User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Can Treating Depression Mitigate CVD Risk?
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
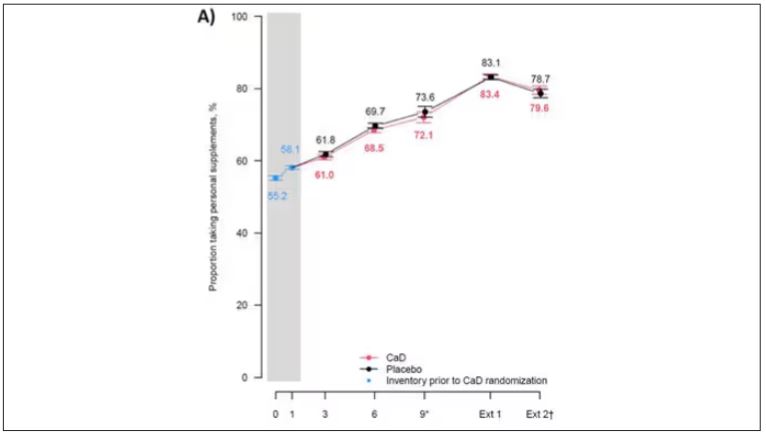
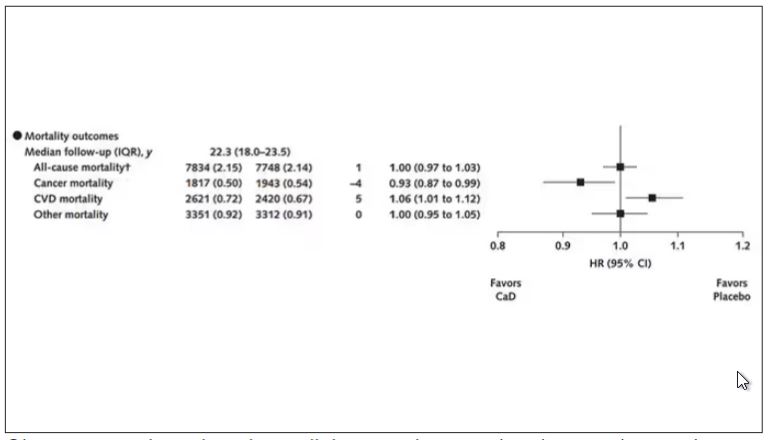
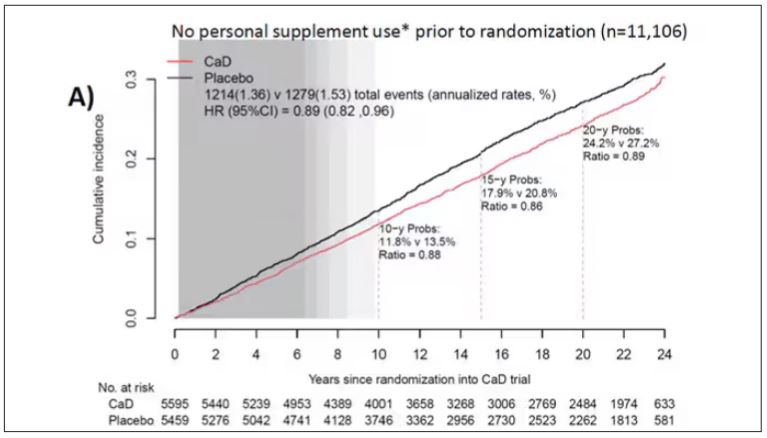
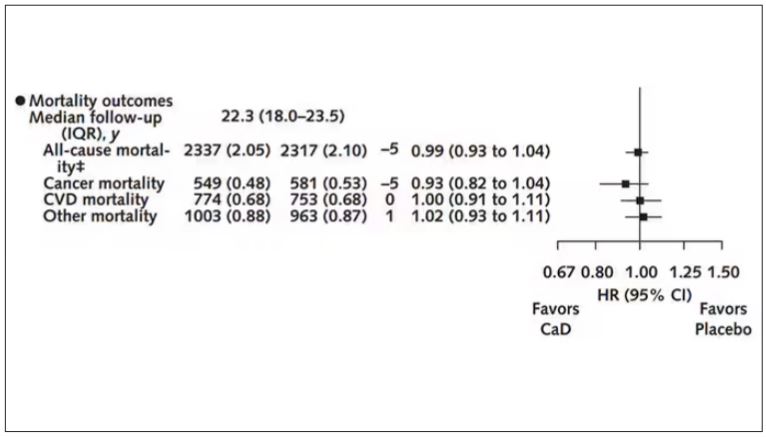
Phase 2 Results: Zerlasiran siRNA Drug Lowers Lp(a) by 90%
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
ACC Consensus Guidance on What’s New in HFrEF Treatment
The American College of Cardiology has published a new update to its consensus decision pathway for the treatment of heart failure with reduced ejection fraction (HFrEF).
Chair of the consensus document Writing Committee Thomas M. Maddox, MD, explained to this news organization that this new Decision Pathway provides a practical, streamlined update to frontline clinicians treating patients with heart failure and incorporates evidence from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“While the AHA/ACC/HFSA Guidelines are wonderful in that they collate all the latest scientific evidence, they don’t speak as much to the practicalities of delivering the care. This is what this Decision Pathway document comes in — it is designed to help frontline clinicians with the practical reality of managing these patients,” Dr. Maddox, who is director of the Healthcare Innovation Lab at BJC HealthCare and the Washington University School of Medicine in St Louis, Missouri, commented.
The document, “Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction,” was published online on March 8 in the Journal of the American College of Cardiology.
The authors provided guidance on introducing the numerous evidence-based therapies now available for HFrEF, improving adherence, overcoming treatment barriers, acknowledging contraindications and situations for which few data exist, affording expensive therapies, treating special cohorts, and making the transition to palliative care.
Rather than focusing on extensive text, the document provided practical tips, tables, and figures to make clear the steps, tools, and provisos needed to treat patients with heart failure successfully and expeditiously, they added.
Dr. Maddox reported that there are three main updated areas of advice on the treatment of heart failure in the new document.
Valsartan/Sacubitril First Line
One of the major changes involves an elevation for the status of the angiotensin receptor-neprilysin inhibitor (ARNI), Entresto (valsartan/sacubitril).
“It is now clear that this agent is superior to ACE-inhibitors or angiotensin receptor blockers in terms of reducing heart failure hospitalization and death, whereas previously it was seen as somewhat equivalent,” Dr. Maddox said. “So, barring a contraindication or another problem with getting the medication, this agent should be one of the first line medicines for all patients with heart failure and a reduced ejection fraction.”
Dual Sodium-Glucose Cotransporter 1/2 (SGLT1/2) Inhibitor
A second update involves the addition of sotagliflozin (a dual inhibitor of both SGLT1 and SGLT2) to the SGLT2 inhibitors as another first-line medication for patients with heart failure and reduced ejection fraction.
“We now have evidence that both SGLT2 and SGLT1 inhibitors are beneficial in reducing heart failure hospitalization and death. Previously we only had evidence on SGLT2 inhibitors — dapagliflozin and empagliflozin. Sotagliflozin is a newer agent, which inhibits both SGLT1 and SGLT2, and it turns out that inhibiting both are beneficial in heart failure. So, this gives us a third med in this category,” Dr. Maddox noted.
Rapid Initiation of the Four Pillars of Therapy
The document stated that more data have emerged recently to support early and rapid initiation and titration of the “four pillars” of medical therapy in heart failure to maximize the benefits of patient-reported outcomes and reduction in hospitalizations and mortality.
The four pillars of therapy are ARNI, a beta-blocker, a mineralocorticoid antagonist, and an SGLT inhibitor.
As an example, four-class medication initiation reduced the hazard of cardiovascular death or hospital admission for heart failure significantly (hazard ratio, 0.38) compared with therapy with just an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker plus a beta-blocker, the document reported.
“What we realize now is that the more quickly we can get patients on all four of these drug classes and escalate to target doses or maximally tolerated doses ideally within 3 months, the better the outcome,” Dr. Maddox said.
“Unfortunately, right now there is very incomplete realization and recognition of that in clinical practice. So, we are trying to highlight the importance of this to encourage clinicians to be more aggressive in making this happen.”
“In all patients with heart failure and reduced ejection fraction, getting them on all four of these medicines as quickly as possible will give the best outcome. We’ve seen evidence in support of this from several broad population trials,” he added. “There are times when they can’t take all four but we should do our best to get there.”
Practical Considerations
Dr. Maddox pointed out that the Consensus Document is also trying to account for practical realities and barriers to heart failure treatment.
“When we think about these recommendations — and evidence that getting patients on all these medicines is valuable, we also focus on the fact that there are three major barriers that can get in the way of this and how to think about overcoming those barriers,” he said.
The barriers are comorbidities/side effects of medications, costs of the medicines, and systems of care that are needed to ensure patients can be treated with multiple medications in a timely fashion.
In terms of comorbidities/side effects, Dr. Maddox explained that patients with heart failure are generally older and are likely to have other comorbidities. “The more medicines we give, the more likely we are to run into side effects. So, we have produced some guidance on how to monitor for adverse effects and ways to mitigate these effects so the guideline recommended therapies can be continued without creating new harms.”
He gave the example of mineralocorticoid antagonists, which can sometimes elevate potassium levels, particularly if there is some underlying kidney disease, so clinicians are advised to recommend a low-potassium diet for these patients or the use of potassium binding agents that will also lower the amount of potassium in the blood stream; in this way, patients are able to continue the mineralocorticoid antagonist.
On costs, Dr. Maddox noted that the valsartan/sacubitril combination drug and SGLT inhibitors are new medicines and are expensive.
“They can be prohibitively expensive for patients who have suboptimal pharmacy benefits or who are uninsured.”
The Consensus Document therefore provided some guidance on ways to identify rebate programs, access insurance, and find different pathways to obtaining those drugs at a more reasonable price. It also advocated for policy changes to allow these medicines to be more accessible to more people.
More Use of Digital Tools
On the issue of systems of care, Dr. Maddox noted that the preexisting model of delivering care, which almost always involves the patient coming into the doctor’s office, invokes a high burden on both the system and most especially, the patient.
“Patients do not want to come back and forth to the doctor’s office multiple times in a few weeks. This is often a nonstarter, particularly for patients with busy lives,” he commented.
The Consensus Document advised more use of digital tools to provide remote care and contact with patients including sensors that can measure variables such as heart rate and blood pressure and video appointments.
“We are still working out what are the right models of care and how they can be performed safely and how they can be funded. But I think at the end of the day, this will give us more practical ways of getting people on multiple heart failure medicines and monitoring them safely without causing an undue burden for them logistically,” Dr. Maddox said.
He pointed out that there are a record number of medicines now available to treat heart failure, and while this is welcome, many of these patients are also on multiple other medications for other comorbidities as well.
“If you start giving patients seven, eight, or nine different medicines that they have to take every day, sometimes multiple times a day — that’s complicated medically, logistically, and financially. The potential for interaction and complications increases with every additional medication.”
Dr. Maddox also noted that patients have limits on how many medications they will accept. “It really helps if we have an engaged patient who has a good relationship with the care team to try to develop the right treatment plan that is going to meet their needs and give them the best possible health outcomes.”
It can take many visits to get the patient on all these medications and then up-titrate to target doses.
“We try and do a couple of things in each appointment. Often, we tend to start one or maybe two drugs at a time at a relatively low dose to avoid side effects, so we can be talking about 12-16 different encounters in total,” he said.
He recommended making a plan and the use of new technologies to manage each incremental step.
A Team Approach
Another issue that is discussed in the document is the use of a healthcare team to manage all the necessary appointments.
“It is no longer practical that one person can be the engineer for all this. It should be a team effort,” Dr. Maddox stated.
Responsibilities can be allocated across physicians, nurses, pharmacists, and even case managers, so that the team can take more of a population approach and develop a system to get patients on the multiple medications as quickly as possible.
“While this can still be quite a big burden for the patient, we need to figure out a system to make this as palatable as possible for them. Practices need to tailor this themselves according to what resources they have,” he added.
While most new patients will be routed to cardiologists to start their treatment plans, once on their initial medications and these have been up titrated to target levels, they should be able to be managed by primary care doctors, who will have the most holistic view of the patient and their other comorbidities, Dr. Maddox advised.
“Following this guidance should lead to more patients receiving evidence-based care which leads to better health outcomes, but delivered in a practical way that fits with their life reality and logistical needs,” he concluded.
A version of this article appeared on Medscape.com.
The American College of Cardiology has published a new update to its consensus decision pathway for the treatment of heart failure with reduced ejection fraction (HFrEF).
Chair of the consensus document Writing Committee Thomas M. Maddox, MD, explained to this news organization that this new Decision Pathway provides a practical, streamlined update to frontline clinicians treating patients with heart failure and incorporates evidence from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“While the AHA/ACC/HFSA Guidelines are wonderful in that they collate all the latest scientific evidence, they don’t speak as much to the practicalities of delivering the care. This is what this Decision Pathway document comes in — it is designed to help frontline clinicians with the practical reality of managing these patients,” Dr. Maddox, who is director of the Healthcare Innovation Lab at BJC HealthCare and the Washington University School of Medicine in St Louis, Missouri, commented.
The document, “Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction,” was published online on March 8 in the Journal of the American College of Cardiology.
The authors provided guidance on introducing the numerous evidence-based therapies now available for HFrEF, improving adherence, overcoming treatment barriers, acknowledging contraindications and situations for which few data exist, affording expensive therapies, treating special cohorts, and making the transition to palliative care.
Rather than focusing on extensive text, the document provided practical tips, tables, and figures to make clear the steps, tools, and provisos needed to treat patients with heart failure successfully and expeditiously, they added.
Dr. Maddox reported that there are three main updated areas of advice on the treatment of heart failure in the new document.
Valsartan/Sacubitril First Line
One of the major changes involves an elevation for the status of the angiotensin receptor-neprilysin inhibitor (ARNI), Entresto (valsartan/sacubitril).
“It is now clear that this agent is superior to ACE-inhibitors or angiotensin receptor blockers in terms of reducing heart failure hospitalization and death, whereas previously it was seen as somewhat equivalent,” Dr. Maddox said. “So, barring a contraindication or another problem with getting the medication, this agent should be one of the first line medicines for all patients with heart failure and a reduced ejection fraction.”
Dual Sodium-Glucose Cotransporter 1/2 (SGLT1/2) Inhibitor
A second update involves the addition of sotagliflozin (a dual inhibitor of both SGLT1 and SGLT2) to the SGLT2 inhibitors as another first-line medication for patients with heart failure and reduced ejection fraction.
“We now have evidence that both SGLT2 and SGLT1 inhibitors are beneficial in reducing heart failure hospitalization and death. Previously we only had evidence on SGLT2 inhibitors — dapagliflozin and empagliflozin. Sotagliflozin is a newer agent, which inhibits both SGLT1 and SGLT2, and it turns out that inhibiting both are beneficial in heart failure. So, this gives us a third med in this category,” Dr. Maddox noted.
Rapid Initiation of the Four Pillars of Therapy
The document stated that more data have emerged recently to support early and rapid initiation and titration of the “four pillars” of medical therapy in heart failure to maximize the benefits of patient-reported outcomes and reduction in hospitalizations and mortality.
The four pillars of therapy are ARNI, a beta-blocker, a mineralocorticoid antagonist, and an SGLT inhibitor.
As an example, four-class medication initiation reduced the hazard of cardiovascular death or hospital admission for heart failure significantly (hazard ratio, 0.38) compared with therapy with just an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker plus a beta-blocker, the document reported.
“What we realize now is that the more quickly we can get patients on all four of these drug classes and escalate to target doses or maximally tolerated doses ideally within 3 months, the better the outcome,” Dr. Maddox said.
“Unfortunately, right now there is very incomplete realization and recognition of that in clinical practice. So, we are trying to highlight the importance of this to encourage clinicians to be more aggressive in making this happen.”
“In all patients with heart failure and reduced ejection fraction, getting them on all four of these medicines as quickly as possible will give the best outcome. We’ve seen evidence in support of this from several broad population trials,” he added. “There are times when they can’t take all four but we should do our best to get there.”
Practical Considerations
Dr. Maddox pointed out that the Consensus Document is also trying to account for practical realities and barriers to heart failure treatment.
“When we think about these recommendations — and evidence that getting patients on all these medicines is valuable, we also focus on the fact that there are three major barriers that can get in the way of this and how to think about overcoming those barriers,” he said.
The barriers are comorbidities/side effects of medications, costs of the medicines, and systems of care that are needed to ensure patients can be treated with multiple medications in a timely fashion.
In terms of comorbidities/side effects, Dr. Maddox explained that patients with heart failure are generally older and are likely to have other comorbidities. “The more medicines we give, the more likely we are to run into side effects. So, we have produced some guidance on how to monitor for adverse effects and ways to mitigate these effects so the guideline recommended therapies can be continued without creating new harms.”
He gave the example of mineralocorticoid antagonists, which can sometimes elevate potassium levels, particularly if there is some underlying kidney disease, so clinicians are advised to recommend a low-potassium diet for these patients or the use of potassium binding agents that will also lower the amount of potassium in the blood stream; in this way, patients are able to continue the mineralocorticoid antagonist.
On costs, Dr. Maddox noted that the valsartan/sacubitril combination drug and SGLT inhibitors are new medicines and are expensive.
“They can be prohibitively expensive for patients who have suboptimal pharmacy benefits or who are uninsured.”
The Consensus Document therefore provided some guidance on ways to identify rebate programs, access insurance, and find different pathways to obtaining those drugs at a more reasonable price. It also advocated for policy changes to allow these medicines to be more accessible to more people.
More Use of Digital Tools
On the issue of systems of care, Dr. Maddox noted that the preexisting model of delivering care, which almost always involves the patient coming into the doctor’s office, invokes a high burden on both the system and most especially, the patient.
“Patients do not want to come back and forth to the doctor’s office multiple times in a few weeks. This is often a nonstarter, particularly for patients with busy lives,” he commented.
The Consensus Document advised more use of digital tools to provide remote care and contact with patients including sensors that can measure variables such as heart rate and blood pressure and video appointments.
“We are still working out what are the right models of care and how they can be performed safely and how they can be funded. But I think at the end of the day, this will give us more practical ways of getting people on multiple heart failure medicines and monitoring them safely without causing an undue burden for them logistically,” Dr. Maddox said.
He pointed out that there are a record number of medicines now available to treat heart failure, and while this is welcome, many of these patients are also on multiple other medications for other comorbidities as well.
“If you start giving patients seven, eight, or nine different medicines that they have to take every day, sometimes multiple times a day — that’s complicated medically, logistically, and financially. The potential for interaction and complications increases with every additional medication.”
Dr. Maddox also noted that patients have limits on how many medications they will accept. “It really helps if we have an engaged patient who has a good relationship with the care team to try to develop the right treatment plan that is going to meet their needs and give them the best possible health outcomes.”
It can take many visits to get the patient on all these medications and then up-titrate to target doses.
“We try and do a couple of things in each appointment. Often, we tend to start one or maybe two drugs at a time at a relatively low dose to avoid side effects, so we can be talking about 12-16 different encounters in total,” he said.
He recommended making a plan and the use of new technologies to manage each incremental step.
A Team Approach
Another issue that is discussed in the document is the use of a healthcare team to manage all the necessary appointments.
“It is no longer practical that one person can be the engineer for all this. It should be a team effort,” Dr. Maddox stated.
Responsibilities can be allocated across physicians, nurses, pharmacists, and even case managers, so that the team can take more of a population approach and develop a system to get patients on the multiple medications as quickly as possible.
“While this can still be quite a big burden for the patient, we need to figure out a system to make this as palatable as possible for them. Practices need to tailor this themselves according to what resources they have,” he added.
While most new patients will be routed to cardiologists to start their treatment plans, once on their initial medications and these have been up titrated to target levels, they should be able to be managed by primary care doctors, who will have the most holistic view of the patient and their other comorbidities, Dr. Maddox advised.
“Following this guidance should lead to more patients receiving evidence-based care which leads to better health outcomes, but delivered in a practical way that fits with their life reality and logistical needs,” he concluded.
A version of this article appeared on Medscape.com.
The American College of Cardiology has published a new update to its consensus decision pathway for the treatment of heart failure with reduced ejection fraction (HFrEF).
Chair of the consensus document Writing Committee Thomas M. Maddox, MD, explained to this news organization that this new Decision Pathway provides a practical, streamlined update to frontline clinicians treating patients with heart failure and incorporates evidence from the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
“While the AHA/ACC/HFSA Guidelines are wonderful in that they collate all the latest scientific evidence, they don’t speak as much to the practicalities of delivering the care. This is what this Decision Pathway document comes in — it is designed to help frontline clinicians with the practical reality of managing these patients,” Dr. Maddox, who is director of the Healthcare Innovation Lab at BJC HealthCare and the Washington University School of Medicine in St Louis, Missouri, commented.
The document, “Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction,” was published online on March 8 in the Journal of the American College of Cardiology.
The authors provided guidance on introducing the numerous evidence-based therapies now available for HFrEF, improving adherence, overcoming treatment barriers, acknowledging contraindications and situations for which few data exist, affording expensive therapies, treating special cohorts, and making the transition to palliative care.
Rather than focusing on extensive text, the document provided practical tips, tables, and figures to make clear the steps, tools, and provisos needed to treat patients with heart failure successfully and expeditiously, they added.
Dr. Maddox reported that there are three main updated areas of advice on the treatment of heart failure in the new document.
Valsartan/Sacubitril First Line
One of the major changes involves an elevation for the status of the angiotensin receptor-neprilysin inhibitor (ARNI), Entresto (valsartan/sacubitril).
“It is now clear that this agent is superior to ACE-inhibitors or angiotensin receptor blockers in terms of reducing heart failure hospitalization and death, whereas previously it was seen as somewhat equivalent,” Dr. Maddox said. “So, barring a contraindication or another problem with getting the medication, this agent should be one of the first line medicines for all patients with heart failure and a reduced ejection fraction.”
Dual Sodium-Glucose Cotransporter 1/2 (SGLT1/2) Inhibitor
A second update involves the addition of sotagliflozin (a dual inhibitor of both SGLT1 and SGLT2) to the SGLT2 inhibitors as another first-line medication for patients with heart failure and reduced ejection fraction.
“We now have evidence that both SGLT2 and SGLT1 inhibitors are beneficial in reducing heart failure hospitalization and death. Previously we only had evidence on SGLT2 inhibitors — dapagliflozin and empagliflozin. Sotagliflozin is a newer agent, which inhibits both SGLT1 and SGLT2, and it turns out that inhibiting both are beneficial in heart failure. So, this gives us a third med in this category,” Dr. Maddox noted.
Rapid Initiation of the Four Pillars of Therapy
The document stated that more data have emerged recently to support early and rapid initiation and titration of the “four pillars” of medical therapy in heart failure to maximize the benefits of patient-reported outcomes and reduction in hospitalizations and mortality.
The four pillars of therapy are ARNI, a beta-blocker, a mineralocorticoid antagonist, and an SGLT inhibitor.
As an example, four-class medication initiation reduced the hazard of cardiovascular death or hospital admission for heart failure significantly (hazard ratio, 0.38) compared with therapy with just an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker plus a beta-blocker, the document reported.
“What we realize now is that the more quickly we can get patients on all four of these drug classes and escalate to target doses or maximally tolerated doses ideally within 3 months, the better the outcome,” Dr. Maddox said.
“Unfortunately, right now there is very incomplete realization and recognition of that in clinical practice. So, we are trying to highlight the importance of this to encourage clinicians to be more aggressive in making this happen.”
“In all patients with heart failure and reduced ejection fraction, getting them on all four of these medicines as quickly as possible will give the best outcome. We’ve seen evidence in support of this from several broad population trials,” he added. “There are times when they can’t take all four but we should do our best to get there.”
Practical Considerations
Dr. Maddox pointed out that the Consensus Document is also trying to account for practical realities and barriers to heart failure treatment.
“When we think about these recommendations — and evidence that getting patients on all these medicines is valuable, we also focus on the fact that there are three major barriers that can get in the way of this and how to think about overcoming those barriers,” he said.
The barriers are comorbidities/side effects of medications, costs of the medicines, and systems of care that are needed to ensure patients can be treated with multiple medications in a timely fashion.
In terms of comorbidities/side effects, Dr. Maddox explained that patients with heart failure are generally older and are likely to have other comorbidities. “The more medicines we give, the more likely we are to run into side effects. So, we have produced some guidance on how to monitor for adverse effects and ways to mitigate these effects so the guideline recommended therapies can be continued without creating new harms.”
He gave the example of mineralocorticoid antagonists, which can sometimes elevate potassium levels, particularly if there is some underlying kidney disease, so clinicians are advised to recommend a low-potassium diet for these patients or the use of potassium binding agents that will also lower the amount of potassium in the blood stream; in this way, patients are able to continue the mineralocorticoid antagonist.
On costs, Dr. Maddox noted that the valsartan/sacubitril combination drug and SGLT inhibitors are new medicines and are expensive.
“They can be prohibitively expensive for patients who have suboptimal pharmacy benefits or who are uninsured.”
The Consensus Document therefore provided some guidance on ways to identify rebate programs, access insurance, and find different pathways to obtaining those drugs at a more reasonable price. It also advocated for policy changes to allow these medicines to be more accessible to more people.
More Use of Digital Tools
On the issue of systems of care, Dr. Maddox noted that the preexisting model of delivering care, which almost always involves the patient coming into the doctor’s office, invokes a high burden on both the system and most especially, the patient.
“Patients do not want to come back and forth to the doctor’s office multiple times in a few weeks. This is often a nonstarter, particularly for patients with busy lives,” he commented.
The Consensus Document advised more use of digital tools to provide remote care and contact with patients including sensors that can measure variables such as heart rate and blood pressure and video appointments.
“We are still working out what are the right models of care and how they can be performed safely and how they can be funded. But I think at the end of the day, this will give us more practical ways of getting people on multiple heart failure medicines and monitoring them safely without causing an undue burden for them logistically,” Dr. Maddox said.
He pointed out that there are a record number of medicines now available to treat heart failure, and while this is welcome, many of these patients are also on multiple other medications for other comorbidities as well.
“If you start giving patients seven, eight, or nine different medicines that they have to take every day, sometimes multiple times a day — that’s complicated medically, logistically, and financially. The potential for interaction and complications increases with every additional medication.”
Dr. Maddox also noted that patients have limits on how many medications they will accept. “It really helps if we have an engaged patient who has a good relationship with the care team to try to develop the right treatment plan that is going to meet their needs and give them the best possible health outcomes.”
It can take many visits to get the patient on all these medications and then up-titrate to target doses.
“We try and do a couple of things in each appointment. Often, we tend to start one or maybe two drugs at a time at a relatively low dose to avoid side effects, so we can be talking about 12-16 different encounters in total,” he said.
He recommended making a plan and the use of new technologies to manage each incremental step.
A Team Approach
Another issue that is discussed in the document is the use of a healthcare team to manage all the necessary appointments.
“It is no longer practical that one person can be the engineer for all this. It should be a team effort,” Dr. Maddox stated.
Responsibilities can be allocated across physicians, nurses, pharmacists, and even case managers, so that the team can take more of a population approach and develop a system to get patients on the multiple medications as quickly as possible.
“While this can still be quite a big burden for the patient, we need to figure out a system to make this as palatable as possible for them. Practices need to tailor this themselves according to what resources they have,” he added.
While most new patients will be routed to cardiologists to start their treatment plans, once on their initial medications and these have been up titrated to target levels, they should be able to be managed by primary care doctors, who will have the most holistic view of the patient and their other comorbidities, Dr. Maddox advised.
“Following this guidance should lead to more patients receiving evidence-based care which leads to better health outcomes, but delivered in a practical way that fits with their life reality and logistical needs,” he concluded.
A version of this article appeared on Medscape.com.
Diet and Exercise in a Pill Are Real: How Mimetics Work
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
If couch-potato lab mice had beach-body dreams and if they could speak, they might tell you they’re thrilled by advances in the science of exercise and calorie-restriction (CR) mimetics.
In recent studies conducted at research centers across the United States, mice have chowed down, fattened up, exercised only if they felt like it, and still managed to lose body fat, improve their blood lipids, increase muscle power, avoid blood sugar problems, and boost heart function.
How did these mice get so lucky? They were given mimetics, experimental drugs that “mimic” the effects of exercise and calorie reduction in the body without the need to break a sweat or eat less.
“The mice looked like they’d done endurance training,” said Thomas Burris, PhD, chair of the Department of Pharmacodynamics at the University of Florida, Gainesville, Florida, and coauthor of a September 2023 study of the exercise mimetic SLU-PP-332, published in The Journal of Pharmacology and Experimental Therapeutics.
Meanwhile, the CR mimetic mannoheptulose (MH) “was incredibly effective at stopping the negative effects of a high-fat diet in mice,” said Donald K. Ingram, PhD, an adjunct professor at Louisiana State University’s Pennington Biomedical Research Center, Baton Rouge, Louisiana, who began studying CR mimetics at the National Institute on Aging in the 1980s. In a 2022 study published in Nutrients, MH also increased insulin sensitivity.
These “have your cake and eat it, too” drugs aren’t on the market for human use — but they’re edging closer. Several have moved into human trials with encouraging results. The National Institutes of Health and the pharmaceutical industry are taking notice, anteing up big research dollars. At the earliest, one could win US Food and Drug Administration (FDA) approval in 4-5 years, Dr. Burris said.
The medical appeal is clear: Mimetics could one day prevent and treat serious conditions such as age- and disease-related muscle loss, diabetes, heart failure, and even neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease, said the scientists studying them.
The commercial appeal is unavoidable: Mimetics have the potential to help nondieters avoid weight gain and allow dieters to build and/or preserve more calorie-burning muscle — a boon because losing weight can reduce muscle, especially with rapid loss.
How do these drugs work? What’s their downside? Like the “miracle” glucagon-like peptide 1 (GLP-1) weight-loss drugs that are now ubiquitous, are mimetics an effective pharmaceutical way to replicate two of society’s biggest lifestyle sticking points — diet and exercise?
It’s possible…
CR Mimetics: The Healthspan Drug?
From nematodes and fruit flies to yeast, Labrador Retrievers, and people, plenty of research shows that reducing calorie intake may improve health and prolong life. By how much? Cutting calories by 25% for 2 years slowed the pace of aging 2%-3% in the landmark CALERIE study of 197 adults, according to a 2023 study in Nature Aging. Sounds small, but the researchers said that equals a 10%-15% lower risk for an early death — on par with the longevity bonus you’d get from quitting smoking.
Trouble is low-cal living isn’t easy. “Diets work,” said George Roth, PhD, of GeroScience, Inc., in Pylesville, MD, who began studying CR at the National Institute on Aging in the 1980s with Ingram. “But it’s hard to sustain.”
That’s where CR mimetics come in. They activate the same health-promoting genes switched on by dieting, fasting, and extended periods of hunger, Dr. Roth said. The end result isn’t big weight loss. Instead, CR mimetics may keep us healthier and younger as we age. “Calorie restriction shifts metabolic processes in the body to protect against damage and stress,” he said.
Dr. Roth and Dr. Ingram are currently focused on the CR mimetic mannoheptulose (MH), a sugar found in unripe avocados. “It works at the first step in carbohydrate metabolism in cells throughout the body, so less energy goes through that pathway,” he said. “Glucose metabolism is reduced by 10%-15%. It’s the closest thing to actually eating less food.”
Their 2022 study found that while mice on an all-you-can-eat high-fat diet gained weight and body fat and saw blood lipids increase while insulin sensitivity decreased, mice that also got MH avoided these problems. A 2023 human study in Nutrients coauthored by Dr. Roth and Dr. Ingram found that a group consuming freeze-dried avocado had lower insulin levels than a placebo group.
Other researchers are looking at ways to stimulate the CR target nicotinamide adenine dinucleotide (NAD+). NAD+ assists sirtuins — a group of seven enzymes central to the beneficial effects of CR on aging — but levels drop with age. University of Colorado researchers are studying the effects of nicotinamide riboside (NR), an NAD+ precursor, in older adults with a $2.5 million National Institute on Aging grant. Small, preliminary human studies have found the compound reduced indicators of insulin resistance in the brain, in a January 2023 study in Aging Cell, and reduced blood pressure and arterial stiffness in a 2018 study published in Nature Communications.
Another NAD+ precursor, nicotinamide mononucleotide, reduced low-density lipoprotein cholesterol, diastolic blood pressure, and body weight in a Harvard Medical School study of 30 midlife and older adults with overweight and obesity, published in August 2023 in The Journal of Clinical Endocrinology & Metabolism. And in an April 2022 study published in Hepatology of people with nonalcoholic fatty liver disease, a proprietary supplement that included NR didn’t reduce liver fat but had a significant (vs placebo) reduction in ceramide and the liver enzyme alanine aminotransferase, a marker of inflammation.
“I think it was a pretty interesting result,” said lead researcher Leonard Guarente, PhD, professor of biology at Massachusetts Institute of Technology and founder of the supplement company Elysium. “Fatty liver progressively damages the liver. This has the potential to slow that down.”
Exercise Mimetics: Fitness in a Pill?
Physical activity builds muscle and fitness, helps keeps bones strong, sharpens thinking and memory, guards against depression, and helps discourage a slew of health concerns from weight gain and high blood pressure to diabetes and heart disease. Muscle becomes more dense, more powerful and may even burn more calories, said Dr. Burris. The problem: That pesky part about actually moving. Fewer than half of American adults get recommended amounts of aerobic exercise and fewer than a quarter fit in strength training, according to the Centers for Disease Control and Prevention.
Enter the exercise mimetics. Unlike CR mimetics, exercise mimetics affect mitochondria — the tiny power plants in muscle and every other cell in the body. They switch on genes that encourage the growth of more mitochondria and encourage them to burn fatty acids, not just glucose, for fuel.
In mice, this can keep them from gaining weight, increase insulin sensitivity, and boost exercise endurance. “We can use a drug to activate the same networks that are activated by physical activity,” said Ronald Evans, PhD, professor and director of the Gene Expression Laboratory at the Salk Institute for Biological Studies in La Jolla, California.
Among notable mimetics moving into human studies is ASP0367, a drug in a class called PPAR delta modulators first developed in Evans’ lab. ASP0367 was licensed to the pharmaceutical company Mitobridge, later acquired by Astellas. Astellas is currently running a phase 2/3 human trial of the investigational drug in people with the rare genetic disorder primary mitochondrial myopathy.
At the University of Florida, Dr. Burris and team hope to soon move the exercise mimetic SLU-PP-332 into human studies. “It targets a receptor called ERR that I’ve been working on since the 1980s,” Dr. Burris said. “We knew from genetic studies that ERR has a role in exercise’s effects on mitochondrial function in muscle.” The calorie mimetics he’s studying also activate genes for making more mitochondria and driving them to burn fatty acids. “This generates a lot of energy,” he said. In a January 2024 study in Circulation, Dr. Burris found the drug restores heart function in mice experiencing heart failure. “Very little heart function was lost,” he said. It’s had no serious side effects.
The Future of Exercise and CR Pills
The field has hit some bumps. Some feel inevitable — such as otherwise healthy people misusing the drugs. GW1516, an early experimental exercise mimetic studied by Dr. Evans and abandoned because it triggered tumor growth in lab studies, is used illegally by elite athletes as a performance-enhancing drug despite warnings from the US Anti-Doping Agency. Dr. Burris worries that future CR mimetics could be misused the same way.
But he and others see plenty of benefits in future, FDA-approved drugs. Exercise mimetics like SLU-PP-332 might one day be given to people alongside weight-loss drugs, such as Mounjaro (tirzepatide) or Ozempic (semaglutide) to prevent muscle loss. “SLU-PP-332 doesn’t affect hunger or food intake the way those drugs do,” he said. “It changes muscle.”
Mimetics may one day help older adults and people with muscle disorders rebuild muscle even when they cannot exercise and to delay a range of age-related diseases without onerous dieting. “The chance to intervene and provide a longer healthspan and lifespan — that’s been the moon shot,” Dr. Roth said.
Dr. Guarente noted that CR mimetics may work best for people who aren’t carrying extra pounds but want the health benefits of slashing calories without sacrificing meals and snacks. “Fat is still going to be a problem for joints, cholesterol, inflammation,” he said. “Calorie mimetics are not a panacea for obesity but could help preserve overall health and vitality.”
And what about the billion-dollar question: What happens when these drugs become available to a general public that has issues with actual exercise and healthy diet?
Evans sees only positives. “Our environment is designed to keep people sitting down and consuming high-calorie foods,” he said. “In the absence of people getting motivated to exercise — and there’s no evidence the country is moving in that direction on its own — a pill is an important option to have.”
A version of this article appeared on Medscape.com.
Medicare Doc Pay Cut Eased, but When Will Serious Revisions Come?
President Joe Biden on March 9 signed into law a measure that softened — but did not completely eliminate — a 2024 cut in a key rate used to determine how physicians are paid for treating Medicare patients.
While physician groups hailed the move as partial relief, they say they’ll continue to press for broader changes in the Medicare physician fee schedule.
The Medicare provision was tucked into a larger spending package approved by the US House and Senate.
The American Academy of Family Physicians (AAFP), the American Medical Association (AMA), and other groups have lobbied Congress for months to undo a 3.4% cut in the base rate, or conversion factor, in the physician fee schedule for 2024.
The conversion factor is used in calculations to determine reimbursement for myriad other services. Federal Medicare officials said the cut would mean a 1.25% decrease in overall payments in 2024, compared with 2023.
“With the passage of this legislation, Congress has offset 2.93% of that payment cut,” said Steven P. Furr, MD, AAFP’s president in a statement. “We appreciate this temporary measure but continue to urge Congress to advance comprehensive, long-term Medicare payment reform.”
In a statement, Representative Larry Bucshon, MD (R-IN), said the payment cut could not be completely eliminated because of budget constraints.
The Medicare physician fee schedule covers much of the care clinicians provide to people older than 65 and those with disabilities. It covers about 8000 different types of services, ranging from office visits to surgical procedures, imaging, and tests, according to the Medicare Payment Advisory Commission (MedPAC).
Along with physicians, the fee schedule sets payments for nurse practitioners, physician assistants, podiatrists, physical therapists, psychologists, and other clinicians.
In 2021, the Medicare program and its beneficiaries paid $92.8 billion for services provided by almost 1.3 million clinicians, MedPAC said.
Larger Changes Ahead?
Rep. Bucshon is among the physicians serving in the House who are pressing for a permanent revamp of the Medicare physician fee schedule. He cosponsored a bill (HR 2474) that would peg future annual increases in the physician fee schedule to the Medicare Economic Index, which would reflect inflation’s effect.
In April, more than 120 state and national medical groups signed onto an AMA-led letter urging Congress to pass this bill.
The measure is a key priority for the AMA. The organization reached out repeatedly last year to federal officials about it through its own in-house lobbyists, this news organization found through a review of congressional lobbying forms submitted by AMA.
These required disclosure forms reveal how much AMA and other organizations spend each quarter to appeal to members of Congress and federal agencies on specific issues. The disclosure forms do not include a detailed accounting of spending on each issue.
But they do show which issues are priorities for an organization. AMA’s in-house lobbyists reported raising dozens of issues in 2024 within contacts in Congress and federal agencies. These issues included abortion access, maternal health, physician burnout, and potential for bias in clinical use of algorithms, as well as Medicare payment for physicians.
AMA reported spending estimated cost of $20.6 million. (AMA spent $6.7 million in the first quarter, $4.75 million in the second quarter, $3.42 million in the third quarter, and $5.74 million in the fourth quarter.)
In a March 6 statement, Jesse M. Ehrenfeld, MD, MPH, AMA president, urged Congress to turn to more serious consideration of Medicare physician pay beyond short-term tweaks attached to other larger bills.
“As physicians, we are trained to run toward emergencies. We urge Congress to do the same,” Dr. Ehrenfeld said. “We encourage Congress to act if this policy decision is an emergency because — in fact — it is. It is well past time to put an end to stopgap measures that fail to address the underlying causes of the continuing decline in Medicare physician payments.”
There’s bipartisan interest in a revamp of the physician fee schedule amid widespread criticism of the last such overhaul, the Medicare Access and CHIP Reauthorization Act of 2015.
For example, Senate Budget Chairman Sheldon Whitehouse (D-RI) has proposed the creation of a technical advisory committee to improve how Medicare sets the physician fee schedule. The existing fee schedule provides too little money for primary care services and primary care provider pay, contributing to shortages, Sen. Whitehouse said.
Sen. Whitehouse on March 6 held a hearing on ways to beef up US primary care. Among the experts who appeared was Amol Navathe, MD, PhD, of the University of Pennsylvania, Philadelphia, Pennsylvania.
Dr. Navathe said the current Medicare physician fee schedule tilts in favor of procedural services, leading to “underinvestment in cognitive, diagnostic, and supportive services such as primary care.”
In addition, much of what primary care clinicians do, “such as addressing social challenges, is not included in the codes of the fee schedule itself,” said Dr. Navathe, who also serves as the vice chairman of MedPAC.
It’s unclear when Congress will attempt a serious revision to the Medicare physician fee schedule. Lawmakers are unlikely to take on such a major challenge in this election year.
There would be significant opposition and challenges for lawmakers in trying to clear a bill that added an inflation adjustment for what’s already seen as an imperfect physician fee schedule, said Mark E. Miller, PhD, executive vice president of healthcare at the philanthropy Arnold Ventures, which studies how payment decisions affect medical care.
“That bill could cost a lot of money and raise a lot of questions,” Dr. Miller said.
A version of this article appeared on Medscape.com.
President Joe Biden on March 9 signed into law a measure that softened — but did not completely eliminate — a 2024 cut in a key rate used to determine how physicians are paid for treating Medicare patients.
While physician groups hailed the move as partial relief, they say they’ll continue to press for broader changes in the Medicare physician fee schedule.
The Medicare provision was tucked into a larger spending package approved by the US House and Senate.
The American Academy of Family Physicians (AAFP), the American Medical Association (AMA), and other groups have lobbied Congress for months to undo a 3.4% cut in the base rate, or conversion factor, in the physician fee schedule for 2024.
The conversion factor is used in calculations to determine reimbursement for myriad other services. Federal Medicare officials said the cut would mean a 1.25% decrease in overall payments in 2024, compared with 2023.
“With the passage of this legislation, Congress has offset 2.93% of that payment cut,” said Steven P. Furr, MD, AAFP’s president in a statement. “We appreciate this temporary measure but continue to urge Congress to advance comprehensive, long-term Medicare payment reform.”
In a statement, Representative Larry Bucshon, MD (R-IN), said the payment cut could not be completely eliminated because of budget constraints.
The Medicare physician fee schedule covers much of the care clinicians provide to people older than 65 and those with disabilities. It covers about 8000 different types of services, ranging from office visits to surgical procedures, imaging, and tests, according to the Medicare Payment Advisory Commission (MedPAC).
Along with physicians, the fee schedule sets payments for nurse practitioners, physician assistants, podiatrists, physical therapists, psychologists, and other clinicians.
In 2021, the Medicare program and its beneficiaries paid $92.8 billion for services provided by almost 1.3 million clinicians, MedPAC said.
Larger Changes Ahead?
Rep. Bucshon is among the physicians serving in the House who are pressing for a permanent revamp of the Medicare physician fee schedule. He cosponsored a bill (HR 2474) that would peg future annual increases in the physician fee schedule to the Medicare Economic Index, which would reflect inflation’s effect.
In April, more than 120 state and national medical groups signed onto an AMA-led letter urging Congress to pass this bill.
The measure is a key priority for the AMA. The organization reached out repeatedly last year to federal officials about it through its own in-house lobbyists, this news organization found through a review of congressional lobbying forms submitted by AMA.
These required disclosure forms reveal how much AMA and other organizations spend each quarter to appeal to members of Congress and federal agencies on specific issues. The disclosure forms do not include a detailed accounting of spending on each issue.
But they do show which issues are priorities for an organization. AMA’s in-house lobbyists reported raising dozens of issues in 2024 within contacts in Congress and federal agencies. These issues included abortion access, maternal health, physician burnout, and potential for bias in clinical use of algorithms, as well as Medicare payment for physicians.
AMA reported spending estimated cost of $20.6 million. (AMA spent $6.7 million in the first quarter, $4.75 million in the second quarter, $3.42 million in the third quarter, and $5.74 million in the fourth quarter.)
In a March 6 statement, Jesse M. Ehrenfeld, MD, MPH, AMA president, urged Congress to turn to more serious consideration of Medicare physician pay beyond short-term tweaks attached to other larger bills.
“As physicians, we are trained to run toward emergencies. We urge Congress to do the same,” Dr. Ehrenfeld said. “We encourage Congress to act if this policy decision is an emergency because — in fact — it is. It is well past time to put an end to stopgap measures that fail to address the underlying causes of the continuing decline in Medicare physician payments.”
There’s bipartisan interest in a revamp of the physician fee schedule amid widespread criticism of the last such overhaul, the Medicare Access and CHIP Reauthorization Act of 2015.
For example, Senate Budget Chairman Sheldon Whitehouse (D-RI) has proposed the creation of a technical advisory committee to improve how Medicare sets the physician fee schedule. The existing fee schedule provides too little money for primary care services and primary care provider pay, contributing to shortages, Sen. Whitehouse said.
Sen. Whitehouse on March 6 held a hearing on ways to beef up US primary care. Among the experts who appeared was Amol Navathe, MD, PhD, of the University of Pennsylvania, Philadelphia, Pennsylvania.
Dr. Navathe said the current Medicare physician fee schedule tilts in favor of procedural services, leading to “underinvestment in cognitive, diagnostic, and supportive services such as primary care.”
In addition, much of what primary care clinicians do, “such as addressing social challenges, is not included in the codes of the fee schedule itself,” said Dr. Navathe, who also serves as the vice chairman of MedPAC.
It’s unclear when Congress will attempt a serious revision to the Medicare physician fee schedule. Lawmakers are unlikely to take on such a major challenge in this election year.
There would be significant opposition and challenges for lawmakers in trying to clear a bill that added an inflation adjustment for what’s already seen as an imperfect physician fee schedule, said Mark E. Miller, PhD, executive vice president of healthcare at the philanthropy Arnold Ventures, which studies how payment decisions affect medical care.
“That bill could cost a lot of money and raise a lot of questions,” Dr. Miller said.
A version of this article appeared on Medscape.com.
President Joe Biden on March 9 signed into law a measure that softened — but did not completely eliminate — a 2024 cut in a key rate used to determine how physicians are paid for treating Medicare patients.
While physician groups hailed the move as partial relief, they say they’ll continue to press for broader changes in the Medicare physician fee schedule.
The Medicare provision was tucked into a larger spending package approved by the US House and Senate.
The American Academy of Family Physicians (AAFP), the American Medical Association (AMA), and other groups have lobbied Congress for months to undo a 3.4% cut in the base rate, or conversion factor, in the physician fee schedule for 2024.
The conversion factor is used in calculations to determine reimbursement for myriad other services. Federal Medicare officials said the cut would mean a 1.25% decrease in overall payments in 2024, compared with 2023.
“With the passage of this legislation, Congress has offset 2.93% of that payment cut,” said Steven P. Furr, MD, AAFP’s president in a statement. “We appreciate this temporary measure but continue to urge Congress to advance comprehensive, long-term Medicare payment reform.”
In a statement, Representative Larry Bucshon, MD (R-IN), said the payment cut could not be completely eliminated because of budget constraints.
The Medicare physician fee schedule covers much of the care clinicians provide to people older than 65 and those with disabilities. It covers about 8000 different types of services, ranging from office visits to surgical procedures, imaging, and tests, according to the Medicare Payment Advisory Commission (MedPAC).
Along with physicians, the fee schedule sets payments for nurse practitioners, physician assistants, podiatrists, physical therapists, psychologists, and other clinicians.
In 2021, the Medicare program and its beneficiaries paid $92.8 billion for services provided by almost 1.3 million clinicians, MedPAC said.
Larger Changes Ahead?
Rep. Bucshon is among the physicians serving in the House who are pressing for a permanent revamp of the Medicare physician fee schedule. He cosponsored a bill (HR 2474) that would peg future annual increases in the physician fee schedule to the Medicare Economic Index, which would reflect inflation’s effect.
In April, more than 120 state and national medical groups signed onto an AMA-led letter urging Congress to pass this bill.
The measure is a key priority for the AMA. The organization reached out repeatedly last year to federal officials about it through its own in-house lobbyists, this news organization found through a review of congressional lobbying forms submitted by AMA.
These required disclosure forms reveal how much AMA and other organizations spend each quarter to appeal to members of Congress and federal agencies on specific issues. The disclosure forms do not include a detailed accounting of spending on each issue.
But they do show which issues are priorities for an organization. AMA’s in-house lobbyists reported raising dozens of issues in 2024 within contacts in Congress and federal agencies. These issues included abortion access, maternal health, physician burnout, and potential for bias in clinical use of algorithms, as well as Medicare payment for physicians.
AMA reported spending estimated cost of $20.6 million. (AMA spent $6.7 million in the first quarter, $4.75 million in the second quarter, $3.42 million in the third quarter, and $5.74 million in the fourth quarter.)
In a March 6 statement, Jesse M. Ehrenfeld, MD, MPH, AMA president, urged Congress to turn to more serious consideration of Medicare physician pay beyond short-term tweaks attached to other larger bills.
“As physicians, we are trained to run toward emergencies. We urge Congress to do the same,” Dr. Ehrenfeld said. “We encourage Congress to act if this policy decision is an emergency because — in fact — it is. It is well past time to put an end to stopgap measures that fail to address the underlying causes of the continuing decline in Medicare physician payments.”
There’s bipartisan interest in a revamp of the physician fee schedule amid widespread criticism of the last such overhaul, the Medicare Access and CHIP Reauthorization Act of 2015.
For example, Senate Budget Chairman Sheldon Whitehouse (D-RI) has proposed the creation of a technical advisory committee to improve how Medicare sets the physician fee schedule. The existing fee schedule provides too little money for primary care services and primary care provider pay, contributing to shortages, Sen. Whitehouse said.
Sen. Whitehouse on March 6 held a hearing on ways to beef up US primary care. Among the experts who appeared was Amol Navathe, MD, PhD, of the University of Pennsylvania, Philadelphia, Pennsylvania.
Dr. Navathe said the current Medicare physician fee schedule tilts in favor of procedural services, leading to “underinvestment in cognitive, diagnostic, and supportive services such as primary care.”
In addition, much of what primary care clinicians do, “such as addressing social challenges, is not included in the codes of the fee schedule itself,” said Dr. Navathe, who also serves as the vice chairman of MedPAC.
It’s unclear when Congress will attempt a serious revision to the Medicare physician fee schedule. Lawmakers are unlikely to take on such a major challenge in this election year.
There would be significant opposition and challenges for lawmakers in trying to clear a bill that added an inflation adjustment for what’s already seen as an imperfect physician fee schedule, said Mark E. Miller, PhD, executive vice president of healthcare at the philanthropy Arnold Ventures, which studies how payment decisions affect medical care.
“That bill could cost a lot of money and raise a lot of questions,” Dr. Miller said.
A version of this article appeared on Medscape.com.
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Long-Term Calcium and Vitamin D: Cancer Deaths Down, CVD Deaths Up in Older Women?
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Look Beyond BMI: Metabolic Factors’ Link to Cancer Explained
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
FROM CANCER
Nurse-Led Strategy Reduces Cholesterol, BP in HIV
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.
Higher Dietary Niacin Tied to Lower Mortality Risk in MASLD
TOPLINE:
Higher dietary niacin intake is associated with a lower risk for all-cause mortality among people with metabolic dysfunction-associated steatotic liver disease (MASLD), but there is no connection between niacin consumption and cardiovascular disease (CVD) mortality, a recent study suggested.
METHODOLOGY:
- Researchers analyzed data from the National Health and Nutrition Examination Survey (2003-2018) for 4315 adults with MASLD (mean age, 52.5 years; 55%, men; 67%, non-Hispanic White).
- Dietary niacin intake levels were based on two 24-hour dietary recall interviews to report the types and quantities of foods that participants consumed in the 24 hours prior to the interviews.
- Participants were categorized by tertile of dietary niacin intake: Tertile 1 (n = 1440), < 18.4 mg; tertile 2 (n = 1441), 18.5-26.6 mg; and tertile 3 (n = 1434), > 26.7 mg.
TAKEAWAY:
- During a median follow-up of 8.8 years, 566 deaths occurred, of which 197 were attributed to CVD.
- Compared with participants with a niacin intake of 18.4 mg or lower (the lowest tertile), the multivariable-adjusted hazard ratios (HRs) for participants with a niacin intake of 26.7 mg or higher (the highest tertile) were 0.70 for all-cause mortality and 0.65 for CVD mortality.
- For the subgroup with diabetes compared with the reference group (the first tertile), the HR of all-cause mortality in the third tertile was 0.82.
- When the subgroup without diabetes was compared with the reference group, the HR of all-cause mortality in the third tertile was 0.58, suggesting a significant interaction between niacin and diabetes with the risk of all-cause mortality.
- An inverse association between dietary niacin intake and all-cause mortality was seen in sensitivity analyses, when excluding a participant who died within 2 years of follow-up.
IN PRACTICE:
“Higher dietary niacin intake was associated with a lower risk of all-cause mortality,” but not CVD, among individuals with MASLD, and “the dose-response association…needs to be further investigated to determine optimal intake level,” the authors wrote.
SOURCE:
The study, led by Jie Pan, MD, Sun Yat-sen University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Physical activity data were missing and could not be adjusted for. The National Death Index used by the researchers has only “modest” ability to accurately classify CVD mortality, and the dietary data were subject to recall bias.
DISCLOSURES:
One author was supported by a grant from the National Nature Science Foundation of China. No other conflicts of interest were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher dietary niacin intake is associated with a lower risk for all-cause mortality among people with metabolic dysfunction-associated steatotic liver disease (MASLD), but there is no connection between niacin consumption and cardiovascular disease (CVD) mortality, a recent study suggested.
METHODOLOGY:
- Researchers analyzed data from the National Health and Nutrition Examination Survey (2003-2018) for 4315 adults with MASLD (mean age, 52.5 years; 55%, men; 67%, non-Hispanic White).
- Dietary niacin intake levels were based on two 24-hour dietary recall interviews to report the types and quantities of foods that participants consumed in the 24 hours prior to the interviews.
- Participants were categorized by tertile of dietary niacin intake: Tertile 1 (n = 1440), < 18.4 mg; tertile 2 (n = 1441), 18.5-26.6 mg; and tertile 3 (n = 1434), > 26.7 mg.
TAKEAWAY:
- During a median follow-up of 8.8 years, 566 deaths occurred, of which 197 were attributed to CVD.
- Compared with participants with a niacin intake of 18.4 mg or lower (the lowest tertile), the multivariable-adjusted hazard ratios (HRs) for participants with a niacin intake of 26.7 mg or higher (the highest tertile) were 0.70 for all-cause mortality and 0.65 for CVD mortality.
- For the subgroup with diabetes compared with the reference group (the first tertile), the HR of all-cause mortality in the third tertile was 0.82.
- When the subgroup without diabetes was compared with the reference group, the HR of all-cause mortality in the third tertile was 0.58, suggesting a significant interaction between niacin and diabetes with the risk of all-cause mortality.
- An inverse association between dietary niacin intake and all-cause mortality was seen in sensitivity analyses, when excluding a participant who died within 2 years of follow-up.
IN PRACTICE:
“Higher dietary niacin intake was associated with a lower risk of all-cause mortality,” but not CVD, among individuals with MASLD, and “the dose-response association…needs to be further investigated to determine optimal intake level,” the authors wrote.
SOURCE:
The study, led by Jie Pan, MD, Sun Yat-sen University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Physical activity data were missing and could not be adjusted for. The National Death Index used by the researchers has only “modest” ability to accurately classify CVD mortality, and the dietary data were subject to recall bias.
DISCLOSURES:
One author was supported by a grant from the National Nature Science Foundation of China. No other conflicts of interest were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher dietary niacin intake is associated with a lower risk for all-cause mortality among people with metabolic dysfunction-associated steatotic liver disease (MASLD), but there is no connection between niacin consumption and cardiovascular disease (CVD) mortality, a recent study suggested.
METHODOLOGY:
- Researchers analyzed data from the National Health and Nutrition Examination Survey (2003-2018) for 4315 adults with MASLD (mean age, 52.5 years; 55%, men; 67%, non-Hispanic White).
- Dietary niacin intake levels were based on two 24-hour dietary recall interviews to report the types and quantities of foods that participants consumed in the 24 hours prior to the interviews.
- Participants were categorized by tertile of dietary niacin intake: Tertile 1 (n = 1440), < 18.4 mg; tertile 2 (n = 1441), 18.5-26.6 mg; and tertile 3 (n = 1434), > 26.7 mg.
TAKEAWAY:
- During a median follow-up of 8.8 years, 566 deaths occurred, of which 197 were attributed to CVD.
- Compared with participants with a niacin intake of 18.4 mg or lower (the lowest tertile), the multivariable-adjusted hazard ratios (HRs) for participants with a niacin intake of 26.7 mg or higher (the highest tertile) were 0.70 for all-cause mortality and 0.65 for CVD mortality.
- For the subgroup with diabetes compared with the reference group (the first tertile), the HR of all-cause mortality in the third tertile was 0.82.
- When the subgroup without diabetes was compared with the reference group, the HR of all-cause mortality in the third tertile was 0.58, suggesting a significant interaction between niacin and diabetes with the risk of all-cause mortality.
- An inverse association between dietary niacin intake and all-cause mortality was seen in sensitivity analyses, when excluding a participant who died within 2 years of follow-up.
IN PRACTICE:
“Higher dietary niacin intake was associated with a lower risk of all-cause mortality,” but not CVD, among individuals with MASLD, and “the dose-response association…needs to be further investigated to determine optimal intake level,” the authors wrote.
SOURCE:
The study, led by Jie Pan, MD, Sun Yat-sen University, Guangzhou, China, was published online in JAMA Network Open.
LIMITATIONS:
Physical activity data were missing and could not be adjusted for. The National Death Index used by the researchers has only “modest” ability to accurately classify CVD mortality, and the dietary data were subject to recall bias.
DISCLOSURES:
One author was supported by a grant from the National Nature Science Foundation of China. No other conflicts of interest were reported.
A version of this article appeared on Medscape.com.