User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.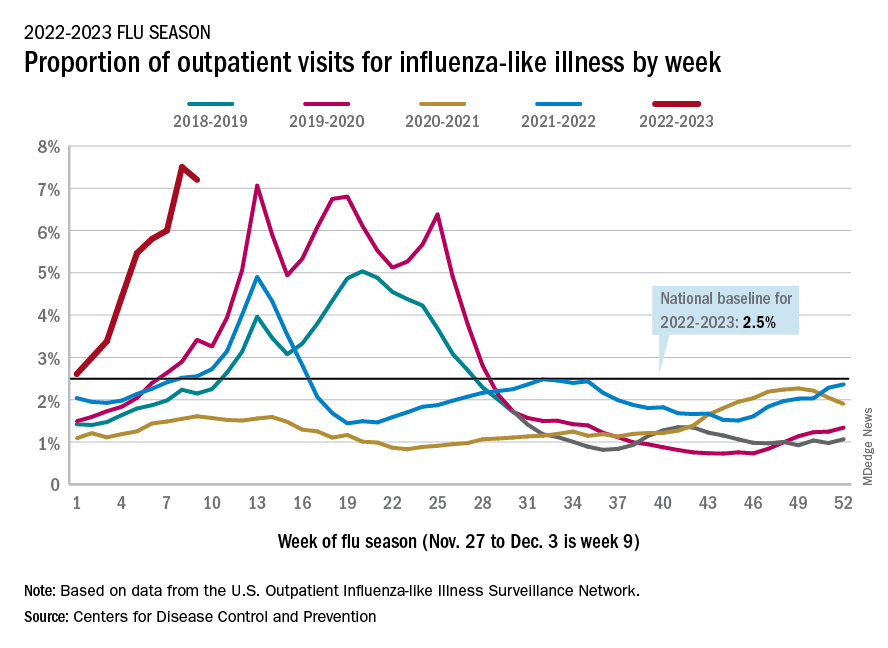
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiologist sues hospital, claims he was fired in retaliation
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
‘Striking’ rate of mental health comorbidities in epilepsy
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
Hospital financial decisions play a role in the critical shortage of pediatric beds for RSV patients
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SSRI tied to improved cognition in comorbid depression, dementia
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM CTAD 2022
Have you heard of VEXAS syndrome?
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
Cognitive behavioral therapy app lowers A1c in type 2 diabetes
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
AT AHA 2022
New AHA statement on complementary medicine in heart failure
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
How your voice could reveal hidden disease
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.





