User login
Neuromyelitis Optica: Historically Misdiagnosed — Now Demands Prompt Treatment
Urgency of treatment is something that many physicians may not fully appreciate when it comes to neuromyelitis optica (NMO), according to experts on this rare autoimmune demyelinating disorder. This may be partly due to its similar presentation to multiple sclerosis (MS), said Michael Levy, MD, PhD, associate professor, Harvard Medical School, research director, Division of Neuroimmunology & Neuroinfectious Disease, and director, Neuroimmunology Clinic and Research Laboratory, at Massachusetts General Hospital in Boston. But while the two conditions share many clinical characteristics, “immunologically, they are about as different as can be,” he warned.
The urgency of distinction is important because where MS is known to have a relatively gradual progression, NMO is now red-flagged to potentially cause rapid and irreversible damage. While the course of MS might be described as a slow burn, NMO should be treated like a wildfire.
“That message has gotten muddled, particularly because acute treatment in MS has never been shown to affect outcome,” said Jeffrey Bennett, MD, PhD, professor of neurology and ophthalmology at the University of Colorado School of Medicine, Aurora. In contrast, rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury,” he said.
First described by Dr. Eugène Devic in 1894, and sometimes known as Devic’s disease, NMO is believed to have a prevalence that varies widely depending on ethnicity and gender. A recent report suggests a prevalence of approximately1/100,000 population among Whites with an annual incidence of less than 1/million in this population, while the prevalence is higher among East Asians (approximately 3.5/100,000), and may reach as high as 10/100,000 in Blacks.1 It has a high female-to-male ratio (up to 9:1) with a mean age of onset of about 40 years, although pediatric cases are described.
It has long been recognized that NMO lacks the “neurocerebritis” of MS, with inflammation predominant in the optic and spinal nerves, but it was not until 2004 that researchers at the Mayo Clinic identified serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG) that could reliably distinguish NMO from MS. In 2015, the international consensus diagnostic criteria for neuromyelitis optica2 cited core clinical characteristics required for patients with AQP4-IgG-positive NMO spectrum disorder (NMOSD) “including clinical syndromes or MRI findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations.” Rarely, NMO patients can be seronegative for AQP4-IgG, but are still considered to have NMOSD for which non-opticospinal clinical and MRI characteristics findings are described. MS patients testing negative for AQP4-IgG should also be tested for the related myelin oligodendrocyte glycoprotein antibody disease (MOGAD), which has a prevalence about four to five times greater than NMO, Dr. Bennett said.
Testing
Because both NMO and MOGAD can be identified by antibodies, they are less commonly misdiagnosed as MS compared to previously. But, prior to the identification of the AQP4-IgG antibody in 2004, the misdiagnosis rate of NMO was probably about 95% said Dr. Levy.
“Of course, before we had the antibody test or clinical criteria, we couldn’t confirm a diagnosis of NMO, so basically everyone had a diagnosis of MS, and after the antibody test became commercially available in 2005/2006, we could confirm the diagnosis, with our study in 2012 showing a much lower misdiagnosis rate of 30%.”3 More recently, the misdiagnosis rates are even lower, he added. A recent study out of Argentina found a rate of only 12%.4
The specificity and sensitivity of cell binding assay serum AQP4-IgG testing is roughly 99% and 90%, respectively, better than ELIZA testing (which has a sensitivity in the 60-65% range), said Dr. Bennett. “That’s why we highly emphasize to physicians, that if you have a suspicion for NMOSD you go to a cell binding assay, and make sure that where you’re sending the serum, the lab can do that procedure.” Still, because of the risk of false positives, he urges restraint in testing for the disorder in the absence of a high suspicion for it. “If you test a lot of people indiscriminately for a rare disorder, you get a lot of false positives because the actual true positives are a very small fraction of that group. So, even with a specificity of around 99% that means 1% of the people you test are falsely positive. And if you’re testing a group of people indiscriminately, then your true positives are less than 1% by far, so then most of the people that you pick up are not truly with disease.”
Acute Treatment
While misdiagnosis of NMO as MS is less common than previously, it is still a concern, not only because of the irreversible risks associated with delayed acute treatment, but also the risks of inappropriate preventive MS therapy, which could be harmful to patients with NMO.
Acute flare-ups of NMO and MOGAD are currently all treated with the same decades-old mainstays for acute MS — intravenous steroids and plasma exchange — but the approach is more aggressive. Retrospective studies have shown that, for NMO, plasma exchange has shown an increased likelihood of improvement versus steroids alone, said Dr. Bennett, but since time is of the essence, treatment should begin before a definitive diagnosis is confirmed.
“What’s limiting our patients is, number one, recognizing NMOSD when the attack is happening in your face. You’ve got to know, hey, this is NMOSD or I’m suspicious of NMOSD and hence, I need to treat it urgently because the outcome has a high probability of not being good. You’ve got to realize that this is NMOSD before the test comes back, because by the time it comes back positive in several days, you’re probably missing the optimal window to treat. The point is to know that the presentation in front of you, the MRI pictures in front of you, the laboratory tests that you might have done in terms of spinal fluid analysis, all highly suggest NMOSD. And so hence, I’m going to take the chance that I might be wrong, but I’m going to treat as if it is and wait for the test to come back.”
Realistically, the risks associated with this approach are minor compared with the potential benefit, Dr. Bennett said. “For plasma exchange, there’s the placement of the central line, and the complications that could happen from that. Plasma exchange can lead to metabolic ionic changes in the blood, fluid shifts that might have to be watched in the hospital setting.”
While waiting for diagnostic results, one clue that may emerge from acute treatment is recovery time. “The recovery from MOGAD attacks is really distinct,” said Dr. Levy. “They get better a lot faster. So, if they’re blind in the hospital, and 3 months later they can see again with treatment, that’s MOGAD.” On the other hand, comorbidities such as lupus strongly favor NMO, he added. And another underrecognized, unique symptom of NMO is that about 10% of people may present with protracted episodes of nausea, vomiting, or hiccups, added Dr. Bennett. “What’s important is not that the neurologist recognize this per se in the emergency department, because they’re not going to be called for that patient — the GI doctors will be called for that patient. But when you’re seeing a patient who may have another presentation: a spinal cord attack, a vision attack with optic neuritis, and you ask them simply ‘have you ever had an episode of protracted nausea, vomiting, or hiccups?’ — I can’t tell you how many times I can have someone say ‘that’s weird I was just in the ED 3 months ago for that.’ And then, I know exactly what’s going on.”
Prevention of Relapse
Treatment of NMO presents some particular challenges for clinicians because the old treatment, rituximab, an anti-CD20 monoclonal antibody which has been used since 2005, has been so affordable and successful. “It’s hard to get people off,” said Dr. Levy. “It’s still the most commonly used drug for NMO in the US, even though it’s not approved. It’s cheap enough, and so people get started on that as a treatment, and then they just continue it, even as an outpatient.”
But since 2019, four new FDA-approved therapies have entered the scene with even better efficacy: the anti-CD-19 targeted medication inebilizumab (Uplizna, Viela Bio, approved in 2020), which requires two 90-minute infusions per year; the interleukin-6 (IL-6) receptor inhibitor satralizumab (Enspryng, Roche, approved in 2020), which is administered subcutaneously once monthly; and the anti-complement C5 inhibitors eculizumab (Soliris, Alexion Pharmaceuticals, approved in 2019), and ravulizumab (Ultomiris, Alexion Pharmaceuticals, approved in 2024), which require infusions every 2 weeks or every 2 months, respectively.
Both experts point to compelling clinical evidence to prescribe the Food and Drug Administration–approved drugs for newly diagnosed NMO, and to switch existing patients from rituximab to the new drugs. “The data is pretty clear that there’s about a 35% failure rate with rituximab, as opposed to less than 5% with the new drugs,” explained Dr. Levy. But ironically, where insurance companies used to balk at covering rituximab because it was not FDA approved for NMO, they are now balking at the FDA-approved options because of the cost. “Even in an academic center, where we get a discount on the drugs, the biosimilar generic of rituximab costs about $890 per dose,” he said. “So overall, it’s less than $4,000 a year for rituximab. Compare that with the most expensive FDA-approved option, which is eculizumab. That’s $715,000 per year. And then the other three drugs are below that, but none are less than about $290,000 a year.”
Patients are also hesitant to switch from rituximab if they’ve been well-controlled on it. “There’s a process to it, and I always talk my patients through it, but I would say less than half make the switch,” said Dr. Levy. “Most people want to stay. It’s a whole different schedule, and mixing two drugs. Are you going to overlap and overly immune suppress? Is the insurance going to approve it? It becomes more complicated.”
“Insurance companies are sometimes inappropriately pushing physicians, asking for patients to fail rituximab before they’ll approve an FDA-approved drug, which is like playing doctor when they’re not a licensed physician,” added Dr. Bennett. “And I think that is absolutely inappropriate, especially in light of the fact that before there were approved drugs, insurance companies used to deny rituximab because it was ‘experimental’ and ‘too expensive’ — and now it’s a cheaper alternative.”
Requiring failure on rituximab is also unethical, given the potential for irreversible damage, Dr. Levy pointed out. “With NMO we don’t tolerate a failure. That’s also how the trials of the new drugs were done. It was considered unethical to have an outcome of annualized relapse rate, like we used to in MS, where we say, OK if you have two attacks a year, then the drug has failed. With NMO, one failure, one breakthrough, and that drug is worthless. We switch.”
A Wealth of Treatment Choices
Patients opting for an FDA-approved treatment now have a “wildly effective” array of new drugs, said Dr. Levy, but choosing can be difficult when each has its own set of advantages and disadvantages. “I have equal numbers of patients on all the drugs, and I show all the data to my patients: efficacy, safety, logistics, cost, and then I ask ‘What are your priorities? Which of these things that I say really rings with you? Is it the infusion schedule? Is it the efficacy? Is it the safety concern? Is it the cost? What are you most concerned about?’ And then we start to have the conversation that way. It’s a shared decision-making process.”
There is definitely an art to finding the best fit for each patient, agreed Dr. Bennett, “both with the urgency of controlling the disease, the particular patient in front of you, their ability to adhere to certain therapies, their ability to have access to infusions, or to self-inject, or to get transported to an infusion center or have access to home infusion.”
Patient empowerment in the decision is very important, added Dr. Levy. “When people make the decision on their own, they’re much more likely to be compliant, rather than me telling them they have to do this. And that’s why I think we haven’t had a single relapse on the new drugs. There have been switches because of intolerance, and cost, and all those issues, but not because of a breakthrough attack.”
Future
Looking ahead in the field, Dr. Bennett sees the biggest potential for improvement is in the management of acute attacks, which he describes as “a major treatment gap.” Although plasma exchange is immediately effective in limiting the amount of circulating pathogenic AQP4-IgG “there are other approaches that could be even more beneficial,” he said. “A promising strategy is to use drugs that act immediately on arms of the immune response that are directly injuring brain tissue. These include serum complement and cells such as neutrophils and natural killer cells that release destructive enzymes and inflammatory mediators,” he explained. “Complement inhibitors, such as the C5 inhibitors eculizumab and ravulizumab, currently approved for NMOSD relapse prevention, act immediately to inhibit complement-mediated tissue injury. Similarly, high doses of antihistamines could be used to rapidly stop the release of the destructive enzyme elastase from neutrophils and natural killer cells, while elastase inhibitors could be given to minimize cell injury. Direct clinical studies are needed to find both the optimal treatment window and regimen.”
References
1. Hor JY et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front Neurol. 2020 Jun 26:11:501. doi: 10.3389/fneur.2020.00501.
2. Wingerchuk DM et al. International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders. Neurology. 2015 Jul 14;85(2):177-89. doi: 10.1212/WNL.0000000000001729.
3. Mealy MA et al. Epidemiology of Neuromyelitis Optica in the United States: A Multicenter Analysis. Arch Neurol. 2012 Sep;69(9):1176-80. doi: 10.1001/archneurol.2012.314.
4. Contentti EC et al. Frequency of NMOSD Misdiagnosis in a Cohort From Latin America: Impact and Evaluation of Different Contributors. Mult Scler. 2023 Feb;29(2):277-286. doi: 10.1177/13524585221136259.
Urgency of treatment is something that many physicians may not fully appreciate when it comes to neuromyelitis optica (NMO), according to experts on this rare autoimmune demyelinating disorder. This may be partly due to its similar presentation to multiple sclerosis (MS), said Michael Levy, MD, PhD, associate professor, Harvard Medical School, research director, Division of Neuroimmunology & Neuroinfectious Disease, and director, Neuroimmunology Clinic and Research Laboratory, at Massachusetts General Hospital in Boston. But while the two conditions share many clinical characteristics, “immunologically, they are about as different as can be,” he warned.
The urgency of distinction is important because where MS is known to have a relatively gradual progression, NMO is now red-flagged to potentially cause rapid and irreversible damage. While the course of MS might be described as a slow burn, NMO should be treated like a wildfire.
“That message has gotten muddled, particularly because acute treatment in MS has never been shown to affect outcome,” said Jeffrey Bennett, MD, PhD, professor of neurology and ophthalmology at the University of Colorado School of Medicine, Aurora. In contrast, rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury,” he said.
First described by Dr. Eugène Devic in 1894, and sometimes known as Devic’s disease, NMO is believed to have a prevalence that varies widely depending on ethnicity and gender. A recent report suggests a prevalence of approximately1/100,000 population among Whites with an annual incidence of less than 1/million in this population, while the prevalence is higher among East Asians (approximately 3.5/100,000), and may reach as high as 10/100,000 in Blacks.1 It has a high female-to-male ratio (up to 9:1) with a mean age of onset of about 40 years, although pediatric cases are described.
It has long been recognized that NMO lacks the “neurocerebritis” of MS, with inflammation predominant in the optic and spinal nerves, but it was not until 2004 that researchers at the Mayo Clinic identified serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG) that could reliably distinguish NMO from MS. In 2015, the international consensus diagnostic criteria for neuromyelitis optica2 cited core clinical characteristics required for patients with AQP4-IgG-positive NMO spectrum disorder (NMOSD) “including clinical syndromes or MRI findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations.” Rarely, NMO patients can be seronegative for AQP4-IgG, but are still considered to have NMOSD for which non-opticospinal clinical and MRI characteristics findings are described. MS patients testing negative for AQP4-IgG should also be tested for the related myelin oligodendrocyte glycoprotein antibody disease (MOGAD), which has a prevalence about four to five times greater than NMO, Dr. Bennett said.
Testing
Because both NMO and MOGAD can be identified by antibodies, they are less commonly misdiagnosed as MS compared to previously. But, prior to the identification of the AQP4-IgG antibody in 2004, the misdiagnosis rate of NMO was probably about 95% said Dr. Levy.
“Of course, before we had the antibody test or clinical criteria, we couldn’t confirm a diagnosis of NMO, so basically everyone had a diagnosis of MS, and after the antibody test became commercially available in 2005/2006, we could confirm the diagnosis, with our study in 2012 showing a much lower misdiagnosis rate of 30%.”3 More recently, the misdiagnosis rates are even lower, he added. A recent study out of Argentina found a rate of only 12%.4
The specificity and sensitivity of cell binding assay serum AQP4-IgG testing is roughly 99% and 90%, respectively, better than ELIZA testing (which has a sensitivity in the 60-65% range), said Dr. Bennett. “That’s why we highly emphasize to physicians, that if you have a suspicion for NMOSD you go to a cell binding assay, and make sure that where you’re sending the serum, the lab can do that procedure.” Still, because of the risk of false positives, he urges restraint in testing for the disorder in the absence of a high suspicion for it. “If you test a lot of people indiscriminately for a rare disorder, you get a lot of false positives because the actual true positives are a very small fraction of that group. So, even with a specificity of around 99% that means 1% of the people you test are falsely positive. And if you’re testing a group of people indiscriminately, then your true positives are less than 1% by far, so then most of the people that you pick up are not truly with disease.”
Acute Treatment
While misdiagnosis of NMO as MS is less common than previously, it is still a concern, not only because of the irreversible risks associated with delayed acute treatment, but also the risks of inappropriate preventive MS therapy, which could be harmful to patients with NMO.
Acute flare-ups of NMO and MOGAD are currently all treated with the same decades-old mainstays for acute MS — intravenous steroids and plasma exchange — but the approach is more aggressive. Retrospective studies have shown that, for NMO, plasma exchange has shown an increased likelihood of improvement versus steroids alone, said Dr. Bennett, but since time is of the essence, treatment should begin before a definitive diagnosis is confirmed.
“What’s limiting our patients is, number one, recognizing NMOSD when the attack is happening in your face. You’ve got to know, hey, this is NMOSD or I’m suspicious of NMOSD and hence, I need to treat it urgently because the outcome has a high probability of not being good. You’ve got to realize that this is NMOSD before the test comes back, because by the time it comes back positive in several days, you’re probably missing the optimal window to treat. The point is to know that the presentation in front of you, the MRI pictures in front of you, the laboratory tests that you might have done in terms of spinal fluid analysis, all highly suggest NMOSD. And so hence, I’m going to take the chance that I might be wrong, but I’m going to treat as if it is and wait for the test to come back.”
Realistically, the risks associated with this approach are minor compared with the potential benefit, Dr. Bennett said. “For plasma exchange, there’s the placement of the central line, and the complications that could happen from that. Plasma exchange can lead to metabolic ionic changes in the blood, fluid shifts that might have to be watched in the hospital setting.”
While waiting for diagnostic results, one clue that may emerge from acute treatment is recovery time. “The recovery from MOGAD attacks is really distinct,” said Dr. Levy. “They get better a lot faster. So, if they’re blind in the hospital, and 3 months later they can see again with treatment, that’s MOGAD.” On the other hand, comorbidities such as lupus strongly favor NMO, he added. And another underrecognized, unique symptom of NMO is that about 10% of people may present with protracted episodes of nausea, vomiting, or hiccups, added Dr. Bennett. “What’s important is not that the neurologist recognize this per se in the emergency department, because they’re not going to be called for that patient — the GI doctors will be called for that patient. But when you’re seeing a patient who may have another presentation: a spinal cord attack, a vision attack with optic neuritis, and you ask them simply ‘have you ever had an episode of protracted nausea, vomiting, or hiccups?’ — I can’t tell you how many times I can have someone say ‘that’s weird I was just in the ED 3 months ago for that.’ And then, I know exactly what’s going on.”
Prevention of Relapse
Treatment of NMO presents some particular challenges for clinicians because the old treatment, rituximab, an anti-CD20 monoclonal antibody which has been used since 2005, has been so affordable and successful. “It’s hard to get people off,” said Dr. Levy. “It’s still the most commonly used drug for NMO in the US, even though it’s not approved. It’s cheap enough, and so people get started on that as a treatment, and then they just continue it, even as an outpatient.”
But since 2019, four new FDA-approved therapies have entered the scene with even better efficacy: the anti-CD-19 targeted medication inebilizumab (Uplizna, Viela Bio, approved in 2020), which requires two 90-minute infusions per year; the interleukin-6 (IL-6) receptor inhibitor satralizumab (Enspryng, Roche, approved in 2020), which is administered subcutaneously once monthly; and the anti-complement C5 inhibitors eculizumab (Soliris, Alexion Pharmaceuticals, approved in 2019), and ravulizumab (Ultomiris, Alexion Pharmaceuticals, approved in 2024), which require infusions every 2 weeks or every 2 months, respectively.
Both experts point to compelling clinical evidence to prescribe the Food and Drug Administration–approved drugs for newly diagnosed NMO, and to switch existing patients from rituximab to the new drugs. “The data is pretty clear that there’s about a 35% failure rate with rituximab, as opposed to less than 5% with the new drugs,” explained Dr. Levy. But ironically, where insurance companies used to balk at covering rituximab because it was not FDA approved for NMO, they are now balking at the FDA-approved options because of the cost. “Even in an academic center, where we get a discount on the drugs, the biosimilar generic of rituximab costs about $890 per dose,” he said. “So overall, it’s less than $4,000 a year for rituximab. Compare that with the most expensive FDA-approved option, which is eculizumab. That’s $715,000 per year. And then the other three drugs are below that, but none are less than about $290,000 a year.”
Patients are also hesitant to switch from rituximab if they’ve been well-controlled on it. “There’s a process to it, and I always talk my patients through it, but I would say less than half make the switch,” said Dr. Levy. “Most people want to stay. It’s a whole different schedule, and mixing two drugs. Are you going to overlap and overly immune suppress? Is the insurance going to approve it? It becomes more complicated.”
“Insurance companies are sometimes inappropriately pushing physicians, asking for patients to fail rituximab before they’ll approve an FDA-approved drug, which is like playing doctor when they’re not a licensed physician,” added Dr. Bennett. “And I think that is absolutely inappropriate, especially in light of the fact that before there were approved drugs, insurance companies used to deny rituximab because it was ‘experimental’ and ‘too expensive’ — and now it’s a cheaper alternative.”
Requiring failure on rituximab is also unethical, given the potential for irreversible damage, Dr. Levy pointed out. “With NMO we don’t tolerate a failure. That’s also how the trials of the new drugs were done. It was considered unethical to have an outcome of annualized relapse rate, like we used to in MS, where we say, OK if you have two attacks a year, then the drug has failed. With NMO, one failure, one breakthrough, and that drug is worthless. We switch.”
A Wealth of Treatment Choices
Patients opting for an FDA-approved treatment now have a “wildly effective” array of new drugs, said Dr. Levy, but choosing can be difficult when each has its own set of advantages and disadvantages. “I have equal numbers of patients on all the drugs, and I show all the data to my patients: efficacy, safety, logistics, cost, and then I ask ‘What are your priorities? Which of these things that I say really rings with you? Is it the infusion schedule? Is it the efficacy? Is it the safety concern? Is it the cost? What are you most concerned about?’ And then we start to have the conversation that way. It’s a shared decision-making process.”
There is definitely an art to finding the best fit for each patient, agreed Dr. Bennett, “both with the urgency of controlling the disease, the particular patient in front of you, their ability to adhere to certain therapies, their ability to have access to infusions, or to self-inject, or to get transported to an infusion center or have access to home infusion.”
Patient empowerment in the decision is very important, added Dr. Levy. “When people make the decision on their own, they’re much more likely to be compliant, rather than me telling them they have to do this. And that’s why I think we haven’t had a single relapse on the new drugs. There have been switches because of intolerance, and cost, and all those issues, but not because of a breakthrough attack.”
Future
Looking ahead in the field, Dr. Bennett sees the biggest potential for improvement is in the management of acute attacks, which he describes as “a major treatment gap.” Although plasma exchange is immediately effective in limiting the amount of circulating pathogenic AQP4-IgG “there are other approaches that could be even more beneficial,” he said. “A promising strategy is to use drugs that act immediately on arms of the immune response that are directly injuring brain tissue. These include serum complement and cells such as neutrophils and natural killer cells that release destructive enzymes and inflammatory mediators,” he explained. “Complement inhibitors, such as the C5 inhibitors eculizumab and ravulizumab, currently approved for NMOSD relapse prevention, act immediately to inhibit complement-mediated tissue injury. Similarly, high doses of antihistamines could be used to rapidly stop the release of the destructive enzyme elastase from neutrophils and natural killer cells, while elastase inhibitors could be given to minimize cell injury. Direct clinical studies are needed to find both the optimal treatment window and regimen.”
References
1. Hor JY et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front Neurol. 2020 Jun 26:11:501. doi: 10.3389/fneur.2020.00501.
2. Wingerchuk DM et al. International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders. Neurology. 2015 Jul 14;85(2):177-89. doi: 10.1212/WNL.0000000000001729.
3. Mealy MA et al. Epidemiology of Neuromyelitis Optica in the United States: A Multicenter Analysis. Arch Neurol. 2012 Sep;69(9):1176-80. doi: 10.1001/archneurol.2012.314.
4. Contentti EC et al. Frequency of NMOSD Misdiagnosis in a Cohort From Latin America: Impact and Evaluation of Different Contributors. Mult Scler. 2023 Feb;29(2):277-286. doi: 10.1177/13524585221136259.
Urgency of treatment is something that many physicians may not fully appreciate when it comes to neuromyelitis optica (NMO), according to experts on this rare autoimmune demyelinating disorder. This may be partly due to its similar presentation to multiple sclerosis (MS), said Michael Levy, MD, PhD, associate professor, Harvard Medical School, research director, Division of Neuroimmunology & Neuroinfectious Disease, and director, Neuroimmunology Clinic and Research Laboratory, at Massachusetts General Hospital in Boston. But while the two conditions share many clinical characteristics, “immunologically, they are about as different as can be,” he warned.
The urgency of distinction is important because where MS is known to have a relatively gradual progression, NMO is now red-flagged to potentially cause rapid and irreversible damage. While the course of MS might be described as a slow burn, NMO should be treated like a wildfire.
“That message has gotten muddled, particularly because acute treatment in MS has never been shown to affect outcome,” said Jeffrey Bennett, MD, PhD, professor of neurology and ophthalmology at the University of Colorado School of Medicine, Aurora. In contrast, rapid diagnosis and treatment of NMO “means potentially preventing future devastating neurologic injury,” he said.
First described by Dr. Eugène Devic in 1894, and sometimes known as Devic’s disease, NMO is believed to have a prevalence that varies widely depending on ethnicity and gender. A recent report suggests a prevalence of approximately1/100,000 population among Whites with an annual incidence of less than 1/million in this population, while the prevalence is higher among East Asians (approximately 3.5/100,000), and may reach as high as 10/100,000 in Blacks.1 It has a high female-to-male ratio (up to 9:1) with a mean age of onset of about 40 years, although pediatric cases are described.
It has long been recognized that NMO lacks the “neurocerebritis” of MS, with inflammation predominant in the optic and spinal nerves, but it was not until 2004 that researchers at the Mayo Clinic identified serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG) that could reliably distinguish NMO from MS. In 2015, the international consensus diagnostic criteria for neuromyelitis optica2 cited core clinical characteristics required for patients with AQP4-IgG-positive NMO spectrum disorder (NMOSD) “including clinical syndromes or MRI findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations.” Rarely, NMO patients can be seronegative for AQP4-IgG, but are still considered to have NMOSD for which non-opticospinal clinical and MRI characteristics findings are described. MS patients testing negative for AQP4-IgG should also be tested for the related myelin oligodendrocyte glycoprotein antibody disease (MOGAD), which has a prevalence about four to five times greater than NMO, Dr. Bennett said.
Testing
Because both NMO and MOGAD can be identified by antibodies, they are less commonly misdiagnosed as MS compared to previously. But, prior to the identification of the AQP4-IgG antibody in 2004, the misdiagnosis rate of NMO was probably about 95% said Dr. Levy.
“Of course, before we had the antibody test or clinical criteria, we couldn’t confirm a diagnosis of NMO, so basically everyone had a diagnosis of MS, and after the antibody test became commercially available in 2005/2006, we could confirm the diagnosis, with our study in 2012 showing a much lower misdiagnosis rate of 30%.”3 More recently, the misdiagnosis rates are even lower, he added. A recent study out of Argentina found a rate of only 12%.4
The specificity and sensitivity of cell binding assay serum AQP4-IgG testing is roughly 99% and 90%, respectively, better than ELIZA testing (which has a sensitivity in the 60-65% range), said Dr. Bennett. “That’s why we highly emphasize to physicians, that if you have a suspicion for NMOSD you go to a cell binding assay, and make sure that where you’re sending the serum, the lab can do that procedure.” Still, because of the risk of false positives, he urges restraint in testing for the disorder in the absence of a high suspicion for it. “If you test a lot of people indiscriminately for a rare disorder, you get a lot of false positives because the actual true positives are a very small fraction of that group. So, even with a specificity of around 99% that means 1% of the people you test are falsely positive. And if you’re testing a group of people indiscriminately, then your true positives are less than 1% by far, so then most of the people that you pick up are not truly with disease.”
Acute Treatment
While misdiagnosis of NMO as MS is less common than previously, it is still a concern, not only because of the irreversible risks associated with delayed acute treatment, but also the risks of inappropriate preventive MS therapy, which could be harmful to patients with NMO.
Acute flare-ups of NMO and MOGAD are currently all treated with the same decades-old mainstays for acute MS — intravenous steroids and plasma exchange — but the approach is more aggressive. Retrospective studies have shown that, for NMO, plasma exchange has shown an increased likelihood of improvement versus steroids alone, said Dr. Bennett, but since time is of the essence, treatment should begin before a definitive diagnosis is confirmed.
“What’s limiting our patients is, number one, recognizing NMOSD when the attack is happening in your face. You’ve got to know, hey, this is NMOSD or I’m suspicious of NMOSD and hence, I need to treat it urgently because the outcome has a high probability of not being good. You’ve got to realize that this is NMOSD before the test comes back, because by the time it comes back positive in several days, you’re probably missing the optimal window to treat. The point is to know that the presentation in front of you, the MRI pictures in front of you, the laboratory tests that you might have done in terms of spinal fluid analysis, all highly suggest NMOSD. And so hence, I’m going to take the chance that I might be wrong, but I’m going to treat as if it is and wait for the test to come back.”
Realistically, the risks associated with this approach are minor compared with the potential benefit, Dr. Bennett said. “For plasma exchange, there’s the placement of the central line, and the complications that could happen from that. Plasma exchange can lead to metabolic ionic changes in the blood, fluid shifts that might have to be watched in the hospital setting.”
While waiting for diagnostic results, one clue that may emerge from acute treatment is recovery time. “The recovery from MOGAD attacks is really distinct,” said Dr. Levy. “They get better a lot faster. So, if they’re blind in the hospital, and 3 months later they can see again with treatment, that’s MOGAD.” On the other hand, comorbidities such as lupus strongly favor NMO, he added. And another underrecognized, unique symptom of NMO is that about 10% of people may present with protracted episodes of nausea, vomiting, or hiccups, added Dr. Bennett. “What’s important is not that the neurologist recognize this per se in the emergency department, because they’re not going to be called for that patient — the GI doctors will be called for that patient. But when you’re seeing a patient who may have another presentation: a spinal cord attack, a vision attack with optic neuritis, and you ask them simply ‘have you ever had an episode of protracted nausea, vomiting, or hiccups?’ — I can’t tell you how many times I can have someone say ‘that’s weird I was just in the ED 3 months ago for that.’ And then, I know exactly what’s going on.”
Prevention of Relapse
Treatment of NMO presents some particular challenges for clinicians because the old treatment, rituximab, an anti-CD20 monoclonal antibody which has been used since 2005, has been so affordable and successful. “It’s hard to get people off,” said Dr. Levy. “It’s still the most commonly used drug for NMO in the US, even though it’s not approved. It’s cheap enough, and so people get started on that as a treatment, and then they just continue it, even as an outpatient.”
But since 2019, four new FDA-approved therapies have entered the scene with even better efficacy: the anti-CD-19 targeted medication inebilizumab (Uplizna, Viela Bio, approved in 2020), which requires two 90-minute infusions per year; the interleukin-6 (IL-6) receptor inhibitor satralizumab (Enspryng, Roche, approved in 2020), which is administered subcutaneously once monthly; and the anti-complement C5 inhibitors eculizumab (Soliris, Alexion Pharmaceuticals, approved in 2019), and ravulizumab (Ultomiris, Alexion Pharmaceuticals, approved in 2024), which require infusions every 2 weeks or every 2 months, respectively.
Both experts point to compelling clinical evidence to prescribe the Food and Drug Administration–approved drugs for newly diagnosed NMO, and to switch existing patients from rituximab to the new drugs. “The data is pretty clear that there’s about a 35% failure rate with rituximab, as opposed to less than 5% with the new drugs,” explained Dr. Levy. But ironically, where insurance companies used to balk at covering rituximab because it was not FDA approved for NMO, they are now balking at the FDA-approved options because of the cost. “Even in an academic center, where we get a discount on the drugs, the biosimilar generic of rituximab costs about $890 per dose,” he said. “So overall, it’s less than $4,000 a year for rituximab. Compare that with the most expensive FDA-approved option, which is eculizumab. That’s $715,000 per year. And then the other three drugs are below that, but none are less than about $290,000 a year.”
Patients are also hesitant to switch from rituximab if they’ve been well-controlled on it. “There’s a process to it, and I always talk my patients through it, but I would say less than half make the switch,” said Dr. Levy. “Most people want to stay. It’s a whole different schedule, and mixing two drugs. Are you going to overlap and overly immune suppress? Is the insurance going to approve it? It becomes more complicated.”
“Insurance companies are sometimes inappropriately pushing physicians, asking for patients to fail rituximab before they’ll approve an FDA-approved drug, which is like playing doctor when they’re not a licensed physician,” added Dr. Bennett. “And I think that is absolutely inappropriate, especially in light of the fact that before there were approved drugs, insurance companies used to deny rituximab because it was ‘experimental’ and ‘too expensive’ — and now it’s a cheaper alternative.”
Requiring failure on rituximab is also unethical, given the potential for irreversible damage, Dr. Levy pointed out. “With NMO we don’t tolerate a failure. That’s also how the trials of the new drugs were done. It was considered unethical to have an outcome of annualized relapse rate, like we used to in MS, where we say, OK if you have two attacks a year, then the drug has failed. With NMO, one failure, one breakthrough, and that drug is worthless. We switch.”
A Wealth of Treatment Choices
Patients opting for an FDA-approved treatment now have a “wildly effective” array of new drugs, said Dr. Levy, but choosing can be difficult when each has its own set of advantages and disadvantages. “I have equal numbers of patients on all the drugs, and I show all the data to my patients: efficacy, safety, logistics, cost, and then I ask ‘What are your priorities? Which of these things that I say really rings with you? Is it the infusion schedule? Is it the efficacy? Is it the safety concern? Is it the cost? What are you most concerned about?’ And then we start to have the conversation that way. It’s a shared decision-making process.”
There is definitely an art to finding the best fit for each patient, agreed Dr. Bennett, “both with the urgency of controlling the disease, the particular patient in front of you, their ability to adhere to certain therapies, their ability to have access to infusions, or to self-inject, or to get transported to an infusion center or have access to home infusion.”
Patient empowerment in the decision is very important, added Dr. Levy. “When people make the decision on their own, they’re much more likely to be compliant, rather than me telling them they have to do this. And that’s why I think we haven’t had a single relapse on the new drugs. There have been switches because of intolerance, and cost, and all those issues, but not because of a breakthrough attack.”
Future
Looking ahead in the field, Dr. Bennett sees the biggest potential for improvement is in the management of acute attacks, which he describes as “a major treatment gap.” Although plasma exchange is immediately effective in limiting the amount of circulating pathogenic AQP4-IgG “there are other approaches that could be even more beneficial,” he said. “A promising strategy is to use drugs that act immediately on arms of the immune response that are directly injuring brain tissue. These include serum complement and cells such as neutrophils and natural killer cells that release destructive enzymes and inflammatory mediators,” he explained. “Complement inhibitors, such as the C5 inhibitors eculizumab and ravulizumab, currently approved for NMOSD relapse prevention, act immediately to inhibit complement-mediated tissue injury. Similarly, high doses of antihistamines could be used to rapidly stop the release of the destructive enzyme elastase from neutrophils and natural killer cells, while elastase inhibitors could be given to minimize cell injury. Direct clinical studies are needed to find both the optimal treatment window and regimen.”
References
1. Hor JY et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front Neurol. 2020 Jun 26:11:501. doi: 10.3389/fneur.2020.00501.
2. Wingerchuk DM et al. International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders. Neurology. 2015 Jul 14;85(2):177-89. doi: 10.1212/WNL.0000000000001729.
3. Mealy MA et al. Epidemiology of Neuromyelitis Optica in the United States: A Multicenter Analysis. Arch Neurol. 2012 Sep;69(9):1176-80. doi: 10.1001/archneurol.2012.314.
4. Contentti EC et al. Frequency of NMOSD Misdiagnosis in a Cohort From Latin America: Impact and Evaluation of Different Contributors. Mult Scler. 2023 Feb;29(2):277-286. doi: 10.1177/13524585221136259.
Diagnosing and Managing Duchenne Muscular Dystrophy: Tips for Practicing Clinicians
Duchenne muscular dystrophy (DMD) is a severe progressive inherited disease characterized by muscle wasting and ultimately culminating in death. It’s a common enough neuromuscular disorder that pediatricians and family practice physicians are likely to see at least a couple of patients with DMD over the course of their career,” John Brandsema, MD, Neuromuscular Section Head, Division of Neurology, Children’s Hospital of Philadelphia in Pennsylvania, said in an interview. Healthcare providers should therefore be familiar with the disorder so as to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers as they traverse the challenging and often heartbreaking journey with this condition.
Pathophysiology and Disease Trajectory
DMD is caused by pathogenic variants in the X-linked DMD gene, leading to reduction in dystrophin, a protein that serves as a cytoskeletal integrator, stabilizing the plasma membrane of striated muscle cells. Dystrophin is critical for muscle membrane stability.2 In particular, mutations in the gene that encodes for dystrophin lead to dysfunction in Dp427m, which is the muscle isoform of dystrophin.3,4
DMD is one of several types of muscular dystrophies. All are progressive disorders. Over time, healthy muscle fibers disappear and are replaced by fibrotic tissue and fat, making the muscles “less able to generate force for everyday activity.”2 Ultimately, the skeletal muscle dysfunction affects not only the patient’s day-to-day mobility but other systems as well. Most patients with DMD eventually die of cardiac and/or respiratory failure between the ages of 20 and 40 years, with a median life expectancy of 22 years — although children born after 1990 have a somewhat higher median life expectancy (28 years), because of the improving standard of care.3,5
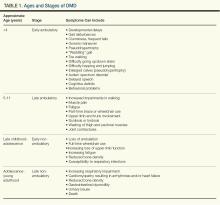
Typically, DMD first presents with developmental delays and weakness in skeletal leg muscles. As the disease goes through stages of progression, it starts involving upper extremities and other systems. (Table 1)
Genetic Causes of DMD
The DMD gene, located on the X chromosome, encodes for the production of dystrophin. Variants of this gene result in the lack of dystrophin protein, leading in turn to muscle fiber degeneration and the progressive symptoms of DMD. Because of the gene’s location on the X chromosome, males (who don’t have a second copy of the X chromosome) cannot compensate for the mutated gene, which is why the disease affects male children. Females with this mutation are carriers and typically do not develop the same severity of symptoms, although they might have milder muscle cramps, weakness, and cardiac issues.3
A female carrier with DMD (or any other X-linked disorder) has a 25% chance to have a carrier daughter, a 25% change of having a noncarrier daughter, a 25% chance of having an affected son, and a 25% chance of having a nonaffected son. A male with the disorder will pass the mutated gene on to his daughters who then become carriers. He cannot pass the disorder on to his sons because males inherit only the Y chromosome from their fathers.3
Diagnosing DMD
“It can take as long as 1-3 years for a child to be diagnosed with DMD,” Dr. Brandsema said. “Parents typically have concerns and know that something is ‘off’ about their child and they’re sent to various specialists, but it usually takes time for an accurate diagnosis to be made.” The mean age at diagnosis of DMD is between ages 4 and 5 years.6
Early identification of infants at risk for developing DMD can help move the needle toward earlier diagnosis. Newborn screening for DMD has been researched and piloted in several programs.6 In 2023, DMD was nominated for inclusion in the Recommended Universal Screening Panel (RUSP) for universal newborn screening. But in May 2024, the advisory committee on Heritable Disorders in Newborns and Children decided to postpone the vote to include DMD in the RUSP, requesting additional information to ensure an evidence-based decision.
In the absence of universal newborn screening for DMD, alternative approaches have been proposed to reduce the delay in clinical diagnosis and specialist referral, including increasing awareness among healthcare providers (eg, pediatricians, pediatric neurologists, and primary care physicians).6
The National Task Force for Early Identification of Childhood Neuromuscular Disorders delineates the steps necessary to identify pediatric muscle weakness and signs of neuromuscular disease. Primary care providers are encouraged to engage in regular developmental surveillance. A surveillance aid lays out the timetable for recommended visits, typical developmental milestones, and components of surveillance. Clinical evaluation includes a detailed patient history, family history, and physical examination.
If a neuromuscular condition is suspected, laboratory work should include creatinine phosphokinase (CK).6 Elevated serum CK points to leakage of CK through the muscle membrane, suggesting muscle damage. If CK is elevated, genetic testing should be performed; and, if negative, it should be followed by genetic sequencing that tests for small-scale mutations in the DMD gene. If that test is negative, a muscle biopsy should be performed to test for deep intronic mutations in the DMD gene.4
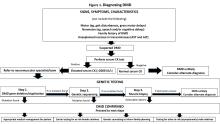
The diagnostic process and immediate steps after a confirmed DMD diagnosis is found in Figure 1.
Targeting Inflammation in DMD
Traditionally, corticosteroids have been the only available medical treatment for DMD and they remain a cornerstone of DMD management. A meta-analysis found “moderate evidence” that corticosteroid therapy improves muscle strength and function in the short term (12 months), and strength up to 2 years.10
The two most common corticosteroids for DMD are prednisone and deflazacort. Deflazacort (Emflaza, PTC Therapeutics) was approved in 2017 to treat patients ages 5 years and older with DMD, subsequently expanded to 2 years and older. Deflazacort has been found to be more effective than prednisone in improving functional outcomes, delaying the onset of cardiomyopathy, and improving overall survival, with fewer adverse effects.11
In 2023, vamorolone (Agamree, Catalyst Pharmaceuticals) was approved by the Food and Drug Administration (FDA) to treat DMD patients (ages 2 years and older). Vamorolone is a dissociative steroidal anti-inflammatory that reduces bone morbidities and is regarded as a safer alternative than prednisone. A clinical trial comparing two doses of vamorolone with prednisone for 24 weeks found that vamorolone 6 mg/kg per day met the primary endpoint (time to stand velocity) and four sequential secondary motor function endpoints, with less bone morbidity, compared to prednisone.12 A more recent trial found improvements in motor outcomes at 48 weeks with a dose of 6 mg/kg per day of vamorolone. Bone morbidities of prednisone were reversed when the patient transitioned to vamorolone.13
“Steroid treatment has been proven to help, usually taken daily, although other schedules have been tried,” Dr. Brandsema said. However, all steroids are fraught with adverse effects and are suboptimal in the long term in reducing the disease burden.
The anti-inflammatory agent givinostat (Duvyzat, ITF Therapeutics), an oral histone deacetylase (HDAC) inhibitor, was approved in March 2024 for the treatment of DMD in patients 6 years of age and older. It is the first nonsteroidal drug to treat patients with all genetic variants of the disease, and it has a unique mechanism of action. Deficits in dystrophin can lead to increased HDAC activity in DMD, reducing the expression of genes involved in muscle regeneration. Givinostat therefore can help to counteract the pathogenic events downstream of dystrophin deficiency by inhibiting HDAC.14
Approval for givinostat was based on the phase 3 EPIDYS trial, which randomized 179 boys with DMD to receive either givinostat or placebo. Although results of a functional task worsened in both groups over the 12-month study period, the decline was significantly smaller with givinostat versus placebo. The most common adverse events were diarrhea and vomiting.14 Dr. Brandsema noted that monitoring of triglycerides and platelet count is required, as hypertriglyceridemia and thrombocytopenia can occur. This treatment was studied in tandem with corticosteroids as a combination approach to muscle stabilization.
New Pharmacotherapeutic Options: Exon-Skipping Agents
Today’s treatments have expanded beyond corticosteroids, with newer therapeutic options that include targeted exon-skipping therapies and, more recently, gene therapies. “These new treatment paradigms have changed the face of DMD treatment,” Dr. Brandsema said.
“Exon-skipping drugs in their current form have only a modest effect, but at least they’re a step in the right direction and a breakthrough, in terms of slowing disease progression,” Dr. Brandsema said.
Current exon-skipping agents use antisense phosphorodiamidate morpholino oligomers (PMOs) to restore a DMD open reading frame. Next-generation drugs called cell-penetrating peptide-conjugated PMOs (PPMOs) are being actively researched, Dr. Brandsema said. These agents have shown enhanced cellular uptake and more efficient dystrophin restoration, compared with unconjugated PMOs.15
There are currently four FDA-approved exon-skipping agents for DMD, all of which are administered via a weekly intravenous infusion: Casimersen (Amondys-45, SRP-4045), approved by the FDA in 2021; Eteplirsen (Exondys 51), approved in 2016; Golodirsen (Vyondys 53,SRP-4053), approved in 2019; and Vitolarsen (Viltepso), approved in 2020. They can be associated with multiple side effects, depending on the drug, including upper respiratory infection, fever, cough, rash, and gastrointestinal issues.16 These agents have the potential to help 30% of DMD patients, restoring low levels of dystrophin.16
Gene Transfer Therapies
Gene transfer therapies, a new class of agents, utilize a nonpathogenic viral vector (adeno-associated virus) to transfer specific genes to patients with DMD. Gene therapy involves overexpressing the micro-dystrophin gene to restore functional dystrophin expression.16
Multiple clinical trials of gene therapy are currently in progress. In 2023, delandistrogene moxeparvovec-rokl (Elevidys, Serepta) was granted accelerated FDA approval for ambulatory individuals with DMD between the ages of 4 and 5 years of age and a confirmed mutation in the DMD gene. It received expanded approval in June 2024 to include ambulatory and nonambulatory individuals aged 4 years and older with DMD and a confirmed mutation in the DMD gene (with the exception of exon 8 or 9 mutations).
The approval was based on preliminary data from two double-blind, placebo-controlled studies and two open-label studies, which enrolled a total of 218 male patients (including those who received placebo) with a confirmed disease-causing mutation in the DMD gene.
Delandistrogene moxeparvovec-rokl is delivered as a one-time infusion and has been associated with side effects and “a lot of potential issues,” Dr. Brandsema said. “We’ve seen cardiac effects, immune system effects, increased muscle inflammation and hepatic complications, and some people who became quite unwell were hospitalized for a long time.”
Fortunately, he added, “these seem to be rare but they do happen. Once the medication has been delivered, it’s permanently in the body, so you’re managing the side effects potentially on a long-term basis.”
It is critical to discuss the risks and benefits of this treatment with the family and caregivers and with the patient as well, if he old enough and able to participate in the decision-making progress. “We don’t want to give unrealistic expectations and we want people to be aware of the potential downside of this treatment,” he said. “This is a very complex discussion because the trajectory of the disease is so devastating and this treatment does hold out hope that other therapies don’t necessarily have.”
Nonpharmacologic Interventions
Physical therapy is a mainstay in DMD treatment, addressing protection of fragile muscles, preservation of strength, and prevention of muscle contractures.16 Given the respiratory impairments that occur with DMD progression, respiratory monitoring and therapy are essential; however, the number and type of evaluations and interventions vary with the stage of the disease, intensifying as the disease progresses.16 Similarly, cardiac monitoring should begin early, with patients screened for cardiac complications, and should intensify through the stages of disease progression.16
Bone health is compromised in patients with DMD, both as a result of corticosteroid treatment and as part of the disease itself. Fractures may be asymptomatic and may go unnoticed. Thus, bone health surveillance and maintenance are critical components of DMD management.16
Patients with DMD often experience gastrointestinal issues. They may experience weight gain because of lack of mobility and corticosteroid use in early stages, or weight loss as a result of diet or fluid imbalance, low bone density, or dysphagia in later stages. Patients should be closely followed by a nutritionist, a gastroenterologist as needed, and a physical therapist.16
Psychosocial support “should be developed and implemented across the lifespan in a manner that promotes thinking about the future and sets expectations that individuals will actively participate in their care and daily activities.”9 This includes psychological care, neuropsychological evaluations, and educational support.
Assisting Patients and Families Through the DMD Journey
DMD care is best delivered in a multidisciplinary setting, where physicians of relevant specialties, physical and occupational therapists, nutritionists, social workers, and genetic counselors collaborate. At Children’s Hospital of Philadelphia, DMD care is delivered through this collaborative model.
Unfortunately, Dr. Brandsema said, many patients don’t have this type of multidisciplinary resource available. “One specialist, such as a pulmonologist or neurologist, might have to be the sole source of care.” Or parents may have to ferry their child to multiple specialists in disparate locations, placing extra stress on an already-stressed family system.
“It’s helpful to connect the family with a comprehensive care center, if possible,” Dr. Brandsema advised. If that’s not available, then he suggests recommending educational opportunities and resources through national organizations such as the Muscular Dystrophy Association; Parent Project MD; NORD; Friends, Family and Duchenne; and Cure Duchenne. Families and caregivers, along with affected individuals, can get education and support from people who understand the day-to-day reality of living with this disease.
One of the major challenges that families face is navigating the high cost of treating DMD, especially the new medications, Dr. Brandsema said. “The authorization process can be intensive and long, and the family may need to take an active role, together with the provider team, in advocating for the patient to get access.”
Among her many responsibilities, Ms. Kaschak engages in care coordination tasks and management, helps patients and caregivers understand care plans, and provides psychosocial support and education about the disease process. She assists families in completing paperwork and navigating specialty authorizations, helping families understand and navigate the complex insurance process. “My role is to bridge gaps in care,” she said.
Dr. Brandsema noted that it’s important for couples to receive genetic counseling if they’re planning to have multiple children because there is a 50% chance that their next boy will be affected. About two thirds of mothers with children who have DMD are carriers, but many are not aware of it. Receiving counseling will enable them to understand their own risks of health complications, as well as the risk to future children.
Managing DMD Across the Lifespan
Another dimension of DMD care is providing resources and help to young people with DMD as they transition into adulthood. “In the past, we had limited treatment and mortality typically took place in the early 20s, so there weren’t a lot of patients who were adults. But as medication options have expanded and management of cardiac and respiratory failure has improved, we see a more significant proportion of adults who require adult-appropriate clinics — or, at the very least, specialists who are conversant in care or can provide care across the lifespan,” Dr. Brandsema said.
The DMD Care Considerations Working Group provides recommendations regarding care across the lifespan,9 as does the Adult North Star Network, of Muscular Dystrophy UK.17,18
Dr. Brandsema emphasized that, despite their disability, many adults with DMD “still engage with the community, and live life to its fullest.” It is to be hoped that, with ongoing research, earlier diagnosis, and improved treatment options, the future will look bright for people with DMD.
Dr. Brandsema has served as a consultant for Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech, Marathon, Momenta/Janssen, NS Pharma, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is on the medical advisory council member for Cure SMA and is a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Biohaven, Catabasis, CSL Behring, Cytokinetics, Dyne, Fibrogen, Genentech, Ionis, Lilly, ML Bio, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Ms. Kaschak has nothing to disclose.
References
1. Venugopal V and Pavlakis S. Duchenne Muscular Dystrophy. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482346/.
2. Gao QQ and McNally EM. Compr Physiol. 2015 Jul 1;5(3):1223-39. doi: 10.1002/cphy.c140048.
3. Duan D et al. Nat Rev Dis Primers. 2021 Feb 18;7(1):13. doi: 10.1038/s41572-021-00248-3.
4. Aartsma-Rus A et al. J Pediatr. 2019 Jan:204:305-313.e14. doi: 10.1016/j.jpeds.2018.10.043.
5. Broomfield J et al. Neurology. 2021 Dec 7;97(23):e2304-e2314. doi: 10.1212/WNL.0000000000012910.
6. Mercuri E et al. Front Pediatr. 2023 Nov 10:11:1276144. doi: 10.1212/WNL.0000000000012910.
7. Birnkrant DJ et al. Lancet Neurol. 2018 Mar;17(3):251-267. doi: 10.1016/S1474-4422(18)30024-3.
8. Birnkrant DJ et al. Lancet Neurol. 2018 Apr;17(4):347-361. doi: 10.1016/S1474-4422(18)30025-5.
9. Birnkrant DJ et al. Lancet Neurol. 2018 May;17(5):445-455. doi: 10.1016/S1474-4422(18)30026-7.
10. Matthews E et al. Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4.
11. Bylo M et al. Ann Pharmacother. 2020 Aug;54(8):788-794. doi: 10.1177/1060028019900500.
12. Guglieri M et al. JAMA Neurol. 2022 Oct 1;79(10):1005-1014. doi: 10.1001/jamaneurol.2022.2480.
13. Dang UJ et al. Neurology. 2024 Mar 12;102(5):e208112. doi: 10.1212/WNL.0000000000208112.
14. Mercuri E et al. Lancet Neurol. 2024 Apr;23(4):393-403. doi: 10.1016/S1474-4422(24)00036-X.
15. Gushchina LV et al. Mol Ther Nucleic Acids. 2022 Nov 9:30:479-492. doi: 10.1016/j.omtn.2022.10.025.
16. Patterson G et al. Eur J Pharmacol. 2023 May 15:947:175675. doi: 10.1016/j.ejphar.2023.175675.
17. Quinlivan R et al. J Neuromuscul Dis. 2021;8(6):899-926. doi: 10.3233/JND-200609.
18. Narayan S et al. J Neuromuscul Dis. 2022;9(3):365-381. doi: 10.3233/JND-210707.
Duchenne muscular dystrophy (DMD) is a severe progressive inherited disease characterized by muscle wasting and ultimately culminating in death. It’s a common enough neuromuscular disorder that pediatricians and family practice physicians are likely to see at least a couple of patients with DMD over the course of their career,” John Brandsema, MD, Neuromuscular Section Head, Division of Neurology, Children’s Hospital of Philadelphia in Pennsylvania, said in an interview. Healthcare providers should therefore be familiar with the disorder so as to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers as they traverse the challenging and often heartbreaking journey with this condition.
Pathophysiology and Disease Trajectory
DMD is caused by pathogenic variants in the X-linked DMD gene, leading to reduction in dystrophin, a protein that serves as a cytoskeletal integrator, stabilizing the plasma membrane of striated muscle cells. Dystrophin is critical for muscle membrane stability.2 In particular, mutations in the gene that encodes for dystrophin lead to dysfunction in Dp427m, which is the muscle isoform of dystrophin.3,4
DMD is one of several types of muscular dystrophies. All are progressive disorders. Over time, healthy muscle fibers disappear and are replaced by fibrotic tissue and fat, making the muscles “less able to generate force for everyday activity.”2 Ultimately, the skeletal muscle dysfunction affects not only the patient’s day-to-day mobility but other systems as well. Most patients with DMD eventually die of cardiac and/or respiratory failure between the ages of 20 and 40 years, with a median life expectancy of 22 years — although children born after 1990 have a somewhat higher median life expectancy (28 years), because of the improving standard of care.3,5
Typically, DMD first presents with developmental delays and weakness in skeletal leg muscles. As the disease goes through stages of progression, it starts involving upper extremities and other systems. (Table 1)
Genetic Causes of DMD
The DMD gene, located on the X chromosome, encodes for the production of dystrophin. Variants of this gene result in the lack of dystrophin protein, leading in turn to muscle fiber degeneration and the progressive symptoms of DMD. Because of the gene’s location on the X chromosome, males (who don’t have a second copy of the X chromosome) cannot compensate for the mutated gene, which is why the disease affects male children. Females with this mutation are carriers and typically do not develop the same severity of symptoms, although they might have milder muscle cramps, weakness, and cardiac issues.3
A female carrier with DMD (or any other X-linked disorder) has a 25% chance to have a carrier daughter, a 25% change of having a noncarrier daughter, a 25% chance of having an affected son, and a 25% chance of having a nonaffected son. A male with the disorder will pass the mutated gene on to his daughters who then become carriers. He cannot pass the disorder on to his sons because males inherit only the Y chromosome from their fathers.3
Diagnosing DMD
“It can take as long as 1-3 years for a child to be diagnosed with DMD,” Dr. Brandsema said. “Parents typically have concerns and know that something is ‘off’ about their child and they’re sent to various specialists, but it usually takes time for an accurate diagnosis to be made.” The mean age at diagnosis of DMD is between ages 4 and 5 years.6
Early identification of infants at risk for developing DMD can help move the needle toward earlier diagnosis. Newborn screening for DMD has been researched and piloted in several programs.6 In 2023, DMD was nominated for inclusion in the Recommended Universal Screening Panel (RUSP) for universal newborn screening. But in May 2024, the advisory committee on Heritable Disorders in Newborns and Children decided to postpone the vote to include DMD in the RUSP, requesting additional information to ensure an evidence-based decision.
In the absence of universal newborn screening for DMD, alternative approaches have been proposed to reduce the delay in clinical diagnosis and specialist referral, including increasing awareness among healthcare providers (eg, pediatricians, pediatric neurologists, and primary care physicians).6
The National Task Force for Early Identification of Childhood Neuromuscular Disorders delineates the steps necessary to identify pediatric muscle weakness and signs of neuromuscular disease. Primary care providers are encouraged to engage in regular developmental surveillance. A surveillance aid lays out the timetable for recommended visits, typical developmental milestones, and components of surveillance. Clinical evaluation includes a detailed patient history, family history, and physical examination.
If a neuromuscular condition is suspected, laboratory work should include creatinine phosphokinase (CK).6 Elevated serum CK points to leakage of CK through the muscle membrane, suggesting muscle damage. If CK is elevated, genetic testing should be performed; and, if negative, it should be followed by genetic sequencing that tests for small-scale mutations in the DMD gene. If that test is negative, a muscle biopsy should be performed to test for deep intronic mutations in the DMD gene.4
The diagnostic process and immediate steps after a confirmed DMD diagnosis is found in Figure 1.
Targeting Inflammation in DMD
Traditionally, corticosteroids have been the only available medical treatment for DMD and they remain a cornerstone of DMD management. A meta-analysis found “moderate evidence” that corticosteroid therapy improves muscle strength and function in the short term (12 months), and strength up to 2 years.10
The two most common corticosteroids for DMD are prednisone and deflazacort. Deflazacort (Emflaza, PTC Therapeutics) was approved in 2017 to treat patients ages 5 years and older with DMD, subsequently expanded to 2 years and older. Deflazacort has been found to be more effective than prednisone in improving functional outcomes, delaying the onset of cardiomyopathy, and improving overall survival, with fewer adverse effects.11
In 2023, vamorolone (Agamree, Catalyst Pharmaceuticals) was approved by the Food and Drug Administration (FDA) to treat DMD patients (ages 2 years and older). Vamorolone is a dissociative steroidal anti-inflammatory that reduces bone morbidities and is regarded as a safer alternative than prednisone. A clinical trial comparing two doses of vamorolone with prednisone for 24 weeks found that vamorolone 6 mg/kg per day met the primary endpoint (time to stand velocity) and four sequential secondary motor function endpoints, with less bone morbidity, compared to prednisone.12 A more recent trial found improvements in motor outcomes at 48 weeks with a dose of 6 mg/kg per day of vamorolone. Bone morbidities of prednisone were reversed when the patient transitioned to vamorolone.13
“Steroid treatment has been proven to help, usually taken daily, although other schedules have been tried,” Dr. Brandsema said. However, all steroids are fraught with adverse effects and are suboptimal in the long term in reducing the disease burden.
The anti-inflammatory agent givinostat (Duvyzat, ITF Therapeutics), an oral histone deacetylase (HDAC) inhibitor, was approved in March 2024 for the treatment of DMD in patients 6 years of age and older. It is the first nonsteroidal drug to treat patients with all genetic variants of the disease, and it has a unique mechanism of action. Deficits in dystrophin can lead to increased HDAC activity in DMD, reducing the expression of genes involved in muscle regeneration. Givinostat therefore can help to counteract the pathogenic events downstream of dystrophin deficiency by inhibiting HDAC.14
Approval for givinostat was based on the phase 3 EPIDYS trial, which randomized 179 boys with DMD to receive either givinostat or placebo. Although results of a functional task worsened in both groups over the 12-month study period, the decline was significantly smaller with givinostat versus placebo. The most common adverse events were diarrhea and vomiting.14 Dr. Brandsema noted that monitoring of triglycerides and platelet count is required, as hypertriglyceridemia and thrombocytopenia can occur. This treatment was studied in tandem with corticosteroids as a combination approach to muscle stabilization.
New Pharmacotherapeutic Options: Exon-Skipping Agents
Today’s treatments have expanded beyond corticosteroids, with newer therapeutic options that include targeted exon-skipping therapies and, more recently, gene therapies. “These new treatment paradigms have changed the face of DMD treatment,” Dr. Brandsema said.
“Exon-skipping drugs in their current form have only a modest effect, but at least they’re a step in the right direction and a breakthrough, in terms of slowing disease progression,” Dr. Brandsema said.
Current exon-skipping agents use antisense phosphorodiamidate morpholino oligomers (PMOs) to restore a DMD open reading frame. Next-generation drugs called cell-penetrating peptide-conjugated PMOs (PPMOs) are being actively researched, Dr. Brandsema said. These agents have shown enhanced cellular uptake and more efficient dystrophin restoration, compared with unconjugated PMOs.15
There are currently four FDA-approved exon-skipping agents for DMD, all of which are administered via a weekly intravenous infusion: Casimersen (Amondys-45, SRP-4045), approved by the FDA in 2021; Eteplirsen (Exondys 51), approved in 2016; Golodirsen (Vyondys 53,SRP-4053), approved in 2019; and Vitolarsen (Viltepso), approved in 2020. They can be associated with multiple side effects, depending on the drug, including upper respiratory infection, fever, cough, rash, and gastrointestinal issues.16 These agents have the potential to help 30% of DMD patients, restoring low levels of dystrophin.16
Gene Transfer Therapies
Gene transfer therapies, a new class of agents, utilize a nonpathogenic viral vector (adeno-associated virus) to transfer specific genes to patients with DMD. Gene therapy involves overexpressing the micro-dystrophin gene to restore functional dystrophin expression.16
Multiple clinical trials of gene therapy are currently in progress. In 2023, delandistrogene moxeparvovec-rokl (Elevidys, Serepta) was granted accelerated FDA approval for ambulatory individuals with DMD between the ages of 4 and 5 years of age and a confirmed mutation in the DMD gene. It received expanded approval in June 2024 to include ambulatory and nonambulatory individuals aged 4 years and older with DMD and a confirmed mutation in the DMD gene (with the exception of exon 8 or 9 mutations).
The approval was based on preliminary data from two double-blind, placebo-controlled studies and two open-label studies, which enrolled a total of 218 male patients (including those who received placebo) with a confirmed disease-causing mutation in the DMD gene.
Delandistrogene moxeparvovec-rokl is delivered as a one-time infusion and has been associated with side effects and “a lot of potential issues,” Dr. Brandsema said. “We’ve seen cardiac effects, immune system effects, increased muscle inflammation and hepatic complications, and some people who became quite unwell were hospitalized for a long time.”
Fortunately, he added, “these seem to be rare but they do happen. Once the medication has been delivered, it’s permanently in the body, so you’re managing the side effects potentially on a long-term basis.”
It is critical to discuss the risks and benefits of this treatment with the family and caregivers and with the patient as well, if he old enough and able to participate in the decision-making progress. “We don’t want to give unrealistic expectations and we want people to be aware of the potential downside of this treatment,” he said. “This is a very complex discussion because the trajectory of the disease is so devastating and this treatment does hold out hope that other therapies don’t necessarily have.”
Nonpharmacologic Interventions
Physical therapy is a mainstay in DMD treatment, addressing protection of fragile muscles, preservation of strength, and prevention of muscle contractures.16 Given the respiratory impairments that occur with DMD progression, respiratory monitoring and therapy are essential; however, the number and type of evaluations and interventions vary with the stage of the disease, intensifying as the disease progresses.16 Similarly, cardiac monitoring should begin early, with patients screened for cardiac complications, and should intensify through the stages of disease progression.16
Bone health is compromised in patients with DMD, both as a result of corticosteroid treatment and as part of the disease itself. Fractures may be asymptomatic and may go unnoticed. Thus, bone health surveillance and maintenance are critical components of DMD management.16
Patients with DMD often experience gastrointestinal issues. They may experience weight gain because of lack of mobility and corticosteroid use in early stages, or weight loss as a result of diet or fluid imbalance, low bone density, or dysphagia in later stages. Patients should be closely followed by a nutritionist, a gastroenterologist as needed, and a physical therapist.16
Psychosocial support “should be developed and implemented across the lifespan in a manner that promotes thinking about the future and sets expectations that individuals will actively participate in their care and daily activities.”9 This includes psychological care, neuropsychological evaluations, and educational support.
Assisting Patients and Families Through the DMD Journey
DMD care is best delivered in a multidisciplinary setting, where physicians of relevant specialties, physical and occupational therapists, nutritionists, social workers, and genetic counselors collaborate. At Children’s Hospital of Philadelphia, DMD care is delivered through this collaborative model.
Unfortunately, Dr. Brandsema said, many patients don’t have this type of multidisciplinary resource available. “One specialist, such as a pulmonologist or neurologist, might have to be the sole source of care.” Or parents may have to ferry their child to multiple specialists in disparate locations, placing extra stress on an already-stressed family system.
“It’s helpful to connect the family with a comprehensive care center, if possible,” Dr. Brandsema advised. If that’s not available, then he suggests recommending educational opportunities and resources through national organizations such as the Muscular Dystrophy Association; Parent Project MD; NORD; Friends, Family and Duchenne; and Cure Duchenne. Families and caregivers, along with affected individuals, can get education and support from people who understand the day-to-day reality of living with this disease.
One of the major challenges that families face is navigating the high cost of treating DMD, especially the new medications, Dr. Brandsema said. “The authorization process can be intensive and long, and the family may need to take an active role, together with the provider team, in advocating for the patient to get access.”
Among her many responsibilities, Ms. Kaschak engages in care coordination tasks and management, helps patients and caregivers understand care plans, and provides psychosocial support and education about the disease process. She assists families in completing paperwork and navigating specialty authorizations, helping families understand and navigate the complex insurance process. “My role is to bridge gaps in care,” she said.
Dr. Brandsema noted that it’s important for couples to receive genetic counseling if they’re planning to have multiple children because there is a 50% chance that their next boy will be affected. About two thirds of mothers with children who have DMD are carriers, but many are not aware of it. Receiving counseling will enable them to understand their own risks of health complications, as well as the risk to future children.
Managing DMD Across the Lifespan
Another dimension of DMD care is providing resources and help to young people with DMD as they transition into adulthood. “In the past, we had limited treatment and mortality typically took place in the early 20s, so there weren’t a lot of patients who were adults. But as medication options have expanded and management of cardiac and respiratory failure has improved, we see a more significant proportion of adults who require adult-appropriate clinics — or, at the very least, specialists who are conversant in care or can provide care across the lifespan,” Dr. Brandsema said.
The DMD Care Considerations Working Group provides recommendations regarding care across the lifespan,9 as does the Adult North Star Network, of Muscular Dystrophy UK.17,18
Dr. Brandsema emphasized that, despite their disability, many adults with DMD “still engage with the community, and live life to its fullest.” It is to be hoped that, with ongoing research, earlier diagnosis, and improved treatment options, the future will look bright for people with DMD.
Dr. Brandsema has served as a consultant for Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech, Marathon, Momenta/Janssen, NS Pharma, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is on the medical advisory council member for Cure SMA and is a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Biohaven, Catabasis, CSL Behring, Cytokinetics, Dyne, Fibrogen, Genentech, Ionis, Lilly, ML Bio, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Ms. Kaschak has nothing to disclose.
References
1. Venugopal V and Pavlakis S. Duchenne Muscular Dystrophy. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482346/.
2. Gao QQ and McNally EM. Compr Physiol. 2015 Jul 1;5(3):1223-39. doi: 10.1002/cphy.c140048.
3. Duan D et al. Nat Rev Dis Primers. 2021 Feb 18;7(1):13. doi: 10.1038/s41572-021-00248-3.
4. Aartsma-Rus A et al. J Pediatr. 2019 Jan:204:305-313.e14. doi: 10.1016/j.jpeds.2018.10.043.
5. Broomfield J et al. Neurology. 2021 Dec 7;97(23):e2304-e2314. doi: 10.1212/WNL.0000000000012910.
6. Mercuri E et al. Front Pediatr. 2023 Nov 10:11:1276144. doi: 10.1212/WNL.0000000000012910.
7. Birnkrant DJ et al. Lancet Neurol. 2018 Mar;17(3):251-267. doi: 10.1016/S1474-4422(18)30024-3.
8. Birnkrant DJ et al. Lancet Neurol. 2018 Apr;17(4):347-361. doi: 10.1016/S1474-4422(18)30025-5.
9. Birnkrant DJ et al. Lancet Neurol. 2018 May;17(5):445-455. doi: 10.1016/S1474-4422(18)30026-7.
10. Matthews E et al. Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4.
11. Bylo M et al. Ann Pharmacother. 2020 Aug;54(8):788-794. doi: 10.1177/1060028019900500.
12. Guglieri M et al. JAMA Neurol. 2022 Oct 1;79(10):1005-1014. doi: 10.1001/jamaneurol.2022.2480.
13. Dang UJ et al. Neurology. 2024 Mar 12;102(5):e208112. doi: 10.1212/WNL.0000000000208112.
14. Mercuri E et al. Lancet Neurol. 2024 Apr;23(4):393-403. doi: 10.1016/S1474-4422(24)00036-X.
15. Gushchina LV et al. Mol Ther Nucleic Acids. 2022 Nov 9:30:479-492. doi: 10.1016/j.omtn.2022.10.025.
16. Patterson G et al. Eur J Pharmacol. 2023 May 15:947:175675. doi: 10.1016/j.ejphar.2023.175675.
17. Quinlivan R et al. J Neuromuscul Dis. 2021;8(6):899-926. doi: 10.3233/JND-200609.
18. Narayan S et al. J Neuromuscul Dis. 2022;9(3):365-381. doi: 10.3233/JND-210707.
Duchenne muscular dystrophy (DMD) is a severe progressive inherited disease characterized by muscle wasting and ultimately culminating in death. It’s a common enough neuromuscular disorder that pediatricians and family practice physicians are likely to see at least a couple of patients with DMD over the course of their career,” John Brandsema, MD, Neuromuscular Section Head, Division of Neurology, Children’s Hospital of Philadelphia in Pennsylvania, said in an interview. Healthcare providers should therefore be familiar with the disorder so as to provide timely diagnosis and early intervention as well as practical and emotional support to the patient and family/caregivers as they traverse the challenging and often heartbreaking journey with this condition.
Pathophysiology and Disease Trajectory
DMD is caused by pathogenic variants in the X-linked DMD gene, leading to reduction in dystrophin, a protein that serves as a cytoskeletal integrator, stabilizing the plasma membrane of striated muscle cells. Dystrophin is critical for muscle membrane stability.2 In particular, mutations in the gene that encodes for dystrophin lead to dysfunction in Dp427m, which is the muscle isoform of dystrophin.3,4
DMD is one of several types of muscular dystrophies. All are progressive disorders. Over time, healthy muscle fibers disappear and are replaced by fibrotic tissue and fat, making the muscles “less able to generate force for everyday activity.”2 Ultimately, the skeletal muscle dysfunction affects not only the patient’s day-to-day mobility but other systems as well. Most patients with DMD eventually die of cardiac and/or respiratory failure between the ages of 20 and 40 years, with a median life expectancy of 22 years — although children born after 1990 have a somewhat higher median life expectancy (28 years), because of the improving standard of care.3,5
Typically, DMD first presents with developmental delays and weakness in skeletal leg muscles. As the disease goes through stages of progression, it starts involving upper extremities and other systems. (Table 1)
Genetic Causes of DMD
The DMD gene, located on the X chromosome, encodes for the production of dystrophin. Variants of this gene result in the lack of dystrophin protein, leading in turn to muscle fiber degeneration and the progressive symptoms of DMD. Because of the gene’s location on the X chromosome, males (who don’t have a second copy of the X chromosome) cannot compensate for the mutated gene, which is why the disease affects male children. Females with this mutation are carriers and typically do not develop the same severity of symptoms, although they might have milder muscle cramps, weakness, and cardiac issues.3
A female carrier with DMD (or any other X-linked disorder) has a 25% chance to have a carrier daughter, a 25% change of having a noncarrier daughter, a 25% chance of having an affected son, and a 25% chance of having a nonaffected son. A male with the disorder will pass the mutated gene on to his daughters who then become carriers. He cannot pass the disorder on to his sons because males inherit only the Y chromosome from their fathers.3
Diagnosing DMD
“It can take as long as 1-3 years for a child to be diagnosed with DMD,” Dr. Brandsema said. “Parents typically have concerns and know that something is ‘off’ about their child and they’re sent to various specialists, but it usually takes time for an accurate diagnosis to be made.” The mean age at diagnosis of DMD is between ages 4 and 5 years.6
Early identification of infants at risk for developing DMD can help move the needle toward earlier diagnosis. Newborn screening for DMD has been researched and piloted in several programs.6 In 2023, DMD was nominated for inclusion in the Recommended Universal Screening Panel (RUSP) for universal newborn screening. But in May 2024, the advisory committee on Heritable Disorders in Newborns and Children decided to postpone the vote to include DMD in the RUSP, requesting additional information to ensure an evidence-based decision.
In the absence of universal newborn screening for DMD, alternative approaches have been proposed to reduce the delay in clinical diagnosis and specialist referral, including increasing awareness among healthcare providers (eg, pediatricians, pediatric neurologists, and primary care physicians).6
The National Task Force for Early Identification of Childhood Neuromuscular Disorders delineates the steps necessary to identify pediatric muscle weakness and signs of neuromuscular disease. Primary care providers are encouraged to engage in regular developmental surveillance. A surveillance aid lays out the timetable for recommended visits, typical developmental milestones, and components of surveillance. Clinical evaluation includes a detailed patient history, family history, and physical examination.
If a neuromuscular condition is suspected, laboratory work should include creatinine phosphokinase (CK).6 Elevated serum CK points to leakage of CK through the muscle membrane, suggesting muscle damage. If CK is elevated, genetic testing should be performed; and, if negative, it should be followed by genetic sequencing that tests for small-scale mutations in the DMD gene. If that test is negative, a muscle biopsy should be performed to test for deep intronic mutations in the DMD gene.4
The diagnostic process and immediate steps after a confirmed DMD diagnosis is found in Figure 1.
Targeting Inflammation in DMD
Traditionally, corticosteroids have been the only available medical treatment for DMD and they remain a cornerstone of DMD management. A meta-analysis found “moderate evidence” that corticosteroid therapy improves muscle strength and function in the short term (12 months), and strength up to 2 years.10
The two most common corticosteroids for DMD are prednisone and deflazacort. Deflazacort (Emflaza, PTC Therapeutics) was approved in 2017 to treat patients ages 5 years and older with DMD, subsequently expanded to 2 years and older. Deflazacort has been found to be more effective than prednisone in improving functional outcomes, delaying the onset of cardiomyopathy, and improving overall survival, with fewer adverse effects.11
In 2023, vamorolone (Agamree, Catalyst Pharmaceuticals) was approved by the Food and Drug Administration (FDA) to treat DMD patients (ages 2 years and older). Vamorolone is a dissociative steroidal anti-inflammatory that reduces bone morbidities and is regarded as a safer alternative than prednisone. A clinical trial comparing two doses of vamorolone with prednisone for 24 weeks found that vamorolone 6 mg/kg per day met the primary endpoint (time to stand velocity) and four sequential secondary motor function endpoints, with less bone morbidity, compared to prednisone.12 A more recent trial found improvements in motor outcomes at 48 weeks with a dose of 6 mg/kg per day of vamorolone. Bone morbidities of prednisone were reversed when the patient transitioned to vamorolone.13
“Steroid treatment has been proven to help, usually taken daily, although other schedules have been tried,” Dr. Brandsema said. However, all steroids are fraught with adverse effects and are suboptimal in the long term in reducing the disease burden.
The anti-inflammatory agent givinostat (Duvyzat, ITF Therapeutics), an oral histone deacetylase (HDAC) inhibitor, was approved in March 2024 for the treatment of DMD in patients 6 years of age and older. It is the first nonsteroidal drug to treat patients with all genetic variants of the disease, and it has a unique mechanism of action. Deficits in dystrophin can lead to increased HDAC activity in DMD, reducing the expression of genes involved in muscle regeneration. Givinostat therefore can help to counteract the pathogenic events downstream of dystrophin deficiency by inhibiting HDAC.14
Approval for givinostat was based on the phase 3 EPIDYS trial, which randomized 179 boys with DMD to receive either givinostat or placebo. Although results of a functional task worsened in both groups over the 12-month study period, the decline was significantly smaller with givinostat versus placebo. The most common adverse events were diarrhea and vomiting.14 Dr. Brandsema noted that monitoring of triglycerides and platelet count is required, as hypertriglyceridemia and thrombocytopenia can occur. This treatment was studied in tandem with corticosteroids as a combination approach to muscle stabilization.
New Pharmacotherapeutic Options: Exon-Skipping Agents
Today’s treatments have expanded beyond corticosteroids, with newer therapeutic options that include targeted exon-skipping therapies and, more recently, gene therapies. “These new treatment paradigms have changed the face of DMD treatment,” Dr. Brandsema said.
“Exon-skipping drugs in their current form have only a modest effect, but at least they’re a step in the right direction and a breakthrough, in terms of slowing disease progression,” Dr. Brandsema said.
Current exon-skipping agents use antisense phosphorodiamidate morpholino oligomers (PMOs) to restore a DMD open reading frame. Next-generation drugs called cell-penetrating peptide-conjugated PMOs (PPMOs) are being actively researched, Dr. Brandsema said. These agents have shown enhanced cellular uptake and more efficient dystrophin restoration, compared with unconjugated PMOs.15
There are currently four FDA-approved exon-skipping agents for DMD, all of which are administered via a weekly intravenous infusion: Casimersen (Amondys-45, SRP-4045), approved by the FDA in 2021; Eteplirsen (Exondys 51), approved in 2016; Golodirsen (Vyondys 53,SRP-4053), approved in 2019; and Vitolarsen (Viltepso), approved in 2020. They can be associated with multiple side effects, depending on the drug, including upper respiratory infection, fever, cough, rash, and gastrointestinal issues.16 These agents have the potential to help 30% of DMD patients, restoring low levels of dystrophin.16
Gene Transfer Therapies
Gene transfer therapies, a new class of agents, utilize a nonpathogenic viral vector (adeno-associated virus) to transfer specific genes to patients with DMD. Gene therapy involves overexpressing the micro-dystrophin gene to restore functional dystrophin expression.16
Multiple clinical trials of gene therapy are currently in progress. In 2023, delandistrogene moxeparvovec-rokl (Elevidys, Serepta) was granted accelerated FDA approval for ambulatory individuals with DMD between the ages of 4 and 5 years of age and a confirmed mutation in the DMD gene. It received expanded approval in June 2024 to include ambulatory and nonambulatory individuals aged 4 years and older with DMD and a confirmed mutation in the DMD gene (with the exception of exon 8 or 9 mutations).
The approval was based on preliminary data from two double-blind, placebo-controlled studies and two open-label studies, which enrolled a total of 218 male patients (including those who received placebo) with a confirmed disease-causing mutation in the DMD gene.
Delandistrogene moxeparvovec-rokl is delivered as a one-time infusion and has been associated with side effects and “a lot of potential issues,” Dr. Brandsema said. “We’ve seen cardiac effects, immune system effects, increased muscle inflammation and hepatic complications, and some people who became quite unwell were hospitalized for a long time.”
Fortunately, he added, “these seem to be rare but they do happen. Once the medication has been delivered, it’s permanently in the body, so you’re managing the side effects potentially on a long-term basis.”
It is critical to discuss the risks and benefits of this treatment with the family and caregivers and with the patient as well, if he old enough and able to participate in the decision-making progress. “We don’t want to give unrealistic expectations and we want people to be aware of the potential downside of this treatment,” he said. “This is a very complex discussion because the trajectory of the disease is so devastating and this treatment does hold out hope that other therapies don’t necessarily have.”
Nonpharmacologic Interventions
Physical therapy is a mainstay in DMD treatment, addressing protection of fragile muscles, preservation of strength, and prevention of muscle contractures.16 Given the respiratory impairments that occur with DMD progression, respiratory monitoring and therapy are essential; however, the number and type of evaluations and interventions vary with the stage of the disease, intensifying as the disease progresses.16 Similarly, cardiac monitoring should begin early, with patients screened for cardiac complications, and should intensify through the stages of disease progression.16
Bone health is compromised in patients with DMD, both as a result of corticosteroid treatment and as part of the disease itself. Fractures may be asymptomatic and may go unnoticed. Thus, bone health surveillance and maintenance are critical components of DMD management.16
Patients with DMD often experience gastrointestinal issues. They may experience weight gain because of lack of mobility and corticosteroid use in early stages, or weight loss as a result of diet or fluid imbalance, low bone density, or dysphagia in later stages. Patients should be closely followed by a nutritionist, a gastroenterologist as needed, and a physical therapist.16
Psychosocial support “should be developed and implemented across the lifespan in a manner that promotes thinking about the future and sets expectations that individuals will actively participate in their care and daily activities.”9 This includes psychological care, neuropsychological evaluations, and educational support.
Assisting Patients and Families Through the DMD Journey
DMD care is best delivered in a multidisciplinary setting, where physicians of relevant specialties, physical and occupational therapists, nutritionists, social workers, and genetic counselors collaborate. At Children’s Hospital of Philadelphia, DMD care is delivered through this collaborative model.
Unfortunately, Dr. Brandsema said, many patients don’t have this type of multidisciplinary resource available. “One specialist, such as a pulmonologist or neurologist, might have to be the sole source of care.” Or parents may have to ferry their child to multiple specialists in disparate locations, placing extra stress on an already-stressed family system.
“It’s helpful to connect the family with a comprehensive care center, if possible,” Dr. Brandsema advised. If that’s not available, then he suggests recommending educational opportunities and resources through national organizations such as the Muscular Dystrophy Association; Parent Project MD; NORD; Friends, Family and Duchenne; and Cure Duchenne. Families and caregivers, along with affected individuals, can get education and support from people who understand the day-to-day reality of living with this disease.
One of the major challenges that families face is navigating the high cost of treating DMD, especially the new medications, Dr. Brandsema said. “The authorization process can be intensive and long, and the family may need to take an active role, together with the provider team, in advocating for the patient to get access.”
Among her many responsibilities, Ms. Kaschak engages in care coordination tasks and management, helps patients and caregivers understand care plans, and provides psychosocial support and education about the disease process. She assists families in completing paperwork and navigating specialty authorizations, helping families understand and navigate the complex insurance process. “My role is to bridge gaps in care,” she said.
Dr. Brandsema noted that it’s important for couples to receive genetic counseling if they’re planning to have multiple children because there is a 50% chance that their next boy will be affected. About two thirds of mothers with children who have DMD are carriers, but many are not aware of it. Receiving counseling will enable them to understand their own risks of health complications, as well as the risk to future children.
Managing DMD Across the Lifespan
Another dimension of DMD care is providing resources and help to young people with DMD as they transition into adulthood. “In the past, we had limited treatment and mortality typically took place in the early 20s, so there weren’t a lot of patients who were adults. But as medication options have expanded and management of cardiac and respiratory failure has improved, we see a more significant proportion of adults who require adult-appropriate clinics — or, at the very least, specialists who are conversant in care or can provide care across the lifespan,” Dr. Brandsema said.
The DMD Care Considerations Working Group provides recommendations regarding care across the lifespan,9 as does the Adult North Star Network, of Muscular Dystrophy UK.17,18
Dr. Brandsema emphasized that, despite their disability, many adults with DMD “still engage with the community, and live life to its fullest.” It is to be hoped that, with ongoing research, earlier diagnosis, and improved treatment options, the future will look bright for people with DMD.
Dr. Brandsema has served as a consultant for Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech, Marathon, Momenta/Janssen, NS Pharma, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is on the medical advisory council member for Cure SMA and is a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Biohaven, Catabasis, CSL Behring, Cytokinetics, Dyne, Fibrogen, Genentech, Ionis, Lilly, ML Bio, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Ms. Kaschak has nothing to disclose.
References
1. Venugopal V and Pavlakis S. Duchenne Muscular Dystrophy. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482346/.
2. Gao QQ and McNally EM. Compr Physiol. 2015 Jul 1;5(3):1223-39. doi: 10.1002/cphy.c140048.
3. Duan D et al. Nat Rev Dis Primers. 2021 Feb 18;7(1):13. doi: 10.1038/s41572-021-00248-3.
4. Aartsma-Rus A et al. J Pediatr. 2019 Jan:204:305-313.e14. doi: 10.1016/j.jpeds.2018.10.043.
5. Broomfield J et al. Neurology. 2021 Dec 7;97(23):e2304-e2314. doi: 10.1212/WNL.0000000000012910.
6. Mercuri E et al. Front Pediatr. 2023 Nov 10:11:1276144. doi: 10.1212/WNL.0000000000012910.
7. Birnkrant DJ et al. Lancet Neurol. 2018 Mar;17(3):251-267. doi: 10.1016/S1474-4422(18)30024-3.
8. Birnkrant DJ et al. Lancet Neurol. 2018 Apr;17(4):347-361. doi: 10.1016/S1474-4422(18)30025-5.
9. Birnkrant DJ et al. Lancet Neurol. 2018 May;17(5):445-455. doi: 10.1016/S1474-4422(18)30026-7.
10. Matthews E et al. Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4.
11. Bylo M et al. Ann Pharmacother. 2020 Aug;54(8):788-794. doi: 10.1177/1060028019900500.
12. Guglieri M et al. JAMA Neurol. 2022 Oct 1;79(10):1005-1014. doi: 10.1001/jamaneurol.2022.2480.
13. Dang UJ et al. Neurology. 2024 Mar 12;102(5):e208112. doi: 10.1212/WNL.0000000000208112.
14. Mercuri E et al. Lancet Neurol. 2024 Apr;23(4):393-403. doi: 10.1016/S1474-4422(24)00036-X.
15. Gushchina LV et al. Mol Ther Nucleic Acids. 2022 Nov 9:30:479-492. doi: 10.1016/j.omtn.2022.10.025.
16. Patterson G et al. Eur J Pharmacol. 2023 May 15:947:175675. doi: 10.1016/j.ejphar.2023.175675.
17. Quinlivan R et al. J Neuromuscul Dis. 2021;8(6):899-926. doi: 10.3233/JND-200609.
18. Narayan S et al. J Neuromuscul Dis. 2022;9(3):365-381. doi: 10.3233/JND-210707.
Promise for Disease-Modifying Therapies to Tame Huntington’s Disease
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
In April 1872, The Medical and Surgical Reporter of Philadelphia published a roughly 3,000-word paper, titled “On Chorea,” by George Huntington, a 22-year-old family practice physician recently graduated from Columbia University, New York City.
“Chorea is essentially a disease of the nervous system. The name ‘chorea’ is given to the disease on account of the dancing propensities of those who are affected by it, and it is a very appropriate designation,” he wrote in the introduction.
Toward the end of the paper Dr. Huntington described a “hereditary chorea” that he had observed while on professional rounds with his father, also a physician, in towns on the eastern end of Long Island in New York.
“It is spoken of by those in whose veins the seeds of the disease are known to exist, with a kind of horror, and not at all alluded to except through dire necessity, when it is mentioned as ‘that disorder,’ ” he wrote, noting later that “I have never known a recovery or even an amelioration of symptoms in this form of chorea; when once it begins it clings to the bitter end.”1
It wasn’t until 1993 that a team of investigators identified the gene responsible for the neurodegenerative disorder we now know as Huntington’s disease.
That discovery sparked hope for better treatments and a cure, but progress over the last 31 years has been incremental. Nonetheless, recent intensive research into novel approaches for treating Huntington’s disease have considerably brightened prospects for patients and caregivers, experts say.
“This is a devastating neurodegenerative disease and in talking to families that I have had the privilege and honor of following, I hear from them that ‘in 1993 when the gene was identified and my family member — parent, grandparent, aunt, uncle — had Huntington’s, we thought that there would be a curative or disease-modifying therapy in no time,’ ” said Erin Furr Stimming, MD, FAAN, FANA professor of neurology and Memorial Hermann Endowed Chair at the McGovern Medical School at University of Texas Health Science Center in Houston.
“Here we are in 2024. I think our families are still so incredibly resilient and courageous, and they are willing and able to participate in clinical trials. Families in the Huntington’s disease community at large are really ready to have trials that do, in fact, demonstrate some evidence of slowing of disease progression. It’s an exciting time,” she said in an interview.
Repeating Nucleotides
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by an expansion of a CAG trinucleotide repeat in exon 1 of the huntingtin gene (HTT), which encodes for the huntingtin protein (HTT). The multiple repeats result in expanded expression of mutant HTT. The mutated protein disrupts normal cellular processes, alters intracellular calcium homeostasis, and interferes with gene transcription, leading to progressive degeneration of neurons, and to a hallmark Huntington’s disease triad consisting of movement disorders, cognitive decline, and mood/behavioral issues.
The number of repeats determines the severity of disease and the age of onset. In nonaffected persons the gene contains about 20 CAG repeats, but as genetics research dating from the 1990s has shown, a single HTT allele containing more than 40 CAG repeats will inevitably result in disease, whereas carriers with fewer than 36 repeats on both alleles will remain unaffected.2
The prevalence of the mutation in Western populations is estimated to be from 4 to 10 per 100,000.3
The disease usually manifests first in adults aged 30-50 years but may also occur in children or adolescents and young adults. Early symptoms often include a decline in executive function that may be noticeable to the patient’s family and friends, mood changes, and chorea.
“Because of the uncontrolled movements (chorea), a person with Huntington’s disease may lose a lot of weight without intending to, and may have trouble walking, balancing, and moving around safely. They will eventually lose the ability to work, drive, and manage tasks at home, and may qualify for disability benefits. Over time, the individual will develop difficulty with speaking and swallowing, and their movements will become slow and stiff. People with advanced Huntington’s disease need full-time care to help with their day-to-day activities, and they ultimately succumb to pneumonia, heart failure, or other complications,” according to the Huntington’s Disease Society of America.4
Managing Symptoms
say neurologists.
There are currently three medications approved for the treatment of chorea, all in the class of agents known as vesicular monoamine transporter 2 inhibitors. These agents are tetrabenazine (Xenazine, Lundbeck), deutetrabenazine (Austedo XR, Teva), and valbenazine (Ingrezza, Neurocrine Biosciences).
“These drugs can treat the chorea, but they also can help with some of the other motor features. For example, if a patient has chorea in the legs and you treat it, then maybe they’re walking will get better, or if they have chorea in the mouth and you treat the chorea, then maybe their speech and swallowing may improve,” Victor Sung, MD, professor of neurology and director of the Huntington’s Disease Clinic at the University of Alabama at Birmingham, said in an interview.
Mood and behavioral symptoms associated with Huntington’s disease – depression, anxiety, irritability, impulsivity, etc – can be managed with off-label use of antidepressants, mood stabilizers, and antipsychotic agents.
“When it comes to the cognitive symptoms, that’s a big gap where we don’t have anything that’s [Food and Drug Administration] approved for Huntington’s disease,” Dr. Sung said.
Agents used to treat Alzheimer’s disease, such as cholinesterase inhibitors and the glutamate receptor antagonist memantine, have been studied extensively for preservation of cognition in Huntington’s disease, but have not shown significant benefit.
Dr. Sung said that one promising approach to the problem of cognitive protection in Huntington’s disease is the investigational agent dalzanemdor (SAGE-718), an N-methyl-D-aspartic (NMDA) acid receptor positive allosteric modulator. The drug is in development for cognitive disorders associated with NMDA receptor dysfunction, including Huntington’s disease and Alzheimer’s disease.
In the phase 2 SURVEYOR trial, which was not powered to show efficacy of dalzanemdor over placebo, patients with Huntington’s disease reportedly tolerated the drug well, with treatment-related adverse events primarily mild or moderate in severity.
As of this writing dalzanemdor is being evaluated for efficacy, compared with placebo, in the phase 2 DIMENSION trial. The primary endpoint of this trial is a change from baseline in composite score of the Huntington’s Disease Cognitive Assessment Battery.
Disease-Modifying Therapies
As previously noted, although there is no cure for Huntington’s disease, neurology investigators are developing new strategies for delaying, stalling, or even preventing disease progression.
“There are so many different approaches, it’s hard to know where to start,” Christopher A. Ross, MD, director of the Huntington’s Disease Center at Johns Hopkins University in Baltimore, Maryland, said in an interview.
The most actively investigated approach may be huntingtin-lowering therapies, based on the supposition that mutant huntingtin protein (mHTT) is the primary toxin in Huntington’s disease.
“What you need to do is in some way lower it, and there are a number of different ways to do that: antinsense olignoclueotides, small interfering RNA, CRISPR Cas, or other gene-editing techniques, as well as gene therapy with an adenoviral vector” he said.
Gene Therapy
Dr. Ross cited as promising a phase 1/2 clinical trial of an investigational gene therapy, AMT-130 (uniQure), which consists of an adeno-associated virus vector and a gene encoding a microRNA that is designed to recognize, bind, and nonselectively lower both mHTT and wild-type HTT.
The compound is injected directly into the corpus striatum. It was demonstrated to decrease signs of Huntington’s disease in animal models, and interim data on 29 patients with Huntington’s disease followed for up to 24 months showed a statically significant, dose-dependent slowing of Huntington’s disease progression and lowering of neurofilament light protein, a marker for neuronal degeneration in Huntington’s disease, in cerebrospinal fluid (CSF).
AMT-130 targets exon 1 of the HTT gene, which appears to be an important target, Dr. Ross commented.
“The huntingtin protein is really big. The polyglutamine expansion, which is what causes the toxicity, is right at the N-terminus of exon 1, and there is increasingly good evidence that you can have an exon 1 misspliced protein product which causes the toxicity, so it may be especially important to lower Huntington by targeting exon 1,” he said.
Oral Splicing Modifier
PTC Therapeutics, based in New Jersey, is developing PTC518, an oral small molecule drug that can cross the blood-brain barrier and is reported to target mutant huntingtin protein.
The drug is a splicing modifier that promotes insertion of a premature stop codon to HTT mRNA, thereby degrading and lowering the HTT levels.5
In June 2024, the company reported that, in the phase 2a PIVOT-HD study of PTC518 in patients with Huntington’s disease, 12 months of treatment was associated with a dose-dependent lowering of mHTT in blood and CSF in an interim cohort.
“In addition, favorable trends were demonstrated on several relevant Huntington’s disease clinical assessments including Total Motor Score and Composite Unified Huntington’s Disease Rating Scale. Furthermore, following 12 months of treatment, PTC518 continues to be safe and well tolerated,” the company stated in a press release.
Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) are short strands of DNA or RNA that bind to RNA sequences in faulty genes to modify production of target proteins.
One such drug, tominersen, developed jointly by Ionis and Roche, initially showed promise in a phase 1/2 trial for lowering mHTT in CSF without serious adverse events. But in a phase 3 trial, the intrathecally delivered agent was halted after an independent data monitoring committee recommended halting the trial, which Roche ended in 2021. The company reported in a letter to The New England Journal of Medicine that people in the high-dosage treatment group did measurably worse – although it remains unclear whether this was caused by excess protein lowering or an off-target effect.6 The tominersen program was the first to demonstrate that it was possible to lower HTT with an intervention, and the companies reported that they are continuing the agent’s development program.
Wave Life Sciences is developing an ASO, labeled WVE-003, designed to target a single-nucleotide polymorphism associated with the mHTT mRNA transcript within HTT. The company says that targeting the single-nucleotide polymorphism should allow lowering of expression of the mHTT will preserving wild-type HTT. This approach has the potential for therapies to prevent disease progression during the prodromal period, the company states.
Somatic Expansion
Dr. Furr Stimming and Dr. Ross both noted that there is considerable research interest into recently identified genetic modifiers that are believed to influence somatic instability, which in turn leads to somatic expansion.
“That seems to happen selectively in the neurons that are affected in Huntington’s disease. So a big puzzle for all of the neurodegenerative diseases is why are certain regions of the brain affected and other regions not? And it looks like, for the repeat expansion, this idea of somatic expansion seems to be increasingly central,” Dr. Ross said.
“The really exciting idea here is that, if somatic expansion is critical to the disease process and you could slow it down or stop it, you could go very early and potentially not just slow the progression of the disease once it starts, but conceivably even delay or possibly prevent the onset of Huntington’s disease,” he said.
Does HTT Lowering Mediate Progression?
“The next question is what does this mean clinically? Does lowering mutant huntingtin protein levels, and wild type for that matter, actually slow disease progression, which can be challenging to measure in a disease that is relatively slowly progressive?,” Dr. Furr Stimming said.
One important tool to help answer this question, she noted, is the Huntington’s Disease Integrated Staging System (HDISS), first described in 2022.7 The consensus-based system incorporates biological, clinical, and functional assessments, and characterizes patients from birth by stages, from stage 0 (persons who carry the mutation but have no detectable pathology), to stage 1 (measurable indicators of underlying pathophysiology), stage 2 (a detectable clinical phenotype), and finally to stage 3 (decline in function).
“The goal of the HDISS, which is designed for clinical research purposes, is to try to enroll individuals into clinical trials earlier rather than later, trying to get pharmaceutical companies and others to take advantage of the period prior to significant neurodegeneration occurring. Like Alzheimer’s disease and Parkinson’s disease, by the time we make a clinical diagnose significant neurodegeneration has occurred, so we really want to take advantage of the prodromal period to intervene with these potential disease-modifying therapies,” Dr. Furr Stimming said.
“At the end of the day, I suspect that we will not have just one effective disease-modifying therapy. I suspect that it will be a multifacted approach. We envision that we would hit the huntingtin protein from a few different angles. I envision that we would not only modify in some form or fashion production of the mutant huntingtin protein, but also try to influence the genetic modifiers that we think are important in somatic instability and expansion, which likely contributes to the rate of progression and symptom severity,” Dr. Furr Stimming said.
References
1. Huntington G. On Chorea. Reprinted in The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(1):109-112. doi: 10.1176/jnp.15.1.109.
2. Kaemmerer WF and Grondin RC. Degener Neurol Neuromuscul Dis. 2019 Mar 8;9:3-17. doi: 10.2147/DNND.S163808.
3. Jimenez-Sanchez M et al. Cold Spring Harb Perspect Med. 2017 Jul 5;7(7):a024240. doi: 10.1101/cshperspect.a024240.
4. Huntington’s Disease Society of America. Overview of Huntington’s Disease. https://hdsa.org/what-is-hd/overview-of-huntingtons-disease/.
5. Beers B et al. J Neurol Neurosurg Psychiatry. 2022;93:A96. https://jnnp.bmj.com/content/93/Suppl_1/A96.1.
6. McColgan P et al. N Engl J Med. 2023 Dec 7;389(23):2203-2205. doi: 10.1056/NEJMc2300400.
7. Tabrizi SJ et al. Lancet Neurol. 2022 Jul;21(7):632-644. doi: 10.1016/S1474-4422(22)00120-X.
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
In April 1872, The Medical and Surgical Reporter of Philadelphia published a roughly 3,000-word paper, titled “On Chorea,” by George Huntington, a 22-year-old family practice physician recently graduated from Columbia University, New York City.
“Chorea is essentially a disease of the nervous system. The name ‘chorea’ is given to the disease on account of the dancing propensities of those who are affected by it, and it is a very appropriate designation,” he wrote in the introduction.
Toward the end of the paper Dr. Huntington described a “hereditary chorea” that he had observed while on professional rounds with his father, also a physician, in towns on the eastern end of Long Island in New York.
“It is spoken of by those in whose veins the seeds of the disease are known to exist, with a kind of horror, and not at all alluded to except through dire necessity, when it is mentioned as ‘that disorder,’ ” he wrote, noting later that “I have never known a recovery or even an amelioration of symptoms in this form of chorea; when once it begins it clings to the bitter end.”1
It wasn’t until 1993 that a team of investigators identified the gene responsible for the neurodegenerative disorder we now know as Huntington’s disease.
That discovery sparked hope for better treatments and a cure, but progress over the last 31 years has been incremental. Nonetheless, recent intensive research into novel approaches for treating Huntington’s disease have considerably brightened prospects for patients and caregivers, experts say.
“This is a devastating neurodegenerative disease and in talking to families that I have had the privilege and honor of following, I hear from them that ‘in 1993 when the gene was identified and my family member — parent, grandparent, aunt, uncle — had Huntington’s, we thought that there would be a curative or disease-modifying therapy in no time,’ ” said Erin Furr Stimming, MD, FAAN, FANA professor of neurology and Memorial Hermann Endowed Chair at the McGovern Medical School at University of Texas Health Science Center in Houston.
“Here we are in 2024. I think our families are still so incredibly resilient and courageous, and they are willing and able to participate in clinical trials. Families in the Huntington’s disease community at large are really ready to have trials that do, in fact, demonstrate some evidence of slowing of disease progression. It’s an exciting time,” she said in an interview.
Repeating Nucleotides
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by an expansion of a CAG trinucleotide repeat in exon 1 of the huntingtin gene (HTT), which encodes for the huntingtin protein (HTT). The multiple repeats result in expanded expression of mutant HTT. The mutated protein disrupts normal cellular processes, alters intracellular calcium homeostasis, and interferes with gene transcription, leading to progressive degeneration of neurons, and to a hallmark Huntington’s disease triad consisting of movement disorders, cognitive decline, and mood/behavioral issues.
The number of repeats determines the severity of disease and the age of onset. In nonaffected persons the gene contains about 20 CAG repeats, but as genetics research dating from the 1990s has shown, a single HTT allele containing more than 40 CAG repeats will inevitably result in disease, whereas carriers with fewer than 36 repeats on both alleles will remain unaffected.2
The prevalence of the mutation in Western populations is estimated to be from 4 to 10 per 100,000.3
The disease usually manifests first in adults aged 30-50 years but may also occur in children or adolescents and young adults. Early symptoms often include a decline in executive function that may be noticeable to the patient’s family and friends, mood changes, and chorea.
“Because of the uncontrolled movements (chorea), a person with Huntington’s disease may lose a lot of weight without intending to, and may have trouble walking, balancing, and moving around safely. They will eventually lose the ability to work, drive, and manage tasks at home, and may qualify for disability benefits. Over time, the individual will develop difficulty with speaking and swallowing, and their movements will become slow and stiff. People with advanced Huntington’s disease need full-time care to help with their day-to-day activities, and they ultimately succumb to pneumonia, heart failure, or other complications,” according to the Huntington’s Disease Society of America.4
Managing Symptoms
say neurologists.
There are currently three medications approved for the treatment of chorea, all in the class of agents known as vesicular monoamine transporter 2 inhibitors. These agents are tetrabenazine (Xenazine, Lundbeck), deutetrabenazine (Austedo XR, Teva), and valbenazine (Ingrezza, Neurocrine Biosciences).
“These drugs can treat the chorea, but they also can help with some of the other motor features. For example, if a patient has chorea in the legs and you treat it, then maybe they’re walking will get better, or if they have chorea in the mouth and you treat the chorea, then maybe their speech and swallowing may improve,” Victor Sung, MD, professor of neurology and director of the Huntington’s Disease Clinic at the University of Alabama at Birmingham, said in an interview.
Mood and behavioral symptoms associated with Huntington’s disease – depression, anxiety, irritability, impulsivity, etc – can be managed with off-label use of antidepressants, mood stabilizers, and antipsychotic agents.
“When it comes to the cognitive symptoms, that’s a big gap where we don’t have anything that’s [Food and Drug Administration] approved for Huntington’s disease,” Dr. Sung said.
Agents used to treat Alzheimer’s disease, such as cholinesterase inhibitors and the glutamate receptor antagonist memantine, have been studied extensively for preservation of cognition in Huntington’s disease, but have not shown significant benefit.
Dr. Sung said that one promising approach to the problem of cognitive protection in Huntington’s disease is the investigational agent dalzanemdor (SAGE-718), an N-methyl-D-aspartic (NMDA) acid receptor positive allosteric modulator. The drug is in development for cognitive disorders associated with NMDA receptor dysfunction, including Huntington’s disease and Alzheimer’s disease.
In the phase 2 SURVEYOR trial, which was not powered to show efficacy of dalzanemdor over placebo, patients with Huntington’s disease reportedly tolerated the drug well, with treatment-related adverse events primarily mild or moderate in severity.
As of this writing dalzanemdor is being evaluated for efficacy, compared with placebo, in the phase 2 DIMENSION trial. The primary endpoint of this trial is a change from baseline in composite score of the Huntington’s Disease Cognitive Assessment Battery.
Disease-Modifying Therapies
As previously noted, although there is no cure for Huntington’s disease, neurology investigators are developing new strategies for delaying, stalling, or even preventing disease progression.
“There are so many different approaches, it’s hard to know where to start,” Christopher A. Ross, MD, director of the Huntington’s Disease Center at Johns Hopkins University in Baltimore, Maryland, said in an interview.
The most actively investigated approach may be huntingtin-lowering therapies, based on the supposition that mutant huntingtin protein (mHTT) is the primary toxin in Huntington’s disease.
“What you need to do is in some way lower it, and there are a number of different ways to do that: antinsense olignoclueotides, small interfering RNA, CRISPR Cas, or other gene-editing techniques, as well as gene therapy with an adenoviral vector” he said.
Gene Therapy
Dr. Ross cited as promising a phase 1/2 clinical trial of an investigational gene therapy, AMT-130 (uniQure), which consists of an adeno-associated virus vector and a gene encoding a microRNA that is designed to recognize, bind, and nonselectively lower both mHTT and wild-type HTT.
The compound is injected directly into the corpus striatum. It was demonstrated to decrease signs of Huntington’s disease in animal models, and interim data on 29 patients with Huntington’s disease followed for up to 24 months showed a statically significant, dose-dependent slowing of Huntington’s disease progression and lowering of neurofilament light protein, a marker for neuronal degeneration in Huntington’s disease, in cerebrospinal fluid (CSF).
AMT-130 targets exon 1 of the HTT gene, which appears to be an important target, Dr. Ross commented.
“The huntingtin protein is really big. The polyglutamine expansion, which is what causes the toxicity, is right at the N-terminus of exon 1, and there is increasingly good evidence that you can have an exon 1 misspliced protein product which causes the toxicity, so it may be especially important to lower Huntington by targeting exon 1,” he said.
Oral Splicing Modifier
PTC Therapeutics, based in New Jersey, is developing PTC518, an oral small molecule drug that can cross the blood-brain barrier and is reported to target mutant huntingtin protein.
The drug is a splicing modifier that promotes insertion of a premature stop codon to HTT mRNA, thereby degrading and lowering the HTT levels.5
In June 2024, the company reported that, in the phase 2a PIVOT-HD study of PTC518 in patients with Huntington’s disease, 12 months of treatment was associated with a dose-dependent lowering of mHTT in blood and CSF in an interim cohort.
“In addition, favorable trends were demonstrated on several relevant Huntington’s disease clinical assessments including Total Motor Score and Composite Unified Huntington’s Disease Rating Scale. Furthermore, following 12 months of treatment, PTC518 continues to be safe and well tolerated,” the company stated in a press release.
Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) are short strands of DNA or RNA that bind to RNA sequences in faulty genes to modify production of target proteins.
One such drug, tominersen, developed jointly by Ionis and Roche, initially showed promise in a phase 1/2 trial for lowering mHTT in CSF without serious adverse events. But in a phase 3 trial, the intrathecally delivered agent was halted after an independent data monitoring committee recommended halting the trial, which Roche ended in 2021. The company reported in a letter to The New England Journal of Medicine that people in the high-dosage treatment group did measurably worse – although it remains unclear whether this was caused by excess protein lowering or an off-target effect.6 The tominersen program was the first to demonstrate that it was possible to lower HTT with an intervention, and the companies reported that they are continuing the agent’s development program.
Wave Life Sciences is developing an ASO, labeled WVE-003, designed to target a single-nucleotide polymorphism associated with the mHTT mRNA transcript within HTT. The company says that targeting the single-nucleotide polymorphism should allow lowering of expression of the mHTT will preserving wild-type HTT. This approach has the potential for therapies to prevent disease progression during the prodromal period, the company states.
Somatic Expansion
Dr. Furr Stimming and Dr. Ross both noted that there is considerable research interest into recently identified genetic modifiers that are believed to influence somatic instability, which in turn leads to somatic expansion.
“That seems to happen selectively in the neurons that are affected in Huntington’s disease. So a big puzzle for all of the neurodegenerative diseases is why are certain regions of the brain affected and other regions not? And it looks like, for the repeat expansion, this idea of somatic expansion seems to be increasingly central,” Dr. Ross said.
“The really exciting idea here is that, if somatic expansion is critical to the disease process and you could slow it down or stop it, you could go very early and potentially not just slow the progression of the disease once it starts, but conceivably even delay or possibly prevent the onset of Huntington’s disease,” he said.
Does HTT Lowering Mediate Progression?
“The next question is what does this mean clinically? Does lowering mutant huntingtin protein levels, and wild type for that matter, actually slow disease progression, which can be challenging to measure in a disease that is relatively slowly progressive?,” Dr. Furr Stimming said.
One important tool to help answer this question, she noted, is the Huntington’s Disease Integrated Staging System (HDISS), first described in 2022.7 The consensus-based system incorporates biological, clinical, and functional assessments, and characterizes patients from birth by stages, from stage 0 (persons who carry the mutation but have no detectable pathology), to stage 1 (measurable indicators of underlying pathophysiology), stage 2 (a detectable clinical phenotype), and finally to stage 3 (decline in function).
“The goal of the HDISS, which is designed for clinical research purposes, is to try to enroll individuals into clinical trials earlier rather than later, trying to get pharmaceutical companies and others to take advantage of the period prior to significant neurodegeneration occurring. Like Alzheimer’s disease and Parkinson’s disease, by the time we make a clinical diagnose significant neurodegeneration has occurred, so we really want to take advantage of the prodromal period to intervene with these potential disease-modifying therapies,” Dr. Furr Stimming said.
“At the end of the day, I suspect that we will not have just one effective disease-modifying therapy. I suspect that it will be a multifacted approach. We envision that we would hit the huntingtin protein from a few different angles. I envision that we would not only modify in some form or fashion production of the mutant huntingtin protein, but also try to influence the genetic modifiers that we think are important in somatic instability and expansion, which likely contributes to the rate of progression and symptom severity,” Dr. Furr Stimming said.
References
1. Huntington G. On Chorea. Reprinted in The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(1):109-112. doi: 10.1176/jnp.15.1.109.
2. Kaemmerer WF and Grondin RC. Degener Neurol Neuromuscul Dis. 2019 Mar 8;9:3-17. doi: 10.2147/DNND.S163808.
3. Jimenez-Sanchez M et al. Cold Spring Harb Perspect Med. 2017 Jul 5;7(7):a024240. doi: 10.1101/cshperspect.a024240.
4. Huntington’s Disease Society of America. Overview of Huntington’s Disease. https://hdsa.org/what-is-hd/overview-of-huntingtons-disease/.
5. Beers B et al. J Neurol Neurosurg Psychiatry. 2022;93:A96. https://jnnp.bmj.com/content/93/Suppl_1/A96.1.
6. McColgan P et al. N Engl J Med. 2023 Dec 7;389(23):2203-2205. doi: 10.1056/NEJMc2300400.
7. Tabrizi SJ et al. Lancet Neurol. 2022 Jul;21(7):632-644. doi: 10.1016/S1474-4422(22)00120-X.
Much progress has been made in managing the symptoms of Huntington’s disease, but the real excitement lies in the development of disease-modifying drugs and genetic therapy.
In April 1872, The Medical and Surgical Reporter of Philadelphia published a roughly 3,000-word paper, titled “On Chorea,” by George Huntington, a 22-year-old family practice physician recently graduated from Columbia University, New York City.
“Chorea is essentially a disease of the nervous system. The name ‘chorea’ is given to the disease on account of the dancing propensities of those who are affected by it, and it is a very appropriate designation,” he wrote in the introduction.
Toward the end of the paper Dr. Huntington described a “hereditary chorea” that he had observed while on professional rounds with his father, also a physician, in towns on the eastern end of Long Island in New York.
“It is spoken of by those in whose veins the seeds of the disease are known to exist, with a kind of horror, and not at all alluded to except through dire necessity, when it is mentioned as ‘that disorder,’ ” he wrote, noting later that “I have never known a recovery or even an amelioration of symptoms in this form of chorea; when once it begins it clings to the bitter end.”1
It wasn’t until 1993 that a team of investigators identified the gene responsible for the neurodegenerative disorder we now know as Huntington’s disease.
That discovery sparked hope for better treatments and a cure, but progress over the last 31 years has been incremental. Nonetheless, recent intensive research into novel approaches for treating Huntington’s disease have considerably brightened prospects for patients and caregivers, experts say.
“This is a devastating neurodegenerative disease and in talking to families that I have had the privilege and honor of following, I hear from them that ‘in 1993 when the gene was identified and my family member — parent, grandparent, aunt, uncle — had Huntington’s, we thought that there would be a curative or disease-modifying therapy in no time,’ ” said Erin Furr Stimming, MD, FAAN, FANA professor of neurology and Memorial Hermann Endowed Chair at the McGovern Medical School at University of Texas Health Science Center in Houston.
“Here we are in 2024. I think our families are still so incredibly resilient and courageous, and they are willing and able to participate in clinical trials. Families in the Huntington’s disease community at large are really ready to have trials that do, in fact, demonstrate some evidence of slowing of disease progression. It’s an exciting time,” she said in an interview.
Repeating Nucleotides
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by an expansion of a CAG trinucleotide repeat in exon 1 of the huntingtin gene (HTT), which encodes for the huntingtin protein (HTT). The multiple repeats result in expanded expression of mutant HTT. The mutated protein disrupts normal cellular processes, alters intracellular calcium homeostasis, and interferes with gene transcription, leading to progressive degeneration of neurons, and to a hallmark Huntington’s disease triad consisting of movement disorders, cognitive decline, and mood/behavioral issues.
The number of repeats determines the severity of disease and the age of onset. In nonaffected persons the gene contains about 20 CAG repeats, but as genetics research dating from the 1990s has shown, a single HTT allele containing more than 40 CAG repeats will inevitably result in disease, whereas carriers with fewer than 36 repeats on both alleles will remain unaffected.2
The prevalence of the mutation in Western populations is estimated to be from 4 to 10 per 100,000.3
The disease usually manifests first in adults aged 30-50 years but may also occur in children or adolescents and young adults. Early symptoms often include a decline in executive function that may be noticeable to the patient’s family and friends, mood changes, and chorea.
“Because of the uncontrolled movements (chorea), a person with Huntington’s disease may lose a lot of weight without intending to, and may have trouble walking, balancing, and moving around safely. They will eventually lose the ability to work, drive, and manage tasks at home, and may qualify for disability benefits. Over time, the individual will develop difficulty with speaking and swallowing, and their movements will become slow and stiff. People with advanced Huntington’s disease need full-time care to help with their day-to-day activities, and they ultimately succumb to pneumonia, heart failure, or other complications,” according to the Huntington’s Disease Society of America.4
Managing Symptoms
say neurologists.
There are currently three medications approved for the treatment of chorea, all in the class of agents known as vesicular monoamine transporter 2 inhibitors. These agents are tetrabenazine (Xenazine, Lundbeck), deutetrabenazine (Austedo XR, Teva), and valbenazine (Ingrezza, Neurocrine Biosciences).
“These drugs can treat the chorea, but they also can help with some of the other motor features. For example, if a patient has chorea in the legs and you treat it, then maybe they’re walking will get better, or if they have chorea in the mouth and you treat the chorea, then maybe their speech and swallowing may improve,” Victor Sung, MD, professor of neurology and director of the Huntington’s Disease Clinic at the University of Alabama at Birmingham, said in an interview.
Mood and behavioral symptoms associated with Huntington’s disease – depression, anxiety, irritability, impulsivity, etc – can be managed with off-label use of antidepressants, mood stabilizers, and antipsychotic agents.
“When it comes to the cognitive symptoms, that’s a big gap where we don’t have anything that’s [Food and Drug Administration] approved for Huntington’s disease,” Dr. Sung said.
Agents used to treat Alzheimer’s disease, such as cholinesterase inhibitors and the glutamate receptor antagonist memantine, have been studied extensively for preservation of cognition in Huntington’s disease, but have not shown significant benefit.
Dr. Sung said that one promising approach to the problem of cognitive protection in Huntington’s disease is the investigational agent dalzanemdor (SAGE-718), an N-methyl-D-aspartic (NMDA) acid receptor positive allosteric modulator. The drug is in development for cognitive disorders associated with NMDA receptor dysfunction, including Huntington’s disease and Alzheimer’s disease.
In the phase 2 SURVEYOR trial, which was not powered to show efficacy of dalzanemdor over placebo, patients with Huntington’s disease reportedly tolerated the drug well, with treatment-related adverse events primarily mild or moderate in severity.
As of this writing dalzanemdor is being evaluated for efficacy, compared with placebo, in the phase 2 DIMENSION trial. The primary endpoint of this trial is a change from baseline in composite score of the Huntington’s Disease Cognitive Assessment Battery.
Disease-Modifying Therapies
As previously noted, although there is no cure for Huntington’s disease, neurology investigators are developing new strategies for delaying, stalling, or even preventing disease progression.
“There are so many different approaches, it’s hard to know where to start,” Christopher A. Ross, MD, director of the Huntington’s Disease Center at Johns Hopkins University in Baltimore, Maryland, said in an interview.
The most actively investigated approach may be huntingtin-lowering therapies, based on the supposition that mutant huntingtin protein (mHTT) is the primary toxin in Huntington’s disease.
“What you need to do is in some way lower it, and there are a number of different ways to do that: antinsense olignoclueotides, small interfering RNA, CRISPR Cas, or other gene-editing techniques, as well as gene therapy with an adenoviral vector” he said.
Gene Therapy
Dr. Ross cited as promising a phase 1/2 clinical trial of an investigational gene therapy, AMT-130 (uniQure), which consists of an adeno-associated virus vector and a gene encoding a microRNA that is designed to recognize, bind, and nonselectively lower both mHTT and wild-type HTT.
The compound is injected directly into the corpus striatum. It was demonstrated to decrease signs of Huntington’s disease in animal models, and interim data on 29 patients with Huntington’s disease followed for up to 24 months showed a statically significant, dose-dependent slowing of Huntington’s disease progression and lowering of neurofilament light protein, a marker for neuronal degeneration in Huntington’s disease, in cerebrospinal fluid (CSF).
AMT-130 targets exon 1 of the HTT gene, which appears to be an important target, Dr. Ross commented.
“The huntingtin protein is really big. The polyglutamine expansion, which is what causes the toxicity, is right at the N-terminus of exon 1, and there is increasingly good evidence that you can have an exon 1 misspliced protein product which causes the toxicity, so it may be especially important to lower Huntington by targeting exon 1,” he said.
Oral Splicing Modifier
PTC Therapeutics, based in New Jersey, is developing PTC518, an oral small molecule drug that can cross the blood-brain barrier and is reported to target mutant huntingtin protein.
The drug is a splicing modifier that promotes insertion of a premature stop codon to HTT mRNA, thereby degrading and lowering the HTT levels.5
In June 2024, the company reported that, in the phase 2a PIVOT-HD study of PTC518 in patients with Huntington’s disease, 12 months of treatment was associated with a dose-dependent lowering of mHTT in blood and CSF in an interim cohort.
“In addition, favorable trends were demonstrated on several relevant Huntington’s disease clinical assessments including Total Motor Score and Composite Unified Huntington’s Disease Rating Scale. Furthermore, following 12 months of treatment, PTC518 continues to be safe and well tolerated,” the company stated in a press release.
Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) are short strands of DNA or RNA that bind to RNA sequences in faulty genes to modify production of target proteins.
One such drug, tominersen, developed jointly by Ionis and Roche, initially showed promise in a phase 1/2 trial for lowering mHTT in CSF without serious adverse events. But in a phase 3 trial, the intrathecally delivered agent was halted after an independent data monitoring committee recommended halting the trial, which Roche ended in 2021. The company reported in a letter to The New England Journal of Medicine that people in the high-dosage treatment group did measurably worse – although it remains unclear whether this was caused by excess protein lowering or an off-target effect.6 The tominersen program was the first to demonstrate that it was possible to lower HTT with an intervention, and the companies reported that they are continuing the agent’s development program.
Wave Life Sciences is developing an ASO, labeled WVE-003, designed to target a single-nucleotide polymorphism associated with the mHTT mRNA transcript within HTT. The company says that targeting the single-nucleotide polymorphism should allow lowering of expression of the mHTT will preserving wild-type HTT. This approach has the potential for therapies to prevent disease progression during the prodromal period, the company states.
Somatic Expansion
Dr. Furr Stimming and Dr. Ross both noted that there is considerable research interest into recently identified genetic modifiers that are believed to influence somatic instability, which in turn leads to somatic expansion.
“That seems to happen selectively in the neurons that are affected in Huntington’s disease. So a big puzzle for all of the neurodegenerative diseases is why are certain regions of the brain affected and other regions not? And it looks like, for the repeat expansion, this idea of somatic expansion seems to be increasingly central,” Dr. Ross said.
“The really exciting idea here is that, if somatic expansion is critical to the disease process and you could slow it down or stop it, you could go very early and potentially not just slow the progression of the disease once it starts, but conceivably even delay or possibly prevent the onset of Huntington’s disease,” he said.
Does HTT Lowering Mediate Progression?
“The next question is what does this mean clinically? Does lowering mutant huntingtin protein levels, and wild type for that matter, actually slow disease progression, which can be challenging to measure in a disease that is relatively slowly progressive?,” Dr. Furr Stimming said.
One important tool to help answer this question, she noted, is the Huntington’s Disease Integrated Staging System (HDISS), first described in 2022.7 The consensus-based system incorporates biological, clinical, and functional assessments, and characterizes patients from birth by stages, from stage 0 (persons who carry the mutation but have no detectable pathology), to stage 1 (measurable indicators of underlying pathophysiology), stage 2 (a detectable clinical phenotype), and finally to stage 3 (decline in function).
“The goal of the HDISS, which is designed for clinical research purposes, is to try to enroll individuals into clinical trials earlier rather than later, trying to get pharmaceutical companies and others to take advantage of the period prior to significant neurodegeneration occurring. Like Alzheimer’s disease and Parkinson’s disease, by the time we make a clinical diagnose significant neurodegeneration has occurred, so we really want to take advantage of the prodromal period to intervene with these potential disease-modifying therapies,” Dr. Furr Stimming said.
“At the end of the day, I suspect that we will not have just one effective disease-modifying therapy. I suspect that it will be a multifacted approach. We envision that we would hit the huntingtin protein from a few different angles. I envision that we would not only modify in some form or fashion production of the mutant huntingtin protein, but also try to influence the genetic modifiers that we think are important in somatic instability and expansion, which likely contributes to the rate of progression and symptom severity,” Dr. Furr Stimming said.
References
1. Huntington G. On Chorea. Reprinted in The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(1):109-112. doi: 10.1176/jnp.15.1.109.
2. Kaemmerer WF and Grondin RC. Degener Neurol Neuromuscul Dis. 2019 Mar 8;9:3-17. doi: 10.2147/DNND.S163808.
3. Jimenez-Sanchez M et al. Cold Spring Harb Perspect Med. 2017 Jul 5;7(7):a024240. doi: 10.1101/cshperspect.a024240.
4. Huntington’s Disease Society of America. Overview of Huntington’s Disease. https://hdsa.org/what-is-hd/overview-of-huntingtons-disease/.
5. Beers B et al. J Neurol Neurosurg Psychiatry. 2022;93:A96. https://jnnp.bmj.com/content/93/Suppl_1/A96.1.
6. McColgan P et al. N Engl J Med. 2023 Dec 7;389(23):2203-2205. doi: 10.1056/NEJMc2300400.
7. Tabrizi SJ et al. Lancet Neurol. 2022 Jul;21(7):632-644. doi: 10.1016/S1474-4422(22)00120-X.
Heat-Related Pediatric ED Visits More Than Double
ORLANDO – according to research presented at the annual meeting of the American Academy of Pediatrics (AAP).
“Our study really highlights the adverse effects that can come from extreme heat, and how increasing heat-related illness is affecting our children,” Taylor Merritt, MD, a pediatric resident at the University of Texas Southwestern Medical Center and Children’s Health in Dallas, said during a press briefing.
Underestimating the Problem?
Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program and was not involved in this research, was not surprised by the findings. “If anything, we’re vastly underestimating it because when people come in with heat exhaustion or heat smoke, that gets coded correctly, but when people come in with heart attacks, asthma attacks, strokes, and other exacerbations of chronic disease, it very rarely gets coded as a heat-related illness.”
Record-breaking summer temperatures from the changing climate have led to increased heat-related morbidity and mortality. Past research suggests that children and teens make up nearly half of all those affected by heat-related illnesses, she noted. 2023, for example, was the hottest year on record, and 2024 is predicted to be hotter, Dr. Merritt said.
A Sharp Increase in Cases
The retrospective study examined emergency department diagnoses during May-September from 2012-2023 at two large children’s hospitals within a north Texas pediatric health care system. The researchers compared heat-specific conditions with rhabdomyolysis encounters based on ICD-10 coding.
Heat-specific conditions include heatstroke/sunstroke, exertion heatstroke, heat syncope, heat crap, heat exhaustion, heat fatigue, heat edema, and exposure to excessive natural heat. Rhabdomyolysis encounters included both exertional and nonexertional rhabdomyolysis as well as non-traumatic rhabdomyolysis and elevated creatine kinase (CK) levels.
Among 542 heat-related encounters, 77% had heat-specific diagnoses and 24% had a rhabdomyolysis diagnosis. Combined, heat-related encounters increased 170% from 2012 to 2023, from 4.3 per 10,000 to 11.6 per 10,000 (P = .1). Summer months with higher peak temperatures were also associated with higher heat-related volume in the emergency department (P < .001).
Teenage boys were most likely to have rhabdomyolysis, with 82% of the cases occurring in boys and 70% in ages 12-18 (P < .001). “Compared to the rhabdomyolysis group, the heat-specific group was more likely to be younger, Hispanic, use government-based insurance, and live in an area with a lower Child Opportunity Index,” Dr. Merritt reported. “Most heat-specific encounters resulted in an ED discharge (96%), while most rhabdomyolysis encounters resulted in hospital admission (63%)” (P < .001).
”Thankfully, pediatric heat-related illness is still relatively rare,” Dr. Merritt said. “However, given the context of increasing temperatures, this is important for us all to know, anyone who cares for children, whether that be families or parents or pediatricians.”
Prevention Is Key
Dr. Byron noted that about half of AAP chapters now have climate committees, many of which have created educational materials on heat and wildfire smoke and on talking with athletes about risk of heat-related illnesses.
“A lot of the state high school sports associations are actually now adopting guidelines on when it’s safe to practice and when it’s safe to play for heat and for smoke, so that’s definitely something that we can talk to parents about and kids about,” Dr. Byron said. “Otherwise, you still have a lot of coaches and a lot of kids out there that think you’re just supposed to be tough and barrel through it.”
Rhabdomyolysis and heat stroke are both potentially deadly illnesses, so the biggest focus needs to be on prevention, Dr. Byron said. “Not just working with individuals in your office, but working within your school or within your state high school sports association is totally within the lane of a pediatrician to get involved.”
The research had no external funding. Dr. Merritt and Dr. Byron had no disclosures.
ORLANDO – according to research presented at the annual meeting of the American Academy of Pediatrics (AAP).
“Our study really highlights the adverse effects that can come from extreme heat, and how increasing heat-related illness is affecting our children,” Taylor Merritt, MD, a pediatric resident at the University of Texas Southwestern Medical Center and Children’s Health in Dallas, said during a press briefing.
Underestimating the Problem?
Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program and was not involved in this research, was not surprised by the findings. “If anything, we’re vastly underestimating it because when people come in with heat exhaustion or heat smoke, that gets coded correctly, but when people come in with heart attacks, asthma attacks, strokes, and other exacerbations of chronic disease, it very rarely gets coded as a heat-related illness.”
Record-breaking summer temperatures from the changing climate have led to increased heat-related morbidity and mortality. Past research suggests that children and teens make up nearly half of all those affected by heat-related illnesses, she noted. 2023, for example, was the hottest year on record, and 2024 is predicted to be hotter, Dr. Merritt said.
A Sharp Increase in Cases
The retrospective study examined emergency department diagnoses during May-September from 2012-2023 at two large children’s hospitals within a north Texas pediatric health care system. The researchers compared heat-specific conditions with rhabdomyolysis encounters based on ICD-10 coding.
Heat-specific conditions include heatstroke/sunstroke, exertion heatstroke, heat syncope, heat crap, heat exhaustion, heat fatigue, heat edema, and exposure to excessive natural heat. Rhabdomyolysis encounters included both exertional and nonexertional rhabdomyolysis as well as non-traumatic rhabdomyolysis and elevated creatine kinase (CK) levels.
Among 542 heat-related encounters, 77% had heat-specific diagnoses and 24% had a rhabdomyolysis diagnosis. Combined, heat-related encounters increased 170% from 2012 to 2023, from 4.3 per 10,000 to 11.6 per 10,000 (P = .1). Summer months with higher peak temperatures were also associated with higher heat-related volume in the emergency department (P < .001).
Teenage boys were most likely to have rhabdomyolysis, with 82% of the cases occurring in boys and 70% in ages 12-18 (P < .001). “Compared to the rhabdomyolysis group, the heat-specific group was more likely to be younger, Hispanic, use government-based insurance, and live in an area with a lower Child Opportunity Index,” Dr. Merritt reported. “Most heat-specific encounters resulted in an ED discharge (96%), while most rhabdomyolysis encounters resulted in hospital admission (63%)” (P < .001).
”Thankfully, pediatric heat-related illness is still relatively rare,” Dr. Merritt said. “However, given the context of increasing temperatures, this is important for us all to know, anyone who cares for children, whether that be families or parents or pediatricians.”
Prevention Is Key
Dr. Byron noted that about half of AAP chapters now have climate committees, many of which have created educational materials on heat and wildfire smoke and on talking with athletes about risk of heat-related illnesses.
“A lot of the state high school sports associations are actually now adopting guidelines on when it’s safe to practice and when it’s safe to play for heat and for smoke, so that’s definitely something that we can talk to parents about and kids about,” Dr. Byron said. “Otherwise, you still have a lot of coaches and a lot of kids out there that think you’re just supposed to be tough and barrel through it.”
Rhabdomyolysis and heat stroke are both potentially deadly illnesses, so the biggest focus needs to be on prevention, Dr. Byron said. “Not just working with individuals in your office, but working within your school or within your state high school sports association is totally within the lane of a pediatrician to get involved.”
The research had no external funding. Dr. Merritt and Dr. Byron had no disclosures.
ORLANDO – according to research presented at the annual meeting of the American Academy of Pediatrics (AAP).
“Our study really highlights the adverse effects that can come from extreme heat, and how increasing heat-related illness is affecting our children,” Taylor Merritt, MD, a pediatric resident at the University of Texas Southwestern Medical Center and Children’s Health in Dallas, said during a press briefing.
Underestimating the Problem?
Lori Byron, MD, a pediatrician from Red Lodge, Montana, who heads the AAP Chapter Climate Advocates program and was not involved in this research, was not surprised by the findings. “If anything, we’re vastly underestimating it because when people come in with heat exhaustion or heat smoke, that gets coded correctly, but when people come in with heart attacks, asthma attacks, strokes, and other exacerbations of chronic disease, it very rarely gets coded as a heat-related illness.”
Record-breaking summer temperatures from the changing climate have led to increased heat-related morbidity and mortality. Past research suggests that children and teens make up nearly half of all those affected by heat-related illnesses, she noted. 2023, for example, was the hottest year on record, and 2024 is predicted to be hotter, Dr. Merritt said.
A Sharp Increase in Cases
The retrospective study examined emergency department diagnoses during May-September from 2012-2023 at two large children’s hospitals within a north Texas pediatric health care system. The researchers compared heat-specific conditions with rhabdomyolysis encounters based on ICD-10 coding.
Heat-specific conditions include heatstroke/sunstroke, exertion heatstroke, heat syncope, heat crap, heat exhaustion, heat fatigue, heat edema, and exposure to excessive natural heat. Rhabdomyolysis encounters included both exertional and nonexertional rhabdomyolysis as well as non-traumatic rhabdomyolysis and elevated creatine kinase (CK) levels.
Among 542 heat-related encounters, 77% had heat-specific diagnoses and 24% had a rhabdomyolysis diagnosis. Combined, heat-related encounters increased 170% from 2012 to 2023, from 4.3 per 10,000 to 11.6 per 10,000 (P = .1). Summer months with higher peak temperatures were also associated with higher heat-related volume in the emergency department (P < .001).
Teenage boys were most likely to have rhabdomyolysis, with 82% of the cases occurring in boys and 70% in ages 12-18 (P < .001). “Compared to the rhabdomyolysis group, the heat-specific group was more likely to be younger, Hispanic, use government-based insurance, and live in an area with a lower Child Opportunity Index,” Dr. Merritt reported. “Most heat-specific encounters resulted in an ED discharge (96%), while most rhabdomyolysis encounters resulted in hospital admission (63%)” (P < .001).
”Thankfully, pediatric heat-related illness is still relatively rare,” Dr. Merritt said. “However, given the context of increasing temperatures, this is important for us all to know, anyone who cares for children, whether that be families or parents or pediatricians.”
Prevention Is Key
Dr. Byron noted that about half of AAP chapters now have climate committees, many of which have created educational materials on heat and wildfire smoke and on talking with athletes about risk of heat-related illnesses.
“A lot of the state high school sports associations are actually now adopting guidelines on when it’s safe to practice and when it’s safe to play for heat and for smoke, so that’s definitely something that we can talk to parents about and kids about,” Dr. Byron said. “Otherwise, you still have a lot of coaches and a lot of kids out there that think you’re just supposed to be tough and barrel through it.”
Rhabdomyolysis and heat stroke are both potentially deadly illnesses, so the biggest focus needs to be on prevention, Dr. Byron said. “Not just working with individuals in your office, but working within your school or within your state high school sports association is totally within the lane of a pediatrician to get involved.”
The research had no external funding. Dr. Merritt and Dr. Byron had no disclosures.
FROM AAP 2024
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
Newborn Screening Programs: What Do Clinicians Need to Know?
Newborn screening programs are public health services aimed at ensuring that the close to 4 million infants born each year in the United States are screened for certain serious disorders at birth. These disorders, albeit rare, are detected in roughly 12,500 newborn babies every year.
Newborn screening isn’t new, although it has expanded and transformed over the decades. The first newborn screening test was developed in the 1960s to detect phenylketonuria (PKU).1 Since then, the number of conditions screened for has increased, with programs in every US state and territory. “Newborn screening is well established now, not experimental or newfangled,” Wendy Chung, MD, PhD, professor of pediatrics, Harvard Medical School, Boston, Massachusetts, told Neurology Reviews.
In newborn screening, blood drawn from the baby’s heel is applied to specialized filter paper, which is then subjected to several analytical methods, including tandem mass spectrometry and molecular analyses to detect biomarkers for the diseases.2 More recently, genomic sequencing is being piloted as part of consented research studies.3
Newborn screening includes not only biochemical and genetic testing, but also includes noninvasive screening for hearing loss or for critical congenital heart disease using pulse oximetry. And newborn screening goes beyond analysis of a single drop of blood. Rather, “it’s an entire system, with the goal of identifying babies with genetic disorders who otherwise have no obvious symptoms,” said Dr. Chung. Left undetected and untreated, these conditions can be associated with serious adverse outcomes and even death.
Dr. Chung described newborn screening as a “one of the most successful public health programs, supporting health equity by screening almost every US baby after birth and then bringing timely treatments when relevant even before the baby develops symptoms of a disorder.” In this way, newborn screening has “saved lives and decreased disease burdens.”
There are at present 38 core conditions that the Department of Health and Human Services (HHS) regards as the most critical to screen for and 26 secondary conditions associated with these core disorders. This is called the Recommended Uniform Screening Panel (RUSP). Guidance regarding the most appropriate application of newborn screening tests, technologies and standards are provided by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC).
Each state “independently determines which screening tests are performed and what follow-up is provided.”4 Information about which tests are provided by which states can be found on the “Report Card” of the National Organization for Rare Diseases (NORD).
Challenges in Expanding the Current Newborn Screening
One of the major drawbacks in the current system is that “we don’t screen for enough diseases,” according to Zhanzhi Hu, PhD, of the Department of Systems Biology and the Department of Biomedical Information, Columbia University, New York City. “There are over 10,000 rare genetic diseases, but we’re currently screening for fewer than 100,” he told Neurology Reviews. Although in the United States, there are about 700-800 drugs approved for genetic diseases, “we can’t identify patients with these diseases early enough for the ideal window when treatments are most effective.”
Moreover, it’s a “lengthy process” to add new diseases to RUSP. “New conditions are added at the pace of less than one per year, on average — even for the hundreds of diseases for which there are treatments,” he said. “If we keep going at the current pace, we won’t be able to screen for those diseases for another few hundred years.”
Speeding up the pace of including new diseases in newborn screening is challenging because “we have more diseases than we have development dollars for,” Dr. Hu said. “Big pharmaceutical companies are reluctant to invest in rare diseases because the population is so small and it’s hard and expensive to develop such drugs. So if we can identify patients first, there will be more interest in developing treatments down the road.”
On the other hand, for trials to take place, these babies have to be identified in a timely manner — which requires testing. “Right now, we have a deadlock,” Dr. Hu said. “To nominate a disease, you need an approved treatment. But to get a treatment developed, you need to identify patients suitable for a clinical trial. If you have to wait for the symptoms to show up, the damage has already manifested and is irreversible. Our chance is to recognize the disease before symptom onset and then start treatment. I would call this a ‘chicken-and-egg’ problem.”
Dr. Hu is passionate about expanding newborn screening, and he has a very personal reason. Two of his children have a rare genetic disease. “My younger son, now 13 years old, was diagnosed at a much earlier age than my older son, although he had very few symptoms at the time, because his older brother was known to have the disease. As a result of this, his outcome was much better.” By contrast, Dr. Hu’s oldest son — now age 16 — wasn’t diagnosed until he became symptomatic.
His quest led him to join forces with Dr. Chung in conducting the Genomic Uniform-screening Against Rare Disease in All Newborns (Guardian) study, which screens newborns for more than 450 genetic conditions not currently screened as part of the standard newborn screening. To date, the study — which focuses on babies born in New York City — has screened about 11,000 infants.
“To accumulate enough evidence requires screening at least 100,000 babies because one requirement for nominating a disease for national inclusion in RUSP is an ‘N of 1’ study — meaning, to identify at least one positive patient using the proposed screening method in a prospective study,” Dr. Hu explained. “Most are rare diseases with an incidence rate of around one in 100,000. So getting to that magic number of 100,000 participants should enable us to hit that ‘N of 1’ for most diseases.”
The most challenging part, according to Dr. Hu, is the requirement of a prospective study, which means that you have to conduct a large-scale study enrolling tens of thousands of families and babies. If done for individual diseases (as has been the case in the past), “this is a huge cost and very inefficient.”
In reality, he added, the true incidence of these diseases is unclear. “Incidence rates are based on historical data rather than prospective studies. We’ve already seen some diseases show up more frequently than previously recorded, while others have shown up less frequently.”
For example, in the 11,000 babies screened to date, at least three girls with Rett syndrome have been identified, which is “quite a bit higher” than what has previously been identified in the literature (ie, one in 10,000-12,000 births). “This is a highly unmet need for these families because if you can initiate early treatment — at age 1, or even younger — the outcome will be better.”
He noted that there is at least one clinical trial underway for treating Rett syndrome, which has yielded “promising” data.5 “We’re hoping that by screening for diseases like Rett and identifying patients early, this will go hand-in-hand with clinical drug development. It can speed both the approval of the treatment and the addition to the newborn screening list,” Dr. Hu stated.
Screening and Drug Development Working in Tandem
Sequencing technologies have advanced and become more sophisticated as well as less costly, so interest in expanding newborn screening through newborn genome sequencing has increased. In fact, many states currently have incorporated genetic testing into newborn screening for conditions without biochemical markers. Additionally, newborn genomic sequencing is also used for further testing in infants with abnormal biochemical screening results.6
Genomic sequencing “identifies nucleotide changes that are the underlying etiology of monogenic disorders.”6 Its use could potentially enable identification of over 500 genetic disorders for which an newborn screening assay is not currently available, said Dr. Hu.
“Molecular DNA analysis has been integrated into newborn testing either as a first- or second-tier test for several conditions, including cystic fibrosis, severe combined immunodeficiency, and spinal muscular atrophy (SMA),” Dr. Hu said.
Dr. Hu pointed to SMA to illustrate the power and potential of newborn screening working hand-in-hand with the development of new treatments. SMA is a neurodegenerative disorder caused by mutations in SMN1, which encodes survival motor neuron protein (SMN).7 Deficiencies in SMN results in loss of motor neurons with muscle weakness and, often, early death.7A pilot study, on which Dr. Chung was the senior author, used both biochemical and genetic testing of close to 4000 newborns and found an SMA carrier frequency of 1.5%. One newborn was identified who had a homozygous SMN1 gene deletion and two copies of SMN2, strongly suggesting the presence of a severe type 1 SMA phenotype.8
At age 15 days, the baby was treated with nusinersen, an injection administered into the fluid surrounding the spinal cord, and the first FDA-approved genetic treatment for SMA. At the time of study publication, the baby was 12 months old, “meeting all developmental milestones and free of any respiratory issues,” the authors report.
“Screening for SMA — which was added to the RUSP in 2018 — has dramatically transformed what used to be the most common genetic cause of death in children under the age of 2,” Dr. Chung said. “Now, a once-and-done IV infusion of genetic therapy right after screening has transformed everything, taking what used to be a lethal condition and allowing children to grow up healthy.”
Advocating for Inclusion of Diseases With No Current Treatment
At present, any condition included in the RUSP is required to have a treatment, which can be dietary, surgical/procedural, or an FDA-approved drug-based agent. Unfortunately, a wide range of neurodevelopmental diseases still have no known treatments. But lack of availability of treatment shouldn’t invalidate a disease from being included in the RUSP, because even if there is no specific treatment for the condition itself, early intervention can still be initiated to prevent some of the manifestations of the condition, said Dr. Hu.
“For example, most patients with these diseases will sooner or later undergo seizures,” Dr. Hu remarked. “We know that repeated seizures can cause brain damage. If we can diagnose the disease before the seizures start to take place, we can put preventive seizure control interventions in place, even if there is no direct ‘treatment’ for the condition itself.”
Early identification can lead to early intervention, which can have other benefits, Dr. Hu noted. “If we train the brain at a young age, when the brain is most receptive, even though a disease may be progressive and will worsen, those abilities acquired earlier will last longer and remain in place longer. When these skills are acquired later, they’re forgotten sooner. This isn’t a ‘cure,’ but it will help with functional improvement.”
Moreover, parents are “interested in knowing that their child has a condition, even if no treatment is currently available for that disorder, according to our research,” Dr. Chung said. “We found that the parents we interviewed endorsed the nonmedical utility of having access to information, even in the absence of a ‘cure,’ so they could prepare for medical issues that might arise down the road and make informed choices.”9
Nina Gold, MD, director of Prenatal Medical Genetics and associate director for Research for Massachusetts General Brigham Personalized Medicine, Boston, obtained similar findings in her own research, which is currently under review for publication. “We conducted focus groups and one-on-one interviews with parents from diverse racial and socioeconomic backgrounds. At least one parent said they didn’t want to compare their child to other children if their child might have a different developmental trajectory. They stressed that the information would be helpful, even if there was no immediate clinical utility.”
Additionally, there are an “increasing number of fetal therapies for rare disorders, so information about a genetic disease in an older child can be helpful for parents who may go on to have another pregnancy,” Dr. Gold noted.
Dr. Hu detailed several other reasons for including a wider range of disorders in the RUSP. Doing so helps families avoid a “stressful and expensive diagnostic odyssey and also provides equitable access to a diagnosis.” And if these patients are identified early, “we can connect the family with clinical trials already underway or connect them to an organization such as the Accelerating Medicines Partnership (AMP) Program Bespoke Gene Therapy Consortium (AMP BGTC). Bespoke “brings together partners from the public, private, and nonprofit sectors to foster development of gene therapies intended to treat rare genetic diseases, which affect populations too small for viable commercial development.”
Next Steps Following Screening
Rebecca Sponberg, NP, of the Children’s Hospital of Orange County, UC Irvine School of Medicine, California, is part of a broader multidisciplinary team that interfaces with parents whose newborns have screened positive for a genetic disorder. The team also includes a biochemical geneticist, a pediatric neurologist, a pediatric endocrinologist, a genetic counselor, and a social worker.
Different states and locations have different procedures for receiving test results, said Dr. Chung. In some, pediatricians are the ones who receive the results, and they are tasked with the responsibility of making sure the children can start getting appropriate care. In particular, these pediatricians are associated with centers of excellence that specialize in working with families around these conditions. Other facilities have multidisciplinary teams.
Ms. Sponberg gave an example of how the process unfolded with X-linked adrenoleukodystrophy, a rare genetic disorder that affects the white matter of the nervous system and the adrenal cortex.10 “This is the most common peroxisomal disorder, affecting one in 20,000 males,” she said. “There are several different forms of the disorder, but males are most at risk for having the cerebral form, which can lead to neurological regression and hasten death. But the regression does not appear until 4 to 12 years of age.”
A baby who screens positive on the initial newborn screening has repeat testing; and if it’s confirmed, the family meets the entire team to help them understand what the disorder is, what to expect, and how it’s monitored and managed. “Children have to be followed closely with a brain MRI every 6 months to detect brain abnormalities quickly,” Ms. Sponberg explained “And we do regular bloodwork to look for adrenocortical insufficiency.”
A child who shows concerning changes on the MRI or abnormal blood test findings is immediately seen by the relevant specialist. “So far, our center has had one patient who had MRI changes consistent with the cerebral form of the disease and the patient was immediately able to receive a bone marrow transplant,” she reported. “We don’t think this child’s condition would have been picked up so quickly or treatment initiated so rapidly if we hadn’t known about it through newborn screening.”
Educating and Involving Families
Part of the role of clinicians is to provide education regarding newborn screening to families, according to Ms. Sponberg. “In my role, I have to call parents to tell them their child screened positive for a genetic condition and that we need to proceed with confirmatory testing,” she said. “We let them know if there’s a high concern that this might be a true positive for the condition, and we offer them information so they know what to expect.”
Unfortunately, Ms. Sponberg said, in the absence of education, some families are skeptical. “When I call families directly, some think it’s a scam and it can be hard to earn their trust. We need to do a better job educating families, especially our pregnant individuals, that testing will occur and if anything is abnormal, they will receive a call.”
References
1. Levy HL. Robert Guthrie and the Trials and Tribulations of Newborn Screening. Int J Neonatal Screen. 2021 Jan 19;7(1):5. doi: 10.3390/ijns7010005.
2. Chace DH et al. Clinical Chemistry and Dried Blood Spots: Increasing Laboratory Utilization by Improved Understanding of Quantitative Challenges. Bioanalysis. 2014;6(21):2791-2794. doi: 10.4155/bio.14.237.
3. Gold NB et al. Perspectives of Rare Disease Experts on Newborn Genome Sequencing. JAMA Netw Open. 2023 May 1;6(5):e2312231. doi: 10.1001/jamanetworkopen.2023.12231.
4. Weismiller DG. Expanded Newborn Screening: Information and Resources for the Family Physician. Am Fam Physician. 2017 Jun 1;95(11):703-709. https://www.aafp.org/pubs/afp/issues/2017/0601/p703.html.
5. Neul JL et al. Trofinetide for the Treatment of Rett Syndrome: A Randomized Phase 3 Study. Nat Med. 2023 Jun;29(6):1468-1475. doi: 10.1038/s41591-023-02398-1.
6. Chen T et al. Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening. JAMA Netw Open. 2023 Sep 5;6(9):e2331162. doi: 10.1001/jamanetworkopen.2023.31162.
7. Mercuri E et al. Spinal Muscular Atrophy. Nat Rev Dis Primers. 2022 Aug 4;8(1):52. doi: 10.1038/s41572-022-00380-8.
8. Kraszewski JN et al. Pilot Study of Population-Based Newborn Screening for Spinal Muscular Atrophy in New York State. Genet Med. 2018 Jun;20(6):608-613. doi: 10.1038/gim.2017.152.
9. Timmins GT et al. Diverse Parental Perspectives of the Social and Educational Needs for Expanding Newborn Screening Through Genomic Sequencing. Public Health Genomics. 2022 Sep 15:1-8. doi: 10.1159/000526382.
10. Turk BR et al. X-linked Adrenoleukodystrophy: Pathology, Pathophysiology, Diagnostic Testing, Newborn Screening and Therapies. Int J Dev Neurosci. 2020 Feb;80(1):52-72. doi: 10.1002/jdn.10003.
Newborn screening programs are public health services aimed at ensuring that the close to 4 million infants born each year in the United States are screened for certain serious disorders at birth. These disorders, albeit rare, are detected in roughly 12,500 newborn babies every year.
Newborn screening isn’t new, although it has expanded and transformed over the decades. The first newborn screening test was developed in the 1960s to detect phenylketonuria (PKU).1 Since then, the number of conditions screened for has increased, with programs in every US state and territory. “Newborn screening is well established now, not experimental or newfangled,” Wendy Chung, MD, PhD, professor of pediatrics, Harvard Medical School, Boston, Massachusetts, told Neurology Reviews.
In newborn screening, blood drawn from the baby’s heel is applied to specialized filter paper, which is then subjected to several analytical methods, including tandem mass spectrometry and molecular analyses to detect biomarkers for the diseases.2 More recently, genomic sequencing is being piloted as part of consented research studies.3
Newborn screening includes not only biochemical and genetic testing, but also includes noninvasive screening for hearing loss or for critical congenital heart disease using pulse oximetry. And newborn screening goes beyond analysis of a single drop of blood. Rather, “it’s an entire system, with the goal of identifying babies with genetic disorders who otherwise have no obvious symptoms,” said Dr. Chung. Left undetected and untreated, these conditions can be associated with serious adverse outcomes and even death.
Dr. Chung described newborn screening as a “one of the most successful public health programs, supporting health equity by screening almost every US baby after birth and then bringing timely treatments when relevant even before the baby develops symptoms of a disorder.” In this way, newborn screening has “saved lives and decreased disease burdens.”
There are at present 38 core conditions that the Department of Health and Human Services (HHS) regards as the most critical to screen for and 26 secondary conditions associated with these core disorders. This is called the Recommended Uniform Screening Panel (RUSP). Guidance regarding the most appropriate application of newborn screening tests, technologies and standards are provided by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC).
Each state “independently determines which screening tests are performed and what follow-up is provided.”4 Information about which tests are provided by which states can be found on the “Report Card” of the National Organization for Rare Diseases (NORD).
Challenges in Expanding the Current Newborn Screening
One of the major drawbacks in the current system is that “we don’t screen for enough diseases,” according to Zhanzhi Hu, PhD, of the Department of Systems Biology and the Department of Biomedical Information, Columbia University, New York City. “There are over 10,000 rare genetic diseases, but we’re currently screening for fewer than 100,” he told Neurology Reviews. Although in the United States, there are about 700-800 drugs approved for genetic diseases, “we can’t identify patients with these diseases early enough for the ideal window when treatments are most effective.”
Moreover, it’s a “lengthy process” to add new diseases to RUSP. “New conditions are added at the pace of less than one per year, on average — even for the hundreds of diseases for which there are treatments,” he said. “If we keep going at the current pace, we won’t be able to screen for those diseases for another few hundred years.”
Speeding up the pace of including new diseases in newborn screening is challenging because “we have more diseases than we have development dollars for,” Dr. Hu said. “Big pharmaceutical companies are reluctant to invest in rare diseases because the population is so small and it’s hard and expensive to develop such drugs. So if we can identify patients first, there will be more interest in developing treatments down the road.”
On the other hand, for trials to take place, these babies have to be identified in a timely manner — which requires testing. “Right now, we have a deadlock,” Dr. Hu said. “To nominate a disease, you need an approved treatment. But to get a treatment developed, you need to identify patients suitable for a clinical trial. If you have to wait for the symptoms to show up, the damage has already manifested and is irreversible. Our chance is to recognize the disease before symptom onset and then start treatment. I would call this a ‘chicken-and-egg’ problem.”
Dr. Hu is passionate about expanding newborn screening, and he has a very personal reason. Two of his children have a rare genetic disease. “My younger son, now 13 years old, was diagnosed at a much earlier age than my older son, although he had very few symptoms at the time, because his older brother was known to have the disease. As a result of this, his outcome was much better.” By contrast, Dr. Hu’s oldest son — now age 16 — wasn’t diagnosed until he became symptomatic.
His quest led him to join forces with Dr. Chung in conducting the Genomic Uniform-screening Against Rare Disease in All Newborns (Guardian) study, which screens newborns for more than 450 genetic conditions not currently screened as part of the standard newborn screening. To date, the study — which focuses on babies born in New York City — has screened about 11,000 infants.
“To accumulate enough evidence requires screening at least 100,000 babies because one requirement for nominating a disease for national inclusion in RUSP is an ‘N of 1’ study — meaning, to identify at least one positive patient using the proposed screening method in a prospective study,” Dr. Hu explained. “Most are rare diseases with an incidence rate of around one in 100,000. So getting to that magic number of 100,000 participants should enable us to hit that ‘N of 1’ for most diseases.”
The most challenging part, according to Dr. Hu, is the requirement of a prospective study, which means that you have to conduct a large-scale study enrolling tens of thousands of families and babies. If done for individual diseases (as has been the case in the past), “this is a huge cost and very inefficient.”
In reality, he added, the true incidence of these diseases is unclear. “Incidence rates are based on historical data rather than prospective studies. We’ve already seen some diseases show up more frequently than previously recorded, while others have shown up less frequently.”
For example, in the 11,000 babies screened to date, at least three girls with Rett syndrome have been identified, which is “quite a bit higher” than what has previously been identified in the literature (ie, one in 10,000-12,000 births). “This is a highly unmet need for these families because if you can initiate early treatment — at age 1, or even younger — the outcome will be better.”
He noted that there is at least one clinical trial underway for treating Rett syndrome, which has yielded “promising” data.5 “We’re hoping that by screening for diseases like Rett and identifying patients early, this will go hand-in-hand with clinical drug development. It can speed both the approval of the treatment and the addition to the newborn screening list,” Dr. Hu stated.
Screening and Drug Development Working in Tandem
Sequencing technologies have advanced and become more sophisticated as well as less costly, so interest in expanding newborn screening through newborn genome sequencing has increased. In fact, many states currently have incorporated genetic testing into newborn screening for conditions without biochemical markers. Additionally, newborn genomic sequencing is also used for further testing in infants with abnormal biochemical screening results.6
Genomic sequencing “identifies nucleotide changes that are the underlying etiology of monogenic disorders.”6 Its use could potentially enable identification of over 500 genetic disorders for which an newborn screening assay is not currently available, said Dr. Hu.
“Molecular DNA analysis has been integrated into newborn testing either as a first- or second-tier test for several conditions, including cystic fibrosis, severe combined immunodeficiency, and spinal muscular atrophy (SMA),” Dr. Hu said.
Dr. Hu pointed to SMA to illustrate the power and potential of newborn screening working hand-in-hand with the development of new treatments. SMA is a neurodegenerative disorder caused by mutations in SMN1, which encodes survival motor neuron protein (SMN).7 Deficiencies in SMN results in loss of motor neurons with muscle weakness and, often, early death.7A pilot study, on which Dr. Chung was the senior author, used both biochemical and genetic testing of close to 4000 newborns and found an SMA carrier frequency of 1.5%. One newborn was identified who had a homozygous SMN1 gene deletion and two copies of SMN2, strongly suggesting the presence of a severe type 1 SMA phenotype.8
At age 15 days, the baby was treated with nusinersen, an injection administered into the fluid surrounding the spinal cord, and the first FDA-approved genetic treatment for SMA. At the time of study publication, the baby was 12 months old, “meeting all developmental milestones and free of any respiratory issues,” the authors report.
“Screening for SMA — which was added to the RUSP in 2018 — has dramatically transformed what used to be the most common genetic cause of death in children under the age of 2,” Dr. Chung said. “Now, a once-and-done IV infusion of genetic therapy right after screening has transformed everything, taking what used to be a lethal condition and allowing children to grow up healthy.”
Advocating for Inclusion of Diseases With No Current Treatment
At present, any condition included in the RUSP is required to have a treatment, which can be dietary, surgical/procedural, or an FDA-approved drug-based agent. Unfortunately, a wide range of neurodevelopmental diseases still have no known treatments. But lack of availability of treatment shouldn’t invalidate a disease from being included in the RUSP, because even if there is no specific treatment for the condition itself, early intervention can still be initiated to prevent some of the manifestations of the condition, said Dr. Hu.
“For example, most patients with these diseases will sooner or later undergo seizures,” Dr. Hu remarked. “We know that repeated seizures can cause brain damage. If we can diagnose the disease before the seizures start to take place, we can put preventive seizure control interventions in place, even if there is no direct ‘treatment’ for the condition itself.”
Early identification can lead to early intervention, which can have other benefits, Dr. Hu noted. “If we train the brain at a young age, when the brain is most receptive, even though a disease may be progressive and will worsen, those abilities acquired earlier will last longer and remain in place longer. When these skills are acquired later, they’re forgotten sooner. This isn’t a ‘cure,’ but it will help with functional improvement.”
Moreover, parents are “interested in knowing that their child has a condition, even if no treatment is currently available for that disorder, according to our research,” Dr. Chung said. “We found that the parents we interviewed endorsed the nonmedical utility of having access to information, even in the absence of a ‘cure,’ so they could prepare for medical issues that might arise down the road and make informed choices.”9
Nina Gold, MD, director of Prenatal Medical Genetics and associate director for Research for Massachusetts General Brigham Personalized Medicine, Boston, obtained similar findings in her own research, which is currently under review for publication. “We conducted focus groups and one-on-one interviews with parents from diverse racial and socioeconomic backgrounds. At least one parent said they didn’t want to compare their child to other children if their child might have a different developmental trajectory. They stressed that the information would be helpful, even if there was no immediate clinical utility.”
Additionally, there are an “increasing number of fetal therapies for rare disorders, so information about a genetic disease in an older child can be helpful for parents who may go on to have another pregnancy,” Dr. Gold noted.
Dr. Hu detailed several other reasons for including a wider range of disorders in the RUSP. Doing so helps families avoid a “stressful and expensive diagnostic odyssey and also provides equitable access to a diagnosis.” And if these patients are identified early, “we can connect the family with clinical trials already underway or connect them to an organization such as the Accelerating Medicines Partnership (AMP) Program Bespoke Gene Therapy Consortium (AMP BGTC). Bespoke “brings together partners from the public, private, and nonprofit sectors to foster development of gene therapies intended to treat rare genetic diseases, which affect populations too small for viable commercial development.”
Next Steps Following Screening
Rebecca Sponberg, NP, of the Children’s Hospital of Orange County, UC Irvine School of Medicine, California, is part of a broader multidisciplinary team that interfaces with parents whose newborns have screened positive for a genetic disorder. The team also includes a biochemical geneticist, a pediatric neurologist, a pediatric endocrinologist, a genetic counselor, and a social worker.
Different states and locations have different procedures for receiving test results, said Dr. Chung. In some, pediatricians are the ones who receive the results, and they are tasked with the responsibility of making sure the children can start getting appropriate care. In particular, these pediatricians are associated with centers of excellence that specialize in working with families around these conditions. Other facilities have multidisciplinary teams.
Ms. Sponberg gave an example of how the process unfolded with X-linked adrenoleukodystrophy, a rare genetic disorder that affects the white matter of the nervous system and the adrenal cortex.10 “This is the most common peroxisomal disorder, affecting one in 20,000 males,” she said. “There are several different forms of the disorder, but males are most at risk for having the cerebral form, which can lead to neurological regression and hasten death. But the regression does not appear until 4 to 12 years of age.”
A baby who screens positive on the initial newborn screening has repeat testing; and if it’s confirmed, the family meets the entire team to help them understand what the disorder is, what to expect, and how it’s monitored and managed. “Children have to be followed closely with a brain MRI every 6 months to detect brain abnormalities quickly,” Ms. Sponberg explained “And we do regular bloodwork to look for adrenocortical insufficiency.”
A child who shows concerning changes on the MRI or abnormal blood test findings is immediately seen by the relevant specialist. “So far, our center has had one patient who had MRI changes consistent with the cerebral form of the disease and the patient was immediately able to receive a bone marrow transplant,” she reported. “We don’t think this child’s condition would have been picked up so quickly or treatment initiated so rapidly if we hadn’t known about it through newborn screening.”
Educating and Involving Families
Part of the role of clinicians is to provide education regarding newborn screening to families, according to Ms. Sponberg. “In my role, I have to call parents to tell them their child screened positive for a genetic condition and that we need to proceed with confirmatory testing,” she said. “We let them know if there’s a high concern that this might be a true positive for the condition, and we offer them information so they know what to expect.”
Unfortunately, Ms. Sponberg said, in the absence of education, some families are skeptical. “When I call families directly, some think it’s a scam and it can be hard to earn their trust. We need to do a better job educating families, especially our pregnant individuals, that testing will occur and if anything is abnormal, they will receive a call.”
References
1. Levy HL. Robert Guthrie and the Trials and Tribulations of Newborn Screening. Int J Neonatal Screen. 2021 Jan 19;7(1):5. doi: 10.3390/ijns7010005.
2. Chace DH et al. Clinical Chemistry and Dried Blood Spots: Increasing Laboratory Utilization by Improved Understanding of Quantitative Challenges. Bioanalysis. 2014;6(21):2791-2794. doi: 10.4155/bio.14.237.
3. Gold NB et al. Perspectives of Rare Disease Experts on Newborn Genome Sequencing. JAMA Netw Open. 2023 May 1;6(5):e2312231. doi: 10.1001/jamanetworkopen.2023.12231.
4. Weismiller DG. Expanded Newborn Screening: Information and Resources for the Family Physician. Am Fam Physician. 2017 Jun 1;95(11):703-709. https://www.aafp.org/pubs/afp/issues/2017/0601/p703.html.
5. Neul JL et al. Trofinetide for the Treatment of Rett Syndrome: A Randomized Phase 3 Study. Nat Med. 2023 Jun;29(6):1468-1475. doi: 10.1038/s41591-023-02398-1.
6. Chen T et al. Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening. JAMA Netw Open. 2023 Sep 5;6(9):e2331162. doi: 10.1001/jamanetworkopen.2023.31162.
7. Mercuri E et al. Spinal Muscular Atrophy. Nat Rev Dis Primers. 2022 Aug 4;8(1):52. doi: 10.1038/s41572-022-00380-8.
8. Kraszewski JN et al. Pilot Study of Population-Based Newborn Screening for Spinal Muscular Atrophy in New York State. Genet Med. 2018 Jun;20(6):608-613. doi: 10.1038/gim.2017.152.
9. Timmins GT et al. Diverse Parental Perspectives of the Social and Educational Needs for Expanding Newborn Screening Through Genomic Sequencing. Public Health Genomics. 2022 Sep 15:1-8. doi: 10.1159/000526382.
10. Turk BR et al. X-linked Adrenoleukodystrophy: Pathology, Pathophysiology, Diagnostic Testing, Newborn Screening and Therapies. Int J Dev Neurosci. 2020 Feb;80(1):52-72. doi: 10.1002/jdn.10003.
Newborn screening programs are public health services aimed at ensuring that the close to 4 million infants born each year in the United States are screened for certain serious disorders at birth. These disorders, albeit rare, are detected in roughly 12,500 newborn babies every year.
Newborn screening isn’t new, although it has expanded and transformed over the decades. The first newborn screening test was developed in the 1960s to detect phenylketonuria (PKU).1 Since then, the number of conditions screened for has increased, with programs in every US state and territory. “Newborn screening is well established now, not experimental or newfangled,” Wendy Chung, MD, PhD, professor of pediatrics, Harvard Medical School, Boston, Massachusetts, told Neurology Reviews.
In newborn screening, blood drawn from the baby’s heel is applied to specialized filter paper, which is then subjected to several analytical methods, including tandem mass spectrometry and molecular analyses to detect biomarkers for the diseases.2 More recently, genomic sequencing is being piloted as part of consented research studies.3
Newborn screening includes not only biochemical and genetic testing, but also includes noninvasive screening for hearing loss or for critical congenital heart disease using pulse oximetry. And newborn screening goes beyond analysis of a single drop of blood. Rather, “it’s an entire system, with the goal of identifying babies with genetic disorders who otherwise have no obvious symptoms,” said Dr. Chung. Left undetected and untreated, these conditions can be associated with serious adverse outcomes and even death.
Dr. Chung described newborn screening as a “one of the most successful public health programs, supporting health equity by screening almost every US baby after birth and then bringing timely treatments when relevant even before the baby develops symptoms of a disorder.” In this way, newborn screening has “saved lives and decreased disease burdens.”
There are at present 38 core conditions that the Department of Health and Human Services (HHS) regards as the most critical to screen for and 26 secondary conditions associated with these core disorders. This is called the Recommended Uniform Screening Panel (RUSP). Guidance regarding the most appropriate application of newborn screening tests, technologies and standards are provided by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC).
Each state “independently determines which screening tests are performed and what follow-up is provided.”4 Information about which tests are provided by which states can be found on the “Report Card” of the National Organization for Rare Diseases (NORD).
Challenges in Expanding the Current Newborn Screening
One of the major drawbacks in the current system is that “we don’t screen for enough diseases,” according to Zhanzhi Hu, PhD, of the Department of Systems Biology and the Department of Biomedical Information, Columbia University, New York City. “There are over 10,000 rare genetic diseases, but we’re currently screening for fewer than 100,” he told Neurology Reviews. Although in the United States, there are about 700-800 drugs approved for genetic diseases, “we can’t identify patients with these diseases early enough for the ideal window when treatments are most effective.”
Moreover, it’s a “lengthy process” to add new diseases to RUSP. “New conditions are added at the pace of less than one per year, on average — even for the hundreds of diseases for which there are treatments,” he said. “If we keep going at the current pace, we won’t be able to screen for those diseases for another few hundred years.”
Speeding up the pace of including new diseases in newborn screening is challenging because “we have more diseases than we have development dollars for,” Dr. Hu said. “Big pharmaceutical companies are reluctant to invest in rare diseases because the population is so small and it’s hard and expensive to develop such drugs. So if we can identify patients first, there will be more interest in developing treatments down the road.”
On the other hand, for trials to take place, these babies have to be identified in a timely manner — which requires testing. “Right now, we have a deadlock,” Dr. Hu said. “To nominate a disease, you need an approved treatment. But to get a treatment developed, you need to identify patients suitable for a clinical trial. If you have to wait for the symptoms to show up, the damage has already manifested and is irreversible. Our chance is to recognize the disease before symptom onset and then start treatment. I would call this a ‘chicken-and-egg’ problem.”
Dr. Hu is passionate about expanding newborn screening, and he has a very personal reason. Two of his children have a rare genetic disease. “My younger son, now 13 years old, was diagnosed at a much earlier age than my older son, although he had very few symptoms at the time, because his older brother was known to have the disease. As a result of this, his outcome was much better.” By contrast, Dr. Hu’s oldest son — now age 16 — wasn’t diagnosed until he became symptomatic.
His quest led him to join forces with Dr. Chung in conducting the Genomic Uniform-screening Against Rare Disease in All Newborns (Guardian) study, which screens newborns for more than 450 genetic conditions not currently screened as part of the standard newborn screening. To date, the study — which focuses on babies born in New York City — has screened about 11,000 infants.
“To accumulate enough evidence requires screening at least 100,000 babies because one requirement for nominating a disease for national inclusion in RUSP is an ‘N of 1’ study — meaning, to identify at least one positive patient using the proposed screening method in a prospective study,” Dr. Hu explained. “Most are rare diseases with an incidence rate of around one in 100,000. So getting to that magic number of 100,000 participants should enable us to hit that ‘N of 1’ for most diseases.”
The most challenging part, according to Dr. Hu, is the requirement of a prospective study, which means that you have to conduct a large-scale study enrolling tens of thousands of families and babies. If done for individual diseases (as has been the case in the past), “this is a huge cost and very inefficient.”
In reality, he added, the true incidence of these diseases is unclear. “Incidence rates are based on historical data rather than prospective studies. We’ve already seen some diseases show up more frequently than previously recorded, while others have shown up less frequently.”
For example, in the 11,000 babies screened to date, at least three girls with Rett syndrome have been identified, which is “quite a bit higher” than what has previously been identified in the literature (ie, one in 10,000-12,000 births). “This is a highly unmet need for these families because if you can initiate early treatment — at age 1, or even younger — the outcome will be better.”
He noted that there is at least one clinical trial underway for treating Rett syndrome, which has yielded “promising” data.5 “We’re hoping that by screening for diseases like Rett and identifying patients early, this will go hand-in-hand with clinical drug development. It can speed both the approval of the treatment and the addition to the newborn screening list,” Dr. Hu stated.
Screening and Drug Development Working in Tandem
Sequencing technologies have advanced and become more sophisticated as well as less costly, so interest in expanding newborn screening through newborn genome sequencing has increased. In fact, many states currently have incorporated genetic testing into newborn screening for conditions without biochemical markers. Additionally, newborn genomic sequencing is also used for further testing in infants with abnormal biochemical screening results.6
Genomic sequencing “identifies nucleotide changes that are the underlying etiology of monogenic disorders.”6 Its use could potentially enable identification of over 500 genetic disorders for which an newborn screening assay is not currently available, said Dr. Hu.
“Molecular DNA analysis has been integrated into newborn testing either as a first- or second-tier test for several conditions, including cystic fibrosis, severe combined immunodeficiency, and spinal muscular atrophy (SMA),” Dr. Hu said.
Dr. Hu pointed to SMA to illustrate the power and potential of newborn screening working hand-in-hand with the development of new treatments. SMA is a neurodegenerative disorder caused by mutations in SMN1, which encodes survival motor neuron protein (SMN).7 Deficiencies in SMN results in loss of motor neurons with muscle weakness and, often, early death.7A pilot study, on which Dr. Chung was the senior author, used both biochemical and genetic testing of close to 4000 newborns and found an SMA carrier frequency of 1.5%. One newborn was identified who had a homozygous SMN1 gene deletion and two copies of SMN2, strongly suggesting the presence of a severe type 1 SMA phenotype.8
At age 15 days, the baby was treated with nusinersen, an injection administered into the fluid surrounding the spinal cord, and the first FDA-approved genetic treatment for SMA. At the time of study publication, the baby was 12 months old, “meeting all developmental milestones and free of any respiratory issues,” the authors report.
“Screening for SMA — which was added to the RUSP in 2018 — has dramatically transformed what used to be the most common genetic cause of death in children under the age of 2,” Dr. Chung said. “Now, a once-and-done IV infusion of genetic therapy right after screening has transformed everything, taking what used to be a lethal condition and allowing children to grow up healthy.”
Advocating for Inclusion of Diseases With No Current Treatment
At present, any condition included in the RUSP is required to have a treatment, which can be dietary, surgical/procedural, or an FDA-approved drug-based agent. Unfortunately, a wide range of neurodevelopmental diseases still have no known treatments. But lack of availability of treatment shouldn’t invalidate a disease from being included in the RUSP, because even if there is no specific treatment for the condition itself, early intervention can still be initiated to prevent some of the manifestations of the condition, said Dr. Hu.
“For example, most patients with these diseases will sooner or later undergo seizures,” Dr. Hu remarked. “We know that repeated seizures can cause brain damage. If we can diagnose the disease before the seizures start to take place, we can put preventive seizure control interventions in place, even if there is no direct ‘treatment’ for the condition itself.”
Early identification can lead to early intervention, which can have other benefits, Dr. Hu noted. “If we train the brain at a young age, when the brain is most receptive, even though a disease may be progressive and will worsen, those abilities acquired earlier will last longer and remain in place longer. When these skills are acquired later, they’re forgotten sooner. This isn’t a ‘cure,’ but it will help with functional improvement.”
Moreover, parents are “interested in knowing that their child has a condition, even if no treatment is currently available for that disorder, according to our research,” Dr. Chung said. “We found that the parents we interviewed endorsed the nonmedical utility of having access to information, even in the absence of a ‘cure,’ so they could prepare for medical issues that might arise down the road and make informed choices.”9
Nina Gold, MD, director of Prenatal Medical Genetics and associate director for Research for Massachusetts General Brigham Personalized Medicine, Boston, obtained similar findings in her own research, which is currently under review for publication. “We conducted focus groups and one-on-one interviews with parents from diverse racial and socioeconomic backgrounds. At least one parent said they didn’t want to compare their child to other children if their child might have a different developmental trajectory. They stressed that the information would be helpful, even if there was no immediate clinical utility.”
Additionally, there are an “increasing number of fetal therapies for rare disorders, so information about a genetic disease in an older child can be helpful for parents who may go on to have another pregnancy,” Dr. Gold noted.
Dr. Hu detailed several other reasons for including a wider range of disorders in the RUSP. Doing so helps families avoid a “stressful and expensive diagnostic odyssey and also provides equitable access to a diagnosis.” And if these patients are identified early, “we can connect the family with clinical trials already underway or connect them to an organization such as the Accelerating Medicines Partnership (AMP) Program Bespoke Gene Therapy Consortium (AMP BGTC). Bespoke “brings together partners from the public, private, and nonprofit sectors to foster development of gene therapies intended to treat rare genetic diseases, which affect populations too small for viable commercial development.”
Next Steps Following Screening
Rebecca Sponberg, NP, of the Children’s Hospital of Orange County, UC Irvine School of Medicine, California, is part of a broader multidisciplinary team that interfaces with parents whose newborns have screened positive for a genetic disorder. The team also includes a biochemical geneticist, a pediatric neurologist, a pediatric endocrinologist, a genetic counselor, and a social worker.
Different states and locations have different procedures for receiving test results, said Dr. Chung. In some, pediatricians are the ones who receive the results, and they are tasked with the responsibility of making sure the children can start getting appropriate care. In particular, these pediatricians are associated with centers of excellence that specialize in working with families around these conditions. Other facilities have multidisciplinary teams.
Ms. Sponberg gave an example of how the process unfolded with X-linked adrenoleukodystrophy, a rare genetic disorder that affects the white matter of the nervous system and the adrenal cortex.10 “This is the most common peroxisomal disorder, affecting one in 20,000 males,” she said. “There are several different forms of the disorder, but males are most at risk for having the cerebral form, which can lead to neurological regression and hasten death. But the regression does not appear until 4 to 12 years of age.”
A baby who screens positive on the initial newborn screening has repeat testing; and if it’s confirmed, the family meets the entire team to help them understand what the disorder is, what to expect, and how it’s monitored and managed. “Children have to be followed closely with a brain MRI every 6 months to detect brain abnormalities quickly,” Ms. Sponberg explained “And we do regular bloodwork to look for adrenocortical insufficiency.”
A child who shows concerning changes on the MRI or abnormal blood test findings is immediately seen by the relevant specialist. “So far, our center has had one patient who had MRI changes consistent with the cerebral form of the disease and the patient was immediately able to receive a bone marrow transplant,” she reported. “We don’t think this child’s condition would have been picked up so quickly or treatment initiated so rapidly if we hadn’t known about it through newborn screening.”
Educating and Involving Families
Part of the role of clinicians is to provide education regarding newborn screening to families, according to Ms. Sponberg. “In my role, I have to call parents to tell them their child screened positive for a genetic condition and that we need to proceed with confirmatory testing,” she said. “We let them know if there’s a high concern that this might be a true positive for the condition, and we offer them information so they know what to expect.”
Unfortunately, Ms. Sponberg said, in the absence of education, some families are skeptical. “When I call families directly, some think it’s a scam and it can be hard to earn their trust. We need to do a better job educating families, especially our pregnant individuals, that testing will occur and if anything is abnormal, they will receive a call.”
References
1. Levy HL. Robert Guthrie and the Trials and Tribulations of Newborn Screening. Int J Neonatal Screen. 2021 Jan 19;7(1):5. doi: 10.3390/ijns7010005.
2. Chace DH et al. Clinical Chemistry and Dried Blood Spots: Increasing Laboratory Utilization by Improved Understanding of Quantitative Challenges. Bioanalysis. 2014;6(21):2791-2794. doi: 10.4155/bio.14.237.
3. Gold NB et al. Perspectives of Rare Disease Experts on Newborn Genome Sequencing. JAMA Netw Open. 2023 May 1;6(5):e2312231. doi: 10.1001/jamanetworkopen.2023.12231.
4. Weismiller DG. Expanded Newborn Screening: Information and Resources for the Family Physician. Am Fam Physician. 2017 Jun 1;95(11):703-709. https://www.aafp.org/pubs/afp/issues/2017/0601/p703.html.
5. Neul JL et al. Trofinetide for the Treatment of Rett Syndrome: A Randomized Phase 3 Study. Nat Med. 2023 Jun;29(6):1468-1475. doi: 10.1038/s41591-023-02398-1.
6. Chen T et al. Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening. JAMA Netw Open. 2023 Sep 5;6(9):e2331162. doi: 10.1001/jamanetworkopen.2023.31162.
7. Mercuri E et al. Spinal Muscular Atrophy. Nat Rev Dis Primers. 2022 Aug 4;8(1):52. doi: 10.1038/s41572-022-00380-8.
8. Kraszewski JN et al. Pilot Study of Population-Based Newborn Screening for Spinal Muscular Atrophy in New York State. Genet Med. 2018 Jun;20(6):608-613. doi: 10.1038/gim.2017.152.
9. Timmins GT et al. Diverse Parental Perspectives of the Social and Educational Needs for Expanding Newborn Screening Through Genomic Sequencing. Public Health Genomics. 2022 Sep 15:1-8. doi: 10.1159/000526382.
10. Turk BR et al. X-linked Adrenoleukodystrophy: Pathology, Pathophysiology, Diagnostic Testing, Newborn Screening and Therapies. Int J Dev Neurosci. 2020 Feb;80(1):52-72. doi: 10.1002/jdn.10003.
Aspects of the Skin Microbiome Remain Elusive
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO — Although it has been known for several years that
In one review of the topic, researchers from the National Institutes of Health wrote that the skin is composed of 1.8 million diverse habitats with an abundance of folds, invaginations, and specialized niches that support a wide range of microorganisms. “Many of these microorganisms are harmless and, in some cases, provide vital functions for us to live and they have not evolved over time,” Jill S. Waibel, MD, medical director of the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium.
“This is complex ecosystem that we don’t really talk about,” she said. “There is wide topographical distribution of bacteria on skin sites. The bacteria we have on our head and neck area is different from that on our feet. There is also a lot of interpersonal variation of the skin microbiome, so one person may have a lot of one type of bacteria and not as much of another.”
A Shield From Foreign Pathogens
At its core, Dr. Waibel continued, the skin microbiome functions as an interface between the human body and the environment, a physical barrier that prevents the invasion of foreign pathogens. The skin also provides a home to commensal microbiota. She likened the skin’s landscape to that of the tundra: “It’s desiccated, has poor nutrients, and it’s very acidic, thus pathogens have a hard time living on it,” she said. “However, our skin microorganisms have adapted to utilize the sparse nutrients available on the skin. That’s why I tell my patients, ‘don’t use a sugar scrub because you’re potentially feeding these bad bacteria.’ ”
According to more recent research, the skin microbiota in healthy adults remains stable over time, despite environmental perturbations, and they have important roles in educating the innate and adaptive arms of the cutaneous immune system. “Some skin diseases are associated with an altered microbial state: dysbiosis,” said Dr. Waibel, subsection chief of dermatology at Baptist Health South Florida, Miami Beach. “Reversion of this may help prevent or treat the disease.”
She cited the following factors that influence the skin microbiome:
- Genetics affects the skin microbiome considerably. Individuals with autoimmune predispositions have different microbiota compared with those who don’t.
- Climate, pollution, and hygiene practices the other influencing factors. “Even clothing can impact the microbiome, by causing the transfer of microorganisms,” she said.
- Age and hormonal changes (particularly during puberty) and senescence alter the microbial landscape.
- Systemic health conditions such as diabetes mellitus and irritable bowel disease, as well as cutaneous conditions like psoriasis and atopic dermatitis can also disrupt the skin microbiome.
Ingredients contained in soaps, antibiotics, and cosmetics can also cause skin dysbiosis, Dr. Waibel said. However, the integrity of the skin’s microbiome following dermatological procedures such as excisions, dermabrasion, laser therapy, and other physical procedures is less understood, according to a recent review of the topic. Phototherapy appears to be the most extensively studied, “and shows an increase in microbial diversity post-treatment,” she said. “Light treatments have been found to kill bacteria by inducing DNA damage. More studies need to be performed on specific wavelengths of light used, conditions being treated and individual patient differences.”
According to the review’s authors, no change in the microbiome was observed in studies of debridement. “That was surprising, as it is a method to remove unhealthy tissue that often contains pathogenic bacteria,” Dr. Waibel said. “The big take-home message is that we need more research.”
Dr. Waibel disclosed that she has conducted clinical trials for several device and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE 2024 MASTERS OF AESTHETICS SYMPOSIUM
Myeloma: Daratumumab Plus Lenalidomide Improves MRD Outcomes
“To date, no randomized trial has directly compared daratumumab-based maintenance therapy vs standard of care lenalidomide maintenance, which is the focus of our trial,” said first author Ashraf Z. Badros, MD, a professor of medicine at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland Medical Center, Baltimore, in presenting the findings at the International Myeloma Society (IMS) 2024.
“These results support the addition of daratumumab not only to induction/consolidation but also to standard of care lenalidomide maintenance for these patients,” he said of the study, which was published concurrently in the journal Blood.
Despite ongoing advancements in regimens for induction, consolidation, and maintenance posttransplant, most patients with MM eventually relapse, driving continuing efforts to optimize treatment strategies and improve long-term outcomes.
While daratumumab, an anti-CD38 monoclonal antibody, is approved in induction and consolidation with ASCT for patients with newly diagnosed MM, the authors sought to investigate the potential benefits of adding it to the standard-of-care therapy lenalidomide in maintenance therapy.
For the phase 3 AURIGA trial, they recruited 200 patients with newly diagnosed MM within 12 months of induction therapy and 6 months of ASCT.
The patients, who were all anti-CD38 naive, received at least four induction cycles, had at least a very good partial response, and were MRD positive following ASCT.
They were randomized 1:1 to receive 28-day lenalidomide maintenance cycles either with (n = 99) or without (n = 101) subcutaneous daratumumab for at least 36 cycles or until disease progression, unacceptable toxicity, or withdrawal.
The patients had similar baseline demographic characteristics; their median age was about 62 years, and 25.3% in the daratumumab and 23.5% in the no-daratumumab group had ISS stage III disease. At the time of diagnosis, 23.9% and 16.9%, respectively, had high cytogenic risk.
Overall, patients received a median of five induction cycles prior to entering the study.
For the primary endpoint, the rate of conversion from MRD positive to MRD negative (at a sensitivity of 10-5 using next-generation sequencing) by 12 months was significantly higher in the daratumumab group than in the lenalidomide-only group, at 50.5% vs 18.8% (odds ratio [OR], 4.51; P < .0001).
A similar benefit with the daratumumab group was observed across all clinically relevant subgroups, including patients with high-risk disease.
The MRD-negative conversion rate was similar at the 10-6 threshold (23.2% vs 5%; OR, 5.97; P = .0002).
At a median follow-up of 32.3 months, the overall rates of MRD negativity were 60.6% and 27.7%, with and without daratumumab, respectively (OR, 4.12; P < .0001)
The achievement of complete response or better also was significantly greater with daratumumab (75.8% vs 61.4%; OR, 2.00; P = .0255).
Likewise, PFS favored daratumumab (hazard ratio, 0.53), and the estimated 30-month PFS rates were 82.7% and 66.4%, respectively.
The daratumumab group received more maintenance cycles than the lenalidomide-only group (median of 33 vs 21.5), and it had higher rates of completion of 12 cycles (88.5% vs 78.6%). Dr. Badros noted that the main reason for discontinuation of therapy in the no-daratumumab arm was disease progression.
Consistent with previous studies, daratumumab was associated with more grade 3/4 treatment-emergent adverse events (TEAEs), occurring in 74.0% patients vs 67.3% patients not receiving daratumumab, including infections (18.8% vs 13.3%), cytopenia (54.2% vs 46.9%), and neutropenia (46.9% vs 41.8%). Dr. Badros noted the significantly longer time of treatment in the daratumumab arm (30 months vs 20 months).
Serious TEAEs occurred in 30.2% daratumumab patients and 22.4% no-daratumumab patients, and fatal TEAEs occurred in 2.1% and 1.0% patients, respectively.
“Overall, there were no new safety concerns for daratumumab,” he said.
The authors noted that the requirement that patients be anti-CD38 naive was partially because of “the D-VRd [daratumumab combined with bortezomib, lenalidomide, and dexamethasone] regimen gaining popularity and increased utilization in the myeloma community for transplant-eligible patients with NDMM, even before the publication of the long-term results of the randomized GRIFFIN and PERSEUS studies.”
A key question, remarked Joseph Mikhael, MD, who is chief medical officer of the International Myeloma Foundation, from the audience, is how applicable the findings are in the modern environment, where most patients now have indeed had prior anti-CD38 treatment.
In response, Dr. Badros explained that “I think this is an important study because it is probably one of the few studies that separates the impact of daratumumab-lenalidomide without prior daratumumab use.”
Dr. Badros noted that results from the PERSEUS trial, of D-VRd, show MRD-positive to MRD-negative conversion rates that are similar to the current trial; “therefore, I really don’t think that using daratumumab up front will prevent using it as maintenance,” he said. “If anything, it actually improves outcomes.”
The findings from continuous treatment “are an important reminder that high-risk patients do not do well if you stop treatment,” he said.
Further commenting on the research at the meeting, María-Victoria Mateos, MD, PhD, an associate professor of medicine at the University of Salamanca in Spain, noted that “the unmet need in maintenance is to upgrade the quality of the response and to increase the conversion of MRD-positivity to MRD negative in order to delay the progression of the disease and prolong the overall survival.”
Regarding the AURIGA trial, “this is very interesting data about the role of daratumumab-lenalidomide maintenance in patients who are MRD positive after autologous stem cell transplantation.”
“What is more important is we are progressing in response-adaptive therapy, and we are generating very useful information to possibly make the majority of patients become MRD negative.
“Developing early endpoints as surrogate markers for long-term outcomes and overall survival is critically important,” she added. “Otherwise, trials may continue for more than 15 years.”
The study was sponsored by Janssen Biotech. Dr. Badros reported relationships with Bristol-Myers Squibb, BeiGene, Roche, Jansen, and GSK. Mateos disclosed ties with AbbVie, Amgen, Bristol-Myers Squibb, GSK, Kite, Johnson & Johnson, Oncopeptides, Pfizer, Regeneron, Roche, and Sanofi.
A version of this article first appeared on Medscape.com.
“To date, no randomized trial has directly compared daratumumab-based maintenance therapy vs standard of care lenalidomide maintenance, which is the focus of our trial,” said first author Ashraf Z. Badros, MD, a professor of medicine at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland Medical Center, Baltimore, in presenting the findings at the International Myeloma Society (IMS) 2024.
“These results support the addition of daratumumab not only to induction/consolidation but also to standard of care lenalidomide maintenance for these patients,” he said of the study, which was published concurrently in the journal Blood.
Despite ongoing advancements in regimens for induction, consolidation, and maintenance posttransplant, most patients with MM eventually relapse, driving continuing efforts to optimize treatment strategies and improve long-term outcomes.
While daratumumab, an anti-CD38 monoclonal antibody, is approved in induction and consolidation with ASCT for patients with newly diagnosed MM, the authors sought to investigate the potential benefits of adding it to the standard-of-care therapy lenalidomide in maintenance therapy.
For the phase 3 AURIGA trial, they recruited 200 patients with newly diagnosed MM within 12 months of induction therapy and 6 months of ASCT.
The patients, who were all anti-CD38 naive, received at least four induction cycles, had at least a very good partial response, and were MRD positive following ASCT.
They were randomized 1:1 to receive 28-day lenalidomide maintenance cycles either with (n = 99) or without (n = 101) subcutaneous daratumumab for at least 36 cycles or until disease progression, unacceptable toxicity, or withdrawal.
The patients had similar baseline demographic characteristics; their median age was about 62 years, and 25.3% in the daratumumab and 23.5% in the no-daratumumab group had ISS stage III disease. At the time of diagnosis, 23.9% and 16.9%, respectively, had high cytogenic risk.
Overall, patients received a median of five induction cycles prior to entering the study.
For the primary endpoint, the rate of conversion from MRD positive to MRD negative (at a sensitivity of 10-5 using next-generation sequencing) by 12 months was significantly higher in the daratumumab group than in the lenalidomide-only group, at 50.5% vs 18.8% (odds ratio [OR], 4.51; P < .0001).
A similar benefit with the daratumumab group was observed across all clinically relevant subgroups, including patients with high-risk disease.
The MRD-negative conversion rate was similar at the 10-6 threshold (23.2% vs 5%; OR, 5.97; P = .0002).
At a median follow-up of 32.3 months, the overall rates of MRD negativity were 60.6% and 27.7%, with and without daratumumab, respectively (OR, 4.12; P < .0001)
The achievement of complete response or better also was significantly greater with daratumumab (75.8% vs 61.4%; OR, 2.00; P = .0255).
Likewise, PFS favored daratumumab (hazard ratio, 0.53), and the estimated 30-month PFS rates were 82.7% and 66.4%, respectively.
The daratumumab group received more maintenance cycles than the lenalidomide-only group (median of 33 vs 21.5), and it had higher rates of completion of 12 cycles (88.5% vs 78.6%). Dr. Badros noted that the main reason for discontinuation of therapy in the no-daratumumab arm was disease progression.
Consistent with previous studies, daratumumab was associated with more grade 3/4 treatment-emergent adverse events (TEAEs), occurring in 74.0% patients vs 67.3% patients not receiving daratumumab, including infections (18.8% vs 13.3%), cytopenia (54.2% vs 46.9%), and neutropenia (46.9% vs 41.8%). Dr. Badros noted the significantly longer time of treatment in the daratumumab arm (30 months vs 20 months).
Serious TEAEs occurred in 30.2% daratumumab patients and 22.4% no-daratumumab patients, and fatal TEAEs occurred in 2.1% and 1.0% patients, respectively.
“Overall, there were no new safety concerns for daratumumab,” he said.
The authors noted that the requirement that patients be anti-CD38 naive was partially because of “the D-VRd [daratumumab combined with bortezomib, lenalidomide, and dexamethasone] regimen gaining popularity and increased utilization in the myeloma community for transplant-eligible patients with NDMM, even before the publication of the long-term results of the randomized GRIFFIN and PERSEUS studies.”
A key question, remarked Joseph Mikhael, MD, who is chief medical officer of the International Myeloma Foundation, from the audience, is how applicable the findings are in the modern environment, where most patients now have indeed had prior anti-CD38 treatment.
In response, Dr. Badros explained that “I think this is an important study because it is probably one of the few studies that separates the impact of daratumumab-lenalidomide without prior daratumumab use.”
Dr. Badros noted that results from the PERSEUS trial, of D-VRd, show MRD-positive to MRD-negative conversion rates that are similar to the current trial; “therefore, I really don’t think that using daratumumab up front will prevent using it as maintenance,” he said. “If anything, it actually improves outcomes.”
The findings from continuous treatment “are an important reminder that high-risk patients do not do well if you stop treatment,” he said.
Further commenting on the research at the meeting, María-Victoria Mateos, MD, PhD, an associate professor of medicine at the University of Salamanca in Spain, noted that “the unmet need in maintenance is to upgrade the quality of the response and to increase the conversion of MRD-positivity to MRD negative in order to delay the progression of the disease and prolong the overall survival.”
Regarding the AURIGA trial, “this is very interesting data about the role of daratumumab-lenalidomide maintenance in patients who are MRD positive after autologous stem cell transplantation.”
“What is more important is we are progressing in response-adaptive therapy, and we are generating very useful information to possibly make the majority of patients become MRD negative.
“Developing early endpoints as surrogate markers for long-term outcomes and overall survival is critically important,” she added. “Otherwise, trials may continue for more than 15 years.”
The study was sponsored by Janssen Biotech. Dr. Badros reported relationships with Bristol-Myers Squibb, BeiGene, Roche, Jansen, and GSK. Mateos disclosed ties with AbbVie, Amgen, Bristol-Myers Squibb, GSK, Kite, Johnson & Johnson, Oncopeptides, Pfizer, Regeneron, Roche, and Sanofi.
A version of this article first appeared on Medscape.com.
“To date, no randomized trial has directly compared daratumumab-based maintenance therapy vs standard of care lenalidomide maintenance, which is the focus of our trial,” said first author Ashraf Z. Badros, MD, a professor of medicine at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland Medical Center, Baltimore, in presenting the findings at the International Myeloma Society (IMS) 2024.
“These results support the addition of daratumumab not only to induction/consolidation but also to standard of care lenalidomide maintenance for these patients,” he said of the study, which was published concurrently in the journal Blood.
Despite ongoing advancements in regimens for induction, consolidation, and maintenance posttransplant, most patients with MM eventually relapse, driving continuing efforts to optimize treatment strategies and improve long-term outcomes.
While daratumumab, an anti-CD38 monoclonal antibody, is approved in induction and consolidation with ASCT for patients with newly diagnosed MM, the authors sought to investigate the potential benefits of adding it to the standard-of-care therapy lenalidomide in maintenance therapy.
For the phase 3 AURIGA trial, they recruited 200 patients with newly diagnosed MM within 12 months of induction therapy and 6 months of ASCT.
The patients, who were all anti-CD38 naive, received at least four induction cycles, had at least a very good partial response, and were MRD positive following ASCT.
They were randomized 1:1 to receive 28-day lenalidomide maintenance cycles either with (n = 99) or without (n = 101) subcutaneous daratumumab for at least 36 cycles or until disease progression, unacceptable toxicity, or withdrawal.
The patients had similar baseline demographic characteristics; their median age was about 62 years, and 25.3% in the daratumumab and 23.5% in the no-daratumumab group had ISS stage III disease. At the time of diagnosis, 23.9% and 16.9%, respectively, had high cytogenic risk.
Overall, patients received a median of five induction cycles prior to entering the study.
For the primary endpoint, the rate of conversion from MRD positive to MRD negative (at a sensitivity of 10-5 using next-generation sequencing) by 12 months was significantly higher in the daratumumab group than in the lenalidomide-only group, at 50.5% vs 18.8% (odds ratio [OR], 4.51; P < .0001).
A similar benefit with the daratumumab group was observed across all clinically relevant subgroups, including patients with high-risk disease.
The MRD-negative conversion rate was similar at the 10-6 threshold (23.2% vs 5%; OR, 5.97; P = .0002).
At a median follow-up of 32.3 months, the overall rates of MRD negativity were 60.6% and 27.7%, with and without daratumumab, respectively (OR, 4.12; P < .0001)
The achievement of complete response or better also was significantly greater with daratumumab (75.8% vs 61.4%; OR, 2.00; P = .0255).
Likewise, PFS favored daratumumab (hazard ratio, 0.53), and the estimated 30-month PFS rates were 82.7% and 66.4%, respectively.
The daratumumab group received more maintenance cycles than the lenalidomide-only group (median of 33 vs 21.5), and it had higher rates of completion of 12 cycles (88.5% vs 78.6%). Dr. Badros noted that the main reason for discontinuation of therapy in the no-daratumumab arm was disease progression.
Consistent with previous studies, daratumumab was associated with more grade 3/4 treatment-emergent adverse events (TEAEs), occurring in 74.0% patients vs 67.3% patients not receiving daratumumab, including infections (18.8% vs 13.3%), cytopenia (54.2% vs 46.9%), and neutropenia (46.9% vs 41.8%). Dr. Badros noted the significantly longer time of treatment in the daratumumab arm (30 months vs 20 months).
Serious TEAEs occurred in 30.2% daratumumab patients and 22.4% no-daratumumab patients, and fatal TEAEs occurred in 2.1% and 1.0% patients, respectively.
“Overall, there were no new safety concerns for daratumumab,” he said.
The authors noted that the requirement that patients be anti-CD38 naive was partially because of “the D-VRd [daratumumab combined with bortezomib, lenalidomide, and dexamethasone] regimen gaining popularity and increased utilization in the myeloma community for transplant-eligible patients with NDMM, even before the publication of the long-term results of the randomized GRIFFIN and PERSEUS studies.”
A key question, remarked Joseph Mikhael, MD, who is chief medical officer of the International Myeloma Foundation, from the audience, is how applicable the findings are in the modern environment, where most patients now have indeed had prior anti-CD38 treatment.
In response, Dr. Badros explained that “I think this is an important study because it is probably one of the few studies that separates the impact of daratumumab-lenalidomide without prior daratumumab use.”
Dr. Badros noted that results from the PERSEUS trial, of D-VRd, show MRD-positive to MRD-negative conversion rates that are similar to the current trial; “therefore, I really don’t think that using daratumumab up front will prevent using it as maintenance,” he said. “If anything, it actually improves outcomes.”
The findings from continuous treatment “are an important reminder that high-risk patients do not do well if you stop treatment,” he said.
Further commenting on the research at the meeting, María-Victoria Mateos, MD, PhD, an associate professor of medicine at the University of Salamanca in Spain, noted that “the unmet need in maintenance is to upgrade the quality of the response and to increase the conversion of MRD-positivity to MRD negative in order to delay the progression of the disease and prolong the overall survival.”
Regarding the AURIGA trial, “this is very interesting data about the role of daratumumab-lenalidomide maintenance in patients who are MRD positive after autologous stem cell transplantation.”
“What is more important is we are progressing in response-adaptive therapy, and we are generating very useful information to possibly make the majority of patients become MRD negative.
“Developing early endpoints as surrogate markers for long-term outcomes and overall survival is critically important,” she added. “Otherwise, trials may continue for more than 15 years.”
The study was sponsored by Janssen Biotech. Dr. Badros reported relationships with Bristol-Myers Squibb, BeiGene, Roche, Jansen, and GSK. Mateos disclosed ties with AbbVie, Amgen, Bristol-Myers Squibb, GSK, Kite, Johnson & Johnson, Oncopeptides, Pfizer, Regeneron, Roche, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM IMS 2024
Diabetic Kidney Disease Therapies Keep on FLOWing
Further data from the FLOW study were presented during the 2024 congress of the European Association for the Study of Diabetes (EASD) in Madrid. The FLOW study was originally presented in May at the European Renal Association’s 2024 congress in Stockholm. It was the first dedicated kidney outcomes trial to examine a GLP-1 receptor agonist.
The FLOW study demonstrated significant kidney, cardiovascular, and mortality benefits with semaglutide 1 mg once weekly in patients with type 2 diabetes and chronic kidney disease (CKD). This study has elevated semaglutide to a new pillar of care for the management of diabetic kidney disease (DKD) alongside RAAS inhibitors, SGLT2 inhibitors, and finerenone.
At first, whether the benefits of semaglutide were independent of baseline SGLT2 inhibitor use was uncertain. The data presented at the EASD congress, however, appeared to confirm the additive benefits of semaglutide, when combined with SGLT2 inhibitor use, in patients with DKD. The authors did acknowledge that study power was limited, given the low use of SGLT2 inhibitors at trial recruitment (no licensed SGLT2 inhibitor was available for CKD at that point), so small, clinically relevant interactions may not have been detected.
So, what are the implications of the FLOW study for primary care?
DKD is a common clinical challenge in primary care; a national diabetes audit in the United Kingdom suggested that over 40% of patients with type 2 diabetes had kidney disease. Moreover, DKD is the most common cause of kidney failure in adults starting renal replacement therapy in the United Kingdom.
Residual renal risk in patients with DKD persists despite optimal use of guideline-directed medical therapy (GDMT) with RAAS inhibitors, SGLT2 inhibitors, and finerenone, as demonstrated in the many landmark kidney outcomes trials over the past 25 years.
So, a new pillar of GDMT is welcome, but I am worried that this widened choice of therapies may worsen therapeutic inertia; baseline use of the newer DKD therapies (specifically SGLT2 inhibitors and finerenone) remains low.
In addition, during the EASD FLOW session, Katherine Tuttle, MD, executive director for research at Providence Inland Northwest Health Services in Spokane, Washington, presented data from the US CURE-CKD registry study showing that baseline ACE inhibitor/ARB use of about 70% dropped to 50% after just 90 days. Baseline use of SGLT2 inhibitors was only about 6% and dropped to 5% after 90 days.
I suspect that much of this reduction in prescribing of ACE inhibitors/ARBs will have been in response to an acute dip in estimated glomerular filtration rate (eGFR) or hyperkalemia, which has been a perennial challenge with RAAS inhibitor use in primary care. Ongoing education in primary care is required to manage hyperkalemia and reductions in eGFR after RAAS inhibitor initiation to prevent premature cessation of these foundational therapies.
On a positive note, there was no acute dip in eGFR after prescribing semaglutide in DKD. This observation will be reassuring for primary care and hopefully prevent unnecessary cessation of therapy.
Also reassuring was the lack of difference in diabetic retinopathy adverse events between the semaglutide and placebo groups. These events raised concerns about semaglutide following the SUSTAIN-6 CVOT study and have affected attitudes in primary care. But the rapidity and magnitude of improvement in glycemic control with semaglutide was believed to be the underlying issue, rather than semaglutide itself. A similar phenomenon has been observed with insulin. The ongoing FOCUS study is exploring the long-term effects of semaglutide on diabetic retinopathy in patients with type 2 diabetes. This study will hopefully provide a definite answer to this issue.
Another useful message from the FLOW study for primary care is the utility of semaglutide for glucose-lowering in the context of CKD. A1c was 0.81% lower in the semaglutide group compared with the placebo group in participants with eGFRs as low as 25 mL/min/1.73 m2. It is well established that SGLT2 inhibitors have negligible glucose-lowering effects once eGFR drops below 45 mL/min/1.73 m2. Indeed, my usual practice in CKD, if additional glucose-lowering is required once renal protection has been established with an SGLT2 inhibitor, was to add a GLP-1 receptor agonist. It is reassuring to have my clinical practice ratified by the FLOW study.
Semaglutide also helpfully provides an alternative therapeutic option for patients who do not tolerate SGLT2 inhibitors because of, for example, recurrent mycotic genital infections or polyuria, or for those in whom SGLT2 inhibitors are contraindicated, such as patients who have experienced an unprovoked episode of diabetic ketoacidosis. Many of these patients still require cardiovascular and kidney protection, so the FLOW study gives me a viable evidence-based alternative.
As a class, semaglutide and GLP-1 receptor agonists are, of course, not without side effects. Gastrointestinal side effects are the most common, and this finding was echoed in the FLOW study. Gastrointestinal disorders led to permanent treatment discontinuation in 4.5% of the semaglutide group compared with 1.1% of the placebo group. The overall safety profile of semaglutide was favorable, however.
Gastrointestinal side effects can be particularly concerning in the context of CKD because of the possibility of clinical dehydration and acute kidney injury with persistent vomiting or diarrhea. Patient education is particularly important when using GLP-1 receptor agonists in this group of individuals. Reassuringly, there was no imbalance in dehydration and acute kidney injury between trial arms in the FLOW study.
Notably, past studies have suggested that patients with CKD are more likely to experience gastrointestinal side effects with GLP-1 receptor agonists; in these patients, the usual mantra of GLP-1 receptor agonist prescribing is particularly important: Start low, go slow.
Finally, medication adherence is a challenge with multiple pillars of GDMT: These evidence-based disease-modifying therapies work only if our patients take them regularly. My senior partner had a lovely turn of phrase when reviewing patients with multiple long-term conditions; he would always start the consultation by asking individuals which medications they were not taking regularly.
Overall, the FLOW study confirms semaglutide’s position as a new therapeutic pillar for DKD. This treatment will help address the residual renal risk for patients with DKD despite optimal use of GDMT. However, education and support will be required in primary care to prevent worsening therapeutic inertia.
Kevin Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, UK, has disclosed the following relevant financial relationships: Received speaker fees from: Amarin; Amgen; AstraZeneca; Bayer; Boehringer Ingelheim; Dexcom; Daiichi Sankyo; Lilly; Menarini; Novartis; Novo Nordisk; Roche Diagnostics; Embecta; Roche Diabetes Care. Received honoraria for participation in advisory boards from: Amarin; Amgen; AstraZen
A version of this article first appeared on Medscape.com.
Further data from the FLOW study were presented during the 2024 congress of the European Association for the Study of Diabetes (EASD) in Madrid. The FLOW study was originally presented in May at the European Renal Association’s 2024 congress in Stockholm. It was the first dedicated kidney outcomes trial to examine a GLP-1 receptor agonist.
The FLOW study demonstrated significant kidney, cardiovascular, and mortality benefits with semaglutide 1 mg once weekly in patients with type 2 diabetes and chronic kidney disease (CKD). This study has elevated semaglutide to a new pillar of care for the management of diabetic kidney disease (DKD) alongside RAAS inhibitors, SGLT2 inhibitors, and finerenone.
At first, whether the benefits of semaglutide were independent of baseline SGLT2 inhibitor use was uncertain. The data presented at the EASD congress, however, appeared to confirm the additive benefits of semaglutide, when combined with SGLT2 inhibitor use, in patients with DKD. The authors did acknowledge that study power was limited, given the low use of SGLT2 inhibitors at trial recruitment (no licensed SGLT2 inhibitor was available for CKD at that point), so small, clinically relevant interactions may not have been detected.
So, what are the implications of the FLOW study for primary care?
DKD is a common clinical challenge in primary care; a national diabetes audit in the United Kingdom suggested that over 40% of patients with type 2 diabetes had kidney disease. Moreover, DKD is the most common cause of kidney failure in adults starting renal replacement therapy in the United Kingdom.
Residual renal risk in patients with DKD persists despite optimal use of guideline-directed medical therapy (GDMT) with RAAS inhibitors, SGLT2 inhibitors, and finerenone, as demonstrated in the many landmark kidney outcomes trials over the past 25 years.
So, a new pillar of GDMT is welcome, but I am worried that this widened choice of therapies may worsen therapeutic inertia; baseline use of the newer DKD therapies (specifically SGLT2 inhibitors and finerenone) remains low.
In addition, during the EASD FLOW session, Katherine Tuttle, MD, executive director for research at Providence Inland Northwest Health Services in Spokane, Washington, presented data from the US CURE-CKD registry study showing that baseline ACE inhibitor/ARB use of about 70% dropped to 50% after just 90 days. Baseline use of SGLT2 inhibitors was only about 6% and dropped to 5% after 90 days.
I suspect that much of this reduction in prescribing of ACE inhibitors/ARBs will have been in response to an acute dip in estimated glomerular filtration rate (eGFR) or hyperkalemia, which has been a perennial challenge with RAAS inhibitor use in primary care. Ongoing education in primary care is required to manage hyperkalemia and reductions in eGFR after RAAS inhibitor initiation to prevent premature cessation of these foundational therapies.
On a positive note, there was no acute dip in eGFR after prescribing semaglutide in DKD. This observation will be reassuring for primary care and hopefully prevent unnecessary cessation of therapy.
Also reassuring was the lack of difference in diabetic retinopathy adverse events between the semaglutide and placebo groups. These events raised concerns about semaglutide following the SUSTAIN-6 CVOT study and have affected attitudes in primary care. But the rapidity and magnitude of improvement in glycemic control with semaglutide was believed to be the underlying issue, rather than semaglutide itself. A similar phenomenon has been observed with insulin. The ongoing FOCUS study is exploring the long-term effects of semaglutide on diabetic retinopathy in patients with type 2 diabetes. This study will hopefully provide a definite answer to this issue.
Another useful message from the FLOW study for primary care is the utility of semaglutide for glucose-lowering in the context of CKD. A1c was 0.81% lower in the semaglutide group compared with the placebo group in participants with eGFRs as low as 25 mL/min/1.73 m2. It is well established that SGLT2 inhibitors have negligible glucose-lowering effects once eGFR drops below 45 mL/min/1.73 m2. Indeed, my usual practice in CKD, if additional glucose-lowering is required once renal protection has been established with an SGLT2 inhibitor, was to add a GLP-1 receptor agonist. It is reassuring to have my clinical practice ratified by the FLOW study.
Semaglutide also helpfully provides an alternative therapeutic option for patients who do not tolerate SGLT2 inhibitors because of, for example, recurrent mycotic genital infections or polyuria, or for those in whom SGLT2 inhibitors are contraindicated, such as patients who have experienced an unprovoked episode of diabetic ketoacidosis. Many of these patients still require cardiovascular and kidney protection, so the FLOW study gives me a viable evidence-based alternative.
As a class, semaglutide and GLP-1 receptor agonists are, of course, not without side effects. Gastrointestinal side effects are the most common, and this finding was echoed in the FLOW study. Gastrointestinal disorders led to permanent treatment discontinuation in 4.5% of the semaglutide group compared with 1.1% of the placebo group. The overall safety profile of semaglutide was favorable, however.
Gastrointestinal side effects can be particularly concerning in the context of CKD because of the possibility of clinical dehydration and acute kidney injury with persistent vomiting or diarrhea. Patient education is particularly important when using GLP-1 receptor agonists in this group of individuals. Reassuringly, there was no imbalance in dehydration and acute kidney injury between trial arms in the FLOW study.
Notably, past studies have suggested that patients with CKD are more likely to experience gastrointestinal side effects with GLP-1 receptor agonists; in these patients, the usual mantra of GLP-1 receptor agonist prescribing is particularly important: Start low, go slow.
Finally, medication adherence is a challenge with multiple pillars of GDMT: These evidence-based disease-modifying therapies work only if our patients take them regularly. My senior partner had a lovely turn of phrase when reviewing patients with multiple long-term conditions; he would always start the consultation by asking individuals which medications they were not taking regularly.
Overall, the FLOW study confirms semaglutide’s position as a new therapeutic pillar for DKD. This treatment will help address the residual renal risk for patients with DKD despite optimal use of GDMT. However, education and support will be required in primary care to prevent worsening therapeutic inertia.
Kevin Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, UK, has disclosed the following relevant financial relationships: Received speaker fees from: Amarin; Amgen; AstraZeneca; Bayer; Boehringer Ingelheim; Dexcom; Daiichi Sankyo; Lilly; Menarini; Novartis; Novo Nordisk; Roche Diagnostics; Embecta; Roche Diabetes Care. Received honoraria for participation in advisory boards from: Amarin; Amgen; AstraZen
A version of this article first appeared on Medscape.com.
Further data from the FLOW study were presented during the 2024 congress of the European Association for the Study of Diabetes (EASD) in Madrid. The FLOW study was originally presented in May at the European Renal Association’s 2024 congress in Stockholm. It was the first dedicated kidney outcomes trial to examine a GLP-1 receptor agonist.
The FLOW study demonstrated significant kidney, cardiovascular, and mortality benefits with semaglutide 1 mg once weekly in patients with type 2 diabetes and chronic kidney disease (CKD). This study has elevated semaglutide to a new pillar of care for the management of diabetic kidney disease (DKD) alongside RAAS inhibitors, SGLT2 inhibitors, and finerenone.
At first, whether the benefits of semaglutide were independent of baseline SGLT2 inhibitor use was uncertain. The data presented at the EASD congress, however, appeared to confirm the additive benefits of semaglutide, when combined with SGLT2 inhibitor use, in patients with DKD. The authors did acknowledge that study power was limited, given the low use of SGLT2 inhibitors at trial recruitment (no licensed SGLT2 inhibitor was available for CKD at that point), so small, clinically relevant interactions may not have been detected.
So, what are the implications of the FLOW study for primary care?
DKD is a common clinical challenge in primary care; a national diabetes audit in the United Kingdom suggested that over 40% of patients with type 2 diabetes had kidney disease. Moreover, DKD is the most common cause of kidney failure in adults starting renal replacement therapy in the United Kingdom.
Residual renal risk in patients with DKD persists despite optimal use of guideline-directed medical therapy (GDMT) with RAAS inhibitors, SGLT2 inhibitors, and finerenone, as demonstrated in the many landmark kidney outcomes trials over the past 25 years.
So, a new pillar of GDMT is welcome, but I am worried that this widened choice of therapies may worsen therapeutic inertia; baseline use of the newer DKD therapies (specifically SGLT2 inhibitors and finerenone) remains low.
In addition, during the EASD FLOW session, Katherine Tuttle, MD, executive director for research at Providence Inland Northwest Health Services in Spokane, Washington, presented data from the US CURE-CKD registry study showing that baseline ACE inhibitor/ARB use of about 70% dropped to 50% after just 90 days. Baseline use of SGLT2 inhibitors was only about 6% and dropped to 5% after 90 days.
I suspect that much of this reduction in prescribing of ACE inhibitors/ARBs will have been in response to an acute dip in estimated glomerular filtration rate (eGFR) or hyperkalemia, which has been a perennial challenge with RAAS inhibitor use in primary care. Ongoing education in primary care is required to manage hyperkalemia and reductions in eGFR after RAAS inhibitor initiation to prevent premature cessation of these foundational therapies.
On a positive note, there was no acute dip in eGFR after prescribing semaglutide in DKD. This observation will be reassuring for primary care and hopefully prevent unnecessary cessation of therapy.
Also reassuring was the lack of difference in diabetic retinopathy adverse events between the semaglutide and placebo groups. These events raised concerns about semaglutide following the SUSTAIN-6 CVOT study and have affected attitudes in primary care. But the rapidity and magnitude of improvement in glycemic control with semaglutide was believed to be the underlying issue, rather than semaglutide itself. A similar phenomenon has been observed with insulin. The ongoing FOCUS study is exploring the long-term effects of semaglutide on diabetic retinopathy in patients with type 2 diabetes. This study will hopefully provide a definite answer to this issue.
Another useful message from the FLOW study for primary care is the utility of semaglutide for glucose-lowering in the context of CKD. A1c was 0.81% lower in the semaglutide group compared with the placebo group in participants with eGFRs as low as 25 mL/min/1.73 m2. It is well established that SGLT2 inhibitors have negligible glucose-lowering effects once eGFR drops below 45 mL/min/1.73 m2. Indeed, my usual practice in CKD, if additional glucose-lowering is required once renal protection has been established with an SGLT2 inhibitor, was to add a GLP-1 receptor agonist. It is reassuring to have my clinical practice ratified by the FLOW study.
Semaglutide also helpfully provides an alternative therapeutic option for patients who do not tolerate SGLT2 inhibitors because of, for example, recurrent mycotic genital infections or polyuria, or for those in whom SGLT2 inhibitors are contraindicated, such as patients who have experienced an unprovoked episode of diabetic ketoacidosis. Many of these patients still require cardiovascular and kidney protection, so the FLOW study gives me a viable evidence-based alternative.
As a class, semaglutide and GLP-1 receptor agonists are, of course, not without side effects. Gastrointestinal side effects are the most common, and this finding was echoed in the FLOW study. Gastrointestinal disorders led to permanent treatment discontinuation in 4.5% of the semaglutide group compared with 1.1% of the placebo group. The overall safety profile of semaglutide was favorable, however.
Gastrointestinal side effects can be particularly concerning in the context of CKD because of the possibility of clinical dehydration and acute kidney injury with persistent vomiting or diarrhea. Patient education is particularly important when using GLP-1 receptor agonists in this group of individuals. Reassuringly, there was no imbalance in dehydration and acute kidney injury between trial arms in the FLOW study.
Notably, past studies have suggested that patients with CKD are more likely to experience gastrointestinal side effects with GLP-1 receptor agonists; in these patients, the usual mantra of GLP-1 receptor agonist prescribing is particularly important: Start low, go slow.
Finally, medication adherence is a challenge with multiple pillars of GDMT: These evidence-based disease-modifying therapies work only if our patients take them regularly. My senior partner had a lovely turn of phrase when reviewing patients with multiple long-term conditions; he would always start the consultation by asking individuals which medications they were not taking regularly.
Overall, the FLOW study confirms semaglutide’s position as a new therapeutic pillar for DKD. This treatment will help address the residual renal risk for patients with DKD despite optimal use of GDMT. However, education and support will be required in primary care to prevent worsening therapeutic inertia.
Kevin Fernando, general practitioner partner, North Berwick Health Centre, North Berwick, UK, has disclosed the following relevant financial relationships: Received speaker fees from: Amarin; Amgen; AstraZeneca; Bayer; Boehringer Ingelheim; Dexcom; Daiichi Sankyo; Lilly; Menarini; Novartis; Novo Nordisk; Roche Diagnostics; Embecta; Roche Diabetes Care. Received honoraria for participation in advisory boards from: Amarin; Amgen; AstraZen
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Flash Drive Versus Paper
“Here’s my records.”
I hear that a lot, usually in the context of a patient handing me a flash drive or (less commonly) trying to plug it into my computer. (I have the USB ports turned toward me to keep that from happening.)
Uh, no.
I love flash drives. They definitely make data transfer easy, compared with the CDs, ZIPs, JAZZ, floppies, paper, and punch cards of past years (I should also, as a childhood TRS-80 user, include cassette tapes).
At this point an encrypted flash drive is pretty much the entire briefcase I carry back and forth to work each day.
But there is no patient I trust enough to plug in one they handed me.
I’m sure most, if not all, are well meaning. But look at how many large corporations have been damaged by someone slipping in a flash drive with a malicious program somewhere in their network. Once in, it’s almost impossible to get out, and can spread quickly.
Even if the patient is benign, I have no idea who formatted the gadget, or put the records on. It could be a relative, or friend, with other motives. It could even be a random flash drive and they don’t even know what else is on it.
My desktop is my chart system. I have to protect the data of all my patients, so I exercise caution about what emails I open and what I plug into it. Even the person offering me the flash drive wants the info guarded.
So I don’t, as a rule, plug in anything a patient hands me. All it takes is one malicious file to compromise it all. Yeah, I pay for software to watch for that sort of thing, but you still can’t be too careful.
This is where paper still shines. It’s readable and it’s transportable (at least for small things like an MRI report and lab results). I can scan it into a PDF without risking any damage to my computer. And it definitely shouldn’t be plugged into a USB drive unless you’re trying to start a fire.
Of course, paper isn’t secure, either. If you have it piled up everywhere it’s pretty easy for an unsupervised person to walk off with it. That actually happened to a doctor I shared space with 20 years ago, albeit unintentionally. A patient had brought in a bunch of his records in a folder and set them down on the counter. When he left he grabbed another patient’s chart by mistake and didn’t realize it until the next day. Fortunately he returned them promptly, and there were no issues. But it had the potential to be worse.
Today my charts on roughly 20,000 patients can all fit on a gadget the size of my thumb instead of a multi-room shelving system and storage closet. That’s pretty cool, actually. But it also opens other vulnerabilities.
It ticks some patients off that I won’t plug in their flash drives, but I don’t care. Most of them understand when I explain it, because it’s to protect them, too.
The odds are that they don’t mean any harm, but I can’t take that chance.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
“Here’s my records.”
I hear that a lot, usually in the context of a patient handing me a flash drive or (less commonly) trying to plug it into my computer. (I have the USB ports turned toward me to keep that from happening.)
Uh, no.
I love flash drives. They definitely make data transfer easy, compared with the CDs, ZIPs, JAZZ, floppies, paper, and punch cards of past years (I should also, as a childhood TRS-80 user, include cassette tapes).
At this point an encrypted flash drive is pretty much the entire briefcase I carry back and forth to work each day.
But there is no patient I trust enough to plug in one they handed me.
I’m sure most, if not all, are well meaning. But look at how many large corporations have been damaged by someone slipping in a flash drive with a malicious program somewhere in their network. Once in, it’s almost impossible to get out, and can spread quickly.
Even if the patient is benign, I have no idea who formatted the gadget, or put the records on. It could be a relative, or friend, with other motives. It could even be a random flash drive and they don’t even know what else is on it.
My desktop is my chart system. I have to protect the data of all my patients, so I exercise caution about what emails I open and what I plug into it. Even the person offering me the flash drive wants the info guarded.
So I don’t, as a rule, plug in anything a patient hands me. All it takes is one malicious file to compromise it all. Yeah, I pay for software to watch for that sort of thing, but you still can’t be too careful.
This is where paper still shines. It’s readable and it’s transportable (at least for small things like an MRI report and lab results). I can scan it into a PDF without risking any damage to my computer. And it definitely shouldn’t be plugged into a USB drive unless you’re trying to start a fire.
Of course, paper isn’t secure, either. If you have it piled up everywhere it’s pretty easy for an unsupervised person to walk off with it. That actually happened to a doctor I shared space with 20 years ago, albeit unintentionally. A patient had brought in a bunch of his records in a folder and set them down on the counter. When he left he grabbed another patient’s chart by mistake and didn’t realize it until the next day. Fortunately he returned them promptly, and there were no issues. But it had the potential to be worse.
Today my charts on roughly 20,000 patients can all fit on a gadget the size of my thumb instead of a multi-room shelving system and storage closet. That’s pretty cool, actually. But it also opens other vulnerabilities.
It ticks some patients off that I won’t plug in their flash drives, but I don’t care. Most of them understand when I explain it, because it’s to protect them, too.
The odds are that they don’t mean any harm, but I can’t take that chance.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
“Here’s my records.”
I hear that a lot, usually in the context of a patient handing me a flash drive or (less commonly) trying to plug it into my computer. (I have the USB ports turned toward me to keep that from happening.)
Uh, no.
I love flash drives. They definitely make data transfer easy, compared with the CDs, ZIPs, JAZZ, floppies, paper, and punch cards of past years (I should also, as a childhood TRS-80 user, include cassette tapes).
At this point an encrypted flash drive is pretty much the entire briefcase I carry back and forth to work each day.
But there is no patient I trust enough to plug in one they handed me.
I’m sure most, if not all, are well meaning. But look at how many large corporations have been damaged by someone slipping in a flash drive with a malicious program somewhere in their network. Once in, it’s almost impossible to get out, and can spread quickly.
Even if the patient is benign, I have no idea who formatted the gadget, or put the records on. It could be a relative, or friend, with other motives. It could even be a random flash drive and they don’t even know what else is on it.
My desktop is my chart system. I have to protect the data of all my patients, so I exercise caution about what emails I open and what I plug into it. Even the person offering me the flash drive wants the info guarded.
So I don’t, as a rule, plug in anything a patient hands me. All it takes is one malicious file to compromise it all. Yeah, I pay for software to watch for that sort of thing, but you still can’t be too careful.
This is where paper still shines. It’s readable and it’s transportable (at least for small things like an MRI report and lab results). I can scan it into a PDF without risking any damage to my computer. And it definitely shouldn’t be plugged into a USB drive unless you’re trying to start a fire.
Of course, paper isn’t secure, either. If you have it piled up everywhere it’s pretty easy for an unsupervised person to walk off with it. That actually happened to a doctor I shared space with 20 years ago, albeit unintentionally. A patient had brought in a bunch of his records in a folder and set them down on the counter. When he left he grabbed another patient’s chart by mistake and didn’t realize it until the next day. Fortunately he returned them promptly, and there were no issues. But it had the potential to be worse.
Today my charts on roughly 20,000 patients can all fit on a gadget the size of my thumb instead of a multi-room shelving system and storage closet. That’s pretty cool, actually. But it also opens other vulnerabilities.
It ticks some patients off that I won’t plug in their flash drives, but I don’t care. Most of them understand when I explain it, because it’s to protect them, too.
The odds are that they don’t mean any harm, but I can’t take that chance.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.