User login
Three Tips to Avoid Common Diagnostic Errors in Primary Care
Common complaints of abdominal pain, fever, shortness of breath, or rash can signal more serious disease that should be referred to specialty care or might be related to benign conditions.
Combine the vague nature of many patients’ descriptions and the pressure of short visits, and clinicians have a recipe for all manner of diagnostic error.
An estimated 5% of US adults who seek outpatient care each year experience a diagnostic error. Most Americans will eventually have this experience, according to a 2015 report from the National Academy of Medicine.
The most frequently missed diagnoses in primary care involve conditions such as pneumonia, decompensated heart failure, acute renal failure, cancer, urinary tract infection, and pyelonephritis.
“It’s not one or two or three types of diagnosis that are missed: We miss a lot of things, especially in primary care,” said Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey VA Medical Center and the Baylor College of Medicine in Houston, Texas.
One of the most serious errors is to miss cancer, with failing to follow‐up on abnormal tests ranking consistently as one of the leading causes of malpractice claims. But most diagnostic errors do not lead to lawsuits, although they often result in missed and delayed opportunities for patients to get needed care.
In interviews with this news organization, experts who have studied the root causes of diagnostic error suggested primary care clinicians focus on several practices to avoid mistakes: Ask questions with humility and curiosity, use checklists, and brainstorm with patients the potential root cause of symptoms.
Humility and Curiosity
Clinicians should remain aware of the potential for errors and reach out for assistance when needed, keeping an open mind that common symptoms may, in rare cases, signal serious illness, Dr. Singh said.
Dr. Singh recommends continual review with what he calls “byte-sized” learning through digital tools such as the Human Diagnosis app and podcasts and webinars offered by Clinical Problem Solvers.
Continuing education activities such as classes for Maintenance of Certification (MOC) can help keep physicians up to date and alert for cases where seemingly common symptoms may turn out to be something serious, said Richard M. Wardrop, III, MD, PhD, an internal medicine physician at the Cleveland Clinic in Ohio, and chair of the internal medicine board at the American Board of Internal Medicine.
“I’ve been in practice for 20 years. I’m double board certified in peds and medicine, and I regularly teach students and residents and mentor other physicians, but the further I go in my career and in practice, the more humble I become,” Dr. Wardrop told this news organization.
He said he recently spent a few hours on MOC for pediatrics and found the review was helpful in his practice in medicine.
“If I find myself taking care of a patient in a newborn nursery anytime soon, I’m going to understand the new hyperbilirubinemia guidelines,” Dr. Wardrop said. “That takes time and energy, but when I was done with the questions for this quarter, I felt good about myself.”
Checking It Twice
Clinicians should incorporate checklists into daily practice. Reviewing these with patients can not only help rule out an illness but also serve as a nonconfrontational method to inquire about issues patients may find uncomfortable, said John Ely, MD, MSPH, professor emeritus of family medicine at the University of Iowa Carver College of Medicine in Iowa City.
Clinicians could benefit from the approach used in aviation, where checklists are a required and routine part of a pilot’s job, Dr. Ely said.
Although clinicians may assume patients expect them to work from memory and knowledge without this aide, many will see using a checklist as a sign of providing thorough care, he said.
Checklists can also open a pathway for discussions about potentially difficult or touchy issues in short visits. For example, a patient might feel defensive if a clinician asks about depression during a visit for abdominal pain. But incorporating a question in a checklist allows for a different framing of the question.
“A clinician could say ‘I didn’t say you were depressed because of your abdominal pain, I brought it up because it’s on the list,’” Dr. Ely said. The checklist is “a very easy way to bring up those things.”
Dr. Ely said he has cared for a few patients who sought help for abdominal pain that turned out to be linked to sexual abuse in their past. Dr. Ely used a checklist with these patients to review possible causes for their illness. He recalled one of these patients who had suffered sexual abuse and had depression, neither of which was readily apparent.
“There was nothing about her affect that appeared to be depressed, and she had seen multiple physicians unable to make a diagnosis,” Dr. Ely said. “She had worked up for multiple other diseases and this had never come up before.”
Cooperation
“Coproduction” is how Kathryn McDonald, PhD, describes an ideal path to getting an accurate diagnosis. The intent is for clinicians to enlist patients in helping them in finding the root cause of symptoms.
“It’s bringing the patient into knowing that they are in a partnership to coproduce, knowing that there is a process going on,” said Dr. McDonald, who is codirector of the Armstrong Institute Center for Diagnostic Excellence at Johns Hopkins University in Baltimore, Maryland.
In many cases, patients seek reassurance for ruling out a suspected condition, which the physician can sometimes provide. In others, clinicians may not be able to offer a concrete diagnosis.
“There are times when uncertainty is more pervasive and I will ask patients, ‘Let’s brainstorm this together,’” Dr. Wardrop said.
A version of this article appeared on Medscape.com.
Common complaints of abdominal pain, fever, shortness of breath, or rash can signal more serious disease that should be referred to specialty care or might be related to benign conditions.
Combine the vague nature of many patients’ descriptions and the pressure of short visits, and clinicians have a recipe for all manner of diagnostic error.
An estimated 5% of US adults who seek outpatient care each year experience a diagnostic error. Most Americans will eventually have this experience, according to a 2015 report from the National Academy of Medicine.
The most frequently missed diagnoses in primary care involve conditions such as pneumonia, decompensated heart failure, acute renal failure, cancer, urinary tract infection, and pyelonephritis.
“It’s not one or two or three types of diagnosis that are missed: We miss a lot of things, especially in primary care,” said Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey VA Medical Center and the Baylor College of Medicine in Houston, Texas.
One of the most serious errors is to miss cancer, with failing to follow‐up on abnormal tests ranking consistently as one of the leading causes of malpractice claims. But most diagnostic errors do not lead to lawsuits, although they often result in missed and delayed opportunities for patients to get needed care.
In interviews with this news organization, experts who have studied the root causes of diagnostic error suggested primary care clinicians focus on several practices to avoid mistakes: Ask questions with humility and curiosity, use checklists, and brainstorm with patients the potential root cause of symptoms.
Humility and Curiosity
Clinicians should remain aware of the potential for errors and reach out for assistance when needed, keeping an open mind that common symptoms may, in rare cases, signal serious illness, Dr. Singh said.
Dr. Singh recommends continual review with what he calls “byte-sized” learning through digital tools such as the Human Diagnosis app and podcasts and webinars offered by Clinical Problem Solvers.
Continuing education activities such as classes for Maintenance of Certification (MOC) can help keep physicians up to date and alert for cases where seemingly common symptoms may turn out to be something serious, said Richard M. Wardrop, III, MD, PhD, an internal medicine physician at the Cleveland Clinic in Ohio, and chair of the internal medicine board at the American Board of Internal Medicine.
“I’ve been in practice for 20 years. I’m double board certified in peds and medicine, and I regularly teach students and residents and mentor other physicians, but the further I go in my career and in practice, the more humble I become,” Dr. Wardrop told this news organization.
He said he recently spent a few hours on MOC for pediatrics and found the review was helpful in his practice in medicine.
“If I find myself taking care of a patient in a newborn nursery anytime soon, I’m going to understand the new hyperbilirubinemia guidelines,” Dr. Wardrop said. “That takes time and energy, but when I was done with the questions for this quarter, I felt good about myself.”
Checking It Twice
Clinicians should incorporate checklists into daily practice. Reviewing these with patients can not only help rule out an illness but also serve as a nonconfrontational method to inquire about issues patients may find uncomfortable, said John Ely, MD, MSPH, professor emeritus of family medicine at the University of Iowa Carver College of Medicine in Iowa City.
Clinicians could benefit from the approach used in aviation, where checklists are a required and routine part of a pilot’s job, Dr. Ely said.
Although clinicians may assume patients expect them to work from memory and knowledge without this aide, many will see using a checklist as a sign of providing thorough care, he said.
Checklists can also open a pathway for discussions about potentially difficult or touchy issues in short visits. For example, a patient might feel defensive if a clinician asks about depression during a visit for abdominal pain. But incorporating a question in a checklist allows for a different framing of the question.
“A clinician could say ‘I didn’t say you were depressed because of your abdominal pain, I brought it up because it’s on the list,’” Dr. Ely said. The checklist is “a very easy way to bring up those things.”
Dr. Ely said he has cared for a few patients who sought help for abdominal pain that turned out to be linked to sexual abuse in their past. Dr. Ely used a checklist with these patients to review possible causes for their illness. He recalled one of these patients who had suffered sexual abuse and had depression, neither of which was readily apparent.
“There was nothing about her affect that appeared to be depressed, and she had seen multiple physicians unable to make a diagnosis,” Dr. Ely said. “She had worked up for multiple other diseases and this had never come up before.”
Cooperation
“Coproduction” is how Kathryn McDonald, PhD, describes an ideal path to getting an accurate diagnosis. The intent is for clinicians to enlist patients in helping them in finding the root cause of symptoms.
“It’s bringing the patient into knowing that they are in a partnership to coproduce, knowing that there is a process going on,” said Dr. McDonald, who is codirector of the Armstrong Institute Center for Diagnostic Excellence at Johns Hopkins University in Baltimore, Maryland.
In many cases, patients seek reassurance for ruling out a suspected condition, which the physician can sometimes provide. In others, clinicians may not be able to offer a concrete diagnosis.
“There are times when uncertainty is more pervasive and I will ask patients, ‘Let’s brainstorm this together,’” Dr. Wardrop said.
A version of this article appeared on Medscape.com.
Common complaints of abdominal pain, fever, shortness of breath, or rash can signal more serious disease that should be referred to specialty care or might be related to benign conditions.
Combine the vague nature of many patients’ descriptions and the pressure of short visits, and clinicians have a recipe for all manner of diagnostic error.
An estimated 5% of US adults who seek outpatient care each year experience a diagnostic error. Most Americans will eventually have this experience, according to a 2015 report from the National Academy of Medicine.
The most frequently missed diagnoses in primary care involve conditions such as pneumonia, decompensated heart failure, acute renal failure, cancer, urinary tract infection, and pyelonephritis.
“It’s not one or two or three types of diagnosis that are missed: We miss a lot of things, especially in primary care,” said Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey VA Medical Center and the Baylor College of Medicine in Houston, Texas.
One of the most serious errors is to miss cancer, with failing to follow‐up on abnormal tests ranking consistently as one of the leading causes of malpractice claims. But most diagnostic errors do not lead to lawsuits, although they often result in missed and delayed opportunities for patients to get needed care.
In interviews with this news organization, experts who have studied the root causes of diagnostic error suggested primary care clinicians focus on several practices to avoid mistakes: Ask questions with humility and curiosity, use checklists, and brainstorm with patients the potential root cause of symptoms.
Humility and Curiosity
Clinicians should remain aware of the potential for errors and reach out for assistance when needed, keeping an open mind that common symptoms may, in rare cases, signal serious illness, Dr. Singh said.
Dr. Singh recommends continual review with what he calls “byte-sized” learning through digital tools such as the Human Diagnosis app and podcasts and webinars offered by Clinical Problem Solvers.
Continuing education activities such as classes for Maintenance of Certification (MOC) can help keep physicians up to date and alert for cases where seemingly common symptoms may turn out to be something serious, said Richard M. Wardrop, III, MD, PhD, an internal medicine physician at the Cleveland Clinic in Ohio, and chair of the internal medicine board at the American Board of Internal Medicine.
“I’ve been in practice for 20 years. I’m double board certified in peds and medicine, and I regularly teach students and residents and mentor other physicians, but the further I go in my career and in practice, the more humble I become,” Dr. Wardrop told this news organization.
He said he recently spent a few hours on MOC for pediatrics and found the review was helpful in his practice in medicine.
“If I find myself taking care of a patient in a newborn nursery anytime soon, I’m going to understand the new hyperbilirubinemia guidelines,” Dr. Wardrop said. “That takes time and energy, but when I was done with the questions for this quarter, I felt good about myself.”
Checking It Twice
Clinicians should incorporate checklists into daily practice. Reviewing these with patients can not only help rule out an illness but also serve as a nonconfrontational method to inquire about issues patients may find uncomfortable, said John Ely, MD, MSPH, professor emeritus of family medicine at the University of Iowa Carver College of Medicine in Iowa City.
Clinicians could benefit from the approach used in aviation, where checklists are a required and routine part of a pilot’s job, Dr. Ely said.
Although clinicians may assume patients expect them to work from memory and knowledge without this aide, many will see using a checklist as a sign of providing thorough care, he said.
Checklists can also open a pathway for discussions about potentially difficult or touchy issues in short visits. For example, a patient might feel defensive if a clinician asks about depression during a visit for abdominal pain. But incorporating a question in a checklist allows for a different framing of the question.
“A clinician could say ‘I didn’t say you were depressed because of your abdominal pain, I brought it up because it’s on the list,’” Dr. Ely said. The checklist is “a very easy way to bring up those things.”
Dr. Ely said he has cared for a few patients who sought help for abdominal pain that turned out to be linked to sexual abuse in their past. Dr. Ely used a checklist with these patients to review possible causes for their illness. He recalled one of these patients who had suffered sexual abuse and had depression, neither of which was readily apparent.
“There was nothing about her affect that appeared to be depressed, and she had seen multiple physicians unable to make a diagnosis,” Dr. Ely said. “She had worked up for multiple other diseases and this had never come up before.”
Cooperation
“Coproduction” is how Kathryn McDonald, PhD, describes an ideal path to getting an accurate diagnosis. The intent is for clinicians to enlist patients in helping them in finding the root cause of symptoms.
“It’s bringing the patient into knowing that they are in a partnership to coproduce, knowing that there is a process going on,” said Dr. McDonald, who is codirector of the Armstrong Institute Center for Diagnostic Excellence at Johns Hopkins University in Baltimore, Maryland.
In many cases, patients seek reassurance for ruling out a suspected condition, which the physician can sometimes provide. In others, clinicians may not be able to offer a concrete diagnosis.
“There are times when uncertainty is more pervasive and I will ask patients, ‘Let’s brainstorm this together,’” Dr. Wardrop said.
A version of this article appeared on Medscape.com.
Heightened Amygdala Activity Tied to Postpartum Depression
MILAN, ITALY — Pregnant women with heightened amygdala activity have a reduced capacity to regulate emotions and report more symptoms of depression than those with lower activity in this brain region, a new imaging study suggested.
If validated, these findings could pave the way for identifying women at higher risk for postpartum depression, said lead researcher Franziska Weinmar, MSc, from the University of Tübingen in Germany.
The study was presented at the 37th European College of Neuropsychopharmacology Congress.
Differences in Brain Activity
During pregnancy and the peripartum period, rising hormone levels create a “psychoneuroendocrinological window of vulnerability” for mental health in which 80% of women can develop transitory “baby blues,” and about one in seven develop more serious postpartum depression, Ms. Weinmar told this news organization.
The study included 47 women — 15 pregnant women and 32 nonpregnant controls. The nonpregnant women had normal menstrual cycles; 16 were in the early follicular phase with low estradiol levels (231.7 pmol/L), and 16 had high estradiol levels (516.6 pmol/L) after administration of estradiol.
To examine brain activity, participants were asked to view negative emotional images while undergoing functional MRI. They were then asked to use cognitive reappraisal to regulate their emotional response to the images.
The findings showed that both pregnant and nonpregnant women were equally successful at emotional regulation, but this process involved different brain activity in pregnant vs their nonpregnant counterpart.
All women had increased left middle frontal gyrus activity when regulating their emotions, but there was a difference in the amygdala between the pregnancy group and controls, Ms. Weinmar noted.
This suggests that pregnant women may have to exert more neural effort in emotional regulation, she said. “And pregnant women with higher amygdala activity were less able to regulate their emotions successfully compared to those with less amygdala activity.”
Linear regression analyses were performed to assess the relation of brain activity during down-regulation, regulation success, and self-reported depression scores, and this showed that higher amygdala activity was also associated with higher depression scores.
“We need to be cautious in interpreting this,” said Ms. Weinmar. “This is a small sample, and we are the first to undertake this work.”
Nonetheless, she said that if the findings are confirmed by larger studies, pregnant women could be assessed “in the waiting room” using existing questionnaires that evaluate emotional regulation.
If a woman has difficulties with emotion regulation, “there are adaptive strategies, like cognitive reappraisal that a counseling psychotherapist can help with,” said Ms. Weinmar.
“I could also imagine group sessions, for example, or online courses,” she said, adding that obstetricians could also be trained to identify these women.
Commenting on the findings in a press release, Susana Carmona, PhD, from Gregorio Marañón Hospital in Madrid, Spain, said research like this is crucial for gaining insight into one of the most intense physiological processes a human can undergo: pregnancy. It’s remarkable how much remains unknown.
“Recently, the FDA [Food and Drug Administration] approved the first treatment for postpartum depression. However, we still have a long way to go in characterizing what happens in the brain during pregnancy, identifying biomarkers that can indicate the risk of developing perinatal mental disorders, and designing strategies to prevent mother and infant suffering during the delicate and critical peripartum period,” Dr. Carmona added.
The study was supported by the Center for Integrative Neuroscience in Tübingen, Germany, and the International Research Training Group “Women’s Mental Health Across the Reproductive Years” (IRTG 2804). Ms. Weinmar and Dr. Carmona reported no relevant disclosures.
A version of this article appeared on Medscape.com.
MILAN, ITALY — Pregnant women with heightened amygdala activity have a reduced capacity to regulate emotions and report more symptoms of depression than those with lower activity in this brain region, a new imaging study suggested.
If validated, these findings could pave the way for identifying women at higher risk for postpartum depression, said lead researcher Franziska Weinmar, MSc, from the University of Tübingen in Germany.
The study was presented at the 37th European College of Neuropsychopharmacology Congress.
Differences in Brain Activity
During pregnancy and the peripartum period, rising hormone levels create a “psychoneuroendocrinological window of vulnerability” for mental health in which 80% of women can develop transitory “baby blues,” and about one in seven develop more serious postpartum depression, Ms. Weinmar told this news organization.
The study included 47 women — 15 pregnant women and 32 nonpregnant controls. The nonpregnant women had normal menstrual cycles; 16 were in the early follicular phase with low estradiol levels (231.7 pmol/L), and 16 had high estradiol levels (516.6 pmol/L) after administration of estradiol.
To examine brain activity, participants were asked to view negative emotional images while undergoing functional MRI. They were then asked to use cognitive reappraisal to regulate their emotional response to the images.
The findings showed that both pregnant and nonpregnant women were equally successful at emotional regulation, but this process involved different brain activity in pregnant vs their nonpregnant counterpart.
All women had increased left middle frontal gyrus activity when regulating their emotions, but there was a difference in the amygdala between the pregnancy group and controls, Ms. Weinmar noted.
This suggests that pregnant women may have to exert more neural effort in emotional regulation, she said. “And pregnant women with higher amygdala activity were less able to regulate their emotions successfully compared to those with less amygdala activity.”
Linear regression analyses were performed to assess the relation of brain activity during down-regulation, regulation success, and self-reported depression scores, and this showed that higher amygdala activity was also associated with higher depression scores.
“We need to be cautious in interpreting this,” said Ms. Weinmar. “This is a small sample, and we are the first to undertake this work.”
Nonetheless, she said that if the findings are confirmed by larger studies, pregnant women could be assessed “in the waiting room” using existing questionnaires that evaluate emotional regulation.
If a woman has difficulties with emotion regulation, “there are adaptive strategies, like cognitive reappraisal that a counseling psychotherapist can help with,” said Ms. Weinmar.
“I could also imagine group sessions, for example, or online courses,” she said, adding that obstetricians could also be trained to identify these women.
Commenting on the findings in a press release, Susana Carmona, PhD, from Gregorio Marañón Hospital in Madrid, Spain, said research like this is crucial for gaining insight into one of the most intense physiological processes a human can undergo: pregnancy. It’s remarkable how much remains unknown.
“Recently, the FDA [Food and Drug Administration] approved the first treatment for postpartum depression. However, we still have a long way to go in characterizing what happens in the brain during pregnancy, identifying biomarkers that can indicate the risk of developing perinatal mental disorders, and designing strategies to prevent mother and infant suffering during the delicate and critical peripartum period,” Dr. Carmona added.
The study was supported by the Center for Integrative Neuroscience in Tübingen, Germany, and the International Research Training Group “Women’s Mental Health Across the Reproductive Years” (IRTG 2804). Ms. Weinmar and Dr. Carmona reported no relevant disclosures.
A version of this article appeared on Medscape.com.
MILAN, ITALY — Pregnant women with heightened amygdala activity have a reduced capacity to regulate emotions and report more symptoms of depression than those with lower activity in this brain region, a new imaging study suggested.
If validated, these findings could pave the way for identifying women at higher risk for postpartum depression, said lead researcher Franziska Weinmar, MSc, from the University of Tübingen in Germany.
The study was presented at the 37th European College of Neuropsychopharmacology Congress.
Differences in Brain Activity
During pregnancy and the peripartum period, rising hormone levels create a “psychoneuroendocrinological window of vulnerability” for mental health in which 80% of women can develop transitory “baby blues,” and about one in seven develop more serious postpartum depression, Ms. Weinmar told this news organization.
The study included 47 women — 15 pregnant women and 32 nonpregnant controls. The nonpregnant women had normal menstrual cycles; 16 were in the early follicular phase with low estradiol levels (231.7 pmol/L), and 16 had high estradiol levels (516.6 pmol/L) after administration of estradiol.
To examine brain activity, participants were asked to view negative emotional images while undergoing functional MRI. They were then asked to use cognitive reappraisal to regulate their emotional response to the images.
The findings showed that both pregnant and nonpregnant women were equally successful at emotional regulation, but this process involved different brain activity in pregnant vs their nonpregnant counterpart.
All women had increased left middle frontal gyrus activity when regulating their emotions, but there was a difference in the amygdala between the pregnancy group and controls, Ms. Weinmar noted.
This suggests that pregnant women may have to exert more neural effort in emotional regulation, she said. “And pregnant women with higher amygdala activity were less able to regulate their emotions successfully compared to those with less amygdala activity.”
Linear regression analyses were performed to assess the relation of brain activity during down-regulation, regulation success, and self-reported depression scores, and this showed that higher amygdala activity was also associated with higher depression scores.
“We need to be cautious in interpreting this,” said Ms. Weinmar. “This is a small sample, and we are the first to undertake this work.”
Nonetheless, she said that if the findings are confirmed by larger studies, pregnant women could be assessed “in the waiting room” using existing questionnaires that evaluate emotional regulation.
If a woman has difficulties with emotion regulation, “there are adaptive strategies, like cognitive reappraisal that a counseling psychotherapist can help with,” said Ms. Weinmar.
“I could also imagine group sessions, for example, or online courses,” she said, adding that obstetricians could also be trained to identify these women.
Commenting on the findings in a press release, Susana Carmona, PhD, from Gregorio Marañón Hospital in Madrid, Spain, said research like this is crucial for gaining insight into one of the most intense physiological processes a human can undergo: pregnancy. It’s remarkable how much remains unknown.
“Recently, the FDA [Food and Drug Administration] approved the first treatment for postpartum depression. However, we still have a long way to go in characterizing what happens in the brain during pregnancy, identifying biomarkers that can indicate the risk of developing perinatal mental disorders, and designing strategies to prevent mother and infant suffering during the delicate and critical peripartum period,” Dr. Carmona added.
The study was supported by the Center for Integrative Neuroscience in Tübingen, Germany, and the International Research Training Group “Women’s Mental Health Across the Reproductive Years” (IRTG 2804). Ms. Weinmar and Dr. Carmona reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ECNP 2024
Purpuric Lesions on the Leg
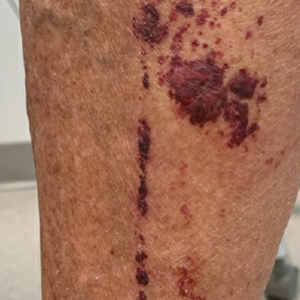
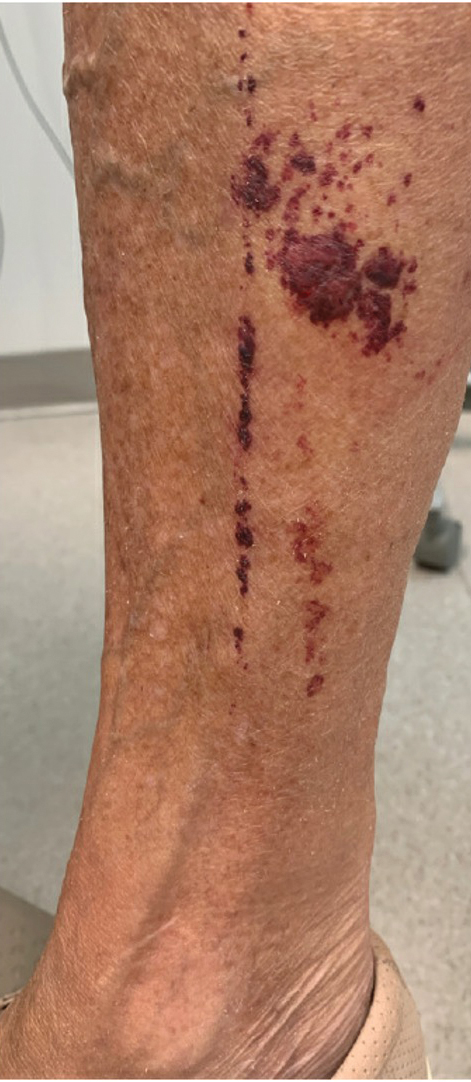
THE DIAGNOSIS: Dengue Hemorrhagic Fever
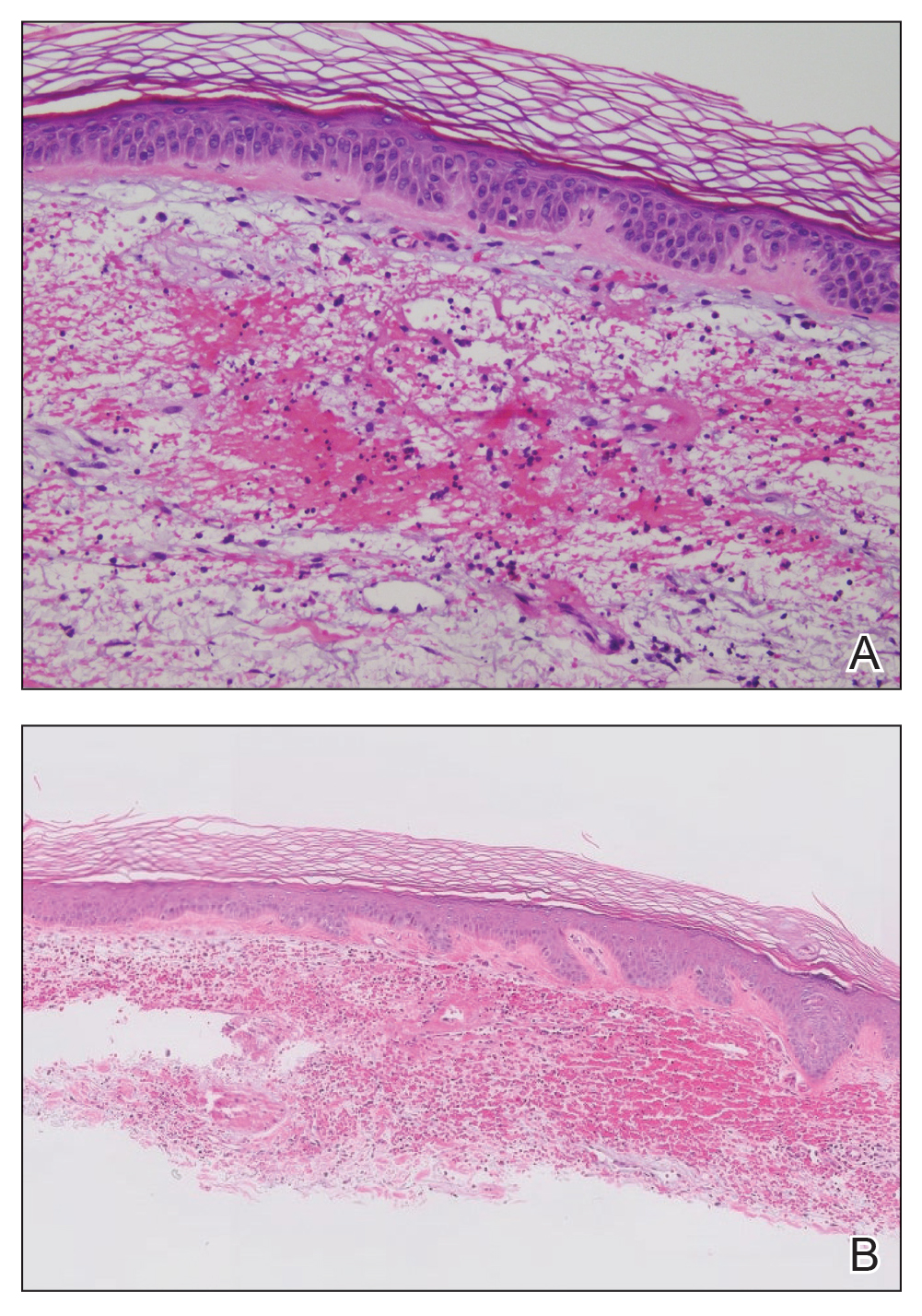
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.
Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1
Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
A 74-year-old woman who frequently traveled abroad presented to the dermatology department with retiform purpura of the lower leg along with gastrointestinal cramps, fatigue, and myalgia. The patient reported that the symptoms had started 10 days after returning from a recent trip to Africa.
Following the Light
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Percutaneous endoscopic gastrostomy (PEG) was first introduced in the early 1980s by surgeons Michael Gauderer and Jeffrey Ponsky as a less-invasive alternative to surgical gastrostomy via open laparotomy. The concept was born after the pair observed that the light from an endoscope in an infant undergoing endoscopy caused the abdominal wall to glow in the darkened operating room.
In fact, PEG was among the first procedures that defined minimally invasive surgery, a concept that has now revolutionized the surgical field. Since that time, PEG has evolved as a preferred method for patients needing long-term nutritional support for various indications. By 2001, approximately 216,000 PEGs were placed annually in the United States. While the volume of PEG procedures has declined in recent years at some institutions as practice patterns have shifted toward interventional radiology–placed gastrostomy tubes, evaluation of patients for PEG insertion, removal, or management of PEG complications remains a core area of gastroenterology practice.
Among the most important roles of the gastroenterologist in considering potential PEG candidates is to determine whether an appropriate indication exists, a decision that requires a detailed understanding of a patient’s overall clinical condition, goals of care, values, and preferences. a common GI consultation that deserves thoughtful consideration and demands effective communication among members of the multidisciplinary team and with patients.
Also in our October issue, we highlight a recently published large multicohort study from Gastroenterology elucidating clinical, serologic, and genetic factors associated with extraintestinal manifestations in IBD. We also review key updates to colonoscopy quality indicators, including modifications to existing indicators such as ADR and the addition of two new “priority indicators” — rate of inadequate bowel prep and sessile serrated lesion detection rate.
In this month’s Member Spotlight, Dr. Stephanie Pointer of Digestive & Liver Health Specialists in Nashville, Tennessee, shares the many ways in which she has given back to her community through music and mentoring while leading a thriving GI practice. We hope you enjoy this, and all the coverage included in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pfizer’s Withdrawal of SCD Drug Raises Questions
The National Alliance of Sickle Cell Centers issued a statement urging patients not to stop voxelotor abruptly. Instead, they should work out plans with their physicians and medical teams for weaning plans.
“Don’t lose faith. This a step backward, but we will stay on the path to better outcomes for everyone,” said the alliance in a statement to patients and clinicians.
On September 25, Pfizer said it would withdraw all lots of voxelotor in all markets where it is approved. The New York–based drugmaker also said it was discontinuing all active voxelotor clinical trials and expanded access programs worldwide. The cause was data that suggested “an imbalance in vaso-occlusive crises and fatal events which require further assessment.”
Pfizer told this news organization in an email exchange that it is focused on analyzing the data and will share updates in the future about presenting or publishing on this issue.
The withdrawal came amid increased scrutiny of the drug by the European Medicines Agency (EMA). The EMA in July began a review of voxelotor after data from a clinical trial showed that a higher number of deaths occurred with the drug than with placebo and another trial showed the total number of deaths was higher than anticipated.
On September 26, the EMA’s human medicines committee recommended suspending the marketing authorization of voxelotor, citing new safety data that emerged during the review. The drug had received marketing authorization for the European Union in 2022, the agency said.
The US Food and Drug Administration (FDA), which first cleared voxelotor for sale in 2019, also said it has been conducting a safety review of the drug. The agency continues to examine post-marketing clinical trial data for voxelotor, the real-world registry studies, and data from the FDA Adverse Event Reporting System. At the conclusion of this review, the FDA will communicate any additional findings, if necessary, the agency said.
The FDA said it appeared that more deaths and a higher rate of vaso-occlusive crisis occurred in patients taking voxelotor vs placebo in post-marketing clinical trials.
“Pfizer also observed a higher rate of vaso-occlusive crisis in patients with sickle cell disease receiving Oxbryta in two real-world registry studies,” the FDA said. “Based on the totality of clinical data, Pfizer has determined the benefit of Oxbryta does not outweigh the risk.”
Gene Therapy, Tried-and-True Hydroxyurea (HU)
As a field, SCD has drawn more interest in recent years, with significant gains made lately in cutting-edge projects.
The FDA in December approved two gene-editing treatments for patients aged 12 years or older. These are considered “milestone treatments” for a debilitating and potentially life-threatening blood disorder that affects about 100,000 people in the United States. Exagamglogene autotemcel (Casgevy, Vertex Pharmaceuticals and CRISPR Therapeutics) is the first to use the gene-editing tool CRISPR. And lovotibeglogene autotemcel (Lyfgenia, bluebird bio) uses a different gene-editing tool called a lentiviral vector.
These advances have been covered widely by the news media but are not expected to be widely available, with the cost of these extensive treatments estimated around $2-$3 million per patient.
“Gene therapy is amazing in that it can offer a cure, but it’s very expensive and not all patients are suitable for it. Some have so much existing organ damage that it’s not an option for them,” said John Wood, MD, PhD, director of cardiovascular MRI at Children’s Hospital Los Angeles, Los Angeles, who does research on SCD.
“So it really is a great treatment for a very few people,” he said in an interview.
The mainstay of treatment for SCD remains a drug that Lydia Pecker, MD, a pediatric hematologist at Johns Hopkins University in Baltimore, describes as the “first, oldest, and best”: HU.
The FDA approved this in 1998 for use in SCD. It reduces the frequency of painful crises and acute chest syndrome and other complications of SCD that otherwise could be serious or even lethal, Pecker said.
“Older doctors can tell you that what they experienced with sickle cell disease in the hospitals has been completely transformed because of the high uptake of the drug,” she said, adding that it made a “profound” change. “We just don’t have data for any other agent that’s quite like that.”
Voxelotor had been a good second drug to add for some patients, in addition to HU and blood transfusions, Dr. Pecker noted. It was a first-line drug for those for whom transfusion and HU were not options, which constitutes a relatively small number of patients, she said.
“So we have, in the last 5 years, felt more hopeful because we had something else to offer,” she said.
Alexis A. Thompson, MD, MPH, chief of the Division of Hematology at Children’s Hospital of Philadelphia in Pennsylvania, said in an interview that her organization also had patients who appeared to benefit from voxelotor, some of whom had been participants in clinical trials.
Dr. Thompson, who has been a top researcher involved in the study of gene therapy, urged the need for companies to keep seeking to expand the options for people with SCD, even after the setback with voxelotor.
“I hope that there’s an appreciation for the need for continued investment in this very serious condition, for which there are insufficient options for treatments,” Dr. Thompson said. “So ongoing investment is really needed if we expect to make progress.”
Dr. Pecker disclosed ties with Novartis, Afimmune, the American Society of Hematology, and the National Institutes of Health. Thompson reported relationships with bluebird bio, Beam, Editas, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The National Alliance of Sickle Cell Centers issued a statement urging patients not to stop voxelotor abruptly. Instead, they should work out plans with their physicians and medical teams for weaning plans.
“Don’t lose faith. This a step backward, but we will stay on the path to better outcomes for everyone,” said the alliance in a statement to patients and clinicians.
On September 25, Pfizer said it would withdraw all lots of voxelotor in all markets where it is approved. The New York–based drugmaker also said it was discontinuing all active voxelotor clinical trials and expanded access programs worldwide. The cause was data that suggested “an imbalance in vaso-occlusive crises and fatal events which require further assessment.”
Pfizer told this news organization in an email exchange that it is focused on analyzing the data and will share updates in the future about presenting or publishing on this issue.
The withdrawal came amid increased scrutiny of the drug by the European Medicines Agency (EMA). The EMA in July began a review of voxelotor after data from a clinical trial showed that a higher number of deaths occurred with the drug than with placebo and another trial showed the total number of deaths was higher than anticipated.
On September 26, the EMA’s human medicines committee recommended suspending the marketing authorization of voxelotor, citing new safety data that emerged during the review. The drug had received marketing authorization for the European Union in 2022, the agency said.
The US Food and Drug Administration (FDA), which first cleared voxelotor for sale in 2019, also said it has been conducting a safety review of the drug. The agency continues to examine post-marketing clinical trial data for voxelotor, the real-world registry studies, and data from the FDA Adverse Event Reporting System. At the conclusion of this review, the FDA will communicate any additional findings, if necessary, the agency said.
The FDA said it appeared that more deaths and a higher rate of vaso-occlusive crisis occurred in patients taking voxelotor vs placebo in post-marketing clinical trials.
“Pfizer also observed a higher rate of vaso-occlusive crisis in patients with sickle cell disease receiving Oxbryta in two real-world registry studies,” the FDA said. “Based on the totality of clinical data, Pfizer has determined the benefit of Oxbryta does not outweigh the risk.”
Gene Therapy, Tried-and-True Hydroxyurea (HU)
As a field, SCD has drawn more interest in recent years, with significant gains made lately in cutting-edge projects.
The FDA in December approved two gene-editing treatments for patients aged 12 years or older. These are considered “milestone treatments” for a debilitating and potentially life-threatening blood disorder that affects about 100,000 people in the United States. Exagamglogene autotemcel (Casgevy, Vertex Pharmaceuticals and CRISPR Therapeutics) is the first to use the gene-editing tool CRISPR. And lovotibeglogene autotemcel (Lyfgenia, bluebird bio) uses a different gene-editing tool called a lentiviral vector.
These advances have been covered widely by the news media but are not expected to be widely available, with the cost of these extensive treatments estimated around $2-$3 million per patient.
“Gene therapy is amazing in that it can offer a cure, but it’s very expensive and not all patients are suitable for it. Some have so much existing organ damage that it’s not an option for them,” said John Wood, MD, PhD, director of cardiovascular MRI at Children’s Hospital Los Angeles, Los Angeles, who does research on SCD.
“So it really is a great treatment for a very few people,” he said in an interview.
The mainstay of treatment for SCD remains a drug that Lydia Pecker, MD, a pediatric hematologist at Johns Hopkins University in Baltimore, describes as the “first, oldest, and best”: HU.
The FDA approved this in 1998 for use in SCD. It reduces the frequency of painful crises and acute chest syndrome and other complications of SCD that otherwise could be serious or even lethal, Pecker said.
“Older doctors can tell you that what they experienced with sickle cell disease in the hospitals has been completely transformed because of the high uptake of the drug,” she said, adding that it made a “profound” change. “We just don’t have data for any other agent that’s quite like that.”
Voxelotor had been a good second drug to add for some patients, in addition to HU and blood transfusions, Dr. Pecker noted. It was a first-line drug for those for whom transfusion and HU were not options, which constitutes a relatively small number of patients, she said.
“So we have, in the last 5 years, felt more hopeful because we had something else to offer,” she said.
Alexis A. Thompson, MD, MPH, chief of the Division of Hematology at Children’s Hospital of Philadelphia in Pennsylvania, said in an interview that her organization also had patients who appeared to benefit from voxelotor, some of whom had been participants in clinical trials.
Dr. Thompson, who has been a top researcher involved in the study of gene therapy, urged the need for companies to keep seeking to expand the options for people with SCD, even after the setback with voxelotor.
“I hope that there’s an appreciation for the need for continued investment in this very serious condition, for which there are insufficient options for treatments,” Dr. Thompson said. “So ongoing investment is really needed if we expect to make progress.”
Dr. Pecker disclosed ties with Novartis, Afimmune, the American Society of Hematology, and the National Institutes of Health. Thompson reported relationships with bluebird bio, Beam, Editas, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The National Alliance of Sickle Cell Centers issued a statement urging patients not to stop voxelotor abruptly. Instead, they should work out plans with their physicians and medical teams for weaning plans.
“Don’t lose faith. This a step backward, but we will stay on the path to better outcomes for everyone,” said the alliance in a statement to patients and clinicians.
On September 25, Pfizer said it would withdraw all lots of voxelotor in all markets where it is approved. The New York–based drugmaker also said it was discontinuing all active voxelotor clinical trials and expanded access programs worldwide. The cause was data that suggested “an imbalance in vaso-occlusive crises and fatal events which require further assessment.”
Pfizer told this news organization in an email exchange that it is focused on analyzing the data and will share updates in the future about presenting or publishing on this issue.
The withdrawal came amid increased scrutiny of the drug by the European Medicines Agency (EMA). The EMA in July began a review of voxelotor after data from a clinical trial showed that a higher number of deaths occurred with the drug than with placebo and another trial showed the total number of deaths was higher than anticipated.
On September 26, the EMA’s human medicines committee recommended suspending the marketing authorization of voxelotor, citing new safety data that emerged during the review. The drug had received marketing authorization for the European Union in 2022, the agency said.
The US Food and Drug Administration (FDA), which first cleared voxelotor for sale in 2019, also said it has been conducting a safety review of the drug. The agency continues to examine post-marketing clinical trial data for voxelotor, the real-world registry studies, and data from the FDA Adverse Event Reporting System. At the conclusion of this review, the FDA will communicate any additional findings, if necessary, the agency said.
The FDA said it appeared that more deaths and a higher rate of vaso-occlusive crisis occurred in patients taking voxelotor vs placebo in post-marketing clinical trials.
“Pfizer also observed a higher rate of vaso-occlusive crisis in patients with sickle cell disease receiving Oxbryta in two real-world registry studies,” the FDA said. “Based on the totality of clinical data, Pfizer has determined the benefit of Oxbryta does not outweigh the risk.”
Gene Therapy, Tried-and-True Hydroxyurea (HU)
As a field, SCD has drawn more interest in recent years, with significant gains made lately in cutting-edge projects.
The FDA in December approved two gene-editing treatments for patients aged 12 years or older. These are considered “milestone treatments” for a debilitating and potentially life-threatening blood disorder that affects about 100,000 people in the United States. Exagamglogene autotemcel (Casgevy, Vertex Pharmaceuticals and CRISPR Therapeutics) is the first to use the gene-editing tool CRISPR. And lovotibeglogene autotemcel (Lyfgenia, bluebird bio) uses a different gene-editing tool called a lentiviral vector.
These advances have been covered widely by the news media but are not expected to be widely available, with the cost of these extensive treatments estimated around $2-$3 million per patient.
“Gene therapy is amazing in that it can offer a cure, but it’s very expensive and not all patients are suitable for it. Some have so much existing organ damage that it’s not an option for them,” said John Wood, MD, PhD, director of cardiovascular MRI at Children’s Hospital Los Angeles, Los Angeles, who does research on SCD.
“So it really is a great treatment for a very few people,” he said in an interview.
The mainstay of treatment for SCD remains a drug that Lydia Pecker, MD, a pediatric hematologist at Johns Hopkins University in Baltimore, describes as the “first, oldest, and best”: HU.
The FDA approved this in 1998 for use in SCD. It reduces the frequency of painful crises and acute chest syndrome and other complications of SCD that otherwise could be serious or even lethal, Pecker said.
“Older doctors can tell you that what they experienced with sickle cell disease in the hospitals has been completely transformed because of the high uptake of the drug,” she said, adding that it made a “profound” change. “We just don’t have data for any other agent that’s quite like that.”
Voxelotor had been a good second drug to add for some patients, in addition to HU and blood transfusions, Dr. Pecker noted. It was a first-line drug for those for whom transfusion and HU were not options, which constitutes a relatively small number of patients, she said.
“So we have, in the last 5 years, felt more hopeful because we had something else to offer,” she said.
Alexis A. Thompson, MD, MPH, chief of the Division of Hematology at Children’s Hospital of Philadelphia in Pennsylvania, said in an interview that her organization also had patients who appeared to benefit from voxelotor, some of whom had been participants in clinical trials.
Dr. Thompson, who has been a top researcher involved in the study of gene therapy, urged the need for companies to keep seeking to expand the options for people with SCD, even after the setback with voxelotor.
“I hope that there’s an appreciation for the need for continued investment in this very serious condition, for which there are insufficient options for treatments,” Dr. Thompson said. “So ongoing investment is really needed if we expect to make progress.”
Dr. Pecker disclosed ties with Novartis, Afimmune, the American Society of Hematology, and the National Institutes of Health. Thompson reported relationships with bluebird bio, Beam, Editas, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Popular Weight Loss Drugs Now for Patients With Cancer?
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Prominent NIH Neuroscientist Fired Over Alleged Research Misconduct
, the NIH said in a statement.
The misconduct involved “falsification and/or fabrication involving reuse and relabel of figure panels representing different experimental results in two publications,” the NIH said.
The agency said it will notify the two journals of its findings so that appropriate action can be taken.
The NIH reportedly launched its probe into potential research misconduct in May 2023 after it received allegations from the Health and Human Service (HHS) Office of Research Integrity (ORI) that month.
The investigation phase began in December 2023 and concluded on September 15, 2024. The institute subsequently notified HHS ORI of its findings.
Dr. Masliah joined the NIH in the summer of 2016 as director of the Division of Neuroscience at the NIA and an NIH intramural researcher investigating synaptic damage in neurodegenerative disorders, publishing “numerous” papers, the NIH said.
Given the findings of their investigation, the NIH said, Dr. Masliah is no longer serving as director of NIA’s Division of Neuroscience.
NIA deputy director Amy Kelley, MD, is now acting director of NIA’s neuroscience division.
Consistent with NIH policies and procedures, any allegations involving Dr. Masliah’s NIH-supported extramural research prior to joining NIH would be referred to HHS ORI, the NIH said.
The NIH announcement came on the same day that Science magazine published an investigative piece suggesting that Dr. Masliah may have fabricated or falsified images or other information in far more than the two studies NIH cited.
According to the article, “scores” of Dr. Masliah’s lab studies conducted at the NIA and the University of California San Diego are “riddled with apparently falsified Western blots — images used to show the presence of proteins — and micrographs of brain tissue. Numerous images seem to have been inappropriately reused within and across papers, sometimes published years apart in different journals, describing divergent experimental conditions.”
The article noted that a neuroscientist and forensic analysts who had previously worked with Science magazine produced a “300-page dossier revealing a steady stream of suspect images between 1997 and 2023 in 132 of his published research papers.”
They concluded that this “pattern of anomalous data raises a credible concern for research misconduct and calls into question a remarkably large body of scientific work,” the Science article stated.
A version of this article appeared on Medscape.com.
, the NIH said in a statement.
The misconduct involved “falsification and/or fabrication involving reuse and relabel of figure panels representing different experimental results in two publications,” the NIH said.
The agency said it will notify the two journals of its findings so that appropriate action can be taken.
The NIH reportedly launched its probe into potential research misconduct in May 2023 after it received allegations from the Health and Human Service (HHS) Office of Research Integrity (ORI) that month.
The investigation phase began in December 2023 and concluded on September 15, 2024. The institute subsequently notified HHS ORI of its findings.
Dr. Masliah joined the NIH in the summer of 2016 as director of the Division of Neuroscience at the NIA and an NIH intramural researcher investigating synaptic damage in neurodegenerative disorders, publishing “numerous” papers, the NIH said.
Given the findings of their investigation, the NIH said, Dr. Masliah is no longer serving as director of NIA’s Division of Neuroscience.
NIA deputy director Amy Kelley, MD, is now acting director of NIA’s neuroscience division.
Consistent with NIH policies and procedures, any allegations involving Dr. Masliah’s NIH-supported extramural research prior to joining NIH would be referred to HHS ORI, the NIH said.
The NIH announcement came on the same day that Science magazine published an investigative piece suggesting that Dr. Masliah may have fabricated or falsified images or other information in far more than the two studies NIH cited.
According to the article, “scores” of Dr. Masliah’s lab studies conducted at the NIA and the University of California San Diego are “riddled with apparently falsified Western blots — images used to show the presence of proteins — and micrographs of brain tissue. Numerous images seem to have been inappropriately reused within and across papers, sometimes published years apart in different journals, describing divergent experimental conditions.”
The article noted that a neuroscientist and forensic analysts who had previously worked with Science magazine produced a “300-page dossier revealing a steady stream of suspect images between 1997 and 2023 in 132 of his published research papers.”
They concluded that this “pattern of anomalous data raises a credible concern for research misconduct and calls into question a remarkably large body of scientific work,” the Science article stated.
A version of this article appeared on Medscape.com.
, the NIH said in a statement.
The misconduct involved “falsification and/or fabrication involving reuse and relabel of figure panels representing different experimental results in two publications,” the NIH said.
The agency said it will notify the two journals of its findings so that appropriate action can be taken.
The NIH reportedly launched its probe into potential research misconduct in May 2023 after it received allegations from the Health and Human Service (HHS) Office of Research Integrity (ORI) that month.
The investigation phase began in December 2023 and concluded on September 15, 2024. The institute subsequently notified HHS ORI of its findings.
Dr. Masliah joined the NIH in the summer of 2016 as director of the Division of Neuroscience at the NIA and an NIH intramural researcher investigating synaptic damage in neurodegenerative disorders, publishing “numerous” papers, the NIH said.
Given the findings of their investigation, the NIH said, Dr. Masliah is no longer serving as director of NIA’s Division of Neuroscience.
NIA deputy director Amy Kelley, MD, is now acting director of NIA’s neuroscience division.
Consistent with NIH policies and procedures, any allegations involving Dr. Masliah’s NIH-supported extramural research prior to joining NIH would be referred to HHS ORI, the NIH said.
The NIH announcement came on the same day that Science magazine published an investigative piece suggesting that Dr. Masliah may have fabricated or falsified images or other information in far more than the two studies NIH cited.
According to the article, “scores” of Dr. Masliah’s lab studies conducted at the NIA and the University of California San Diego are “riddled with apparently falsified Western blots — images used to show the presence of proteins — and micrographs of brain tissue. Numerous images seem to have been inappropriately reused within and across papers, sometimes published years apart in different journals, describing divergent experimental conditions.”
The article noted that a neuroscientist and forensic analysts who had previously worked with Science magazine produced a “300-page dossier revealing a steady stream of suspect images between 1997 and 2023 in 132 of his published research papers.”
They concluded that this “pattern of anomalous data raises a credible concern for research misconduct and calls into question a remarkably large body of scientific work,” the Science article stated.
A version of this article appeared on Medscape.com.
Editor's Note: 2024 Rare Neurological Disease Report
EDITOR’S NOTE
This year, we again focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust. Let’s hope the work of many dedicated researchers adds to the list of rare neurological diseases for which treatment is available.
This year also marks a change of leadership at NORD, our publishing partner in this annual supplement. We here at Neurology Reviews salute the leadership and accomplishments of former NORD CEO Peter Saltonstall and also welcome incoming CEO Pamela Gavin, who has spent many years in NORD leadership roles and was essential in the planning, launch, and early years of this annual supplement. I can think of no one better than Pamela Gavin to continue NORD’s mission into the future.
And finally, a recap of accolades for this annual supplement. For the second year in a row, the Rare Neurological Disease Special Report has won an Azbee award in the category of annual supplement from the American Society of Business Publication Editors. The 2023 issue won a National Gold Award and a Regional Gold Award.
—Glenn Williams, VP, Group Editor, Neurology Reviews and MDedge Neurology
A NOTE FROM NORD
Hello, and Welcome! The National Organization for Rare Disorders (NORD) is pleased to partner with Neurology Reviews to bring you the 2024 edition of the Rare Neurological Disease Report. Through this collaboration, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
As healthcare providers, you play a key role in catalyzing advancements and bringing new hope and possibilities to the rare disease community. Your efforts can contribute to shortening the diagnostic odyssey and improving day-to-day care for people living with rare disorders in crucial ways:
Identifying patients: Healthcare providers can recognize the possible signs of a rare disease and initiate further investigation or referral to specialists. Early detection is key as it can lead to a quicker, more accurate diagnosis, better management, and improved outcomes.
Educating other physicians: Many rare diseases are not well-known or understood by the general medical community. Healthcare providers can help bridge this knowledge gap by educating other physicians about rare conditions. They can raise awareness through clinical teaching, seminars, medical literature, or continuing medical education (CME) sessions focused on rare diseases. Raising awareness and providing up-to-date information about rare diseases bolsters diagnostic and treatment capabilities within the medical field.
Providing information to patients: Once a rare disease is identified, healthcare providers can offer valuable support to patients and their families. They can provide potential treatments and management strategies. They can also connect patients with support groups, support programs, educational resources, and specialists with expertise in specific rare conditions. Clear communication and guidance on support resources can positively impact patients’ well-being, empower them to make informed decisions, and help them navigate a complex rare condition.
This issue of the Rare Disease Neurological Special Report features articles by rare disease medical experts on specific diseases with updates on clinical management. Topics include the diagnosis and management of Duchenne muscular dystrophy, the promise of disease-modifying therapies for Huntington’s disease, patient choices and cultural changes around myasthenia gravis, advances in neuromyelitis optica, and untangling chronic inflammatory demyelinating polyneuropathy. In addition, two online-only articles offer timely insights from key opinion leaders on the pros and cons of genetic testing and what clinicians need to know about newborn screening.
You will also find information about the NORD Rare Diseases and Orphan Products Breakthrough Summit. This annual event convenes thought leaders from patient advocacy organizations, industry, academia, medical and research institutions, and government to discuss critical topics facing the rare disease community.
NORD is deeply appreciative of healthcare professionals like you, who despite long hours and demanding workloads, remain committed to staying up to date on the latest medical advances for the benefit of their patients. Your dedication and hard work make a significant difference to the patients and families we serve, and your commitment does not go unnoticed. Thank you for all that you do.
—Pamela Gavin, NORD Chief Executive Officer
EDITOR’S NOTE
This year, we again focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust. Let’s hope the work of many dedicated researchers adds to the list of rare neurological diseases for which treatment is available.
This year also marks a change of leadership at NORD, our publishing partner in this annual supplement. We here at Neurology Reviews salute the leadership and accomplishments of former NORD CEO Peter Saltonstall and also welcome incoming CEO Pamela Gavin, who has spent many years in NORD leadership roles and was essential in the planning, launch, and early years of this annual supplement. I can think of no one better than Pamela Gavin to continue NORD’s mission into the future.
And finally, a recap of accolades for this annual supplement. For the second year in a row, the Rare Neurological Disease Special Report has won an Azbee award in the category of annual supplement from the American Society of Business Publication Editors. The 2023 issue won a National Gold Award and a Regional Gold Award.
—Glenn Williams, VP, Group Editor, Neurology Reviews and MDedge Neurology
A NOTE FROM NORD
Hello, and Welcome! The National Organization for Rare Disorders (NORD) is pleased to partner with Neurology Reviews to bring you the 2024 edition of the Rare Neurological Disease Report. Through this collaboration, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
As healthcare providers, you play a key role in catalyzing advancements and bringing new hope and possibilities to the rare disease community. Your efforts can contribute to shortening the diagnostic odyssey and improving day-to-day care for people living with rare disorders in crucial ways:
Identifying patients: Healthcare providers can recognize the possible signs of a rare disease and initiate further investigation or referral to specialists. Early detection is key as it can lead to a quicker, more accurate diagnosis, better management, and improved outcomes.
Educating other physicians: Many rare diseases are not well-known or understood by the general medical community. Healthcare providers can help bridge this knowledge gap by educating other physicians about rare conditions. They can raise awareness through clinical teaching, seminars, medical literature, or continuing medical education (CME) sessions focused on rare diseases. Raising awareness and providing up-to-date information about rare diseases bolsters diagnostic and treatment capabilities within the medical field.
Providing information to patients: Once a rare disease is identified, healthcare providers can offer valuable support to patients and their families. They can provide potential treatments and management strategies. They can also connect patients with support groups, support programs, educational resources, and specialists with expertise in specific rare conditions. Clear communication and guidance on support resources can positively impact patients’ well-being, empower them to make informed decisions, and help them navigate a complex rare condition.
This issue of the Rare Disease Neurological Special Report features articles by rare disease medical experts on specific diseases with updates on clinical management. Topics include the diagnosis and management of Duchenne muscular dystrophy, the promise of disease-modifying therapies for Huntington’s disease, patient choices and cultural changes around myasthenia gravis, advances in neuromyelitis optica, and untangling chronic inflammatory demyelinating polyneuropathy. In addition, two online-only articles offer timely insights from key opinion leaders on the pros and cons of genetic testing and what clinicians need to know about newborn screening.
You will also find information about the NORD Rare Diseases and Orphan Products Breakthrough Summit. This annual event convenes thought leaders from patient advocacy organizations, industry, academia, medical and research institutions, and government to discuss critical topics facing the rare disease community.
NORD is deeply appreciative of healthcare professionals like you, who despite long hours and demanding workloads, remain committed to staying up to date on the latest medical advances for the benefit of their patients. Your dedication and hard work make a significant difference to the patients and families we serve, and your commitment does not go unnoticed. Thank you for all that you do.
—Pamela Gavin, NORD Chief Executive Officer
EDITOR’S NOTE
This year, we again focus on rare neurological diseases that have new therapies that have been recently approved as well as conditions for which the treatment pipeline is robust. Let’s hope the work of many dedicated researchers adds to the list of rare neurological diseases for which treatment is available.
This year also marks a change of leadership at NORD, our publishing partner in this annual supplement. We here at Neurology Reviews salute the leadership and accomplishments of former NORD CEO Peter Saltonstall and also welcome incoming CEO Pamela Gavin, who has spent many years in NORD leadership roles and was essential in the planning, launch, and early years of this annual supplement. I can think of no one better than Pamela Gavin to continue NORD’s mission into the future.
And finally, a recap of accolades for this annual supplement. For the second year in a row, the Rare Neurological Disease Special Report has won an Azbee award in the category of annual supplement from the American Society of Business Publication Editors. The 2023 issue won a National Gold Award and a Regional Gold Award.
—Glenn Williams, VP, Group Editor, Neurology Reviews and MDedge Neurology
A NOTE FROM NORD
Hello, and Welcome! The National Organization for Rare Disorders (NORD) is pleased to partner with Neurology Reviews to bring you the 2024 edition of the Rare Neurological Disease Report. Through this collaboration, we share cutting-edge research and insights from leading medical experts, including specialists from the NORD Rare Disease Centers of Excellence network, about the latest advances in the treatment of rare neurological conditions.
As healthcare providers, you play a key role in catalyzing advancements and bringing new hope and possibilities to the rare disease community. Your efforts can contribute to shortening the diagnostic odyssey and improving day-to-day care for people living with rare disorders in crucial ways:
Identifying patients: Healthcare providers can recognize the possible signs of a rare disease and initiate further investigation or referral to specialists. Early detection is key as it can lead to a quicker, more accurate diagnosis, better management, and improved outcomes.
Educating other physicians: Many rare diseases are not well-known or understood by the general medical community. Healthcare providers can help bridge this knowledge gap by educating other physicians about rare conditions. They can raise awareness through clinical teaching, seminars, medical literature, or continuing medical education (CME) sessions focused on rare diseases. Raising awareness and providing up-to-date information about rare diseases bolsters diagnostic and treatment capabilities within the medical field.
Providing information to patients: Once a rare disease is identified, healthcare providers can offer valuable support to patients and their families. They can provide potential treatments and management strategies. They can also connect patients with support groups, support programs, educational resources, and specialists with expertise in specific rare conditions. Clear communication and guidance on support resources can positively impact patients’ well-being, empower them to make informed decisions, and help them navigate a complex rare condition.
This issue of the Rare Disease Neurological Special Report features articles by rare disease medical experts on specific diseases with updates on clinical management. Topics include the diagnosis and management of Duchenne muscular dystrophy, the promise of disease-modifying therapies for Huntington’s disease, patient choices and cultural changes around myasthenia gravis, advances in neuromyelitis optica, and untangling chronic inflammatory demyelinating polyneuropathy. In addition, two online-only articles offer timely insights from key opinion leaders on the pros and cons of genetic testing and what clinicians need to know about newborn screening.
You will also find information about the NORD Rare Diseases and Orphan Products Breakthrough Summit. This annual event convenes thought leaders from patient advocacy organizations, industry, academia, medical and research institutions, and government to discuss critical topics facing the rare disease community.
NORD is deeply appreciative of healthcare professionals like you, who despite long hours and demanding workloads, remain committed to staying up to date on the latest medical advances for the benefit of their patients. Your dedication and hard work make a significant difference to the patients and families we serve, and your commitment does not go unnoticed. Thank you for all that you do.
—Pamela Gavin, NORD Chief Executive Officer
FDA Panel Votes for Limits on Gastric, Esophageal Cancer Immunotherapy
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
Balancing Act: Weighing the Pros and Cons of Genetic Testing in Rare Diseases
The overwhelming majority of rare diseases have a genetic origin, with estimates varying from 71.9% to 80% of rare diseases. Although a rare disease is defined as a condition that affects fewer than 200,000 people domestically, collectively, rare diseases impact approximately 30 million US residents, with at least one of the more than 7,000 rare genetic disorders. In fact, the population of patients with at least one rare disease mirrors the prevalence of people who have type 2 diabetes, or one in every 10 people. Despite their prevalence, most rare conditions are treated only when symptomatic, as many cases remain either misdiagnosed or undiagnosed. As with most health conditions, it is imperative to have a prompt and accurate diagnosis to improve outcomes and avoid inappropriate or unnecessary treatments that may pose severe side effects to the patient.
To that end, this report weighs the less obvious pros and cons of genetic testing in rare diseases of which neurologists should be aware.
The Path to Accurate Diagnosis Remains Long Despite Increased Genetic Testing
When it comes to identifying the greatest challenge in rare genetic disease testing for the neurology community, experts have different opinions. For Kiley Boone Quintana, MD, assistant professor of pediatrics at the University of New Mexico in Albuquerque, the greatest challenge for neurologists navigating this space lies in becoming comfortable with the unknown.
“Many neurologists think genetic testing will certainly find an answer or that the answers will be black and white — which is not true,” said Dr. Quintana. “Instead of clear answers, we often find variants of unknown significance and genetic changes like a deletion or duplication that can have reduced penetrance, so clinicians have to become comfortable with not always having an answer or not knowing exactly how the answer will impact the person.”
One reason for late diagnosis is the need for more knowledge or familiarity a clinician may have with a certain disease, given its rarity.
Perhaps the nebulous nature of genetic testing for people living with rare diseases unveils another drawback, which centers around what researchers refer to as the “diagnostic odyssey.” While the concept describing the average time to diagnosis as 5 years, the time to diagnosis can vary greatly in the rare disease community. In some cases, patients may experience diagnostic delays of only a few months. For others, the time frame could be a decade or greater. The time frame often depends on the patient’s age, phenotype, and accessibility to resources.
Despite these diagnostic challenges, Debra Regier, MD, PhD, chief, genetics and metabolism, at Children’s National Hospital in Washington, DC, sees the silver lining in identifying the underlying cause of a patient’s symptoms of illness. In some cases, a diagnosis leads a patient to access disease-specific medication. However, in the rare genetic disease space, the occurrence is low, as only approximately 10% of these diagnosed conditions have an available treatment.
Despite the small selection of disease-specific therapies for this patient population, patients may still have options, especially when it comes to palliating symptoms.
“We often look toward disease experts to consider what medications are more likely to be supportive,” Dr. Regier said. “This might mean considering a pain regimen, a seizure regimen, other type of symptomatic treatment, or even using some information learned to support the current patient from cases where other families may have preceded them in the odyssey.”
Whole Exome and Whole Genome Testing Continues Growing in Prevalence, But Neither Offers a Panacea
Historically, genetic testing was expensive, with only a few genes interrogated at a time. However, the past decade has seen prices simmer down with the introduction of next-generation sequencing — a technology that improves both the accuracy and utility of genetic testing.
One form of genetic testing, called whole exome sequencing, has proven especially helpful in recent years because it looks at all 20,000 genes and spelling changes that can cause mutations and genetic diseases. However, whole exome testing comes with its own limitations. It tests at the DNA loci that produce the actual protein blueprints but does not look at the DNA between those spaces. In addition, the medical community lacks a comprehensive understanding of all 20,000 genes, as scientists have yet to understand all their functions.
Unfortunately, the drawbacks do not stop there.
“Whole exome sequencing is not good at detecting conditions such as Huntington’s disease or Fragile X syndrome,” Dr. Quintana said. “It also fails to pick up spelling changes in DNA of noncoding regions, which we are learning do have functions in epigenetics.”
Quality also can limit reliability of both exome and genome testing. According to Dr. Regier, trustworthiness of results depends on several factors, including the lab conducting the test and the analysis performed. To help ensure quality, Dr. Regier and her colleagues use only CLIA-certified labs and labs that follow the American College of Medical Genetics (ACMG) guidelines. Furthermore, they allow only qualified experts to analyze the results, experts who hold board certifications with either the American College of Medical Genetics and Genomics or the American Board of Pathology.
Familial and Societal Stigma Surrounding Rare Diseases Engenders Emotional, Psychological, and Financial Distress
Ultimately, traversing the trajectory of delayed diagnosis and its ambiguity also leaves questions regarding how it will impact the person. All too often, these mysteries transcend the patient with the condition, affecting relatives and other loved ones, as the familial and societal stigma surrounding rare diseases engenders emotional and psychological distress.
In cases with prolonged or delayed diagnostics, Dr. Quintana said that neurologists should advise patients to prepare themselves for the potential of arduous workups — some of which may also come at a high price. Not only does a circuitous path to diagnosis impede treatment initiation, but it often results in major trauma for patients and their caregivers, who encounter significant emotional, psychological, and financial distress in the fallout. Emotional distress of misdiagnosis or lack of a diagnosis remains a significant pain point for patients and their family members alike.
Emotional distress presents the greatest drawback for the rare disease community, according to Dr. Regier. She described the cons of navigating a rare genetic disease diagnosis as “very personal” for families.
“Sometimes, there can be guilt or shame associated with a genetic illness,” Dr. Regier noted. “Understanding the ‘why’ or knowing better how to use nonspecific treatments can be incredibly important to reduce guilt and shame, but it also allows the family to feel like there is a reason and encourages inclusion in the social setting.”
Diagnosis typically results in inclusion in a patient and family group, which increases understanding while easing some of the psychological and emotional stress associated with not knowing the cause.
Establishing Social Support Networks Typically Falls on the Patient and Loved Ones
Another con in rare genetic diseases is the lack of adoption across the community.
Because of the long haul, neurologists and other clinicians should recognize the need for patients to have support. Both Dr. Regier and Dr. Quintana agreed that communal support is a critical component of managing the rare genetic disease population. However, finding one’s tribe is easier said than done. Due to the diagnostic hurdles and low number of people with confirmed diagnoses, patient communities and patient advocacy groups for people with individual rare diseases can be underdeveloped. However, the importance of family-based support groups should not be understated. The low community head counts and high level of time investment for care also contributes to poor recruitment turnouts for clinical trials and, subsequently, the sparse number of therapies for such conditions in the pipeline. However, it is also worth noting that, in the case of rare diseases, insufficient disease state knowledge, antiquated policies, lack of funding, and poor research and development diagnostic infrastructure also amplify such cons.
Patients can form communities of support by finding other families and knowing what to expect in terms of complications. While clinicians may not always have the resources to help the patient establish support systems, they can increase the patients’ awareness and encourage them to search for groups that align with their needs. Dr. Quintana reported that many of her patients find support groups of people with the same rare conditions through social media outlets such as Facebook.
Lack of Widespread Genetic Testing Adoption Remains a Barrier in Rare Diseases
As Dr. Quintana told Neurology Reviews, geneticists are more likely to order exome testing, despite the fact that genome-wide testing is slightly more likely to find a diagnosis. However, she anticipates that genome-wide testing will gain wider adoption in the future.
In terms of cost and feasibility, genetic testing can identify roughly 50% of the underlying etiology of a rare disease, including phenotyping to make a clinical diagnosis and using genetic testing, according to Dr. Regier.
Regarding the broad use of whole genome sequencing, Dr. Regier foresees that the more we learn about all the diagnostic and prognostic information rare disease testing can give us, “the more this number will grow.”
As an example of the true impact, she shared how new research indicates that changes to one’s DNA can lead to intellectual disability.
Dr. Quintana agreed that genetic testing will increase, noting an increase in genetic testing ordered from neonatal intensive care units. However, that uptick comes with the caveat of an ever-evolving landscape as genetic companies continue undergoing mergers, acquisitions, and other structural changes that can complicate service availability, provision, and acceptance.
Even if the clinician orders a comprehensive workup, he or she may still encounter resistance at the hands of insurance companies, which can prolong an accurate and prompt diagnosis while hindering families’ access to a thorough investigation.
“Genetic testing is advantageous for insurance companies as well and can prevent unnecessary lab tests to find an answer,” said Dr. Quintana.
Accessibility and Lack of Geneticists Often a Rate-Limiting Step
The paucity of geneticists also creates another hurdle. “Where I practice in New Mexico and in many other places in this country, there’s a shortage of geneticists,” Dr. Quintana said. “For 3 years, the state had only one geneticist, and that’s a lot of ground to cover.”
Dr. Quintana went on to stress the importance of neurologists and other clinicians conducting outreach in rural areas despite the logistical barriers; oftentimes, families cannot travel to big cities. Despite these geographical challenges, prenatal genetic testing is becoming more accessible for both rural and urban areas. For that reason, some babies are born with a diagnosis, allowing the parents and healthcare providers to take immediate action.
Moreover, risks and uncertainty exist around genetic testing results and access to long-term life insurance and disability insurance coverage. “Obtaining proper consent prior to genetic testing is very important,” said Dr. Quintana.
In many cases, genetic counseling may be beneficial because it offers patients some additional information and resources that help them understand not only the results of their genetic tests but also the consequences of their conditions.
Ultimately, Genetic Testing in Rare Diseases Requires All Stakeholders to Have Patience and Tenacity
Dr. Regier summarized some of the nuances of genetic testing in the rare disease community. “Families understand that you might not be able to make the diagnosis,” Dr. Regier said. “It is more important to them that you stay on the journey with them, even if there is not a diagnosis.”
Another critical element of the diagnostic voyage hinges on clinicians recognizing and honoring that every family — and patient — is different.
“Some families want to do testing while others want to take one thing at a time and start with symptom management,” Dr. Regier said. “Both of these approaches are good, and every family has the right to decide when and if genetic testing should be part of their diagnostic odyssey.”
Suggested Reading
Baynam G et al. Stigma Associated With Genetic Testing for Rare Diseases — Causes and Recommendations. Front Genet. 2024 Apr 4:15:1335768. doi: 10.3389/fgene.2024.1335768.
Marwaha S et al. A Guide for the Diagnosis of Rare and Undiagnosed Disease: Beyond the Exome. Genome Med. 2022 Feb 28;14(1):23. doi: 10.1186/s13073-022-01026-w.
The overwhelming majority of rare diseases have a genetic origin, with estimates varying from 71.9% to 80% of rare diseases. Although a rare disease is defined as a condition that affects fewer than 200,000 people domestically, collectively, rare diseases impact approximately 30 million US residents, with at least one of the more than 7,000 rare genetic disorders. In fact, the population of patients with at least one rare disease mirrors the prevalence of people who have type 2 diabetes, or one in every 10 people. Despite their prevalence, most rare conditions are treated only when symptomatic, as many cases remain either misdiagnosed or undiagnosed. As with most health conditions, it is imperative to have a prompt and accurate diagnosis to improve outcomes and avoid inappropriate or unnecessary treatments that may pose severe side effects to the patient.
To that end, this report weighs the less obvious pros and cons of genetic testing in rare diseases of which neurologists should be aware.
The Path to Accurate Diagnosis Remains Long Despite Increased Genetic Testing
When it comes to identifying the greatest challenge in rare genetic disease testing for the neurology community, experts have different opinions. For Kiley Boone Quintana, MD, assistant professor of pediatrics at the University of New Mexico in Albuquerque, the greatest challenge for neurologists navigating this space lies in becoming comfortable with the unknown.
“Many neurologists think genetic testing will certainly find an answer or that the answers will be black and white — which is not true,” said Dr. Quintana. “Instead of clear answers, we often find variants of unknown significance and genetic changes like a deletion or duplication that can have reduced penetrance, so clinicians have to become comfortable with not always having an answer or not knowing exactly how the answer will impact the person.”
One reason for late diagnosis is the need for more knowledge or familiarity a clinician may have with a certain disease, given its rarity.
Perhaps the nebulous nature of genetic testing for people living with rare diseases unveils another drawback, which centers around what researchers refer to as the “diagnostic odyssey.” While the concept describing the average time to diagnosis as 5 years, the time to diagnosis can vary greatly in the rare disease community. In some cases, patients may experience diagnostic delays of only a few months. For others, the time frame could be a decade or greater. The time frame often depends on the patient’s age, phenotype, and accessibility to resources.
Despite these diagnostic challenges, Debra Regier, MD, PhD, chief, genetics and metabolism, at Children’s National Hospital in Washington, DC, sees the silver lining in identifying the underlying cause of a patient’s symptoms of illness. In some cases, a diagnosis leads a patient to access disease-specific medication. However, in the rare genetic disease space, the occurrence is low, as only approximately 10% of these diagnosed conditions have an available treatment.
Despite the small selection of disease-specific therapies for this patient population, patients may still have options, especially when it comes to palliating symptoms.
“We often look toward disease experts to consider what medications are more likely to be supportive,” Dr. Regier said. “This might mean considering a pain regimen, a seizure regimen, other type of symptomatic treatment, or even using some information learned to support the current patient from cases where other families may have preceded them in the odyssey.”
Whole Exome and Whole Genome Testing Continues Growing in Prevalence, But Neither Offers a Panacea
Historically, genetic testing was expensive, with only a few genes interrogated at a time. However, the past decade has seen prices simmer down with the introduction of next-generation sequencing — a technology that improves both the accuracy and utility of genetic testing.
One form of genetic testing, called whole exome sequencing, has proven especially helpful in recent years because it looks at all 20,000 genes and spelling changes that can cause mutations and genetic diseases. However, whole exome testing comes with its own limitations. It tests at the DNA loci that produce the actual protein blueprints but does not look at the DNA between those spaces. In addition, the medical community lacks a comprehensive understanding of all 20,000 genes, as scientists have yet to understand all their functions.
Unfortunately, the drawbacks do not stop there.
“Whole exome sequencing is not good at detecting conditions such as Huntington’s disease or Fragile X syndrome,” Dr. Quintana said. “It also fails to pick up spelling changes in DNA of noncoding regions, which we are learning do have functions in epigenetics.”
Quality also can limit reliability of both exome and genome testing. According to Dr. Regier, trustworthiness of results depends on several factors, including the lab conducting the test and the analysis performed. To help ensure quality, Dr. Regier and her colleagues use only CLIA-certified labs and labs that follow the American College of Medical Genetics (ACMG) guidelines. Furthermore, they allow only qualified experts to analyze the results, experts who hold board certifications with either the American College of Medical Genetics and Genomics or the American Board of Pathology.
Familial and Societal Stigma Surrounding Rare Diseases Engenders Emotional, Psychological, and Financial Distress
Ultimately, traversing the trajectory of delayed diagnosis and its ambiguity also leaves questions regarding how it will impact the person. All too often, these mysteries transcend the patient with the condition, affecting relatives and other loved ones, as the familial and societal stigma surrounding rare diseases engenders emotional and psychological distress.
In cases with prolonged or delayed diagnostics, Dr. Quintana said that neurologists should advise patients to prepare themselves for the potential of arduous workups — some of which may also come at a high price. Not only does a circuitous path to diagnosis impede treatment initiation, but it often results in major trauma for patients and their caregivers, who encounter significant emotional, psychological, and financial distress in the fallout. Emotional distress of misdiagnosis or lack of a diagnosis remains a significant pain point for patients and their family members alike.
Emotional distress presents the greatest drawback for the rare disease community, according to Dr. Regier. She described the cons of navigating a rare genetic disease diagnosis as “very personal” for families.
“Sometimes, there can be guilt or shame associated with a genetic illness,” Dr. Regier noted. “Understanding the ‘why’ or knowing better how to use nonspecific treatments can be incredibly important to reduce guilt and shame, but it also allows the family to feel like there is a reason and encourages inclusion in the social setting.”
Diagnosis typically results in inclusion in a patient and family group, which increases understanding while easing some of the psychological and emotional stress associated with not knowing the cause.
Establishing Social Support Networks Typically Falls on the Patient and Loved Ones
Another con in rare genetic diseases is the lack of adoption across the community.
Because of the long haul, neurologists and other clinicians should recognize the need for patients to have support. Both Dr. Regier and Dr. Quintana agreed that communal support is a critical component of managing the rare genetic disease population. However, finding one’s tribe is easier said than done. Due to the diagnostic hurdles and low number of people with confirmed diagnoses, patient communities and patient advocacy groups for people with individual rare diseases can be underdeveloped. However, the importance of family-based support groups should not be understated. The low community head counts and high level of time investment for care also contributes to poor recruitment turnouts for clinical trials and, subsequently, the sparse number of therapies for such conditions in the pipeline. However, it is also worth noting that, in the case of rare diseases, insufficient disease state knowledge, antiquated policies, lack of funding, and poor research and development diagnostic infrastructure also amplify such cons.
Patients can form communities of support by finding other families and knowing what to expect in terms of complications. While clinicians may not always have the resources to help the patient establish support systems, they can increase the patients’ awareness and encourage them to search for groups that align with their needs. Dr. Quintana reported that many of her patients find support groups of people with the same rare conditions through social media outlets such as Facebook.
Lack of Widespread Genetic Testing Adoption Remains a Barrier in Rare Diseases
As Dr. Quintana told Neurology Reviews, geneticists are more likely to order exome testing, despite the fact that genome-wide testing is slightly more likely to find a diagnosis. However, she anticipates that genome-wide testing will gain wider adoption in the future.
In terms of cost and feasibility, genetic testing can identify roughly 50% of the underlying etiology of a rare disease, including phenotyping to make a clinical diagnosis and using genetic testing, according to Dr. Regier.
Regarding the broad use of whole genome sequencing, Dr. Regier foresees that the more we learn about all the diagnostic and prognostic information rare disease testing can give us, “the more this number will grow.”
As an example of the true impact, she shared how new research indicates that changes to one’s DNA can lead to intellectual disability.
Dr. Quintana agreed that genetic testing will increase, noting an increase in genetic testing ordered from neonatal intensive care units. However, that uptick comes with the caveat of an ever-evolving landscape as genetic companies continue undergoing mergers, acquisitions, and other structural changes that can complicate service availability, provision, and acceptance.
Even if the clinician orders a comprehensive workup, he or she may still encounter resistance at the hands of insurance companies, which can prolong an accurate and prompt diagnosis while hindering families’ access to a thorough investigation.
“Genetic testing is advantageous for insurance companies as well and can prevent unnecessary lab tests to find an answer,” said Dr. Quintana.
Accessibility and Lack of Geneticists Often a Rate-Limiting Step
The paucity of geneticists also creates another hurdle. “Where I practice in New Mexico and in many other places in this country, there’s a shortage of geneticists,” Dr. Quintana said. “For 3 years, the state had only one geneticist, and that’s a lot of ground to cover.”
Dr. Quintana went on to stress the importance of neurologists and other clinicians conducting outreach in rural areas despite the logistical barriers; oftentimes, families cannot travel to big cities. Despite these geographical challenges, prenatal genetic testing is becoming more accessible for both rural and urban areas. For that reason, some babies are born with a diagnosis, allowing the parents and healthcare providers to take immediate action.
Moreover, risks and uncertainty exist around genetic testing results and access to long-term life insurance and disability insurance coverage. “Obtaining proper consent prior to genetic testing is very important,” said Dr. Quintana.
In many cases, genetic counseling may be beneficial because it offers patients some additional information and resources that help them understand not only the results of their genetic tests but also the consequences of their conditions.
Ultimately, Genetic Testing in Rare Diseases Requires All Stakeholders to Have Patience and Tenacity
Dr. Regier summarized some of the nuances of genetic testing in the rare disease community. “Families understand that you might not be able to make the diagnosis,” Dr. Regier said. “It is more important to them that you stay on the journey with them, even if there is not a diagnosis.”
Another critical element of the diagnostic voyage hinges on clinicians recognizing and honoring that every family — and patient — is different.
“Some families want to do testing while others want to take one thing at a time and start with symptom management,” Dr. Regier said. “Both of these approaches are good, and every family has the right to decide when and if genetic testing should be part of their diagnostic odyssey.”
Suggested Reading
Baynam G et al. Stigma Associated With Genetic Testing for Rare Diseases — Causes and Recommendations. Front Genet. 2024 Apr 4:15:1335768. doi: 10.3389/fgene.2024.1335768.
Marwaha S et al. A Guide for the Diagnosis of Rare and Undiagnosed Disease: Beyond the Exome. Genome Med. 2022 Feb 28;14(1):23. doi: 10.1186/s13073-022-01026-w.
The overwhelming majority of rare diseases have a genetic origin, with estimates varying from 71.9% to 80% of rare diseases. Although a rare disease is defined as a condition that affects fewer than 200,000 people domestically, collectively, rare diseases impact approximately 30 million US residents, with at least one of the more than 7,000 rare genetic disorders. In fact, the population of patients with at least one rare disease mirrors the prevalence of people who have type 2 diabetes, or one in every 10 people. Despite their prevalence, most rare conditions are treated only when symptomatic, as many cases remain either misdiagnosed or undiagnosed. As with most health conditions, it is imperative to have a prompt and accurate diagnosis to improve outcomes and avoid inappropriate or unnecessary treatments that may pose severe side effects to the patient.
To that end, this report weighs the less obvious pros and cons of genetic testing in rare diseases of which neurologists should be aware.
The Path to Accurate Diagnosis Remains Long Despite Increased Genetic Testing
When it comes to identifying the greatest challenge in rare genetic disease testing for the neurology community, experts have different opinions. For Kiley Boone Quintana, MD, assistant professor of pediatrics at the University of New Mexico in Albuquerque, the greatest challenge for neurologists navigating this space lies in becoming comfortable with the unknown.
“Many neurologists think genetic testing will certainly find an answer or that the answers will be black and white — which is not true,” said Dr. Quintana. “Instead of clear answers, we often find variants of unknown significance and genetic changes like a deletion or duplication that can have reduced penetrance, so clinicians have to become comfortable with not always having an answer or not knowing exactly how the answer will impact the person.”
One reason for late diagnosis is the need for more knowledge or familiarity a clinician may have with a certain disease, given its rarity.
Perhaps the nebulous nature of genetic testing for people living with rare diseases unveils another drawback, which centers around what researchers refer to as the “diagnostic odyssey.” While the concept describing the average time to diagnosis as 5 years, the time to diagnosis can vary greatly in the rare disease community. In some cases, patients may experience diagnostic delays of only a few months. For others, the time frame could be a decade or greater. The time frame often depends on the patient’s age, phenotype, and accessibility to resources.
Despite these diagnostic challenges, Debra Regier, MD, PhD, chief, genetics and metabolism, at Children’s National Hospital in Washington, DC, sees the silver lining in identifying the underlying cause of a patient’s symptoms of illness. In some cases, a diagnosis leads a patient to access disease-specific medication. However, in the rare genetic disease space, the occurrence is low, as only approximately 10% of these diagnosed conditions have an available treatment.
Despite the small selection of disease-specific therapies for this patient population, patients may still have options, especially when it comes to palliating symptoms.
“We often look toward disease experts to consider what medications are more likely to be supportive,” Dr. Regier said. “This might mean considering a pain regimen, a seizure regimen, other type of symptomatic treatment, or even using some information learned to support the current patient from cases where other families may have preceded them in the odyssey.”
Whole Exome and Whole Genome Testing Continues Growing in Prevalence, But Neither Offers a Panacea
Historically, genetic testing was expensive, with only a few genes interrogated at a time. However, the past decade has seen prices simmer down with the introduction of next-generation sequencing — a technology that improves both the accuracy and utility of genetic testing.
One form of genetic testing, called whole exome sequencing, has proven especially helpful in recent years because it looks at all 20,000 genes and spelling changes that can cause mutations and genetic diseases. However, whole exome testing comes with its own limitations. It tests at the DNA loci that produce the actual protein blueprints but does not look at the DNA between those spaces. In addition, the medical community lacks a comprehensive understanding of all 20,000 genes, as scientists have yet to understand all their functions.
Unfortunately, the drawbacks do not stop there.
“Whole exome sequencing is not good at detecting conditions such as Huntington’s disease or Fragile X syndrome,” Dr. Quintana said. “It also fails to pick up spelling changes in DNA of noncoding regions, which we are learning do have functions in epigenetics.”
Quality also can limit reliability of both exome and genome testing. According to Dr. Regier, trustworthiness of results depends on several factors, including the lab conducting the test and the analysis performed. To help ensure quality, Dr. Regier and her colleagues use only CLIA-certified labs and labs that follow the American College of Medical Genetics (ACMG) guidelines. Furthermore, they allow only qualified experts to analyze the results, experts who hold board certifications with either the American College of Medical Genetics and Genomics or the American Board of Pathology.
Familial and Societal Stigma Surrounding Rare Diseases Engenders Emotional, Psychological, and Financial Distress
Ultimately, traversing the trajectory of delayed diagnosis and its ambiguity also leaves questions regarding how it will impact the person. All too often, these mysteries transcend the patient with the condition, affecting relatives and other loved ones, as the familial and societal stigma surrounding rare diseases engenders emotional and psychological distress.
In cases with prolonged or delayed diagnostics, Dr. Quintana said that neurologists should advise patients to prepare themselves for the potential of arduous workups — some of which may also come at a high price. Not only does a circuitous path to diagnosis impede treatment initiation, but it often results in major trauma for patients and their caregivers, who encounter significant emotional, psychological, and financial distress in the fallout. Emotional distress of misdiagnosis or lack of a diagnosis remains a significant pain point for patients and their family members alike.
Emotional distress presents the greatest drawback for the rare disease community, according to Dr. Regier. She described the cons of navigating a rare genetic disease diagnosis as “very personal” for families.
“Sometimes, there can be guilt or shame associated with a genetic illness,” Dr. Regier noted. “Understanding the ‘why’ or knowing better how to use nonspecific treatments can be incredibly important to reduce guilt and shame, but it also allows the family to feel like there is a reason and encourages inclusion in the social setting.”
Diagnosis typically results in inclusion in a patient and family group, which increases understanding while easing some of the psychological and emotional stress associated with not knowing the cause.
Establishing Social Support Networks Typically Falls on the Patient and Loved Ones
Another con in rare genetic diseases is the lack of adoption across the community.
Because of the long haul, neurologists and other clinicians should recognize the need for patients to have support. Both Dr. Regier and Dr. Quintana agreed that communal support is a critical component of managing the rare genetic disease population. However, finding one’s tribe is easier said than done. Due to the diagnostic hurdles and low number of people with confirmed diagnoses, patient communities and patient advocacy groups for people with individual rare diseases can be underdeveloped. However, the importance of family-based support groups should not be understated. The low community head counts and high level of time investment for care also contributes to poor recruitment turnouts for clinical trials and, subsequently, the sparse number of therapies for such conditions in the pipeline. However, it is also worth noting that, in the case of rare diseases, insufficient disease state knowledge, antiquated policies, lack of funding, and poor research and development diagnostic infrastructure also amplify such cons.
Patients can form communities of support by finding other families and knowing what to expect in terms of complications. While clinicians may not always have the resources to help the patient establish support systems, they can increase the patients’ awareness and encourage them to search for groups that align with their needs. Dr. Quintana reported that many of her patients find support groups of people with the same rare conditions through social media outlets such as Facebook.
Lack of Widespread Genetic Testing Adoption Remains a Barrier in Rare Diseases
As Dr. Quintana told Neurology Reviews, geneticists are more likely to order exome testing, despite the fact that genome-wide testing is slightly more likely to find a diagnosis. However, she anticipates that genome-wide testing will gain wider adoption in the future.
In terms of cost and feasibility, genetic testing can identify roughly 50% of the underlying etiology of a rare disease, including phenotyping to make a clinical diagnosis and using genetic testing, according to Dr. Regier.
Regarding the broad use of whole genome sequencing, Dr. Regier foresees that the more we learn about all the diagnostic and prognostic information rare disease testing can give us, “the more this number will grow.”
As an example of the true impact, she shared how new research indicates that changes to one’s DNA can lead to intellectual disability.
Dr. Quintana agreed that genetic testing will increase, noting an increase in genetic testing ordered from neonatal intensive care units. However, that uptick comes with the caveat of an ever-evolving landscape as genetic companies continue undergoing mergers, acquisitions, and other structural changes that can complicate service availability, provision, and acceptance.
Even if the clinician orders a comprehensive workup, he or she may still encounter resistance at the hands of insurance companies, which can prolong an accurate and prompt diagnosis while hindering families’ access to a thorough investigation.
“Genetic testing is advantageous for insurance companies as well and can prevent unnecessary lab tests to find an answer,” said Dr. Quintana.
Accessibility and Lack of Geneticists Often a Rate-Limiting Step
The paucity of geneticists also creates another hurdle. “Where I practice in New Mexico and in many other places in this country, there’s a shortage of geneticists,” Dr. Quintana said. “For 3 years, the state had only one geneticist, and that’s a lot of ground to cover.”
Dr. Quintana went on to stress the importance of neurologists and other clinicians conducting outreach in rural areas despite the logistical barriers; oftentimes, families cannot travel to big cities. Despite these geographical challenges, prenatal genetic testing is becoming more accessible for both rural and urban areas. For that reason, some babies are born with a diagnosis, allowing the parents and healthcare providers to take immediate action.
Moreover, risks and uncertainty exist around genetic testing results and access to long-term life insurance and disability insurance coverage. “Obtaining proper consent prior to genetic testing is very important,” said Dr. Quintana.
In many cases, genetic counseling may be beneficial because it offers patients some additional information and resources that help them understand not only the results of their genetic tests but also the consequences of their conditions.
Ultimately, Genetic Testing in Rare Diseases Requires All Stakeholders to Have Patience and Tenacity
Dr. Regier summarized some of the nuances of genetic testing in the rare disease community. “Families understand that you might not be able to make the diagnosis,” Dr. Regier said. “It is more important to them that you stay on the journey with them, even if there is not a diagnosis.”
Another critical element of the diagnostic voyage hinges on clinicians recognizing and honoring that every family — and patient — is different.
“Some families want to do testing while others want to take one thing at a time and start with symptom management,” Dr. Regier said. “Both of these approaches are good, and every family has the right to decide when and if genetic testing should be part of their diagnostic odyssey.”
Suggested Reading
Baynam G et al. Stigma Associated With Genetic Testing for Rare Diseases — Causes and Recommendations. Front Genet. 2024 Apr 4:15:1335768. doi: 10.3389/fgene.2024.1335768.
Marwaha S et al. A Guide for the Diagnosis of Rare and Undiagnosed Disease: Beyond the Exome. Genome Med. 2022 Feb 28;14(1):23. doi: 10.1186/s13073-022-01026-w.