User login
Should Opioids Be Used for Chronic Cancer Pain?
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
FROM CANCER
A Banned Chemical That Is Still Causing Cancer
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These types of stories usually end with a call for regulation — to ban said chemical or substance, or to regulate it — but in this case, that has already happened. This new carcinogen I’m telling you about is actually an old chemical. And it has not been manufactured or legally imported in the US since 2013.
So, why bother? Because in this case, the chemical — or, really, a group of chemicals called polybrominated diphenyl ethers (PBDEs) — are still around: in our soil, in our food, and in our blood.
PBDEs are a group of compounds that confer flame-retardant properties to plastics, and they were used extensively in the latter part of the 20th century in electronic enclosures, business equipment, and foam cushioning in upholstery.
But there was a problem. They don’t chemically bond to plastics; they are just sort of mixed in, which means they can leach out. They are hydrophobic, meaning they don’t get washed out of soil, and, when ingested or inhaled by humans, they dissolve in our fat stores, making it difficult for our normal excretory systems to excrete them.
PBDEs biomagnify. Small animals can take them up from contaminated soil or water, and those animals are eaten by larger animals, which accumulate higher concentrations of the chemicals. This bioaccumulation increases as you move up the food web until you get to an apex predator — like you and me.
This is true of lots of chemicals, of course. The concern arises when these chemicals are toxic. To date, the toxicity data for PBDEs were pretty limited. There were some animal studies where rats were exposed to extremely high doses and they developed liver lesions — but I am always very wary of extrapolating high-dose rat toxicity studies to humans. There was also some suggestion that the chemicals could be endocrine disruptors, affecting breast and thyroid tissue.
What about cancer? In 2016, the International Agency for Research on Cancer concluded there was “inadequate evidence in humans for the carcinogencity of” PBDEs.
In the same report, though, they suggested PBDEs are “probably carcinogenic to humans” based on mechanistic studies.
In other words, we can’t prove they’re cancerous — but come on, they probably are.
Finally, we have some evidence that really pushes us toward the carcinogenic conclusion, in the form of this study, appearing in JAMA Network Open. It’s a nice bit of epidemiology leveraging the population-based National Health and Nutrition Examination Survey (NHANES).
Researchers measured PBDE levels in blood samples from 1100 people enrolled in NHANES in 2003 and 2004 and linked them to death records collected over the next 20 years or so.
The first thing to note is that the researchers were able to measure PBDEs in the blood samples. They were in there. They were detectable. And they were variable. Dividing the 1100 participants into low, medium, and high PBDE tertiles, you can see a nearly 10-fold difference across the population.
Importantly, not many baseline variables correlated with PBDE levels. People in the highest group were a bit younger but had a fairly similar sex distribution, race, ethnicity, education, income, physical activity, smoking status, and body mass index.
This is not a randomized trial, of course — but at least based on these data, exposure levels do seem fairly random, which is what you would expect from an environmental toxin that percolates up through the food chain. They are often somewhat indiscriminate.
This similarity in baseline characteristics between people with low or high blood levels of PBDE also allows us to make some stronger inferences about the observed outcomes. Let’s take a look at them.
After adjustment for baseline factors, individuals in the highest PBDE group had a 43% higher rate of death from any cause over the follow-up period. This was not enough to achieve statistical significance, but it was close.
But the key finding is deaths due to cancer. After adjustment, cancer deaths occurred four times as frequently among those in the high PBDE group, and that is a statistically significant difference.
To be fair, cancer deaths were rare in this cohort. The vast majority of people did not die of anything during the follow-up period regardless of PBDE level. But the data are strongly suggestive of the carcinogenicity of these chemicals.
I should also point out that the researchers are linking the PBDE level at a single time point to all these future events. If PBDE levels remain relatively stable within an individual over time, that’s fine, but if they tend to vary with intake of different foods for example, this would not be captured and would actually lead to an underestimation of the cancer risk.
The researchers also didn’t have granular enough data to determine the type of cancer, but they do show that rates are similar between men and women, which might point away from the more sex-specific cancer etiologies. Clearly, some more work is needed.
Of course, I started this piece by telling you that these chemicals are already pretty much banned in the United States. What are we supposed to do about these findings? Studies have examined the primary ongoing sources of PBDE in our environment and it seems like most of our exposure will be coming from the food we eat due to that biomagnification thing: high-fat fish, meat and dairy products, and fish oil supplements. It may be worth some investigation into the relative adulteration of these products with this new old carcinogen.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Computer-Aided Colonoscopy Falls Short in Real-World Practice
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Active Surveillance for Cancer Doesn’t Increase Malpractice Risk
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
Liquid Biopsy for Colorectal Cancer Appears Promising But Still Lacks Robust Efficacy
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
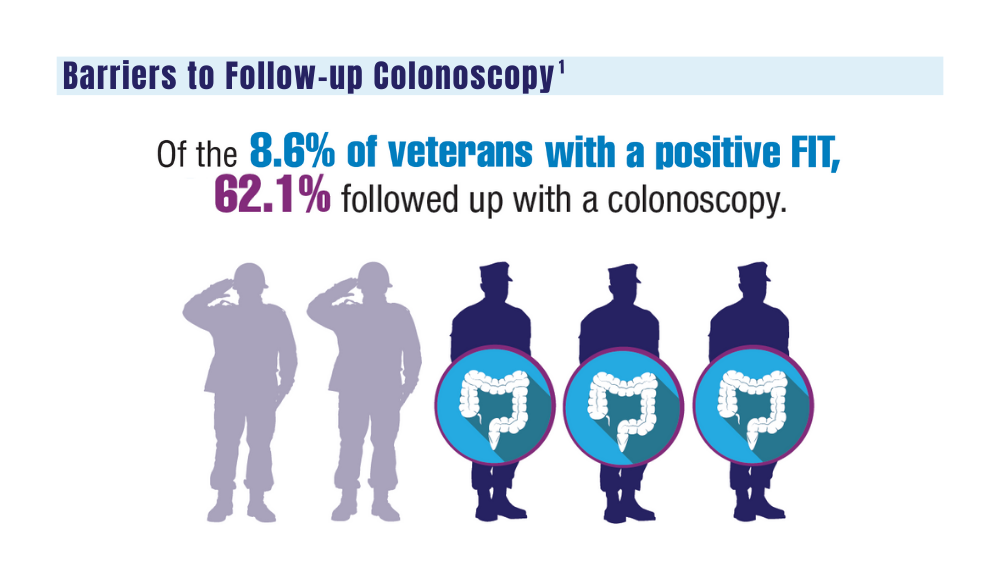
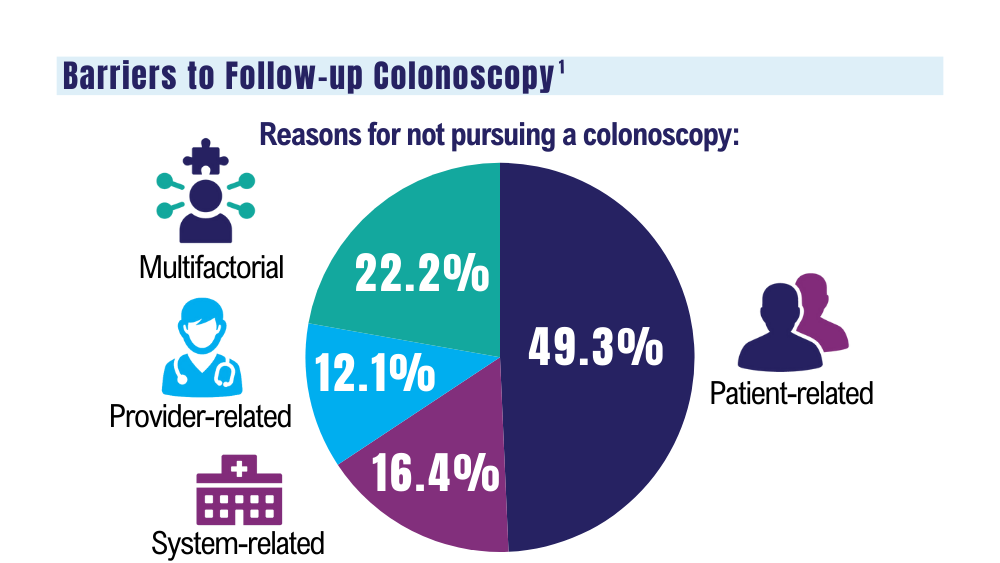
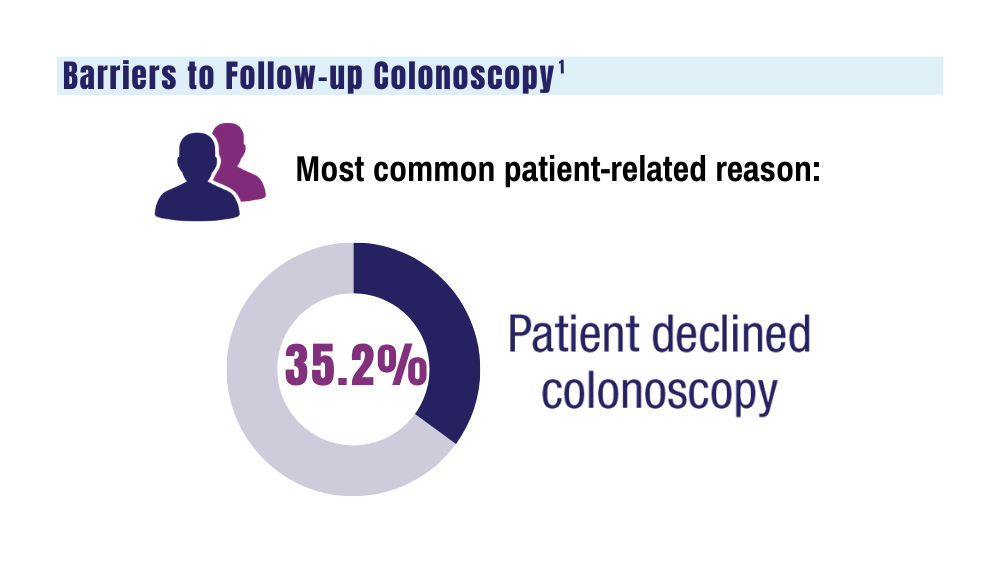
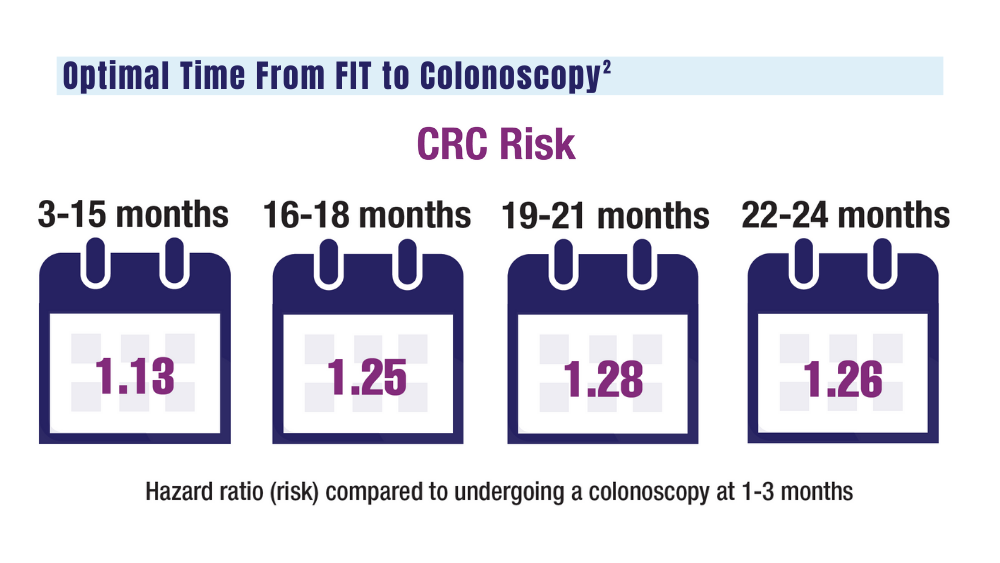
VA to Expand Cancer Prevention Services
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
New CRC Risk Prediction Model Outperforms Polyp-Based Model
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A comprehensive model considering patient age, diabetes, colonoscopy indications, and polyp findings can predict colorectal cancer (CRC) risk more accurately than the solely polyp-based model in patients with a first diagnosis of adenoma on colonoscopy.
METHODOLOGY:
- Because colonoscopy surveillance guidelines relying solely on previous polyp findings to assess CRC risk are imprecise, researchers developed and tested a comprehensive risk prediction model from a list of CRC-related predictors that included patient characteristics and clinical factors in addition to polyp findings.
- The comprehensive model included baseline colonoscopy indication, age group, diabetes diagnosis, and polyp findings (adenoma with advanced histology, polyp size ≥ 10 mm, and sessile serrated or traditional serrated adenoma).
- They randomly assigned 95,001 patients (mean age, 61.9 years; 45.5% women) who underwent colonoscopy with polypectomy to remove a conventional adenoma into two cohorts: Model development (66,500) and internal validation (28,501).
- In both cohorts, researchers compared the performance of the polyp findings-only method against the comprehensive model in predicting CRC, defined as an adenocarcinoma of the colon or rectum diagnosed a year after the baseline colonoscopy.
TAKEAWAY:
- During the follow-up period starting 1 year after colonoscopy, 495 patients were diagnosed with CRC; 354 were in the development cohort and 141 were in the validation cohort.
- The comprehensive model demonstrated better predictive performance than the traditional polyp-based model in the development cohort (area under the curve [AUC], 0.71 vs 0.61) and in the validation cohort (AUC, 0.7 vs 0.62).
- The difference in the Akaike Information Criterion values between the comprehensive and polyp models was 45.7, much above the threshold of 10, strongly indicating the superior performance of the comprehensive model.
IN PRACTICE:
“Improving the ability to accurately predict the patients at highest risk for CRC after polypectomy is critically important, given the considerable costs and resources associated with treating CRC and the better prognosis associated with early cancer detection. The current findings provide proof of concept that inclusion of CRC risk factors beyond prior polyp findings has the potential to improve post-colonoscopy risk stratification,” the authors wrote.
SOURCE:
The study, led by Jeffrey K. Lee, MD, MPH, Division of Research, Kaiser Permanente Northern California, Oakland, California, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
External validation of the model’s performance is needed in different practice settings. The generalizability of the findings is limited because the study population did not include individuals without a prior adenoma or those with an isolated serrated polyp. Moreover, the examination of polyp size > 20 mm as a potential predictor of CRC was precluded due to incomplete data.
DISCLOSURES:
The study was conducted within the National Cancer Institute–funded Population-Based Research to Optimize the Screening Process II consortium and funded by a career development grant from the National Cancer Institute to Lee. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
New CRC stool test beats FIT for sensitivity but not specificity
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Cell-Free DNA Blood Test Has High Accuracy for Detecting Colorectal Cancer
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM NEJM
Cancer Data Trends 2024: Colorectal Cancer
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.