User login
Immediate-completion lymph node dissection in metastatic melanoma
In patients who have melanoma with sentinel node metastasis, immediate-completion lymph node dissection doesn’t improve melanoma-specific survival, compared with nodal observation using ultrasound, according to a report published online June 8 in the New England Journal of Medicine.
Immediate-completion lymph node dissection – removal of the remaining regional lymph nodes after sentinel node excision – is usually recommended for patients found to have sentinel node metastasis, even though the evidence supporting this practice is inconclusive. A large prospective phase III trial was performed to compare outcomes with this approach against outcomes in patients who instead underwent observation using frequent nodal ultrasound and had lymph node dissection only if nodal recurrence developed, said Mark B. Faries, MD, of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
The second Multicenter Selective Lymphadenectomy Trial (MSLT-II) involved 1,939 adults at 63 medical centers who had clinically localized cutaneous melanoma of intermediate thickness, at least one tumor-positive sentinel node as determined by standard pathological assessment or a quantitative reverse transcriptase–polymerase chain reaction assay, and a life expectancy of 10 years or more. These participants were randomly assigned to immediate-completion node dissection (971 patients) or nodal observation (931 patients).
At 3 years of follow-up, the primary end point – the rate of melanoma-specific survival – was the same in the immediate-dissection group as in the observation group (86%). Further analyses showed that no subgroup of patients, including those defined by tumor burden, showed a significant melanoma-specific benefit from immediate completion lymph node dissection. However, the immediate-dissection group had a significant disadvantage regarding adverse events; 24.1% developed lymphedema, compared with only 6.3% of the observation group.
Secondary end points slightly favored immediate dissection. At 3 years, the rate of disease-free survival was slightly higher in that group (68%) than in the observation group (63%), and the rate of disease control in the regional nodes was higher (92% vs. 77%). However, “differences with respect to the secondary end points must be interpreted with caution,” Dr. Faries and his associates said (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMoa1613210).
“Overall, some value may be derived from immediate-completion lymph node dissection with regard to staging and an increased rate of regional disease control. However, this value comes at the cost of increased complications,” the investigators said.
This study was supported by the National Cancer Institute, the Borstein Family Foundation, Amy’s Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the John Wayne Cancer Institute Auxiliary. Dr. Faries reported serving on advisory boards for Myriad Genetic Laboratories, Amgen, and Immune Design; his associates reported ties to numerous industry sources.
The findings of Dr. Faries and his associates are definitive, unequivocal, and completely consistent with previously published results of retrospective series and one other prospective randomized trial: Immediate completion lymph node dissection doesn’t increase melanoma-specific survival, compared with active ultrasound surveillance of the nodal basin.
These findings should be construed as practice changing.
It appears that in melanoma, as in so many other cancers, the elective removal of clinically negative nodes has rarely if ever been shown to improve disease-specific survival.
Daniel Coit, MD, is at Memorial Sloan Kettering Cancer Center in New York. He reported receiving personal fees for serving as an advisory board member for the MSLT-II trial. Dr. Coit made these remarks in an editorial accompanying Dr. Faries’ report (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMe1704290 ).
The findings of Dr. Faries and his associates are definitive, unequivocal, and completely consistent with previously published results of retrospective series and one other prospective randomized trial: Immediate completion lymph node dissection doesn’t increase melanoma-specific survival, compared with active ultrasound surveillance of the nodal basin.
These findings should be construed as practice changing.
It appears that in melanoma, as in so many other cancers, the elective removal of clinically negative nodes has rarely if ever been shown to improve disease-specific survival.
Daniel Coit, MD, is at Memorial Sloan Kettering Cancer Center in New York. He reported receiving personal fees for serving as an advisory board member for the MSLT-II trial. Dr. Coit made these remarks in an editorial accompanying Dr. Faries’ report (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMe1704290 ).
The findings of Dr. Faries and his associates are definitive, unequivocal, and completely consistent with previously published results of retrospective series and one other prospective randomized trial: Immediate completion lymph node dissection doesn’t increase melanoma-specific survival, compared with active ultrasound surveillance of the nodal basin.
These findings should be construed as practice changing.
It appears that in melanoma, as in so many other cancers, the elective removal of clinically negative nodes has rarely if ever been shown to improve disease-specific survival.
Daniel Coit, MD, is at Memorial Sloan Kettering Cancer Center in New York. He reported receiving personal fees for serving as an advisory board member for the MSLT-II trial. Dr. Coit made these remarks in an editorial accompanying Dr. Faries’ report (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMe1704290 ).
In patients who have melanoma with sentinel node metastasis, immediate-completion lymph node dissection doesn’t improve melanoma-specific survival, compared with nodal observation using ultrasound, according to a report published online June 8 in the New England Journal of Medicine.
Immediate-completion lymph node dissection – removal of the remaining regional lymph nodes after sentinel node excision – is usually recommended for patients found to have sentinel node metastasis, even though the evidence supporting this practice is inconclusive. A large prospective phase III trial was performed to compare outcomes with this approach against outcomes in patients who instead underwent observation using frequent nodal ultrasound and had lymph node dissection only if nodal recurrence developed, said Mark B. Faries, MD, of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
The second Multicenter Selective Lymphadenectomy Trial (MSLT-II) involved 1,939 adults at 63 medical centers who had clinically localized cutaneous melanoma of intermediate thickness, at least one tumor-positive sentinel node as determined by standard pathological assessment or a quantitative reverse transcriptase–polymerase chain reaction assay, and a life expectancy of 10 years or more. These participants were randomly assigned to immediate-completion node dissection (971 patients) or nodal observation (931 patients).
At 3 years of follow-up, the primary end point – the rate of melanoma-specific survival – was the same in the immediate-dissection group as in the observation group (86%). Further analyses showed that no subgroup of patients, including those defined by tumor burden, showed a significant melanoma-specific benefit from immediate completion lymph node dissection. However, the immediate-dissection group had a significant disadvantage regarding adverse events; 24.1% developed lymphedema, compared with only 6.3% of the observation group.
Secondary end points slightly favored immediate dissection. At 3 years, the rate of disease-free survival was slightly higher in that group (68%) than in the observation group (63%), and the rate of disease control in the regional nodes was higher (92% vs. 77%). However, “differences with respect to the secondary end points must be interpreted with caution,” Dr. Faries and his associates said (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMoa1613210).
“Overall, some value may be derived from immediate-completion lymph node dissection with regard to staging and an increased rate of regional disease control. However, this value comes at the cost of increased complications,” the investigators said.
This study was supported by the National Cancer Institute, the Borstein Family Foundation, Amy’s Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the John Wayne Cancer Institute Auxiliary. Dr. Faries reported serving on advisory boards for Myriad Genetic Laboratories, Amgen, and Immune Design; his associates reported ties to numerous industry sources.
In patients who have melanoma with sentinel node metastasis, immediate-completion lymph node dissection doesn’t improve melanoma-specific survival, compared with nodal observation using ultrasound, according to a report published online June 8 in the New England Journal of Medicine.
Immediate-completion lymph node dissection – removal of the remaining regional lymph nodes after sentinel node excision – is usually recommended for patients found to have sentinel node metastasis, even though the evidence supporting this practice is inconclusive. A large prospective phase III trial was performed to compare outcomes with this approach against outcomes in patients who instead underwent observation using frequent nodal ultrasound and had lymph node dissection only if nodal recurrence developed, said Mark B. Faries, MD, of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
The second Multicenter Selective Lymphadenectomy Trial (MSLT-II) involved 1,939 adults at 63 medical centers who had clinically localized cutaneous melanoma of intermediate thickness, at least one tumor-positive sentinel node as determined by standard pathological assessment or a quantitative reverse transcriptase–polymerase chain reaction assay, and a life expectancy of 10 years or more. These participants were randomly assigned to immediate-completion node dissection (971 patients) or nodal observation (931 patients).
At 3 years of follow-up, the primary end point – the rate of melanoma-specific survival – was the same in the immediate-dissection group as in the observation group (86%). Further analyses showed that no subgroup of patients, including those defined by tumor burden, showed a significant melanoma-specific benefit from immediate completion lymph node dissection. However, the immediate-dissection group had a significant disadvantage regarding adverse events; 24.1% developed lymphedema, compared with only 6.3% of the observation group.
Secondary end points slightly favored immediate dissection. At 3 years, the rate of disease-free survival was slightly higher in that group (68%) than in the observation group (63%), and the rate of disease control in the regional nodes was higher (92% vs. 77%). However, “differences with respect to the secondary end points must be interpreted with caution,” Dr. Faries and his associates said (N Engl J Med. 2017 Jun 8. doi: 10.1056/NEJMoa1613210).
“Overall, some value may be derived from immediate-completion lymph node dissection with regard to staging and an increased rate of regional disease control. However, this value comes at the cost of increased complications,” the investigators said.
This study was supported by the National Cancer Institute, the Borstein Family Foundation, Amy’s Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the John Wayne Cancer Institute Auxiliary. Dr. Faries reported serving on advisory boards for Myriad Genetic Laboratories, Amgen, and Immune Design; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: At 3 years of follow-up, the primary end point – the rate of melanoma-specific survival – was the same in the immediate-dissection group as in the observation group (86%).
Data source: A prospective international randomized phase-III trial involving 1,939 adults followed for a median of 43 months at 63 medical centers.
Disclosures: This study was supported by the National Cancer Institute, the Borstein Family Foundation, Amy’s Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the John Wayne Cancer Institute Auxiliary. Dr. Faries reported serving on advisory boards for Myriad Genetic Laboratories, Amgen, and Immune Design; his associates reported ties to numerous industry sources.
Weight gain in pregnancy: Too much and too little can be harmful
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
FROM JAMA
Key clinical point: Both high and low gestational weight gain are associated with increased risks of adverse perinatal outcomes.
Major finding: Low gestational weight gain was associated with a 5% higher risk of an SGA neonate and a 5% higher risk of preterm birth, while high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of an LGA neonate, and a 6% higher risk of macrosomia.
Data source: A systematic review and meta-analysis of 23 studies involving 1,309,136 pregnancies from diverse international cohorts.
Disclosures: The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
Cirrhosis linked to increased risk of stroke
reported online June 5 in JAMA Neurology.
Cirrhosis is known to be associated with “extrahepatic hemorrhagic and thrombotic processes, such as GI bleeding and venous thromboembolism. [But] the cerebrovascular complications of cirrhosis are comparatively less well understood.” Previous studies of the association with stroke have been small and have yielded conflicting results, with some finding a reduced incidence of stroke and others finding an increase among cirrhosis patients, said Neal S. Parikh, MD, of the Fell Family Brain and Mind Research Institute and Weill Cornell Medicine, both in New York, and his associates.
After the data were adjusted to account for stroke risk factors, relevant comorbidities, and demographic traits, the annual incidence of any type of stroke was significantly higher with cirrhosis than without cirrhosis (hazard ratio, 1.4). The association was stronger for intracranial hemorrhage (HR, 1.9) and subarachnoid hemorrhage (HR, 2.4) than for ischemic stroke (HR, 1.3).
The results of several secondary and sensitivity analyses were consistent with those of the primary analysis, regardless of whether the cirrhosis was alcohol-related or the stroke was fatal. The association was strongest among patients who had decompensated cirrhosis and was not evident at all among patients who had mild liver disease, Dr. Parikh and his associates said (JAMA Neurol. 2017 Jun 5 [doi: 10.1001/jamaneurol.2017.0923).
This study was not designed to explore the reasons for an association between cirrhosis and stroke, but the investigators noted many possible explanations. First, “cirrhosis is accompanied by a mixed coagulopathy, with potential implications for hemorrhagic and thrombotic processes.” It has been linked to many bleeding complications, including, most recently, cerebral microhemorrhages detectable on brain MRI. In addition, the underlying causes of cirrhosis, including alcohol abuse, hepatitis infection, and metabolic disease, may also contribute to stroke risk.
Alternatively, clinicians caring for patients with cirrhosis “may limit the aggressiveness of stroke prevention” – for example, by limiting antithrombotic medications or statins – because they are mindful of the patient’s increased risk of bleeding and hepatic toxicity, the investigators said.
reported online June 5 in JAMA Neurology.
Cirrhosis is known to be associated with “extrahepatic hemorrhagic and thrombotic processes, such as GI bleeding and venous thromboembolism. [But] the cerebrovascular complications of cirrhosis are comparatively less well understood.” Previous studies of the association with stroke have been small and have yielded conflicting results, with some finding a reduced incidence of stroke and others finding an increase among cirrhosis patients, said Neal S. Parikh, MD, of the Fell Family Brain and Mind Research Institute and Weill Cornell Medicine, both in New York, and his associates.
After the data were adjusted to account for stroke risk factors, relevant comorbidities, and demographic traits, the annual incidence of any type of stroke was significantly higher with cirrhosis than without cirrhosis (hazard ratio, 1.4). The association was stronger for intracranial hemorrhage (HR, 1.9) and subarachnoid hemorrhage (HR, 2.4) than for ischemic stroke (HR, 1.3).
The results of several secondary and sensitivity analyses were consistent with those of the primary analysis, regardless of whether the cirrhosis was alcohol-related or the stroke was fatal. The association was strongest among patients who had decompensated cirrhosis and was not evident at all among patients who had mild liver disease, Dr. Parikh and his associates said (JAMA Neurol. 2017 Jun 5 [doi: 10.1001/jamaneurol.2017.0923).
This study was not designed to explore the reasons for an association between cirrhosis and stroke, but the investigators noted many possible explanations. First, “cirrhosis is accompanied by a mixed coagulopathy, with potential implications for hemorrhagic and thrombotic processes.” It has been linked to many bleeding complications, including, most recently, cerebral microhemorrhages detectable on brain MRI. In addition, the underlying causes of cirrhosis, including alcohol abuse, hepatitis infection, and metabolic disease, may also contribute to stroke risk.
Alternatively, clinicians caring for patients with cirrhosis “may limit the aggressiveness of stroke prevention” – for example, by limiting antithrombotic medications or statins – because they are mindful of the patient’s increased risk of bleeding and hepatic toxicity, the investigators said.
reported online June 5 in JAMA Neurology.
Cirrhosis is known to be associated with “extrahepatic hemorrhagic and thrombotic processes, such as GI bleeding and venous thromboembolism. [But] the cerebrovascular complications of cirrhosis are comparatively less well understood.” Previous studies of the association with stroke have been small and have yielded conflicting results, with some finding a reduced incidence of stroke and others finding an increase among cirrhosis patients, said Neal S. Parikh, MD, of the Fell Family Brain and Mind Research Institute and Weill Cornell Medicine, both in New York, and his associates.
After the data were adjusted to account for stroke risk factors, relevant comorbidities, and demographic traits, the annual incidence of any type of stroke was significantly higher with cirrhosis than without cirrhosis (hazard ratio, 1.4). The association was stronger for intracranial hemorrhage (HR, 1.9) and subarachnoid hemorrhage (HR, 2.4) than for ischemic stroke (HR, 1.3).
The results of several secondary and sensitivity analyses were consistent with those of the primary analysis, regardless of whether the cirrhosis was alcohol-related or the stroke was fatal. The association was strongest among patients who had decompensated cirrhosis and was not evident at all among patients who had mild liver disease, Dr. Parikh and his associates said (JAMA Neurol. 2017 Jun 5 [doi: 10.1001/jamaneurol.2017.0923).
This study was not designed to explore the reasons for an association between cirrhosis and stroke, but the investigators noted many possible explanations. First, “cirrhosis is accompanied by a mixed coagulopathy, with potential implications for hemorrhagic and thrombotic processes.” It has been linked to many bleeding complications, including, most recently, cerebral microhemorrhages detectable on brain MRI. In addition, the underlying causes of cirrhosis, including alcohol abuse, hepatitis infection, and metabolic disease, may also contribute to stroke risk.
Alternatively, clinicians caring for patients with cirrhosis “may limit the aggressiveness of stroke prevention” – for example, by limiting antithrombotic medications or statins – because they are mindful of the patient’s increased risk of bleeding and hepatic toxicity, the investigators said.
FROM JAMA NEUROLOGY
Key clinical point: Cirrhosis was associated with an increased risk of stroke, especially hemorrhagic stroke, in a large nationally representative cohort study.
Major finding: The annual incidence of any type of stroke was significantly higher with cirrhosis than without cirrhosis (HR, 1.4), and this association was stronger for intracranial hemorrhage (HR, 1.9) and subarachnoid hemorrhage (HR, 2.4) than for ischemic stroke (HR, 1.3).
Data source: A retrospective cohort study involving a nationally representative sample of 1.6 million Medicare patients.
Disclosures: This study was supported by the National Institute of Neurological Disorders and Stroke and the Florence Gould Endowment for Discovery in Stroke. Dr. Parikh and his associates reported having no relevant financial disclosures.
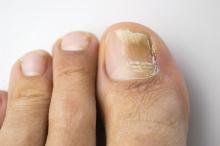
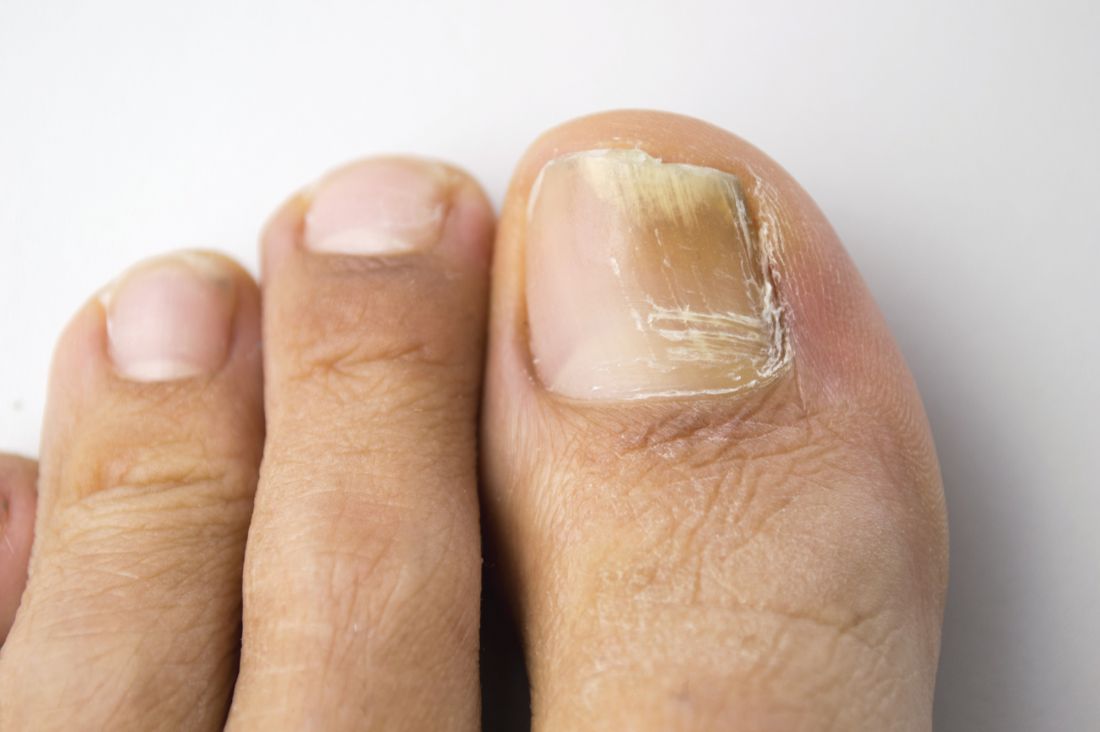
Direct microscopy plus nail clipping identifies onychomycosis
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
FROM MYCOSES
Key clinical point: In the absence of a typical clinical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis.
Major finding: Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis, while histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases.
Data source: A single-center prospective cross-sectional study involving 212 adults suspected of having onychomycosis during a 2-year period.
Disclosures: The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
Minocycline may delay conversion to MS
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The primary outcome, conversion to MS within 6 months of randomization, occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo.
Data source: A multicenter, randomized, double-blind, placebo-controlled trial involving 142 adults treated for up to 24 months.
Disclosures: This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
Large-scale ERAS program reduces postoperative LOS, complications
Enhanced Recovery After Surgery (ERAS), a program implemented by Kaiser Permanente Northern California – a multihospital integrated health system – significantly reduced length of stay and complication rates, according to a report published in JAMA Surgery.
Beginning in 2014, when the ERAS program was implemented in 20 Kaiser hospitals, progress was made on the goal of improving inpatient safety, as well as improvements in-hospital mortality, rates of early ambulation, patient nutrition, and reduced opioid use, said Vincent X. Liu, MD, of the division of research, Kaiser Permanente Oakland, and his associates. Those outcomes were studied in the context of a similar group of patients in other, non-ERAS hospitals to determine the degree of change in each area.
ERAS aimed to reduce opioid use by encouraging multimodal analgesia, which included pre- and postoperative IV acetaminophen and NSAIDs, perioperative IV lidocaine, or peripheral nerve blocks. It encouraged ambulation within 12 hours of surgery completion and a daily goal of walking at least 21 feet during the first 3 postoperative days.
The program enhanced patient nutrition by reducing prolonged preoperative fasting, providing a high-carbohydrate beverage 2-4 hours before surgery, and allowing solids 8-12 hours before surgery. It also provided food within 12 hours of completing surgery. ERAS also encouraged patient engagement in care by use of educational materials and a calendar that detailed what the care process would entail. For clinicians, ERAS provided new electronic tools such as electronic medical record order sets to facilitate standardized practice.
In the first phase of their study, Dr. Liu and his associates assessed changes over time in patient safety outcomes among 3,768 patients undergoing elective colorectal resection and 5,002 undergoing emergency hip fracture repair.
Hospital length of stay decreased significantly after implementation of ERAS, from 5.1 to 4.2 days in the colorectal resection group and from 3.6 to 3.2 days in the hip fracture group. Complication rates decreased from 18.1% to 14.7% and from 30.8% to 24.9%, respectively. Early ambulation rates increased substantially, from 22.3% to 56.5% and from 2.8% to 21.2%, respectively.
The rate of improved nutrition rose from 13.0% to 39.2% in the colorectal resection group and from 45.6% to 57.1% in the hip repair group. And the total dose of morphine equivalents dropped from 52.4 to 30.6 and from 38.9 to 27.0, respectively (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1032).
In the second phase of the study, the investigators compared these changes against the outcomes of two comparator groups who underwent similar surgeries (5,556 resection comparators and 1,523 hip repair comparators) during the same time frame but in hospitals that did not implement the ERAS program.
In this analysis, LOS was significantly shorter and complication rates were significantly lower for both procedures at the hospitals where the intervention was implemented, compared with the other hospitals. In-hospital mortality, opioid use, early ambulation, and discharge to home rather than a rehabilitation facility also favored the intervention groups.
“This study demonstrates the effectiveness of a systems-level approach to ERAS program implementation, even across widely divergent target populations,” Dr. Liu and his associates said.
The Gordon and Betty Moore Foundation, the Permanente Medical Group, the Kaiser Foundation Health Plan, and the National Institutes of Health funded the study. Dr. Liu and his associates reported having no relevant financial disclosures.
Findings from Liu et al. have clinical, research, and policy relevance. First, they went beyond select surgical procedures from single hospitals. The investigators have robustly taken implementation science to the next level, thus showing that thoughtfully planned quality-improvement endeavors that are integrated with robust research evaluation measures can positively affect our surgical patients. In a similar vein, these results underscore the value proposition of research conducted in large health care systems that goes beyond the limitation of traditional stand-alone hospitals, such as small sample size and referral and practice biases. [In addition,] this investigation raises many and exciting future research opportunities to an eager audience of stakeholders. What are the cost implications of such efforts? How can we better leverage electronic health records with smart tools to better implement and measure the effects of the ERAS program and other quality and safety initiatives? [We also] need to be mindful of its unintended consequences on vulnerable populations and financially strained hospitals.
Mohammed Bayasi, MD, FACS, and Waddah Al-Refaie, MD, FACS, are with the department of surgery, MedStar Georgetown University Hospital, Washington. Their comments are from an editorial (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1051). They had no disclosures.
Findings from Liu et al. have clinical, research, and policy relevance. First, they went beyond select surgical procedures from single hospitals. The investigators have robustly taken implementation science to the next level, thus showing that thoughtfully planned quality-improvement endeavors that are integrated with robust research evaluation measures can positively affect our surgical patients. In a similar vein, these results underscore the value proposition of research conducted in large health care systems that goes beyond the limitation of traditional stand-alone hospitals, such as small sample size and referral and practice biases. [In addition,] this investigation raises many and exciting future research opportunities to an eager audience of stakeholders. What are the cost implications of such efforts? How can we better leverage electronic health records with smart tools to better implement and measure the effects of the ERAS program and other quality and safety initiatives? [We also] need to be mindful of its unintended consequences on vulnerable populations and financially strained hospitals.
Mohammed Bayasi, MD, FACS, and Waddah Al-Refaie, MD, FACS, are with the department of surgery, MedStar Georgetown University Hospital, Washington. Their comments are from an editorial (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1051). They had no disclosures.
Findings from Liu et al. have clinical, research, and policy relevance. First, they went beyond select surgical procedures from single hospitals. The investigators have robustly taken implementation science to the next level, thus showing that thoughtfully planned quality-improvement endeavors that are integrated with robust research evaluation measures can positively affect our surgical patients. In a similar vein, these results underscore the value proposition of research conducted in large health care systems that goes beyond the limitation of traditional stand-alone hospitals, such as small sample size and referral and practice biases. [In addition,] this investigation raises many and exciting future research opportunities to an eager audience of stakeholders. What are the cost implications of such efforts? How can we better leverage electronic health records with smart tools to better implement and measure the effects of the ERAS program and other quality and safety initiatives? [We also] need to be mindful of its unintended consequences on vulnerable populations and financially strained hospitals.
Mohammed Bayasi, MD, FACS, and Waddah Al-Refaie, MD, FACS, are with the department of surgery, MedStar Georgetown University Hospital, Washington. Their comments are from an editorial (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1051). They had no disclosures.
Enhanced Recovery After Surgery (ERAS), a program implemented by Kaiser Permanente Northern California – a multihospital integrated health system – significantly reduced length of stay and complication rates, according to a report published in JAMA Surgery.
Beginning in 2014, when the ERAS program was implemented in 20 Kaiser hospitals, progress was made on the goal of improving inpatient safety, as well as improvements in-hospital mortality, rates of early ambulation, patient nutrition, and reduced opioid use, said Vincent X. Liu, MD, of the division of research, Kaiser Permanente Oakland, and his associates. Those outcomes were studied in the context of a similar group of patients in other, non-ERAS hospitals to determine the degree of change in each area.
ERAS aimed to reduce opioid use by encouraging multimodal analgesia, which included pre- and postoperative IV acetaminophen and NSAIDs, perioperative IV lidocaine, or peripheral nerve blocks. It encouraged ambulation within 12 hours of surgery completion and a daily goal of walking at least 21 feet during the first 3 postoperative days.
The program enhanced patient nutrition by reducing prolonged preoperative fasting, providing a high-carbohydrate beverage 2-4 hours before surgery, and allowing solids 8-12 hours before surgery. It also provided food within 12 hours of completing surgery. ERAS also encouraged patient engagement in care by use of educational materials and a calendar that detailed what the care process would entail. For clinicians, ERAS provided new electronic tools such as electronic medical record order sets to facilitate standardized practice.
In the first phase of their study, Dr. Liu and his associates assessed changes over time in patient safety outcomes among 3,768 patients undergoing elective colorectal resection and 5,002 undergoing emergency hip fracture repair.
Hospital length of stay decreased significantly after implementation of ERAS, from 5.1 to 4.2 days in the colorectal resection group and from 3.6 to 3.2 days in the hip fracture group. Complication rates decreased from 18.1% to 14.7% and from 30.8% to 24.9%, respectively. Early ambulation rates increased substantially, from 22.3% to 56.5% and from 2.8% to 21.2%, respectively.
The rate of improved nutrition rose from 13.0% to 39.2% in the colorectal resection group and from 45.6% to 57.1% in the hip repair group. And the total dose of morphine equivalents dropped from 52.4 to 30.6 and from 38.9 to 27.0, respectively (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1032).
In the second phase of the study, the investigators compared these changes against the outcomes of two comparator groups who underwent similar surgeries (5,556 resection comparators and 1,523 hip repair comparators) during the same time frame but in hospitals that did not implement the ERAS program.
In this analysis, LOS was significantly shorter and complication rates were significantly lower for both procedures at the hospitals where the intervention was implemented, compared with the other hospitals. In-hospital mortality, opioid use, early ambulation, and discharge to home rather than a rehabilitation facility also favored the intervention groups.
“This study demonstrates the effectiveness of a systems-level approach to ERAS program implementation, even across widely divergent target populations,” Dr. Liu and his associates said.
The Gordon and Betty Moore Foundation, the Permanente Medical Group, the Kaiser Foundation Health Plan, and the National Institutes of Health funded the study. Dr. Liu and his associates reported having no relevant financial disclosures.
Enhanced Recovery After Surgery (ERAS), a program implemented by Kaiser Permanente Northern California – a multihospital integrated health system – significantly reduced length of stay and complication rates, according to a report published in JAMA Surgery.
Beginning in 2014, when the ERAS program was implemented in 20 Kaiser hospitals, progress was made on the goal of improving inpatient safety, as well as improvements in-hospital mortality, rates of early ambulation, patient nutrition, and reduced opioid use, said Vincent X. Liu, MD, of the division of research, Kaiser Permanente Oakland, and his associates. Those outcomes were studied in the context of a similar group of patients in other, non-ERAS hospitals to determine the degree of change in each area.
ERAS aimed to reduce opioid use by encouraging multimodal analgesia, which included pre- and postoperative IV acetaminophen and NSAIDs, perioperative IV lidocaine, or peripheral nerve blocks. It encouraged ambulation within 12 hours of surgery completion and a daily goal of walking at least 21 feet during the first 3 postoperative days.
The program enhanced patient nutrition by reducing prolonged preoperative fasting, providing a high-carbohydrate beverage 2-4 hours before surgery, and allowing solids 8-12 hours before surgery. It also provided food within 12 hours of completing surgery. ERAS also encouraged patient engagement in care by use of educational materials and a calendar that detailed what the care process would entail. For clinicians, ERAS provided new electronic tools such as electronic medical record order sets to facilitate standardized practice.
In the first phase of their study, Dr. Liu and his associates assessed changes over time in patient safety outcomes among 3,768 patients undergoing elective colorectal resection and 5,002 undergoing emergency hip fracture repair.
Hospital length of stay decreased significantly after implementation of ERAS, from 5.1 to 4.2 days in the colorectal resection group and from 3.6 to 3.2 days in the hip fracture group. Complication rates decreased from 18.1% to 14.7% and from 30.8% to 24.9%, respectively. Early ambulation rates increased substantially, from 22.3% to 56.5% and from 2.8% to 21.2%, respectively.
The rate of improved nutrition rose from 13.0% to 39.2% in the colorectal resection group and from 45.6% to 57.1% in the hip repair group. And the total dose of morphine equivalents dropped from 52.4 to 30.6 and from 38.9 to 27.0, respectively (JAMA Surg. 2017 May 10. doi: 10.1001/jamasurg.2017.1032).
In the second phase of the study, the investigators compared these changes against the outcomes of two comparator groups who underwent similar surgeries (5,556 resection comparators and 1,523 hip repair comparators) during the same time frame but in hospitals that did not implement the ERAS program.
In this analysis, LOS was significantly shorter and complication rates were significantly lower for both procedures at the hospitals where the intervention was implemented, compared with the other hospitals. In-hospital mortality, opioid use, early ambulation, and discharge to home rather than a rehabilitation facility also favored the intervention groups.
“This study demonstrates the effectiveness of a systems-level approach to ERAS program implementation, even across widely divergent target populations,” Dr. Liu and his associates said.
The Gordon and Betty Moore Foundation, the Permanente Medical Group, the Kaiser Foundation Health Plan, and the National Institutes of Health funded the study. Dr. Liu and his associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: An Enhanced Recovery After Surgery program aimed at improving inpatient safety significantly reduced length of stay and complication rates at 20 California hospitals.
Major finding: After the ERAS program was implemented, hospital LOS decreased from 5.1 to 4.2 days in the colorectal resection group and from 3.6 to 3.2 days in the hip fracture group, and complication rates decreased from 18.1% to 14.7% and from 30.8% to 24.9%, respectively.
Data source: A “pre-post” comparison study of patients’ safety outcomes after implementation of an ERAS program, which involved 15,849 surgical patients at 20 hospitals.
Disclosures: The Gordon and Betty Moore Foundation, the Permanente Medical Group, the Kaiser Foundation Health Plan, and the National Institutes of Health funded the study. Dr. Liu and his associates reported having no relevant financial disclosures.
Framingham: Arterial stiffening not inevitable with aging
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
FROM HYPERTENSION
Key clinical point: Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 years and older participating in certain cohorts of the Framingham Heart Study.
Major finding: The prevalence of healthy vascular aging was 18% overall, 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older.
Data source: A secondary analysis of data accumulated for 3,196 adults participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study.
Disclosures: The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
Biologics after methotrexate fails: Huge cost, minimal benefit
Prescribing rheumatoid arthritis patients a biologic agent rather than triple therapy, when they fail to respond adequately to methotrexate, costs more than half a million dollars per quality-adjusted life year gained over a lifetime but provides only a minimal health benefit beyond triple therapy, according to a report published online May 30 in Annals of Internal Medicine.
The evidence is clear that triple therapy using sulfasalazine, hydroxychloroquine, and methotrexate is at least as effective as methotrexate plus an anti–tumor necrosis factor biologic (etanercept, adalimumab, infliximab, golimumab, or certolizumab pegol). Nevertheless, few RA patients are transitioned to triple therapy before escalating to biologics. This occurred in only 2.5% of patients in one Veterans Affairs study, said Nick Bansback, PhD, of the University of British Columbia and St. Paul’s Hospital, Vancouver, and his associates.
Dr. Bansback and his colleagues performed a cost-effectiveness study of proceeding directly to etanercept when RA fails to respond to methotrexate, using data from the Rheumatoid Arthritis Comparison of Active Therapies (RACAT) trial. The RACAT study, a large international, randomized, double-blind trial, confirmed the clinical noninferiority of triple therapy versus etanercept plus methotrexate. In their analysis, Dr. Bansback and his associates calculated the costs and QALYs for both treatment strategies for 324 RACAT participants.
Switching directly to etanercept-methotrexate instead of triple therapy provided only a marginal advantage in QALYs, a difference of only 0.004 QALY over 24 weeks of treatment (0.358 with etanercept-methotrexate vs. 0.353 with triple therapy) and of only 0.016 QALY over 48 weeks of treatment (0.743 with etanercept-methotrexate vs. 0.726 with triple therapy). The associated costs were $11,295 for 24 weeks of etanercept-methotrexate, vs. $343 for 24 weeks of triple therapy, and $19,634 for 48 weeks of etanercept-methotrexate, vs. $3,680 for 48 weeks of triple therapy.
“The resultant ICER [incremental cost-effectiveness ratio] for first-line etanercept-methotrexate, vs. triple therapy, was $2.7 million per QALY gained over 24 weeks,” the investigators said (Ann Intern Med. 2017 May 29. doi: 10.7326/M16-0713).
When they extrapolated the data to determine the cost-effectiveness of the two treatment strategies over the course of the average patient’s lifetime, the model predicted an ICER of $521,520 per QALY gained for etanercept-methotrexate instead of triple therapy. These findings remained robust in numerous sensitivity and scenario analyses. For example, when the model assumed that radiographic and quality of life benefits of triple therapy were far lower than observed in the RACAT trial and far lower than what has been reported in the literature and that the tolerability of triple therapy was far worse than observed in the RACAT trial and reported in the literature, switching to etanercept-methotrexate instead of triple therapy still predicted an ICER of $350,000 per QALY per patient.
All of these ICERs far exceed the standard cost-effectiveness acceptability threshold ICER of $100,000 per QALY, Dr. Bansback and his associates noted.
This study demonstrates the substantial cost savings of prescribing triple therapy before a biologic. It shows that “for every patient who tries triple therapy before a biologic, payers will save an average of $78,000 over the patient’s lifetime, and most of the savings will accrue within the first 10 years,” the investigators wrote.
“Patients who receive triple therapy before a biologic will miss out on a benefit of approximately 0.15 QALY over their lifetime or a benefit of approximately 0.05 HAQ [Health Assessment Questionnaire] point at any point in time. To put these numbers in perspective, total hip arthroplasty in a patient with osteoarthritis who is approximately the same age as an average patient with RA provides an additional 6.9 QALYs. In terms of HAQ score, only differences greater than 0.2 points are considered minimally important to patients,” Dr. Bansback and his associates wrote.
Changing health care policy to require, rather than to just recommend, that triple therapy be prescribed before biologics would save millions of dollars in health care expenditures, they added.
This study was supported by the U.S. Department of Veterans Affairs Office of Research and Development, the Canadian Institutes for Health Research, the National Institutes of Health, and the American Recovery and Reinvestment Act. Dr. Bansback reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
The findings of Bansback et al., consistent with those of several other researchers, indicate that patients who have RA and no contraindications to triple-conventional, disease-modifying antirheumatic drug therapy should use therapy instead of biologics as the next regimen if methotrexate alone fails to control symptoms and radiographic progression.
Elena Losina, PhD, and Jeffrey N. Katz, MD, are at Brigham and Women’s Hospital in Boston. They disclosed having no conflicts of interest. Dr. Losina and Dr. Katz made these remarks in an editorial accompanying Dr. Bansback’s report (Ann Intern Med. 2017 May 29. doi: 10.7326/M17-1176).
The findings of Bansback et al., consistent with those of several other researchers, indicate that patients who have RA and no contraindications to triple-conventional, disease-modifying antirheumatic drug therapy should use therapy instead of biologics as the next regimen if methotrexate alone fails to control symptoms and radiographic progression.
Elena Losina, PhD, and Jeffrey N. Katz, MD, are at Brigham and Women’s Hospital in Boston. They disclosed having no conflicts of interest. Dr. Losina and Dr. Katz made these remarks in an editorial accompanying Dr. Bansback’s report (Ann Intern Med. 2017 May 29. doi: 10.7326/M17-1176).
The findings of Bansback et al., consistent with those of several other researchers, indicate that patients who have RA and no contraindications to triple-conventional, disease-modifying antirheumatic drug therapy should use therapy instead of biologics as the next regimen if methotrexate alone fails to control symptoms and radiographic progression.
Elena Losina, PhD, and Jeffrey N. Katz, MD, are at Brigham and Women’s Hospital in Boston. They disclosed having no conflicts of interest. Dr. Losina and Dr. Katz made these remarks in an editorial accompanying Dr. Bansback’s report (Ann Intern Med. 2017 May 29. doi: 10.7326/M17-1176).
Prescribing rheumatoid arthritis patients a biologic agent rather than triple therapy, when they fail to respond adequately to methotrexate, costs more than half a million dollars per quality-adjusted life year gained over a lifetime but provides only a minimal health benefit beyond triple therapy, according to a report published online May 30 in Annals of Internal Medicine.
The evidence is clear that triple therapy using sulfasalazine, hydroxychloroquine, and methotrexate is at least as effective as methotrexate plus an anti–tumor necrosis factor biologic (etanercept, adalimumab, infliximab, golimumab, or certolizumab pegol). Nevertheless, few RA patients are transitioned to triple therapy before escalating to biologics. This occurred in only 2.5% of patients in one Veterans Affairs study, said Nick Bansback, PhD, of the University of British Columbia and St. Paul’s Hospital, Vancouver, and his associates.
Dr. Bansback and his colleagues performed a cost-effectiveness study of proceeding directly to etanercept when RA fails to respond to methotrexate, using data from the Rheumatoid Arthritis Comparison of Active Therapies (RACAT) trial. The RACAT study, a large international, randomized, double-blind trial, confirmed the clinical noninferiority of triple therapy versus etanercept plus methotrexate. In their analysis, Dr. Bansback and his associates calculated the costs and QALYs for both treatment strategies for 324 RACAT participants.
Switching directly to etanercept-methotrexate instead of triple therapy provided only a marginal advantage in QALYs, a difference of only 0.004 QALY over 24 weeks of treatment (0.358 with etanercept-methotrexate vs. 0.353 with triple therapy) and of only 0.016 QALY over 48 weeks of treatment (0.743 with etanercept-methotrexate vs. 0.726 with triple therapy). The associated costs were $11,295 for 24 weeks of etanercept-methotrexate, vs. $343 for 24 weeks of triple therapy, and $19,634 for 48 weeks of etanercept-methotrexate, vs. $3,680 for 48 weeks of triple therapy.
“The resultant ICER [incremental cost-effectiveness ratio] for first-line etanercept-methotrexate, vs. triple therapy, was $2.7 million per QALY gained over 24 weeks,” the investigators said (Ann Intern Med. 2017 May 29. doi: 10.7326/M16-0713).
When they extrapolated the data to determine the cost-effectiveness of the two treatment strategies over the course of the average patient’s lifetime, the model predicted an ICER of $521,520 per QALY gained for etanercept-methotrexate instead of triple therapy. These findings remained robust in numerous sensitivity and scenario analyses. For example, when the model assumed that radiographic and quality of life benefits of triple therapy were far lower than observed in the RACAT trial and far lower than what has been reported in the literature and that the tolerability of triple therapy was far worse than observed in the RACAT trial and reported in the literature, switching to etanercept-methotrexate instead of triple therapy still predicted an ICER of $350,000 per QALY per patient.
All of these ICERs far exceed the standard cost-effectiveness acceptability threshold ICER of $100,000 per QALY, Dr. Bansback and his associates noted.
This study demonstrates the substantial cost savings of prescribing triple therapy before a biologic. It shows that “for every patient who tries triple therapy before a biologic, payers will save an average of $78,000 over the patient’s lifetime, and most of the savings will accrue within the first 10 years,” the investigators wrote.
“Patients who receive triple therapy before a biologic will miss out on a benefit of approximately 0.15 QALY over their lifetime or a benefit of approximately 0.05 HAQ [Health Assessment Questionnaire] point at any point in time. To put these numbers in perspective, total hip arthroplasty in a patient with osteoarthritis who is approximately the same age as an average patient with RA provides an additional 6.9 QALYs. In terms of HAQ score, only differences greater than 0.2 points are considered minimally important to patients,” Dr. Bansback and his associates wrote.
Changing health care policy to require, rather than to just recommend, that triple therapy be prescribed before biologics would save millions of dollars in health care expenditures, they added.
This study was supported by the U.S. Department of Veterans Affairs Office of Research and Development, the Canadian Institutes for Health Research, the National Institutes of Health, and the American Recovery and Reinvestment Act. Dr. Bansback reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
Prescribing rheumatoid arthritis patients a biologic agent rather than triple therapy, when they fail to respond adequately to methotrexate, costs more than half a million dollars per quality-adjusted life year gained over a lifetime but provides only a minimal health benefit beyond triple therapy, according to a report published online May 30 in Annals of Internal Medicine.
The evidence is clear that triple therapy using sulfasalazine, hydroxychloroquine, and methotrexate is at least as effective as methotrexate plus an anti–tumor necrosis factor biologic (etanercept, adalimumab, infliximab, golimumab, or certolizumab pegol). Nevertheless, few RA patients are transitioned to triple therapy before escalating to biologics. This occurred in only 2.5% of patients in one Veterans Affairs study, said Nick Bansback, PhD, of the University of British Columbia and St. Paul’s Hospital, Vancouver, and his associates.
Dr. Bansback and his colleagues performed a cost-effectiveness study of proceeding directly to etanercept when RA fails to respond to methotrexate, using data from the Rheumatoid Arthritis Comparison of Active Therapies (RACAT) trial. The RACAT study, a large international, randomized, double-blind trial, confirmed the clinical noninferiority of triple therapy versus etanercept plus methotrexate. In their analysis, Dr. Bansback and his associates calculated the costs and QALYs for both treatment strategies for 324 RACAT participants.
Switching directly to etanercept-methotrexate instead of triple therapy provided only a marginal advantage in QALYs, a difference of only 0.004 QALY over 24 weeks of treatment (0.358 with etanercept-methotrexate vs. 0.353 with triple therapy) and of only 0.016 QALY over 48 weeks of treatment (0.743 with etanercept-methotrexate vs. 0.726 with triple therapy). The associated costs were $11,295 for 24 weeks of etanercept-methotrexate, vs. $343 for 24 weeks of triple therapy, and $19,634 for 48 weeks of etanercept-methotrexate, vs. $3,680 for 48 weeks of triple therapy.
“The resultant ICER [incremental cost-effectiveness ratio] for first-line etanercept-methotrexate, vs. triple therapy, was $2.7 million per QALY gained over 24 weeks,” the investigators said (Ann Intern Med. 2017 May 29. doi: 10.7326/M16-0713).
When they extrapolated the data to determine the cost-effectiveness of the two treatment strategies over the course of the average patient’s lifetime, the model predicted an ICER of $521,520 per QALY gained for etanercept-methotrexate instead of triple therapy. These findings remained robust in numerous sensitivity and scenario analyses. For example, when the model assumed that radiographic and quality of life benefits of triple therapy were far lower than observed in the RACAT trial and far lower than what has been reported in the literature and that the tolerability of triple therapy was far worse than observed in the RACAT trial and reported in the literature, switching to etanercept-methotrexate instead of triple therapy still predicted an ICER of $350,000 per QALY per patient.
All of these ICERs far exceed the standard cost-effectiveness acceptability threshold ICER of $100,000 per QALY, Dr. Bansback and his associates noted.
This study demonstrates the substantial cost savings of prescribing triple therapy before a biologic. It shows that “for every patient who tries triple therapy before a biologic, payers will save an average of $78,000 over the patient’s lifetime, and most of the savings will accrue within the first 10 years,” the investigators wrote.
“Patients who receive triple therapy before a biologic will miss out on a benefit of approximately 0.15 QALY over their lifetime or a benefit of approximately 0.05 HAQ [Health Assessment Questionnaire] point at any point in time. To put these numbers in perspective, total hip arthroplasty in a patient with osteoarthritis who is approximately the same age as an average patient with RA provides an additional 6.9 QALYs. In terms of HAQ score, only differences greater than 0.2 points are considered minimally important to patients,” Dr. Bansback and his associates wrote.
Changing health care policy to require, rather than to just recommend, that triple therapy be prescribed before biologics would save millions of dollars in health care expenditures, they added.
This study was supported by the U.S. Department of Veterans Affairs Office of Research and Development, the Canadian Institutes for Health Research, the National Institutes of Health, and the American Recovery and Reinvestment Act. Dr. Bansback reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: For every RA patient who tries triple therapy before a biologic, payers will save an average of $78,000 over the patient’s lifetime without sacrificing their health or quality of life.
Data source: A cost-effectiveness analysis using data from 324 participants in an international, randomized, double-blind trial.
Disclosures: This study was supported by the U.S. Department of Veterans Affairs Office of Research and Development, the Canadian Institutes for Health Research, the National Institutes of Health, and the American Recovery and Reinvestment Act. Dr. Bansback reported having no relevant financial disclosures. His associates reported ties to numerous industry sources.
Statins: No benefit as primary prevention in elderly
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes.
Major finding: For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care (HR, 1.08), and for those aged 75 years and older, there were 92 deaths with pravastatin vs. 65 with usual care (HR, 1.34).
Data source: A secondary analysis of data in the randomized, open-label ALLHAT trial, focusing on 2,867 elderly participants followed for 6 years.
Disclosures: This work was supported by the National Institutes of Health. Pfizer, AstraZeneca, and Bristol-Myers Squibb provided study medications free of charge. Dr. Han and his associates reported having no relevant financial disclosures.
Mepolizumab proves effective for eosinophilic granulomatosis with polyangiitis
Adding mepolizumab to standard-of-care glucocorticoids with or without immunosuppressive agents can induce remission in many patients who have eosinophilic granulomatosis with polyangiitis (EGPA), according to a report published online May 18 in the New England Journal of Medicine.
EGPA, a rare disorder characterized by asthma, sinusitis, pulmonary infiltrates, neuropathy, and eosinophilic vasculitis in at least one end-organ, frequently relapses despite glucocorticoid therapy or fails to respond adequately to the treatment. Patients have elevated levels of the cytokine interleukin-5, which regulates eosinophil maturation, differentiation, and proliferation. Neutralizing this cytokine is thought to be a potential therapeutic approach, said Michael E. Wechsler, MD, of National Jewish Health, Denver, and his associates.
Proof-of-concept studies have demonstrated the efficacy of subcutaneous mepolizumab, an anti–interleukin-5 monoclonal antibody, in EGPA, so Dr. Wechsler and his colleagues assessed the safety and efficacy of a 1-year course of mepolizumab (300 mg) as add-on therapy in a double-blind, randomized, phase III trial, which involved 136 adults treated at 31 academic medical centers in nine countries. The study was sponsored by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases.
The first of two primary efficacy endpoints was the total accrued weeks of remission. A total of 28% of the mepolizumab group achieved remission for at least 24 weeks, compared with only 3% of the placebo group, for an odds ratio of 5.91.
The second primary efficacy endpoint was the proportion of patients in remission at both week 36 and week 48. Again, significantly more patients in the mepolizumab group (32%) than in the placebo group (3%) met this end point (OR, 16.74).
Mepolizumab also proved superior to placebo regarding numerous secondary endpoints, the investigators said (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1702079). More patients who received active treatment achieved remission within the first 6 months of treatment and remained in remission for a full year (19% vs. 1%; OR, 19.65). The time to first relapse was significantly longer for mepolizumab, with only 56% of that group experiencing a relapse within 1 year, compared with 82% of the placebo group. The annualized relapse rate was half as high with mepolizumab (1.14) as with placebo (2.27).
In addition, patients in the mepolizumab group were more likely to reduce their doses of glucocorticoids (OR, 0.20) or discontinue the drugs altogether (18% vs. 3% taking placebo).
Mepolizumab was most effective among the 79 patients who had a high absolute eosinophil count (150 or more cells per cubic millimeter) at baseline. In this subgroup, 33% of patients taking mepolizumab achieved remission for 6 months or more, compared with none of the patients taking placebo (OR, 26.1).
Although the effectiveness of mepolizumab in this difficult-to-treat population was noteworthy, only about half of the patients given the active treatment achieved remission as defined by the study protocol. It is unclear why the drug was not effective in the other half of patients. One possible reason is that some manifestations of the disorder are not driven by eosinophils. Another is that nonresponsive patients may have sustained longstanding, irreversible vasculitic damage that is no longer amenable to anti–interleukin-5 therapy.
Alternatively, it’s possible that mepolizumab reduced eosinophils in the blood but not those in the body tissues of nonresponsive patients or that the patients who didn’t respond well simply required a higher dose of the drug, Dr. Wechsler and his associates said.
The NIAID is now supporting a study of blood, urine, sputum, and tissue samples from some of these participants “to address questions related to disease risk and pathological features, as well as response to treatment,” they added.
Many authors reported receiving payments from pharmaceutical companies, including several from GlaxoSmithKline. Four authors are employees of the company.
The study by Michael E. Wechsler, MD, and his associates can be considered proof of concept. Now, researchers must turn to identifying biomarkers that predict the success or failure of mepolizumab in patients.
Researchers must also elucidate the fate of eosinophils in the tissues, especially in vasculitic lesions, after treatment with mepolizumab. And they should address possible synergistic activity when the drug is given together with immunosuppressants such as azathioprine and cyclophosphamide.
In addition, future studies should include patients who have organ-threatening or life-threatening eosinophilic granulomatosis with polyangiitis, who were excluded from this trial but who are most in need of novel treatments.
Ratko Djukanovic, MD, is with the University of Southampton (England) and the National Institute for Health Research Southampton Biomedical Research Centre. Paul M. O’Byrne, MD, is with the Firestone Institute for Respiratory Health within St. Joseph’s Healthcare and McMaster University in Hamilton, Ont. Dr. Djukanovic and Dr. O’Byrne both reported financial relationships with pharmaceutical companies outside their editorial. They made these remarks in an editorial accompanying Dr. Wechsler and colleagues’ report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1704402).
The study by Michael E. Wechsler, MD, and his associates can be considered proof of concept. Now, researchers must turn to identifying biomarkers that predict the success or failure of mepolizumab in patients.
Researchers must also elucidate the fate of eosinophils in the tissues, especially in vasculitic lesions, after treatment with mepolizumab. And they should address possible synergistic activity when the drug is given together with immunosuppressants such as azathioprine and cyclophosphamide.
In addition, future studies should include patients who have organ-threatening or life-threatening eosinophilic granulomatosis with polyangiitis, who were excluded from this trial but who are most in need of novel treatments.
Ratko Djukanovic, MD, is with the University of Southampton (England) and the National Institute for Health Research Southampton Biomedical Research Centre. Paul M. O’Byrne, MD, is with the Firestone Institute for Respiratory Health within St. Joseph’s Healthcare and McMaster University in Hamilton, Ont. Dr. Djukanovic and Dr. O’Byrne both reported financial relationships with pharmaceutical companies outside their editorial. They made these remarks in an editorial accompanying Dr. Wechsler and colleagues’ report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1704402).
The study by Michael E. Wechsler, MD, and his associates can be considered proof of concept. Now, researchers must turn to identifying biomarkers that predict the success or failure of mepolizumab in patients.
Researchers must also elucidate the fate of eosinophils in the tissues, especially in vasculitic lesions, after treatment with mepolizumab. And they should address possible synergistic activity when the drug is given together with immunosuppressants such as azathioprine and cyclophosphamide.
In addition, future studies should include patients who have organ-threatening or life-threatening eosinophilic granulomatosis with polyangiitis, who were excluded from this trial but who are most in need of novel treatments.
Ratko Djukanovic, MD, is with the University of Southampton (England) and the National Institute for Health Research Southampton Biomedical Research Centre. Paul M. O’Byrne, MD, is with the Firestone Institute for Respiratory Health within St. Joseph’s Healthcare and McMaster University in Hamilton, Ont. Dr. Djukanovic and Dr. O’Byrne both reported financial relationships with pharmaceutical companies outside their editorial. They made these remarks in an editorial accompanying Dr. Wechsler and colleagues’ report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1704402).
Adding mepolizumab to standard-of-care glucocorticoids with or without immunosuppressive agents can induce remission in many patients who have eosinophilic granulomatosis with polyangiitis (EGPA), according to a report published online May 18 in the New England Journal of Medicine.
EGPA, a rare disorder characterized by asthma, sinusitis, pulmonary infiltrates, neuropathy, and eosinophilic vasculitis in at least one end-organ, frequently relapses despite glucocorticoid therapy or fails to respond adequately to the treatment. Patients have elevated levels of the cytokine interleukin-5, which regulates eosinophil maturation, differentiation, and proliferation. Neutralizing this cytokine is thought to be a potential therapeutic approach, said Michael E. Wechsler, MD, of National Jewish Health, Denver, and his associates.
Proof-of-concept studies have demonstrated the efficacy of subcutaneous mepolizumab, an anti–interleukin-5 monoclonal antibody, in EGPA, so Dr. Wechsler and his colleagues assessed the safety and efficacy of a 1-year course of mepolizumab (300 mg) as add-on therapy in a double-blind, randomized, phase III trial, which involved 136 adults treated at 31 academic medical centers in nine countries. The study was sponsored by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases.
The first of two primary efficacy endpoints was the total accrued weeks of remission. A total of 28% of the mepolizumab group achieved remission for at least 24 weeks, compared with only 3% of the placebo group, for an odds ratio of 5.91.
The second primary efficacy endpoint was the proportion of patients in remission at both week 36 and week 48. Again, significantly more patients in the mepolizumab group (32%) than in the placebo group (3%) met this end point (OR, 16.74).
Mepolizumab also proved superior to placebo regarding numerous secondary endpoints, the investigators said (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1702079). More patients who received active treatment achieved remission within the first 6 months of treatment and remained in remission for a full year (19% vs. 1%; OR, 19.65). The time to first relapse was significantly longer for mepolizumab, with only 56% of that group experiencing a relapse within 1 year, compared with 82% of the placebo group. The annualized relapse rate was half as high with mepolizumab (1.14) as with placebo (2.27).
In addition, patients in the mepolizumab group were more likely to reduce their doses of glucocorticoids (OR, 0.20) or discontinue the drugs altogether (18% vs. 3% taking placebo).
Mepolizumab was most effective among the 79 patients who had a high absolute eosinophil count (150 or more cells per cubic millimeter) at baseline. In this subgroup, 33% of patients taking mepolizumab achieved remission for 6 months or more, compared with none of the patients taking placebo (OR, 26.1).
Although the effectiveness of mepolizumab in this difficult-to-treat population was noteworthy, only about half of the patients given the active treatment achieved remission as defined by the study protocol. It is unclear why the drug was not effective in the other half of patients. One possible reason is that some manifestations of the disorder are not driven by eosinophils. Another is that nonresponsive patients may have sustained longstanding, irreversible vasculitic damage that is no longer amenable to anti–interleukin-5 therapy.
Alternatively, it’s possible that mepolizumab reduced eosinophils in the blood but not those in the body tissues of nonresponsive patients or that the patients who didn’t respond well simply required a higher dose of the drug, Dr. Wechsler and his associates said.
The NIAID is now supporting a study of blood, urine, sputum, and tissue samples from some of these participants “to address questions related to disease risk and pathological features, as well as response to treatment,” they added.
Many authors reported receiving payments from pharmaceutical companies, including several from GlaxoSmithKline. Four authors are employees of the company.
Adding mepolizumab to standard-of-care glucocorticoids with or without immunosuppressive agents can induce remission in many patients who have eosinophilic granulomatosis with polyangiitis (EGPA), according to a report published online May 18 in the New England Journal of Medicine.
EGPA, a rare disorder characterized by asthma, sinusitis, pulmonary infiltrates, neuropathy, and eosinophilic vasculitis in at least one end-organ, frequently relapses despite glucocorticoid therapy or fails to respond adequately to the treatment. Patients have elevated levels of the cytokine interleukin-5, which regulates eosinophil maturation, differentiation, and proliferation. Neutralizing this cytokine is thought to be a potential therapeutic approach, said Michael E. Wechsler, MD, of National Jewish Health, Denver, and his associates.
Proof-of-concept studies have demonstrated the efficacy of subcutaneous mepolizumab, an anti–interleukin-5 monoclonal antibody, in EGPA, so Dr. Wechsler and his colleagues assessed the safety and efficacy of a 1-year course of mepolizumab (300 mg) as add-on therapy in a double-blind, randomized, phase III trial, which involved 136 adults treated at 31 academic medical centers in nine countries. The study was sponsored by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases.
The first of two primary efficacy endpoints was the total accrued weeks of remission. A total of 28% of the mepolizumab group achieved remission for at least 24 weeks, compared with only 3% of the placebo group, for an odds ratio of 5.91.
The second primary efficacy endpoint was the proportion of patients in remission at both week 36 and week 48. Again, significantly more patients in the mepolizumab group (32%) than in the placebo group (3%) met this end point (OR, 16.74).
Mepolizumab also proved superior to placebo regarding numerous secondary endpoints, the investigators said (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1702079). More patients who received active treatment achieved remission within the first 6 months of treatment and remained in remission for a full year (19% vs. 1%; OR, 19.65). The time to first relapse was significantly longer for mepolizumab, with only 56% of that group experiencing a relapse within 1 year, compared with 82% of the placebo group. The annualized relapse rate was half as high with mepolizumab (1.14) as with placebo (2.27).
In addition, patients in the mepolizumab group were more likely to reduce their doses of glucocorticoids (OR, 0.20) or discontinue the drugs altogether (18% vs. 3% taking placebo).
Mepolizumab was most effective among the 79 patients who had a high absolute eosinophil count (150 or more cells per cubic millimeter) at baseline. In this subgroup, 33% of patients taking mepolizumab achieved remission for 6 months or more, compared with none of the patients taking placebo (OR, 26.1).
Although the effectiveness of mepolizumab in this difficult-to-treat population was noteworthy, only about half of the patients given the active treatment achieved remission as defined by the study protocol. It is unclear why the drug was not effective in the other half of patients. One possible reason is that some manifestations of the disorder are not driven by eosinophils. Another is that nonresponsive patients may have sustained longstanding, irreversible vasculitic damage that is no longer amenable to anti–interleukin-5 therapy.
Alternatively, it’s possible that mepolizumab reduced eosinophils in the blood but not those in the body tissues of nonresponsive patients or that the patients who didn’t respond well simply required a higher dose of the drug, Dr. Wechsler and his associates said.
The NIAID is now supporting a study of blood, urine, sputum, and tissue samples from some of these participants “to address questions related to disease risk and pathological features, as well as response to treatment,” they added.
Many authors reported receiving payments from pharmaceutical companies, including several from GlaxoSmithKline. Four authors are employees of the company.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Of the mepolizumab group, 28% achieved remission for at least 24 weeks, compared with only 3% of the placebo group (OR, 5.91).
Data source: An international double-blind randomized placebo-controlled phase III trial involving 136 adults treated for 1 year.
Disclosures: This study was supported by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases. Many authors reported receiving payments from pharmaceutical companies, including several from GlaxoSmithKline. Four authors are employees of the company.