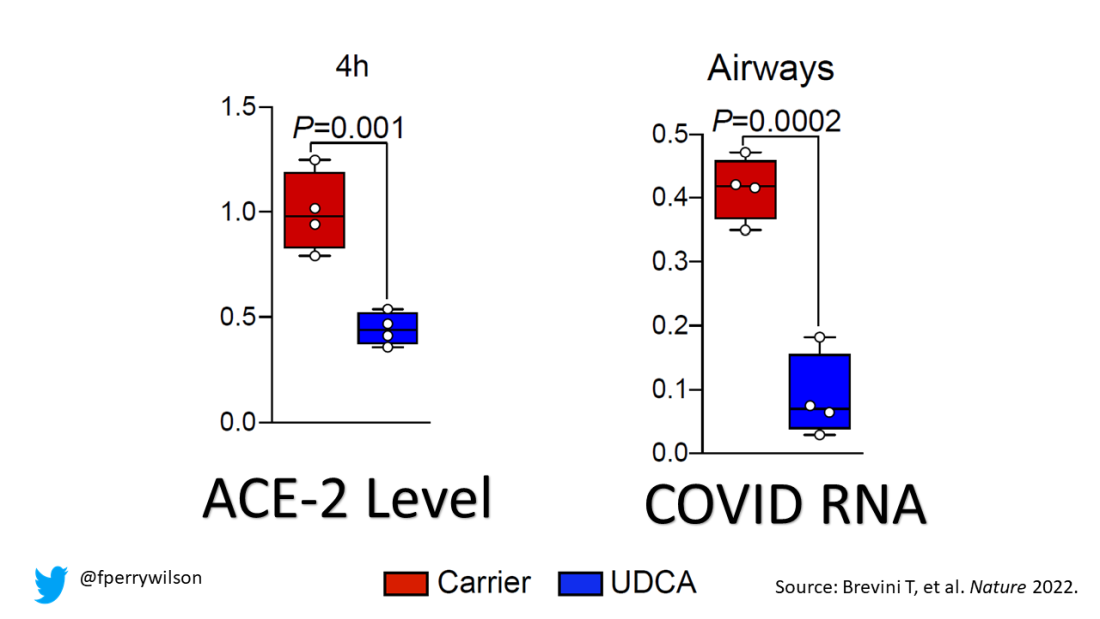
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.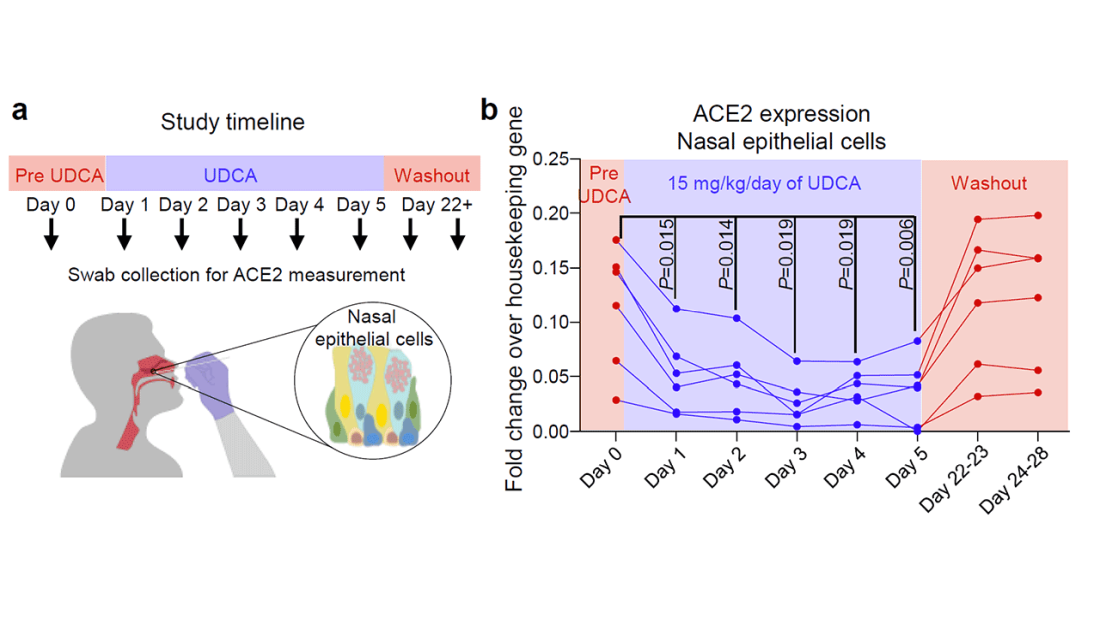
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Mind the geriatrician gap
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.





